INTRODUCTION
The introduction of vaccination into the clinical field at the beginning of the 20th century was a remarkable success story. Vaccinations have efficaciously minimized the spread of diseases and alleviated mortality and morbidity caused by severe infectious diseases. For example, the World Health Organization (WHO) reported that 2–3 million deaths are avoided every year by vaccination against diphtheria, tetanus, pertussis, influenza, and measles (World Health Organization, 2019). However, if global vaccine coverage develops, an additional 1.5 million deaths may be avoided. Indeed, vaccination is an efficient and economical public health strategy for maintaining and improving healthy communities. The introduction of effective global vaccination campaigns in 1979, for instance, allowed the eradication of smallpox, which was one of the most feared diseases at that time (Vincent et al., 2003).
Most vaccines are traditionally produced from a dead, attenuated, or inactivated disease-causing pathogen (Lemoine et al., 2020). Vaccines are administered via various routes, primarily intradermal, subcutaneous, or intramuscular (IM) injection (Hickling et al., 2011). However, there are certain drawbacks faced by these conventional vaccines where the production is time-consuming, and the risk of contaminations is high and undetected (Kurup and Thomas, 2020). Furthermore, safety concerns, philosophical assumptions, religious beliefs, infliction of pain, and fear of needles were the main reasons behind the refusal or delay of parenteral vaccines (McKee and Bohannon, 2016).
Recently, novel vaccine designs, including virus-like particles (VLP), conjugate vaccines, cellular vaccines, and nucleic acid vaccines, have been made and showed higher public compliance (Wallis et al., 2019). They are also safer substitutes to conventional vaccines due to their complete inability to replicate and enable faster production (D’Amico et al., 2021). For example, DNA vaccines are an excellent example of advanced biological products produced at a low cost on a large scale (Ingolotti et al., 2010). Also, various studies with RNA had been performed on oncogenes that are currently in phase I–III clinical trials on specific pathogens like human immune deficiency virus (HIV) (Bobbin et al., 2015), Zika (Richner et al., 2017), and rabies (Alberer et al., 2017). In addition, different biomolecules and synthetic inhibitors against COVID-19 are being tested by worldwide scientists, where nucleic acid-based molecules can be viewed as promising drug candidates (Piyush et al., 2020).
Although new vaccine designs are very successful, they require a vector or carrier to be transported to the target sites. Some examples of these carriers are liposomes, polymeric nanoparticles (NPs), inorganic particles, infectious material like bacteria and viruses, immune-stimulating complex (ISCOM) mucosal delivery systems, and microemulsions (Yang et al., 2016). Due to the benefits of vaccine delivery systems such as self-administration, biocompatibility, stability, and cost-effectiveness, they have become prominent in delivering vaccines efficiently and improving general immunization (Criscuolo et al., 2019). Thus, the current review provides an overview of the emerging new delivery system platforms in vaccine development and their significant roles in managing vaccine formulations and their applications. The review also covers recent research on delivery systems loaded with the COVID-19 vaccines and discusses the current status and prospects in this area.
VESICULAR DELIVERY SYSTEMS (VDSS)
In the last three decades, VDSs have seen exponential development in several clinical areas. Various vesicular devices explored to date could accomplish differing achievements (Kapoor et al., 2019). They play an essential role in simulating biological membranes and transport and targeting active agents. The VDSs are highly organized structures consisting of several concentric bilayers formed in water due to self-assembling amphiphilic building blocks. Due to their improved bioavailability and ability to target the drug to a specific site of action, they are chiefly essential for selective vaccine delivery, thus increasing their safety and efficacy (Jain et al., 2014b, 2014c). The VDSs possess structural flexibility; therefore, they can enclose hydrophilic drugs in the central aqueous space and are adsorbed at the bilayer’s surface.
In contrast, hydrophobic drugs are retained in the lipophilic partitioning of the bilayer. By encapsulating a drug in the vesicular structure, the drug’s half-life can be prolonged in the systemic circulation, and toxicity can be reduced. These carriers are also candidates for the development of sustained-release products for rapidly metabolized drugs. They preserve drug activity at a predetermined rate, relatively constant (zero-order kinetics), and reduce unwanted side effects of drugs. Numerous VDSs had been developed like liposomes, virosomes, iccosomes, ethosomes, niosomes, and bilosomes (Jain et al., 2014b, 2014c).
Liposomes
They are primary vesicular carriers studied for the delivery of drugs. In 1964, the liposome tale started with an electronic microscopic illustration of multilamellar phospholipid vesicles published in the Molecular Biology Journal by Bangham and Horne of the Cambridge Babraham Institute (Siler-Marinkovic, 2016). A liposome is a spherical-shaped vesicle composed of one or more phospholipid bilayers. Liposomes were initially used as models for the biological membranes due to their similarities in lipid composition and shape (Siler-Marinkovic, 2016). Multiple administration routes have then been thoroughly studied for liposomes as potential carriers for various medications. The basic structure of a liposome and its component is shown in Figure 1 (Kalepu et al., 2013).
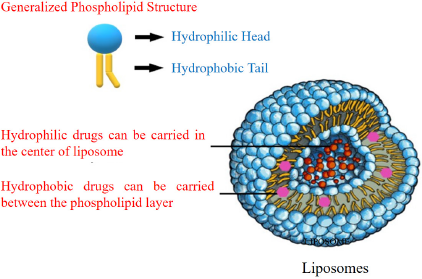 | Figure 1. The structural components of the liposomes presenting the location of hydrophilic/hydrophobic drugs loading into the carrier (Kalepu et al., 2013 ). [Click here to view] |
Liposomal vaccine carriers
As a vaccine carrier, liposomes have inherent adjuvant properties established as early by Allison and Gregoriadis (1974) and Wang et al. (2017). Thus, they have widely been used as vehicles to deliver vaccine antigens. The main advantages of these formulations include antigen stabilization, sustained release of antigens, and promotion of antigen uptake by antigen-presenting cells (APCs). Also, liposomal physicochemical properties are highly adaptable, and their charge, size, and lamellarity can be customized to suit the vaccine requirements (Bernasconi et al., 2016).
Liposomes can elicit cell-mediated and humoral immunity against a broad spectrum of antigens such as ovalbumin (OVA), bacterial polysaccharide, influenza subunit vaccines, and bovine serum albumin (BSA) (Wang et al., 2019). A previous study approved that strong humoral immune responses were observed in mice after injection with liposomes entrapped diphtheria toxoid. No hypersensitivity reactions were reported in the preimmunized animals when the antigen was incorporated in the liposomes and given by intravenous or intrafootpad injection. In addition, the granulomas were formed at the injection site when adjuvants other than liposomes were used (Gregoriadis and Allison, 1974).
Lipid content affects liposome stability; a more stable formulation can lead to a higher volume of antigen that is bioavailable and with depot benefit. One of these researches was reported by Han et al. (1997), who used various combinations of cholesterol, di-palmitoyl phosphatidylserine, di-stearoyl phosphatidylcholine, and di-palmitoyl phosphatidylcholine to prepare the liposomes formulations. Their results showed that liposomes with di-stearoyl phosphatidylcholine were the most stable formula and could better shield the antigen from gastrointestinal tract (GIT) degradation.
Despite the advantages of liposomes, they have shown some limitations as insufficient adjuvant activity, expensive preparation techniques, special storage conditions and handling, and variable purity (Sercombe et al., 2015).
Surface-modified liposomes
The first generation of liposomes based on phospholipid bilayer membranes demonstrated inadequate stability and rapid clearance following their injection. Thus, long-circulating liposomes, also known as stealth liposomes, were developed. The liposome shell is coated with hydrophilic polymers like polyethylene glycol (PEG), resulting in decreased blood protein adsorption and prolonged circulation time (Zylberberg and Matosevic, 2016). It was reported that the dose of the PEG-modified liposomes containing antigen is an essential factor for controlling the response of mucosal and systemic immune systems in an oral vaccine. Unmodified liposomes tend to induce a more robust systemic immune response than the PEGylated liposomes, especially at high concentrations. In contrast, the mucosal immune response was more substantial for the PEG-modified liposomes than the unmodified ones at the lower liposome concentration (Minato et al., 2003).
Interestingly, the liposomes coupled with IgG showed improved transmucosal transport and were even more immunogenic than the plain liposomes when administrated intranasally. Therefore, studies were performed in vivo to determine the potential surface-engineered vesicular carriers for mucosal immunization via the nasal route. For example, the IgG antibodies were immobilized at the liposomes’ surface loaded with hepatitis B antigen (HBsAg). Serum containing anti-HBsAg titer collected from the postnasal administration of IgG-coupled liposomes was slightly elevated than that of plain liposomes. Besides, the IgG-coupled liposomes induced both immune responses and humoral (systemic and mucosal) immune responses, while the alum-adsorbed antigen showed neither mucosal nor cellular response (Tiwari et al., 2011).
Furthermore, the utility of new mucoadhesive liposomes coated with polymer in conjunction with DNA vaccine showed promising results in mice. The prospective ability of liposomes coated with glycol chitosan as a nasal vaccine delivery system to trigger viral-specific humoral mucosal and cellular immune responses was evaluated and compared with plain liposomes. The adjuvanted chitosan vaccine remained homogeneously dispersed in the mucus following the intranasal administration, demonstrating sufficient mucosal immunity activation (Khatri et al., 2008).
Several studies have tested using Ulex europaeus agglutinin I (UEA-I) for vaccination through M-cells targeting. When UEA-I was conjugated onto the surface of liposomes, it demonstrated better intestinal absorption of antigen, resulting in robust mucosal and systemic immune responses. In addition, experiments on bovine BSA that encapsulated UEA-I modified liposomes (UEAI-LIP) provided sufficient data supporting UEAI-LIP as a carrier for oral vaccines compared to the unmodified ones (Li et al., 2011b).
Gene-loaded liposomes
Another emerging area of liposome research involves inserting RNA- and DNA-encoding antigens into APCs to provide a renewable and continuous source of immunization (McEntee et al., 2015). The negatively charged DNA molecules were complexed to the cationic liposomes. Results were promising, yet further studies are required to optimize the liposomes formulations for effective gene delivery. For such tasks, cancer vaccine potency was improved by targeting vaccine antigens to APCs and increasing T cells activation.
Moreover, CD169-expressing splenic macrophages could successfully capture particulate antigens from the blood and deliver them to dendritic cells (DCs), leading to CD8+ T cells activation. In addition, potent immune responses were established when ganglioside M3, a physiological ligand for CD169, was incorporated inside the liposomes because of the enhanced uptake of liposomes by CD169. These results proposed liposomes as a candidate delivery strategy for cancer vaccination (Nijen Twilhaar et al., 2020). In another study, the M1 influenza A virus gene was used to create a cationic liposome/DNA vaccine and an M1 encoding plasmid for oral vaccination, which resulted in the expression of the M1 gene in the intestine of the vaccinated mice and showed potent immune responses (Liu et al., 2014).
Immune-stimulating complexes
ISCOMs are open cage-like spherical self-assembled particles around 40 nm in diameter. ISCOMs formed spontaneously when phospholipids, cholesterol, and immune-stimulating Quil A saponin were mixed under a particular stoichiometry with the vaccine antigen (Pedersen et al., 2012). The antigen-free particle was recognized as the ISCOMs matrix (Nevagi et al., 2018). The Quil A saponin possesses an affinity to cholesterol that causes stabilization in the ISCOM matrix. The technology was vastly evolved where adjuvant-containing vaccines had been tried in clinical trials ever since the primary description of ISCOMs potency as an adjuvant by Hook and Rades (2013).
Following the vaccination with ISCOM adjuvants and antigens, both humoral and cellular immune responses had been documented (Morelli et al., 2012). Therefore, various research works are underway to boost the formulation of ISCOMs-based vaccines and adjuvants to control the specificity, type, and effectiveness of the immune response generated. For example, ISCOMs could significantly increase the Th1 type of response, IL-2, and interferon-gamma production, which had been proven to cause a potent immune response in newborns and used as an important delivery mechanism for mucosal administration (Brito et al., 2013).
Furthermore, the main advantages of ISCOMs are that they improve the quality of immune responses, activate the humoral and cell-mediated immune response, show an acceptable safety profile with only minor local side effects reported upon injection, provide long-lasting immune responses and permit reduction of antigen dose, and possess flexibility in vaccine design due to the simplicity of the preparation method that involves mixing antigen postmanufacturing. ISCOMs are also vital to future adjuvants and vaccines’ success in cancer treatment for veterinary and human use (Nielsen et al., 2015). Diphtheria toxin was introduced into the ISCOMs, which was well tolerated and of excellent efficacy when administered mucosally and parenterally. Although oral and intranasal immunizations could lead to systemic responses, the significant IgA response was induced after the nasal administration rather than the oral (McEntee et al., 2015).
Current trials had demonstrated positive outcomes when ISCOMs were used as adjuvants and given by injection. One of these trials was a phase I clinical trial in adults that showed the influenza vaccine’s loaded ISCOMs effectiveness. Also, chickens were successfully vaccinated by the IM route, resulting in elevated levels of antigen-specific intestinal IgA, CD4, and CD8 positive intestinal intraepithelial T lymphocytes (Nielsen et al., 2015). ISCOMs were also used to deliver the mutant V3 loop peptide of HIV-1, nonameric peptides I, II, and III from foot and mouth disease virus capsid VP1 proteins, among many others (Nevagi et al., 2018). In addition, when ISCOMs-based vaccines were used in horses and other nonhuman primates, active immunity was produced in the presence of maternal antibodies against equine herpes II virus and measles virus, but this was not the case with the traditional vaccines (Osterhaus et al., 1998).
Niosomes
Niosomes were applied as vaccines adjuvants or carriers to assess the immune response elicited by antigen since they possess specific immunological response, decreased toxicity, and better stability than liposomes (Ge et al., 2019). The initial studies of niosomes as a vaccine adjuvant were conducted by Brewer and Alexander (1992). They found that niosomes encompassing bovine BSA produced antibody titers of similar concentrations to that produced by the same amount of BSA emulsified in Freund’s complete adjuvant (FCA). Niosomes were found to be better IgG2a stimulators than FCA upon examining the IgG subclass specific to BSA stimulated by FCA and niosomes. The efficacy of niosomes as vaccine adjuvants was entirely reliant on their ability to encompass the antigen. Besides, BSA not enclosed in niosomes was found to be ineffective.
Vangala et al. (2006) developed a vaccine-loaded vesicular adjuvant system by incorporating dioctadecyl dimethyl ammonium into the niosomal component. The modified niosomes were stable and were suggested for the prevention and treatment of infections. Another promising research suggested the modified polysaccharide O-palmitoyl Mannon (OPM) coated niosomes as a topical vaccine carrier and adjuvant. In addition, the BSA loaded into the mannosylated niosomes was found to possess the ability to elicit humoral and cellular immunity.
Interestingly, niosomes targeted the loaded antigen to the Langerhans cells located below the stratum corneum. For the mannosylated niosomes, the serum IgG levels were significantly higher relative to plain uncoated niosomes (Jain and Vyas, 2005). Another application of mannosylated niosomes is as an oral vaccine carrier and adjuvant. The tetanus toxoid antigen is entrapped within the OPM-coated niosomes. The OPM coat played a significant role in shielding the niosomes from the digestive enzymes present in the GIT and directed the vesicle towards the APCs existing in Peyer’s patches. The OPM-coated niosomes induced higher IgG levels than uncoated niosomes and alum adsorbed tetanus toxoid administered orally. They could also evoke humoral and cellular mediated immunity through their combined IgG2a and IgG1 activity (Jain and Vyas, 2006).
In another work, the soluble parasite antigen, Toxoplasma gondii, was encompassed in the nonionic surfactant vesicles (NISV) and utilized for vaccination in the murine congenital toxoplasmosis model. As assessed by the lowered abortions and parasite burdens in the vaccinated groups, not only did the niosomes-based vaccine elicit a defensive immune response, but the vaccinated groups’ splenocytes also improved the production of antigen-stimulated interferon-γ compared with FCA (Roberts et al., 1994).
Moreover, a study of the immune response triggered by type 1 herpes simplex (HSV-1) antigen incorporated within NISV exhibited significant serum antibody production following the vaccination with adjuvant formulations, indicating that NISV effectively enhanced the body’s immune response to antigens relevant to disease (Hassan et al., 1996).
One more example is that niosomes were evaluated for topical vaccine administration using cholera toxin B as an adjuvant and hepatitis B surface protein as an antigen. The optimal niosomal formulation showed good entrapment efficiency and vesicular size range of 2.83 ± 0.29 μm. In addition, the immune response after three consecutive topical administrations was more successful than the single-dose administration. Thus, cholera toxin B was proved to be a potential adjuvant for topical vaccination when coadministered with the HBsAg encapsulated niosomes, and niosomes are effective vaccine carriers for topical immunization (Maheshwari et al., 2011). Further examples of research on niosomes-based vaccines are displayed in Table 1.
Bilosomes
The bilosomes delivery system is a novel vaccine oral carrier formed by altering lipid-based vesicles (niosomes), resulting in greater stability, targeted delivery, and enhanced absorption through the gut. The niosomal formulation integrates bile salts such as sodium deoxycholate which are known as bilosomes. Incorporating bile salts in the vesicles shields the vaccine molecules from degradation due to bile acids and digestive enzymes present in the GIT, consequently improving the immunological adjuvant property of the antigen encompassed within the vesicles (Rajput and Chauhan, 2017).
In the last few decades, bilosomes potential utilization for oral vaccine delivery has been investigated in several studies (Aburahma, 2016; Thakur and Foged, 2020). It was reported that bilosomes allowed the antigen to be directed to the mucosal tissue without premature release; thus, minor antigens concentrations are required for mucosal immunization with bilosomes (Wilkhu et al., 2013). Also, the vesicular size plays an essential role in constructing efficient vaccine vehicles with targeted site delivery to exert therapeutic action. Through experimental studies, this property is displayed, where Peyer’s patches absorb a vesicle size of 10 µm or less. In comparison, vesicles with a size of less than 5 µm are transported by the lymphoid drainage method to other cells. In influenza vaccine-bilosomal formulations, the more giant vesicles (6 µm in size) exhibited enhanced uptake within Peyer’s patches. In addition, they supported viral cell load reduction better than smaller vesicles (Azuar et al., 2019; Wilkhu et al., 2013).
Various ligands like lectins, microbial adhesions, and immunoglobulins were conjugated to the vehicles to enhance the targeting efficacy of M-cell receptors. Furthermore, recombinant virus-bilosomes complexes were also examined to increase the transmucosal uptake through M-cells targeting. For example, the recombinant baculovirus (Bac-VP1) was complexed with bilosomes for oral vaccination against human enterovirus 71 in mice (Premanand et al., 2013).
Novel surfaced modified bilosomes were developed to improve the stability and entrapment of cholera toxin B subunit complexed to the bilosomes which was favorable to deliver recombinant HBsAg (Shukla et al., 2010). Also, glucomannan bilosomes (GM-bilosomes) were developed to improve stability and mucosal immunization of vaccine-loaded bilosomes. The surface-modified GM bilosomes were found as a promising vehicle and adjuvant for oral vaccination (Jain et al., 2014a).
Also, Jain et al. (2014b, 2014c) compared three types of vesicular systems, bilosomes, GM-bilosomes, and niosomes, for oral immunization efficiency and functionality of vaccine-loaded vesicular-type nanocarrier. For oral immunization of tetanus toxoid in mice, the GM-bilosomes are superior in their immune response compared to the standard bilosomes and the niosomes obtained. The immunological improvement was attributed to the GM’s polymeric nature that increased mannose molecules density at the surface of bilosomes; consequently, the APCs uptake of mannosylated bilosomes was improved. Moreover, the mannosylated bilosomes were found more stable against digestive enzymes.
Ethosomes
Transcutaneous vaccination has several advantages compared to conventional oral or injection immunization such as avoiding hepatic first-pass effect, good compliance, and convenient use. However, the skin’s stratum corneum is the main barrier limiting antigen molecules’ permeation to activate DCs in the epidermis. Thereby lipid colloidal carries like transfersomes and ethosomes were developed to improve the transdermal permeation of molecules (Al Shuwaili et al., 2016).
Ethosomes are flexible, spherical vesicles that deliver drugs into deep tissues and systemic circulation via topical administration. Most ethosomes are composed of ethanol, phospholipids, and water. Ethosomes sometimes contain other excipients, such as surfactants, to better penetrate the membranes and reach the desired target site (Abdulbaqi et al., 2016). The ethosomes also interact with the skin’s lipid components, resulting in high absorption of lipophilic drugs at drug-specific levels (Sinico and Fadda, 2009). They possess several advantages like extending skin contact time, increasing the therapeutic effectiveness of the enclosed drug, enhancing the final dosage form’s consistency and shelf life, and patient adherence. Thereby, novel pharmaceutical formulations such as transdermal patches, gels, and creams were developed by incorporating ethosomes. Besides, ethanol acts as a preservative that enhances ethosomic stability and prolongs its shelf life (Ainbinder et al., 2010).
After two decades of extensive research work, ethosomes exhibited efficient drug entrapment, good stability, and improved transdermal drug delivery, hence making them a potential transdermal delivery system for vaccines. In a previous study, ethosomal-based gels were prepared using different solvents for transdermal antigen delivery. The in vitro stability analysis indicated that the phosphate buffer solvent-containing gel was more stable than the aqueous preparations. Furthermore, a prompt IgG release and appreciated in vivo immunogenic effect were observed with phosphate buffer solvent gel (Zhang et al., 2018). Also, ethosomes for transcutaneous immunization against hepatitis B were investigated. The flow cytometric analysis and spectral bioimaging results revealed that the in vitro uptake of hepatitis B-loaded ethosomes by murine DCs peaked by 180 minutes.
An in vitro permeation study through the dermis of a human cadaver showed an increased transcutaneous delivery of ethosomal antigen compared to traditional liposomes. Moreover, a robust systemic and mucosal humoral immune response was obtained from the topical ethosomes following the IM administration compared to the control, alum-adsorbed antigen suspension (Mishra et al., 2008). Besides, ethosomes transcutaneous permeation enhancement was significantly higher than other vesicular vectors of a peptide vaccine through the tested piglet skin (Rattanapak et al., 2012).
 | Table 1. Examples of niosomes-based vaccines formulations. [Click here to view] |
Structurally modified ethosomes were also tried for vaccines delivery. For example, modified hyaluronic acid/galactosylated chitosan ethosomes (HA-GC-Eth) were prepared by layer-by-layer self-assembly techniques and loaded with OVA antigen. The modified ethosomes were stable and showed promising activity in vitro. Moreover, the novel (OVA@HA-GC-Eth) loaded silk fibroin nanofibrous mats effectively stimulated the antigen’s immune response following the transdermal administration (Yang et al., 2020). Also, the structural modification of the deformable vesicles ethosomes with the cationic lipids octadecylamine (Wang et al., 2007) or stearylamine (Zhang et al., 2017) can evoke immune responses in transcutaneous immunization.
NANOPARTICULATE DELIVERY SYSTEMS
Nanotechnology was first introduced in the 1950s by Feynman, after which successive attempts were made to develop nanomedicine in medical research and treatments. However, nanomedicine was first developed as a modern emerging science only in the early 1990s (Krukemeyer et al., 2015). NPs are a colloidal-based drug delivery system that comprises nanometer range polymeric particles ranging in diameter from 1 to 1,000 nanometers (Van et al., 2019). In general, NPs are classified into two types, nanocapsules, based on their morphology. The nanocapsules are made up of a core in which the drug is usually dissolved and a polymeric layer that regulates the drug release profile of the core. On the other hand, nanospheres were made up of a continuous polymeric network that allows the substance to be stored within or adsorbed onto its surface (Salatin et al., 2017).
The main advantages of NPs are improved drugs bioavailability and therapeutic activity by enhancing aqueous solubility, prolonging of the biological half-life, and drug targeting to a specific body location. One more considerable merit is that NPs have a high binding affinity to small molecules due to their large surface area (Patra et al., 2018). The high drug-NPs loading resulted in decreased drug dose, consequently allowing the safe delivery of therapeutic agents to the target and protecting nontarget tissues and cells from harmful side effects. Because of the high drug-NPs storage, the drug dosage is reduced, allowing for safe therapeutic agent distribution to the desired area of action and shielding cells and tissues that are not on target from adverse effects (Mahapatro and Singh, 2011).
Polymeric NPs
During the last decades, polymeric NPs have attracted great interest because of their property, resulting in smaller particle sizes. Polymer-based NPs (50–300 nm in size) were used as adjuvants and vaccine carriers. The loading of vaccines into NPs showed crucial merits in enhancing their immunogenicity via delivering the vaccine to particular intracellular compartments to activate receptors for immune pathways over an extended period (Diaz-Arévalo and Zeng, 2020) stability in the biological fluids and prolong their circulation time in the blood. A particle’s size, for example, played a critical role in vaccine efficacy. Particles smaller than 5mm produced more immune responses than larger ones. This is because macrophages take up small nanoparticles more readily than microparticles (Sung and Kim, 2020).
Moreover, the NPs-consisting polymers’ characteristics, such as biocompatibility, biodegradability, stability, and toxicity, must be evaluated before their formulation. Several polymers were used for the production of NPs. For example, poly-(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer (Elmowafy et al., 2019) that the United States Food and Drug Administration (FDA) newly approved for parenteral administration (Makadia and Siegel, 2011). Polystyrene synthetic is biodegradable, safe, and easy to functionalize (Loos et al., 2014). Synthetic poly(propylene)sulfide is a biodegradable and oxidation-sensitive homopolymer (Hirosue et al., 2010). Chitosan is a cationic, biodegradable, biocompatible, and not toxic natural biopolymer (Ragelle et al., 2013).
One of the challenging tasks is finding an effective method for antigen loading into the NPs, which is highly affected by both antigen and polymer (Lambricht et al., 2017). Vaccine molecules loaded NPs can be surface conjugated, encapsulated, or surface adsorbed. Surface adsorption of the antigen at the NPs’ surface is mediated by hydrogen bonds, ionic or hydrophobic interaction. For example, dextran sulfate and chitosan polymers were efficiently used to prepare vaccine-loaded NPs. The viral antigen loading into these NPs was mainly by immobilization and hydrogen bond interaction (Pati et al., 2018). The antigen molecules must remain intact during the encapsulation process and be adequately released after administration.
Many techniques have been applied to conjugate/incorporate vaccine molecules into the NPs. However, nanovaccine production and manufacturing are still complicated and require modern machines, making this technique costly compared to the conventional one. Furthermore, nanovaccines administration via topical, inhalation, or ocular routes and incorporating multiple antigens into the same particle to defend against more than one disease were also investigated (Deaguero et al., 2020). The nanosystem configuration directly influences antigens and adjuvants’ release profile from the carrier matrix (Mahapatro and Singh, 2011). Different polymeric nanostructures, varying from particles, nanogels, and micelles to polymerases and core-shell particles, can be synthesized using a wide variety of materials, including natural and synthetic polymers (De Geest, 2020; Moran et al., 2018).
Moreover, many studies strongly confirmed that the setting of physicochemical properties of NPs could be used as an essential tool to target vaccine molecules to specific sites and trigger the desired immune responses. Surface modification of NPs altered the interaction extent with APCs and ligand specificity (Pati et al., 2018). For example, 47 treelike DCs were anchored to the surface of NPs which could modulate the downstream signaling cascades and reduce NPs engulfment by phagocytic cells (Kawai and Akira, 2011). Other studies used toll-like receptors (TLRs) 7, 8, and 9 that stimulate innate immune responses when identifying pathogenic nucleic acids. Authors found that complexing NPs surfaces achieved increased cytokine production and the expression of immunoregulatory genes with the TLRs 7, 8, and 9 agonists (Kawasaki and Kawai, 2014).
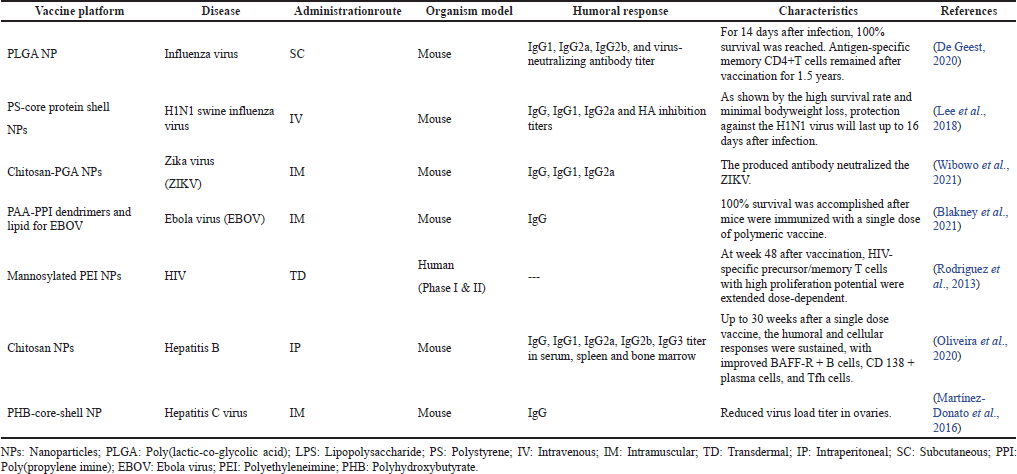
In a comparative study, Pawar et al. (2013) fabricated glycol chitosan-coated PLGA (GC-PLGA) NPs and compared their immunoadjuvant ability compared to chitosan-coated and uncoated PLGA NPs. The results proved that GC-PLGA demonstrated higher systemic and mucosal immune response and lower clearance than chitosan-coated and uncoated NPs. Figure 2 demonstrates loading the drug onto the NP carrier where the antigen is encapsulated inside the particle’s core or conjugated to the surface of the NPs (Pati et al., 2018). Various targeting molecules were conjugated to the surface of NPs, e.g., antibodies. Fab-fragments, peptides, and PEG or poloxamer surface coating. They could enhance particle distribution to APCs and trigger innate and adaptive immune responses (Parhiz et al., 2018). Studies utilizing polymeric NPs for vaccine development against various infective diseases are presented in Table 2.
Inorganic NPs
Recent studies focus on using the inorganic NPs as both carriers and adjuvants in vaccine production and approval preclinical setup. Due to the distinctive features of inorganic NPs such as small particle size, enhanced stability, high loading capacity, and improved permeability, these nanocarriers have occupied a leading place in antigen/vaccine delivery and targeting (Turner et al., 2015). Inorganic NPs had been applied in biomedicines to deliver and target drugs to a specific site in the body. They were also used in cancer therapy and are considered as excellent carriers for tumor vaccines (Khan et al., 2014). Gold NPs (AuNPs), iron oxide NPs (IONPs), quantum NPs, and carbon nanotubes are typical examples of inorganic NPs.
Gold NPs
In 1996, Demenev and his team were the first to apply AuNPs in designing a vaccine against tick-borne encephalitis. AuNPs are of a wide range of particle sizes (from 1 nm to 8 μm) and display various shapes like spherical, triangular, hexagonal, nanocube, nanoshell, and nanorods (Bansal et al., 2020). Owing to their small size, they can cross the cell membrane, interact with the nucleus, and perform their therapeutic action. In addition, AuNPs possess a high degree of stability and biocompatibility. Because of their exciting advantages, AuNPs have been used to prepare antibodies and vaccines against many microorganisms like viruses, bacteria, and parasites. Recently, AuNPs exhibited the best antigen carrier in cancer vaccination (Dykman et al., 2018). However, multifunctionalized AuNPs became progressively more complicated, and a balance must be attained between suitable in vivo stability, tumor localization, limited cytotoxicity, and efficacy (Nicol et al., 2015).
Another point to be considered is that AuNPs are nonbiodegradable; therefore, their pharmacokinetics and elimination fate must be studied and determined. For example, the elimination time of AuNPs from the spleen and liver takes up to 4 months, leading to potential toxicity. Another study reported that AuNPs of 10 nm in size were widely spread in various body compartments where larger particles were detected only in the liver and spleen and blood (De Jong et al., 2008).
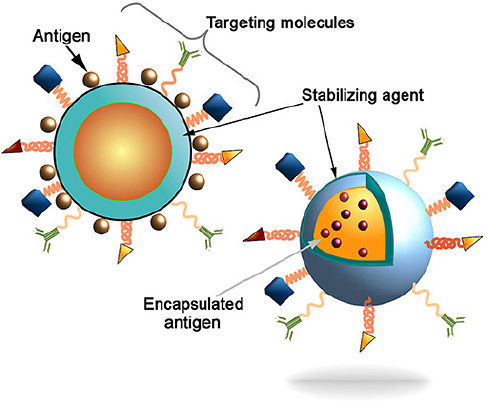 | Figure 2. A schematic representation of the nanocarriers illustrates the antigen conjugation to the surface of the NPs or antigen entrapment into the particle’s core. The targeting molecules, e.g. antibodies, Fab-fragments, peptides, etc., could further improve the delivery of particles into the APCs to induce characteristic and robust immune responses (Pati et al., 2018 ). [Click here to view] |
 | Table 2. Studies utilizing polymeric NPs for vaccine development against various infective diseases. [Click here to view] |
Also, to design biocompatible and biostable AuNPs, it was necessary to include a hydrophilic excipient to modify the monolayer core’s hydrophobic nature. For example, PEG was widely employed to passivate the NPs’ surfaces and prevent nonspecific interaction, especially with biomolecules (Fratoddi, 2017). In a previous study conducted by Zhang et al. (2009), it was observed that the 20 nm PEG5000 anchored NPs exhibited the lowest uptake by the reticuloendothelial system and showed the most extended biological half-life comparing to the large-sized AuNPs. Another novel delivery system encompassing chitosan-functionalized AuNPs was developed to enhance better systemic and mucosal immune responses and was considered as a sound approach for oral vaccine delivery against various pathogens (Barhate et al., 2014).
Iron oxide NPs
Recently, magnetic (Fe3O4 and γ-Fe2O3) IONPs have attracted a lot of attention and become particularly appreciated in medical applications such as thermal therapy, protein immobilization, diagnostic tools, and drug delivery. This is because IONPs possess excellent magnetic properties and a high surface-to-volume ratio for functionalization and are easily prepared. In addition, they are considered safe, bioavailable, and biocompatible (Wu et al., 2015).
Various chemical and physical methods were used to prepare IONPs, like dry processes and wet chemical or microbiological techniques (Hasany et al., 2013). However, the physical techniques suffered from some problems associated with controlling the particles’ size, and they need highly complex and costly machines (Cuenya, 2010).
In 1996, the FDA approved IONPs in the clinical field (Cortajarena et al., 2014). The use of IONPs as a diagnostic tool (e.g., magnetic resonance imaging (MRI) and malignant hyperthermia) garnered interest in the healthcare sector. Their wide application was due to some intrinsic parameters such as the small particle size, saturation magnetization, shape, and chemical composition (Blanco-Andujar et al., 2016). The currently approved product GastroMARK® (AMAG Pharmaceuticals) is an aqueous dispersion of silicone-coated superparamagnetic IONPs (SPIONs) administered orally before the radiological examination (Cortajarena et al., 2014).
Additionally, IONPs showed great promise to serve as both an adjuvant and vaccine delivery platform. It can elicit the immune system as an adjuvant where it induces both humoral and cell-mediated immune responses. Researches had explored the IONPs-immune interactions as vaccines or immune therapies for infectious diseases and cancers (Neto et al., 2018). For instance, Zhao et al. (2018) reported that OVA-loaded iron NPs produced significant immune responses and tumor inhibition compared to the soluble OVA alone. Thus, they suggested the IONPs as a novel vaccine delivery platform and immune inducer. In another work by Pusic et al. (2013), IONPs had been used as a vaccine carrier against malaria. They noticed that vaccination with these NPs induced higher titers of parasite-inhibitory antibodies than immunization with the adjuvant, Montanide.
Fortunately, the surface-modified polymer-coated IONPs were also investigated for antigen delivery. For example, the immunogenic melanoma antigen (hgp10025-33-loaded to dextran-coated SPIONs) displayed an intact homogeneous structure used for functionalization with imaging or drugs or dyes (Kim et al., 2012). In addition, chitosan and its derivatives were the most common polysaccharides for producing surface-coated iron NPs (Kumar et al., 2010). The surface modification of the NPs was proved via the electrostatic interactions and physical adsorption between iron and the free hydroxyl and amino groups in chitosan molecules (Abarca-Cabrera et al., 2021).
A modified version of the nanomagnetic particles was developed and is known as SPIONs with surface functionalization. These NPs were synthesized by different methods to obtain uniform particle size and narrow size distribution (Wallyn et al., 2019). As a result, SPIONs exhibit great promise in clinical and preclinical diagnostic testing/imaging and targeted therapy. Currently, the FDA approved using SPIONs clinically as an imaging agent, demonstrating safety and efficacy (Thakor et al., 2016). Of note, SPIONs had a distinguished application in vaccine delivery and cancer therapy, and many studies investigated the possibility of delivering antigens by these nanocarriers. For example, the malaria DNA vaccine’s transfection efficacy and vector targeting were improved via magnetofection by the SPIONs. In addition, the synthetic SPIONs showed high DNA binding capacity and were considered an excellent gene transfection platform (Balcells et al., 2019).
Still, SPIONs have some issues with aggregation and stability and are currently under development to overcome these drawbacks. Furthermore, SPIONs-based immunotherapy is given by intracranial injection, enabling the possibility of controlled magnetic delivery of activated immune cells to treat deep brain tumors and multifocal diseases (Champagne et al., 2018). Keeping this in mind, although cancer immunotherapy is undergoing rapid progress, there are still many challenges in this area, and further future studies are required.
Carbon nanotubes
Carbon nanotubes are nontoxic and biocompatible NPs (Hasnain and Nayak, 2019). They are relatively inert, nonimmunogenic, and stable on the shelf and in vivo (Scheinberg et al., 2010). Nanotubes have a length of up to millimeters and a diameter ranging from 1 to 100 nm. Two types of carbon nanotubes are present: a) single-walled carbon nanotubes and b) multiwalled carbon nanotubes (Divekar, 2020). The high tensile strength of carbon nanotubes mainly accounts for the high-frequency strong carbon-carbon bond, making them thermostable (Saifuddin et al., 2013).
Studies reported that carbon nanotubes were appreciated as candidate adjuvants and platforms to target specific antigens and facilitate their delivery to the immune system. Hence high binding capacity is one of the carbon nanotubes delivery systems’ critical advantages; therefore, different molecules can conjugate to their surface simultaneously (Scheinberg et al., 2010). Based on this, there are still significant studies that have to be conducted to understand the role of carbon nanotubes in vaccine formulations compared to more conventional vectors.
Dendrimers
Dendrimers are NPs of homogeneous, well-defined, and monodispersed structures with a typically symmetric core and inner and outer shells (Abbasi et al., 2014). One of the most critical aspects that need to be considered for dendrimers’ biomedical application is their pharmacokinetics features (Kaminskas et al., 2011). Several trials were conducted in dendrimers’ structural modification to function as an effective drug delivery system. For example, dendrimers’ peripheral groups’ alteration resulted in establishing different antibody-dendrimer (Szyma?ski et al., 2011) and peptide-dendrimer conjugates (Kojima et al., 2018). Also, dendritic boxes that encapsulate guest molecules were developed (Noriega-Luna et al., 2014). Many dendrimers were synthesized based on their structure modifications, although five families are commonly used (Pedziwiatr-Werbicka et al., 2019).
The variety of their applications in medicine is leading to an increased interest in this field. Currently, dendrimers are used in drug delivery, photodynamic therapy, imaging, and neutron capture therapy (Abbasi et al., 2014). They also have ideal characteristics required for potent adjuvants to enhance vaccines’ effectiveness (Yadav et al., 2018). A dendrimer-based carrier system was developed to deliver peptide-based vaccines and demonstrated its antimicrobial efficacy in the Chlamydia trachomatis mouse model. The dendrimers (G4OH) comprise a peptide mimic of chlamydial glycopeptides antigen and generation 4, hydroxyl-terminated polyamidoamine (PAMAM). The peptide was conjugated through an ester bond. Serum antibodies specific for Chlamydia were produced following immunizations with Pep4-conjugated dendrimers via the subcutaneous route.
Moreover, this vaccine formulation provided substantial protection for immunized animals against vaginal infection with C. trachomatis by decreasing a load of infection and genital tissue damage. Pep4 conjugated to G4OH showed improved safety relative to Pep4 and adjuvant (alum), indicating a powerful adjuvant effect of the PAMAM dendrimer (Ganda et al., 2017). It was reported that the PEG-citrate G2 dendrimer containing several epitopes developed significant cellular immune responses in vivo and a more robust Th1 response compared to Th2, thus contributing to the production of potent HIV vaccines (Abdoli et al., 2017). PAMAM dendrimers are composed of an ethylene diamine nucleus with their methyl acrylate and ethylene diamine branches (Pedziwiatr-Werbicka et al., 2019). Many PAMAM dendrimers have carboxyl groups on their surface, while other generations possess amino groups on their surfaces (Gillani et al., 2020). Lysine-conjugated PAMAM dendrimers demonstrated outstanding activity as a vector vaccine, boosting the immunoreactivity and vaccine effectiveness against Schistosoma japonicum (helminthic parasites) (Wang et al., 2014).
The researchers also designed a vaccine platform based on the dendrimer that embodies the antigen-expressing replicon mRNAs and produces a single-dose protective response toward viruses such as H1N1 and Ebola and other parasites like T. gondii (Chahal et al., 2016). The vaccine consisted of an ionizable G1-PAMAM dendrimer, the lipid-anchored PEG, and the RNA. These experiments highlight the dendrimers’ role in producing new generation vaccines for various infections. It is known that Middle East respiratory syndrome coronavirus (MERS-CoV) and coronavirus disease are developing rapidly. This pandemic’s global transmission poses a significant threat to public health and the global economy (Gurunathan et al., 2020). Polycationic dendrimers with a primary amine and three forms of polyanionic dendrimers with terminal hydroxyl, succinic acid, and sodium carboxylate were used to test their antiviral function by using the Vero cells model. The most potent cytotoxicity was observed with terminal sodium carboxylate, followed by the cationic dendrimers indicating the polyanionic dendrimers’ safety and antiviral activity against the respiratory syndrome coronavirus in the Middle East (Kandeel et al., 2020).
In conclusion, dendrimers nowadays are considered as the typical carriers in biomedical applications such as imaging as MRI contrast agents to diagnose diseases, tissue engineering and delivery, and targeting of drugs, vaccines, and other biological products. However, research is underway to create new dendrimers scaffolds that need to be designed with better physicochemical, mechanical, and biological properties (Noriega-Luna et al., 2014).
MICROPARTICULATE DELIVERY SYSTEMS
One of the most convenient ways of administering the drug is by mouth. However, some drugs have shown limited oral absorption or a short half-life; therefore, repeated doses were required to achieve its therapeutic effect. This problem created pressure on many patients and led to noncompliance. Thus, drug delivery systems as microparticles were introduced to deliver the drugs to a particular site in the body (Lengyel et al., 2019).
Indeed, microparticles can incorporate solid, liquid, or gaseous drugs moieties to form intact entities of particle size ranges between 1 and 1,000 µm. They comprise two main parts, the core and the shell. Usually, the core includes the API Active Pharmaceutical Ingredients (API) and other excipients, while the shell is the coating material surrounding the core. Microparticulate Drug Delivery Systems (MDDSs) were formulated in various systems, including microparticles, microspheres, and microcapsules, which can provide therapeutic activity via different administration routes. The term “microparticles” was used in the literature for the matrix biodegradable microparticles, while the microspheres refer to the shell-enclosed core particles (Lengyel et al., 2019).
Microparticles are currently applied in the biomedical field for many advantages regarding the API, such as reducing adverse effects and dosing frequency, masking the unpleasant taste and odor, modifying drugs release profile, avoiding drug interaction to excipients, improving stability, and increasing bioavailability (Jeevana and Jyosna, 2014).
The microparticles improvement journey as an adjuvant and carrier of vaccines began a long time ago. The incorporation of vaccine antigens into such vectors is due to their exciting advantages. For example, the vaccine antigen’s thermostability was improved by loading it into the microparticles in a dry powder containing sugar as a stabilizing agent (Foged, 2016). Delayed and controlled drug release from the microparticles was achieved using smart biodegradable polymers (Park, 2014). PLGA is one of the preferred biodegradable polymers for preparing microparticles. PLGA polymer was approved by the FDA to formulate microparticles using various techniques, double emulsion solvent evaporation being the most common. The polymeric microparticles’ size and structure can be modified to resemble the pathogens or deliver the vaccine antigens and trigger a humoral and cell-mediated immune response (Silva et al., 2016). This technique paved the way for the preparation of PLGA/polysaccharides or polysaccharides vaccine delivery systems. Mata et al. (2011) loaded the malaria peptides into PLGA/sodium alginate microparticles for intradermal injection, which induced a balanced Th1/Th2 response BALB/c in mice compared to the antigen alone or antigen-loaded PLGA microparticles.
Anionic polymers like sodium alginate were employed to formulate live Mycobacterium-loaded microparticles as aerosol inhalable vaccine formulations. The coated microparticles were more immunogenic than the liquid aerosols in mice against Mycobacterium tuberculosis infection (Nagpal et al., 2019). Cationic polymers, such as chitosan, have also been investigated to formulate microparticles. However, these required another preparation technique known as ionotropic gelation and, when combined with PLGA polymer, chitosan showed an adjuvant effect due to its positive charge (Li et al., 2016). The novel multifunctionalized dextrans microparticles were also examined as a vaccine adjuvant and carrier, resulting in robust immune responses (Bolandparvaz et al., 2020).
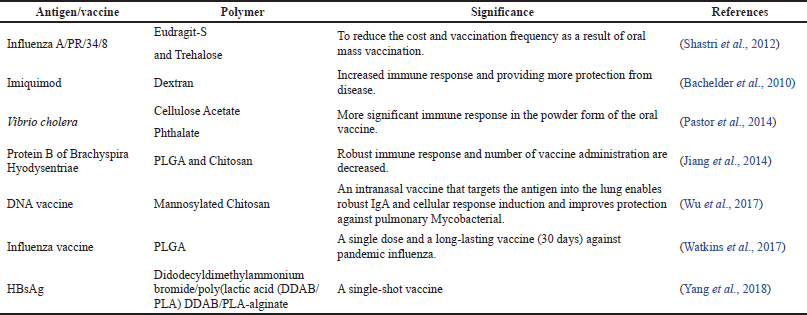
The coloading of antigen and adjuvant into microparticles was a new vaccine development strategy in the last decade. The combining systems showed robust immune responses after single-dose vaccination compared to multidose scheduled immunization with the conventional preparations (Zhang et al., 2015a, 2015b). Finally, stability is a critical issue in antigens encapsulation which should be optimized for each vaccine formulation. Antigen’s stability can be maintained by incorporating stabilizing additives into the core, such as hydrophilic polymers, surface-active agents, sugars, or protein BSA (Han et al., 2016). Eudragit E, poly(L-lysine), and branched polyethyleneimine are cationic additives, enhancing immunological response when coencapsulated with attenuated polio vaccine into PLGA microparticles (Tzeng et al., 2018). Some examples of antigen vaccines incorporated into MDDSs for better immune response are listed in Table 3.
EMULSIONS AND MICROEMULSIONS
Emulsion-based vaccines
Emulsion adjuvants have a long history of clinical application as vaccines adjuvant. In the 1940s, Freund prepared the first adjuvant emulsion containing killed mycobacteria called complete Freund’s adjuvant (CFA). He then developed the incomplete Freund’s adjuvant (IFA), similar to CFA but free of the killed mycobacteria. The oil used in the CFA is nonbiodegradable mineral oil with a mannide monooleate emulsifier. Later studies proved that the IFA was also an active adjuvant and had better tolerability than the CFA (Shah et al., 2015).
Interestingly, besides their role as vaccines adjuvants, emulsions were employed effectively as a vaccine delivery system. The amphiphilic bioresorbable polymer poly(ethylene glycol)-block-poly(lactide-co-epsilon-caprolactone) (PEG-b-PLACL) was developed by Huang et al. (2009) and loaded with OVA as a model antigen. The findings showed that PEG-b-PLACL-emulsified formulations comprised small homogeneous particles and were stable, reproducible, stable, and thus advantageous over the conventional vaccines (Huang et al., 2009). In Italy, squalene oil-in-water (O/W) emulsion for influenza vaccine was approved in 1997, and it was approved in various other countries in 2000. Furthermore, squalene-based emulsion with murabutide as an immune potentiator could enhance the antigen’s uptake by immune cells and evoke a humoral and cell-mediated immune response in mice (Garçon et al., 2012; Kantipakala et al., 2019).
Microemulsion-based vaccines
Microemulsions are low viscosity liquid mixtures consisting of oil, water, and surfactant. They are thermodynamically stable and transparent. Based on the phase, microemulsions are present in two forms: O/W and water-in-oil (W/O) (Mohanty et al., 2019). Due to their unique properties, these systems have become interesting in pharmaceutical product development and design, including vaccines. For example, a novel ethanol-in-fluorocarbon microemulsion for topical genetic immunization was reported by Cui et al. (2003). The pDNA was incorporated into the fluorocarbon-based microemulsion system, and it significantly improved the mouse skin’s luciferase expression compared to pDNA in normal saline or ethanol.
The microemulsion preparations had shown promising candidates for adjuvant systems aimed at intranasal immunization (Lee et al., 2016). Microemulsion adjuvants have been developed to induce a humoral response in mice and have shown effective in vivo protection. Moreover, in a previous study, microemulsion adjuvant had shown to be a better candidate for rabies immunization than other tested adjuvants since it provided strong potency against the virus and did not seem to cause any local reaction (Leclercq et al., 2011).
Also, cationic microemulsions could serve as a novel adjuvant to administer a parenteral vaccine (Lamaisakul et al., 2020). Influenza vaccine was prepared from Al(OH)3 hydrogel with monophosphoryl lipid A, and dioctadecyldimethyl ammonium bromide and mineral oil are examples of potent and safe microemulsion adjuvants (Lamaisakul et al., 2020). The microemulsions and water-in-oil-in-water (W/O/W) multiple emulsions were also investigated as potential adjuvants for the bluetongue vaccine in rabbits. The microemulsion adjuvant displayed a lower titer; however, it was simpler to prepare, stable at 4°C, and did not show any local reactions (Macedo et al., 2013).
VIROSOMES
Virosomes or VLP were prepared first by Almeida et al. (1975). Virosomes are reconstituted viral envelopes that comprise viral spike glycoproteins and membrane lipids but lack the root virus’s genetic material. They can be used as vaccines or adjuvants to deliver nucleic acids, genes, drugs, and macromolecules for therapeutic uses (Asadi and Gholami, 2021; BioPharm International, 2011).
Virosome-based vaccination is successful in regulatory and safety reports and the viability of upgrading development. It has also been approved in more than 40 countries with use in elderly patients and infants. Typically, the virus used to develop virosomes is the influenza virus (Nair et al., 2020).
Besides, studies proved that virosomes-enclosed proteins like influenza virus hemagglutinin (HA) and neuraminidase allow the membrane of virosomes to bind with the immune system cells, where it transports the specific antigen to the targeted site, thus eliciting an immune response with poor immunogenic antigens, and is fully degraded inside the cells after antigens delivery (Hamilton et al., 2012). So virosomal HA greatly enhances cytosolic delivery of vaccines.
 | Table 3. Antigen vaccines incorporated into microparticulate delivery systems for better immune response. [Click here to view] |
Furthermore, the pharmaceutically active compounds are protected from proteolytic degradation and low pH of endosomes until they enter the cytoplasm, which can be considered an essential advantage of virosomes over liposomal delivery systems (Siraj et al., 2019). Virosome adjuvants may also enable APCs to acquire antigens, present them in significant histocompatibility complexes, and allow CD4+ T cells and CD8+ T cells to induce cellular and humoral immune responses (Blom et al., 2017).
On the other hand, the Sendai virus or hemagglutinating virus in Japan has been documented to be used as a virosome in treating tuberculosis (Kaneda et al., 2002). The DNA encoding of the Ag85A antigen is conjugated with the Plasmid Adeno-associated viruses-cytomegalovirus (PAAV-CMV) plasmid. It is then embedded with Sendai virosomes and injected IM into the mice. After the mice immunization, the results obtained showed an elevated Th1 mediated cytokine reaction and activated cytotoxic activity of cytotoxic T lymphocytes and natural killer cells.
Recent research in mice immunized with influenza A virus (H1N1) DNA adsorbed on the surface of cationic influenza virosomes showed complete protection against the homogeneous challenge strain and substantial protection against intrasubtype drift virus challenge (Raska and Turanek, 2015). In the virosomal hepatitis A vaccine, the antigen is bound to virosomes by reacting with phospholipids to be delivered to the normal hepatocyte receptor. The pharmacokinetics, safety profile, and immune responses caused by various hepatitis A virus vaccines, alum-adsorbed, fluid, and virosome vaccines, were recorded in initial experiments, all carrying the same quantity of inactivated hepatitis A virus. Local responses were produced in the virosome-HAV community at a slightly lower rate than alum-adsorbed vaccine recipients (Glück et al., 1992). A faster immune response was triggered with the virosome-HAV vaccine with a higher seroconversion rate on day 14, followed by a higher geometric mean titer. Comparable immune responses were observed within 4 weeks of immunization. Interestingly, the levels of antibodies in the groups immunized with alum-adsorbed or fluid vaccinations had declined dramatically. After 1 year of vaccination with virosomes-HAV, all subjects had anti-HAV levels >20 m IU/ml. Thus, compared to other traditional methods like the aluminum-adsorbed hepatitis A vaccine, the virosome-based vaccines can provide increased safety with lower local adverse events. Single-dose immunization successfully controlled outbreaks of hepatitis A and endemic diseases (Poovorawan et al., 1995).
Virosomes were also used as carriers and adjuvants for vaccine production, cancer prevention, and immunotherapy. For instance, cervical cancer was treated successfully with virosome-formulated cervical cancer vaccines (Liu et al., 2015). In addition, virosome-formulated antimalarial peptides exhibit a strong adaptive immune response and high permissibility in humans (Nair et al., 2020). The AMA-1 merozoite and NPNA region are two peptide regions that can act as antigens for a vaccine against malaria (Rodig, 2020).
CYCLODEXTRIN (CD)-BASED VACCINE DELIVERY SYSTEM
For over 100 years, CDs have been known as excipients of significant importance in the pharmaceutical industry (Agrawal and Gupta, 2012). CDs have received tremendous interest in the pharmaceutical field because of their potential to entrap a wide range of drug molecules totally or at least partially, including branched and unbranched toroidal structures (Abdul Rasool and Salmo, 2012). There are three types of CDs: α-, β-, and γ-CDs, also known as parent CDs or first-generation CDs. Chemically modified CDs are developed from the native ones by functionalizing their hydroxyl groups with various substituents and combinations (Braga et al., 2021).
Recent research reported that CDs could be altered and used as virucidal agents (Braga, 2019). For example, methylated β-CD had reduced influenza A virus and coronavirus infectivity by sequestering or depleting viral cholesterol from the host cell membranes (Pratelli and Colao, 2015). Meanwhile, Jones et al. (2020) developed the idea of CDs modified with mercaptoundecanoic sulfonic acids to provide essential nontoxic virucidal activity. The results were promising since the modified sugar molecules attract viruses before they are irreversibly inactivated.
Moreover, Daiichi Sankyo, a Japanese pharmaceutical company, developed an advanced influenza vaccine that incorporates hydroxypropyl beta-CD (HPβCD) as an adjuvant agent. During vaccine development studies with various animal models, HPβCD was shown to induce a synergic immune response by interacting with immunoglobulins. It also enhanced antibody production by 30% and induced the production of Th2 cells responsible for long-term immune “memory” and the generation of follicular B helper T cells (Thf), which stimulate B cells (responsible for long-term immune “memory”) (Kim et al., 2016; Onishi et al., 2015). Suvaxyn PCV, an inactivated recombinant porcine circovirus type 1 vaccine, one of the viral proteins containing the adjuvants squalene and sulfolipo-CD (SL-β-CD), was developed Suvaxyn PCV®, an inactivated recombinant porcine circovirus type 1 vaccine, one of the viral proteins containing the adjuvants squalene and sulfolipo-CD (SL-β-CD), was developed by Zoetis (European Medicines Agency Suvaxyn PCV Product Information). SL-β-CD was shown to cause a faster onset of immunity and a more incredible immune response than Carbopol® (polyacrylic acid), used in previous vaccine formulations (Braga et al., 2021).
Recently, β-CD and branched polyethyleneimine (CD-PEI) for mRNA vectorization was reported. CD-PEI and mRNA interacted to form nanocomplexes, which were shown to allow mRNA to enter the cell cytoplasm, transfect DCs efficiently, and induce high encoded protein levels expression. The administration of the nanocomplexes was tested in mice through various routes. With an optimized mRNA structure and a very suitable administered route, this CD-PEI-based mRNA vaccine model held promise for future application to specific antigens (Tan et al., 2020). Researchers also reported that, with a clinically isolated pandemic H1N1 influenza virus, the HPβCD-adjuvanted influenza HA split vaccine protected against a lethal challenge in cynomolgus macaques. The findings suggested that HPβCD can be used in various vaccines (Onishi et al., 2015).
VACCINE DELIVERY SYSTEMS IN COVID-19
COVID-19 is a virus found in China in 2019. In March 2020, the WHO described COVID-19 as a pandemic disease, which has created a dramatic loss of human life globally. It is spread by inhalation or ingestion of the droplets of an affected individual (WHO, 2020).
The spike protein present on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus’s surface binds with the ACE 2 receptor. This binding activity helps the virus to enter into the target cell and causes infection. Therefore, antibodies that can inhibit the binding of spike protein to the angiotensin-converting enzyme II (ACE2) are required (Lan et al., 2020 and Wrapp et al., 2020). However, to prevent from getting affected by this disease is only by developing an effective vaccine. Many vaccines have already been marketed (Table 4), but those vaccines were not 100% effective. Hence, researchers are still trying to develop an effective vaccine using different delivery systems.
Implementing VDSs in the medical sector would be very promising, especially in developing new therapeutic approaches to COVID-19 (Itani et al., 2020). Lipid carriers can be used to deliver the COVID vaccines via the IM route of administration (Fanciullino et al., 2021). Also, preclinical data reported systemic distribution of proteins when loaded to the liposomal carriers and administered via IM injection (Li et al., 2011a).
A novel mRNA vaccine candidate based on liposome for SARS-CoV-2, EG-COVID, encodes the complete spike protein (S) of the European variant (D614G), 2P-3Q substitution. By using liposome-based technology, lyophilization of the mRNA vaccine platform can be carried out. Strong humoral and cellular immune responses to SARS-CoV-2 were produced with this vaccine, and the infection of SARS-CoV-2 into Vero cells was also inhibited successfully. A clinical trial is supposed to begin soon (Hong et al., 2021). Thus, liposomes offer excellent potential in healthcare firms’ current clinical trials to treat COVID-19.
On the other hand, the Mymetics corporation, an international biotechnology company, continues to progress its COVID-19 vaccine production plan based on its virosome-vaccine platform. The corporation has received a grant from the Swiss Innovation Agency to begin preclinical studies on Mymetics intranasal virosome-based COVID-19 vaccine candidate for its ability to induce protective respiratory immunity to prevent nasal infection and stop the spread of the virus into the lungs and brain. Since April 2020, the corporation has been striving to design a safe, tolerant, and highly potent virosome-based COVID-19 vaccine administered to individuals of all age groups, especially the immunocompromised and older adults (Bloomberg, 2020). Therefore, virosomes are essential in producing vaccines for intracellular infectious diseases like SARS-CoV-2.
ISCOM is another safe and potent adjuvant called cage-like nanospheres formed when a saponin is mixed with fat. Matrix M is an example of the COVID-19 vaccine designed by the US Biotech company Novavax (Borrell, 2020). The matrix M comprises 40 nm NPs based on saponin extracted from Quillaja saponaria Molina’s bark with phospholipid and cholesterol. It significantly increased the biological functions and elicited potent, robust, and long-lasting immune responses with vaccine dose sparing properties (Novavax addresses urgent global public health needs with innovative technology, 2021). Preclinical studies conducted in baboons and mice and phase I/II human clinical trials with NVX-CoV2373 revealed high anti-S protein IgG titers with a potent neutralizing effect (Tian et al., 2020). The vaccine was also well tolerated among healthy individuals (between 18 and 59 years) (Park et al., 2021).
However, recent studies were carried out to assess the in vitro SARS-CoV-2 antiviral activity of astodrimer sodium, a dendrimer with a broad spectrum of antimicrobial activity. The astodrimer inhibits the replication of SARS-CoV-2 in Vero E6 cells with a 50% reduction in virus-induced cytopathic effect. Thus researchers suggested the potential use of astodrimer sodium as an antiviral agent, either inhaled or nasally administrated for treatment and prevention of SARS-CoV-2 (Paull et al., 2021). When developing a vaccine, the antigen, adjuvant, production method, and distribution strategy are essential considerations.
The genome and structural details of SARS-COV-2 were allocated in record time, allowing for rapid vaccine development (Andersen et al., 2020; Benvenuto et al., 2020; Hwang et al., 2020; Yan et al., 2020; Yuan et al., 2020). Nanotechnology platforms are beneficial in current vaccine design and have accelerated new candidate vaccines from clinical trials. Evolving nanotechnology such as mRNA vaccines provided by the lipid NPs and viral vector vaccines have already passed phase II and phase III trials, in addition to inactivated vaccines (Shin et al., 2020). To produce an effective COVID-19 therapy, one must first consider the virus’s mechanism of action.
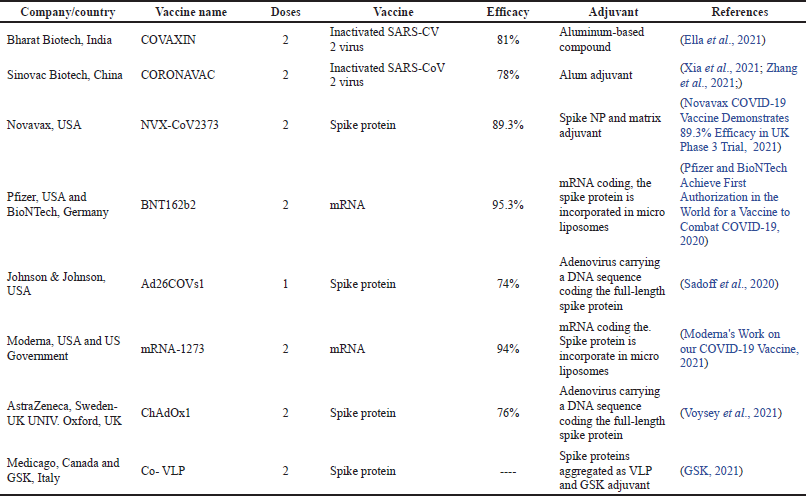 | Table 4. COVID-19 vaccines are currently available in the market. [Click here to view] |
The novel SARS-CoV-2 utilizes a “lock and key” system, similar to SARS and Middle East respiratory syndrome coronavirus (MERS-CoV) coronaviruses, where ACE2 acts as a “key” to target specialized cells that retain its “lock” (Yu et al., 2020). The lung, heart, arteries, kidneys, and intestine cells all contain these target areas. Once released, the virus can multiply and kill other cells using the organelles of the host cell.
Consequently, a therapy that’s top the virus from entering the cell may be helpful (Itani et al., 2020). The shape, size, and surface charge of the engineered nanocarriers are essential factors that should be considered when optimizing for intranasal delivery and thus play a crucial role in the procedure’s effectiveness. Several experiments were recently analyzed to determine the optimum characteristics of theranostic NPs for pulmonary and intranasal administration (Marasini and Kaminskas, 2019). According to the results, an optimal lung delivery mechanism is smaller than 100–200 nm for better immune responses. The slight positive charges are to increase cell association. It is synthesized with a mixture of NP-loaded and surface-conjugates therapeutic moieties and is hydrophobic (Itani et al., 2020).
The inorganic NPs prominent role is targeted toward their potential in diagnostics, therapeutics, and vaccination. The most commonly employed inorganic NPs used are AuNPs. AuNPs are used due to their exceptional characteristic that might be useful in COVID-19 since AuNPs can act as carriers of antigen for SARS-CoV spike S protein and adjuvant. It was found that AuNPs when binding with the S protein through a powerful electrostatic force and high surface energy formed spontaneous protein corona around these NPs. Protein corona loading around the AuNPs increased the size, which remained stable for 7 days, where each particle retained 200–250 spike proteins on the surface. The giant virus-like AuNPs exhibited a more robust antibody response, better stability, and lowered cytotoxicity (Medhi et al., 2020). AuNPs are currently used as a viral diagnostic and for inhibition of the viral activity. AuNPs detected the presence of COVID-19 viral antigen through a simple colorimetric shift in a short time using a naked eye assay (approximately within 5 minutes). The anti-spike antibody is attached to the surface of AuNPs to control the viral infection. The AuNPs-antibodies complex were then supposed to bind to pseudo-SARS-CoV-2 and inhibit the virus from binding to the cells’ receptors which inhibits viral replication and leads to the destruction of the virus’s lipid membrane (Pramanik et al., 2021).
At present, researchers have proposed quantum dots NPs to deliver vaccines and fight viral infections. Quantum dots can bind to ligands and consequently prevent the virus from binding to the surface of host cells. The underlying mechanism of action of COVID-19-loaded quantum dots is mainly explained by the ionic interaction between the quantum dots’ positive surface charge and the negative charge present of the viral RNA, which leads to the production of oxygen species within SARS-CoV-2, and ultimately the virus inactivation, as shown in Figure 3 (Manivannan and Ponnuchamy, 2020).
Recent studies on the antiviral activity of astodrimer sodium, a dendrimer with a broad spectrum of antimicrobial activity, in vitro against SARS-CoV-2 have been performed. The astodrimer inhibits the replication of SARS-CoV-2 in Vero E6 cells with a 50% reduction in virus-induced cytopathic effect. Thus, according to the researchers, astodrimer sodium is potentially used in inhaled or nasal routes to treat and prevent SARS-CoV-2 (Paull et al., 2021).
Microparticles are another carrier that is extensively tried for COVID-19 vaccines. Martin D’Souza is still developing a vaccine against COVID-19 by incorporating the antigen into a microparticulate carrier and administering it via transdermal route microneedle (Beimfohr, 2020). Similarly, antigen-adjuvant-loaded microparticles combination was found to be successful in manufacturing vaccines for the influenza virus. The influenza vaccine showed many benefits. Most notably, long-lasting immunity decreased viral load, and less time was taken for clearing the virus (Ting, 2021). As a result, researchers are trying to manufacture the COVID-19 vaccine using antigen-adjuvant-loaded microparticles to achieve the similar benefits shown in the influenza vaccine.
Emulsions/microemulsions had also been tested preclinically in the coronavirus vaccine, and researches were conducted. Some examples are described in this section. The MERS-CoV receptor-binding domain subunit vaccine (MERS-CoV RBD) induced a detectable level of neutralizing antibodies and T cell responses in mice (Zhang et al., 2016). In addition, mice developed a high neutralizing antibody titer upon administering two doses of inactivated SARS-CoV whole virus vaccine. However, the adjuvanted vaccine demonstrated early and robust antiviral responses. In another study, the adjuvanted vaccine provided complete protection against wild-type SARS-CoV infection in hamsters (Roberts et al., 2010). In mice, when paired with montanide ISA51, the MERS-CoV RBD domain induced a high neutralizing antibody titer and protected against viral challenge (Du et al., 2013).
Lately, a saponin-based microemulsion adjuvant has been investigated for use in the COVID-19 vaccine. A SARS-CoV-2 S1 protein with Fc region of human IgG1 vaccine candidate with saponin-based microemulsion adjuvant had led to the development of high titers of S1- (recombinant protein-) specific neutralizing antibodies. The study was performed using cynomolgus monkeys (Ren et al., 2020). Indeed, Roquette has a long history of supplying KLEPTOSE® HPβCD as an excipient. The EU, the US, and Chinese regulatory authorities have all licensed it for oral and parenteral administration. However, subunit vaccines are poorly immunogenic and require an additional adjuvant to stimulate immunity as they are nonliving vaccine antigens. HPβCD, as an adjuvant, induces Th2 cell response, enhances antigen- (vaccine-) specific antibody titers, and provides a more prolonged immune response. Also, Roquette suggested that HPβCD can be a safe and effective adjuvant in producing COVID-19 vaccines (Combating Coronavirus: Cyclodextrins in Treatment & Prevention, 2020).
Johnson & Johnson is developing virus subunit vaccines and has HPβCD as an adjuvant (Administration, no date). The vaccine engineered for the new SARS-CoV-2 pandemic was based on viral DNA. The vaccine was carried by a synthetic adenovirus vector and incorporated HPβCD as a cryopreservative. The new formulation stabilized proteins and prevented them from aggregating and adhering to the container wall (Daily Med, 2021).
Besides, the CDs application’s core objective is to enhance drugs dissolution and develop controlled drug release systems (Tan et al., 2014, 2020). The formation of β-CD and soluble (sACE2) complex effectively enhances the solubility in water of sACE2 to satisfy inhalation drug atomization criteria. After entering the body by atomization or other drug delivery, the inclusion conjugates release sACE2, which interacts with the SARS-CoV-2 S protein and inhibits the virus’s entry into the human cells (Sun et al., 2020).
 | Figure 3. Characteristics and applications of quantum dots loaded with COVID-19 vaccination (Manivannan and Ponnuchamy, 2020 ). [Click here to view] |
CURRENT STATUS AND FUTURE PROSPECTS
Currently, two licensed ISCOMs-based vaccines are available for veterinary purposes, and both are used in horses. The first is an influenza vaccine. More than 1 million horses were immunized with this vaccine, and no adverse effects were recorded. The second is a novel peptide vaccine, known as Equity® (Pfizer Australia, Melbourne), which is newly registered to control the estrous activity in mares and fillies (Sanders et al., 2005). Some examples of ISCOMs delivery systems in the clinical trial phase for vaccines and veterinary applications are demonstrated in Table 5.
Regarding liposomes drug carriers, they successfully entered the market in 1995 by producing PEGylated liposomal formulation Doxia® (Bulbake et al., 2017). Another product for the vaccine adjuvant delivery system is Shingrix®, manufactured by the pharmaceutical company GSK and approved in October 2017 by the FDA to be used in elderly patients aged 50 and above, a prophylactic for shingles (herpes zoster). It has a strong safety profile to induce strong antipathogen immunity compared to conventional microbial vaccines (Wang et al., 2017). Further examples of the novel promising, vaccine-loaded liposomal formulations are displayed in Table 6.
Furthermore, hepatitis A (Epaxal®) and influenza (Inflexal® V) vaccines have been used successfully as a carrier device and also as an adjuvant, and it validates the unique features of virosomes (Herzog et al., 2009). More than 45 countries have gained approval for the vaccine and have used this to immunize more than 10 million patients worldwide to date (Saroja et al., 2011).
Several emulsion formulations had been identified, the prominent of which is MONTANIDE® developed by SEPPIC. Montanides are W/O emulsion adjuvants similar to FIA evaluated in AIDS, cancer, malaria, and other autoimmune diseases for therapeutic application. Another example is MF59; an emulsion adjuvant was initially produced by Ciba Geigy and Chiron Corporation in the 1990s and then developed by Novartis Vaccines and Diagnostics. MF59 has been the most popular emulsion adjuvant in more than 35 countries, with more than 150 million administered doses (O’Hagan et al., 2013). Clinical trials confirmed the efficiency of MF59 in the immunogenicity improvement in vaccines for influenza, HIV, hepatitis B/hepatitis C, para-virus, HSV, human papillomavirus, and cytomegalovirus. In addition, MF59 offered enhanced protection and tolerated and lasting immunity to various populations from children to older age groups (O’Hagan et al., 2012). Table 7 summarizes emulsion adjuvants in approved vaccines in the preclinical and clinical settings.
However, consistent high-throughput technologies integrated with well-designed analytical tools at all stages of vaccine delivery systems could be a possible approach to obtaining approval for novel vaccines by reducing costs and confirming the highest level of patient safety. The field of vaccine development suffers from a shortage of potent adjuvants and advanced formulations-based delivery systems and carriers (Zieli?ska et al., 2020). Such novel vaccine formulations should be discovered and developed more rationally than in the past and licensed for human use. For example, suppose more challenges and concerns related to polymeric NPs, such as synthetic material protection, expansion manufacturing, and product quality, are resolved. In that case, more polymeric NP-based vaccines will be produced and approved to enter the market (Liu et al., 2018 and Nanomedicine and the COVID-19 vaccines, 2020). Also, new techniques such as the reverse microemulsion underwent rapid growth over the past few decades and were applied for NPs manipulating and designing. For example, a bicontinuous reverse microemulsion technique was used to synthesize Al-nano sticks, a new aluminum salt-based material consisting mainly of monodisperse amorphous aluminum (oxy)hydroxide (ALOOH) particles with a nanometer-scale size (80 nm) and sticklike shape. These nanosticks showed favorable physicochemical properties and potent vaccine adjuvant actions (Li et al., 2017).
 | Table 5. ISCOMs delivery system in the clinical trial phase for vaccines and veterinary applications. [Click here to view] |
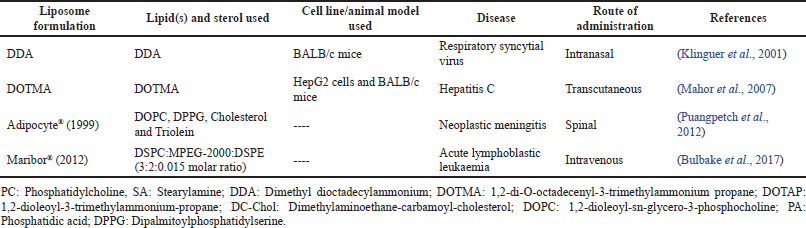 | Table 6. Novel promising vaccine-loaded liposomal formulations. [Click here to view] |
 | Table 7. Emulsion adjuvants in licensed vaccines approved in clinical and preclinical stages. [Click here to view] |
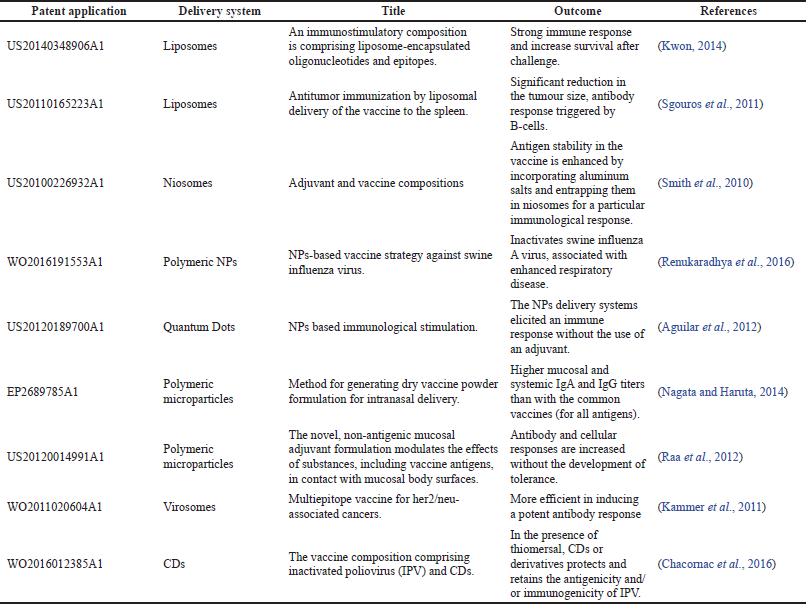 | Table 8. Patents published on vaccines loaded to various delivery platforms (cited from patents.google.com). [Click here to view] |
Several patenting activities and innovations in vaccine research and manufacturing to facilitate access to vaccine technologies that can be used in developing countries are displayed in Table 8.
CONCLUSION
The application of vaccine delivery platforms is gaining importance these days. Owing to the many potential features of these delivery systems, such as reduced dose frequency, improved biocompatibility, compliance, and increased stability, they can be employed to produce vaccines as a carrier or an adjuvant. Furthermore, most of the delivery systems discussed in this review have proven to elicit robust and successful immune responses. Hence these are used to develop more efficient vaccines to fight against various infectious diseases.
Recently many vaccines have been marketed as a preventive measure to curb the infection caused by COVID-19. Although the world has been grappling with the pandemic, researchers and pharmaceutical firms are working around the clock to develop a vaccine that could restore normalcy to everyday life. As a matter of fact, based on a large number of recent publications in this area and the promising clinical results published so far, it is fair to expect that innovative vaccine delivery technologies will become more widely used in the near future, resulting in significant increases in global immunization coverage.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in this work.
FUNDING
Dubai Pharmacy College for Girls provided partial funding for the publication of this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article
AUTHORS’ CONTRIBUTIONS
All authors have made significant contributions in analyzing and interpreting data, collecting references, and writing the article. They agreed to submit to the current journal, approved the final version for publication, and agreed to be responsible for all aspects of the work. The corresponding author was also responsible for designing and reviewing the article for intellectual and scholarly content.
PUBLISHER’S NOTE
This journal remains neutral concerning jurisdictional claims in published institutional affiliation.
REFERENCES
Abarca-Cabrera L, Fraga-García P, Berensmeier S. Bio-nano interactions: binding proteins, polysaccharides, lipids and nucleic acids onto magnetic nanoparticles. Biomater Res, 2021; 25(12):1–18; doi:10.1186/s40824-021-00212-y. CrossRef
Abbasi E, Aval SF, Akbarzadeh A, Morteza Milani, Nasrabadi1 HT, Joo SW, Hanifehpour Y, Koshki KN, Asl RP. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett, 2014; 9(247):1–10; doi:10.1186/1556-276X-9-247. CrossRef
Abdoli A, Radmehr N, Bolhassani A, Eidi A, Mehrbod P, Motevalli F, Kianmehr Z, Chiani M, Mahdavi M, Yazdani S, Ardestani MS, Kandi MR, Aghasadeghi MR. Conjugated anionic PEG-citrate G2 dendrimer with multi-epitopic HIV-1 vaccine candidate enhance the cellular immune responses in mice. Artif Cells Nanomed Biotechnol, 2017; 45(8):1762–8; doi:10.1080/21691401.2017.1290642. CrossRef
Abdul Rasool BK, Salmo HM. Development and clinical evaluation of clotrimazole-β-cyclodextrin eyedrops for the treatment of fungal keratitis. AAPS PharmSciTech, 2012; 13(3):883–9; doi:10.1208/s12249-012-9813-4. CrossRef
Abdulbaqi I, Darwis Y, Abdul Karim Khan N, Abou Assi R, Khan A. Ethosomal nanocarriers: the impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int J Nanomed, 2016; 2016(11):279–304; doi:10.2147/IJN.S105016. CrossRef
Aburahma MH. Bile salts-containing vesicles: promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv, 2016; 23(6):1847–67; doi:10.3109/10717544.2014.976892.
Agrawal R, Gupta V. Cyclodextrins-a review on pharmaceutical application for drug delivery. Int J Pharm Front Res, 2012; 2(1):95–112.
Aguilar Z, Wang Y, Xu H, Hui G, Pusic K, inventors. Ocean Nanotech LLC University of Hawaii, assignee nanoparticle based immunological stimulation. United States Patent 20120189700A1. 2012-07-26.
Ainbinder D, Paolino D, Fresta M, Touitou E. Drug delivery applications with ethosomes. J Biomed Nanotechnol, 2010; 6(5):558–68; doi:10.1166/jbn.2010.1152. CrossRef
Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, Fotin-Mleczek M, Hoerr I, Clemens R, von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet, 2017; 390(10101):1511–20; doi:10.1016/S0140-6736(17)31665-3. CrossRef
Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature, 1974; 252(5480):252; doi:10.1038/252252a0. CrossRef
Almeida JD, Edwards DC, Brand CM, Heath TD. Formation of virosomes from influenza subunits and liposomes. Lancet, 1975; 2(7941):899–901; doi:10.1016/s0140-6736(75)92130-3. CrossRef
Al Shuwaili AH, Abdul Rasool BK, Abdulrasool AA. Optimisation of elastic transfersomes formulations for transdermal delivery of pentoxifylline. Eur J Pharm Biopharm, 2016; 102:101–14; doi:10.1016/j.ejpb.2016.02.013. CrossRef
Andersen KG, Rambaut A, Lipkin WI, Edward CH, Robert FG. The proximal origin of SARS-CoV-2. Nat Med, 2020; 26:450–2; doi:10.1038/s41591-020-0820-9. CrossRef
Asadi K, Gholami A. Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: a review [Published Online ahead of Print, 2021 Apr 15]. Int J Biol Macromol, 2021; 182:648–58; doi:10.1016/j.ijbiomac.2021.04.005. CrossRef
Azuar A, Zhao L, Hei TT, Nevagi RJ, Bartlett S, Hussein WM, Khalil ZG, Capon RJ, Toth I, Skwarczynski M. Cholic acid-based delivery system for vaccine candidates against group a Streptococcus. ACS Med Chem Lett, 2019; 10(9):1253–9; doi:10.1021/acsmedchemlett.9b00239. CrossRef
Bachelder EM, Beaudette TT, Broaders KE, Fréchet JM, Albrecht MT, Mateczun AJ, Ainslie KM, Pesce JT, Keane-Myers AM. In vitro analysis of acetalated dextran microparticles as a potent delivery platform for vaccine adjuvants. Mol Pharm, 2010; 7(3):826–35; doi:10.1021/mp900311x. CrossRef
Balcells L, Fornaguera C, Brugada-Vilà P, Guerra-Rebollo M, Meca-Cortés O, Martínez G, Rubio N, Blanco J, Santamaría J, Cascante A, Borrós S. SPIONs’ enhancer effect on cell transfection: an unexpected advantage for an improved gene delivery system. ACS Omega, 2019; 4(2):2728–40; doi:10.1021/acsomega.8b02905. CrossRef
Bansal SA, Kumar V, Karimi J, Singha AP, Kumar S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv, 2020; 2:3764–87; doi:10.1039/d0na00472c. CrossRef
Barhate G, Gautam M, Gairola S, Jadhav S, Pokharkar V. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalised gold nanoparticles and botanical adjuvant: characterisation, immunogenicity, and stability assessment. J Pharm Sci, 2014; 103(11):3448–56; doi:10.1002/jps.24161. CrossRef
Behzad H, Huckriede ALW, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis, 2012; 205(3):466–73; doi:10.1093/infdis/jir769. CrossRef
Beimfohr C. Mercer pharmacy professor researching COVID-19 vaccine. 2020. Available via https://www.wnep.com/article/news/health/coronavirus/mercer-professor-researching-vaccine-covid19-microparticles/93-27f9b79c-eba6-4801-ba36-adbbc01eae4e (Accessed 24 April 2021).
Benvenuto D, Giovanetti M, Ciccozzi A, Spoto S, Angeletti S, Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J Med Virol, 2020; 92(4):455–9; doi:10.1002/jmv.25688. CrossRef
Bernasconi V, Norling K, Bally M, Höök F, Lycke NY. Mucosal vaccine development based on liposome technology. J Immunol Res, 2016; 2016:5482087; doi:10.1155/2016/5482087. CrossRef
BioPharm International. Virosomes: a novel strategy for drug delivery and targeting. BioPharm International, 2011. Available via https://www.biopharminternational.com/view/virosomes-novel-strategy-drug-delivery-and-targeting (Accessed 11 April 2021).
Blakney AK, Ip S, Geall AJ. An update on self-amplifying mRNA vaccine development. Vaccines (Basel), 2021; 9(2):97; doi:10.3390/vaccines9020097. CrossRef
Blanco-Andujar C, Walter A, Cotin G, Bordeianu C, Mertz D, Felder-Flesch D, Begin-Colin S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine (Lond), 2016; 11(14):1889–910; doi:10.2217/nnm-2016-5001. CrossRef
Blom RAM, Amacker M, van Dijk RM, Moser C, Stumbles PA, Blank F, von Garnier C. Pulmonary delivery of virosome-bound antigen enhances antigen-specific CD4+ T cell proliferation compared to liposome-bound or soluble antigen. Front Immunol, 2017; 8:359; doi:10.3389/fimmu.2017.00359. CrossRef
Bobbin ML, Burnett JC, Rossi JJ. RNA interference approaches for treatment of HIV-1 infection. Genome Med, 2015; 7(1):50; doi:10.1186/s13073-015-0174-y. CrossRef
Bolandparvaz A, Vapniarsky N, Harriman R, Alvarez K, Saini J, Zang Z, Van De Water J, Lewis JS. Biodistribution and toxicity of epitope-functionalised dextran iron oxide nanoparticles in a pregnant murine model. J Biomed Mater Res A, 2020; 108(5):1186–202; doi:10.1002/jbm.a.36893. CrossRef
Borrell B. The Tree That Could Help Stop the Pandemic. The Atlantic. 2020. Available at: https://www.theatlantic.com/science/archive/2020/10/single-tree-species-may-hold-key-coronavirus-vaccine/616792/
Bloomberg. Mymetics to advance preclinical studies for virosome-based COVID-19 vaccine through Innosuisse Grant. November 18, 2020. Available via https://www.bloomberg.com/press-releases/2020-11-18/mymetics-to-advance-preclinical-studies-for-virosome-based-covid-19-vaccine-through-innosuisse-grant (Accessed 18 May 2021).
Braga SS. Cyclodextrins: emerging medicines of the new millennium. Biomolecules, 2019; 9(12):801; doi:10.3390/biom9120801. CrossRef
Braga SS, Barbosa JS, Santos NE, El-Saleh F, Paz FAA. Cyclodextrins in antiviral therapeutics and vaccines. Pharmaceutics, 2021; 13(3):409; doi:10.3390/pharmaceutics13030409. CrossRef
Brewer JM, Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology, 1992; 75(4):570–5.
Brito LA, Malyala P, O’Hagan DT. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol, 2013; 25(2):130–45; doi:10.1016/j.smim.2013.05.007. CrossRef
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics, 2017; 9(2):12; doi:10.3390/pharmaceutics9020012. CrossRef
Chacornac I, Francon A, Vacus P, inventors. Vaccine composition comprising ipv and cyclodextrins. French Patent WO2016012385A1. 2016-01-28. CrossRef
Chahal JS, Khan OF, Cooper CL, McPartlan JS, Tsosie JK, Tilley LD, Sidik SM, Lourido S, Langer R, Bavari S, Ploegh HL, Anderson DG. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci USA, 2016; 113(29):E4133–42; doi:10.1073/pnas.1600299113. CrossRef
Champagne PO, Westwick H, Bouthillier A, Sawan M. Colloidal stability of superparamagnetic iron oxide nanoparticles in the central nervous system: a review. Nanomedicine, 2018; 13(11):1385–400; doi:10.2217/nnm-2018-0021. CrossRef
Combating coronavirus: cyclodextrins in treatment & prevention. 2020. Available via https://ondrugdelivery.com/combating-coronavirus-key-role-of-cyclodextrins-in-treatment-and-prevention/ (Accessed 8 May 2021). CrossRef
Cortajarena AL, Ortega D, Ocampo SM, Gonzalez-García A, Couleaud P, Miranda R, Belda-Iniesta C, Ayuso-Sacido A. Engineering iron oxide nanoparticles for clinical settings. Nanobiomedicine, 2014; 1:2; doi:10.5772/58841. CrossRef
Criscuolo E, Caputo V, Diotti RA, Sautto GA, Kirchenbaum GA, Clementi N. Alternative methods of vaccine delivery: an overview of edible and intradermal vaccines. J Immunol Res, 2019; 2019:13; doi:10.1155/2019/8303648. CrossRef
Cuenya BR. Synthesis and catalytic properties of metal nanoparticles: size, shape, support, composition, and oxidation state effects. Thin Solid Films, 2010; 518(12):3127–50; doi:10.1016/j.tsf.2010.01.018. CrossRef
Cui Z, Fountain W, Clark M, Jay M, Mumper RJ. Novel ethanol-in-fluorocarbon microemulsions for topical genetic immunization. Pharm Res, 2003; 20(1):16–23; doi:10.1023/a:1022234305600.
Daily Med. Label: Janssen COVID-19 vaccine-ad26.cov2.s injection. 2021. Available via https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=14a822ff-f353-49f9-a7f2-21424b201e3b (Accessed 18 May 2021). CrossRef
D’Amico C, Fontana F, Cheng R, Santos HA. Development of vaccine formulations: past, present, and future. Drug Deliv Transl Res, 2021; 11(2):353–72; doi:10.1007/s13346-021-00924-7. CrossRef
Deaguero IG, Huda MN, Rodriguez V, Zicari J, Al-Hilal TA, Badruddoza AZM, Nurunnabi M. Nano-vesicle based anti-fungal formulation shows higher stability, skin diffusion, biosafety and anti-fungal efficacy in vitro. Pharmaceutics, 2020; 12(6):516; doi:10.3390/pharmaceutics12060516. CrossRef
De Geest BG. Fusing antigens and adjuvants into traceless nanogels. ACS Cent Sci, 2020; 6(3):342–3; doi:10.1021/acscentsci.0c00098. CrossRef
De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials, 2008; 29(12):1912–9; doi:10.1016/j.biomaterials.2007.12.037. CrossRef
De Vries P, Uytdehaag FG, Osterhaus AD. Canine distemper virus (CDV) immune-stimulating complexes (Iscoms), but not measles virus iscoms, protect dogs against CDV infection. J Gen Virol, 1988; 69(Pt 8):2071–83; doi:10.1099/0022-1317-69-8-2071. CrossRef
Diaz-Arévalo D, Zeng M. Nanoparticle-based vaccines: opportunities and limitations. Nanopharmaceuticals, 2020; 135–50; doi:10.1016/B978-0-12-817778-5.00007-5.
Divekar SR. Carbon nanotubes an advanced drug delivery system—a review. IJPSR, 2020; 11(8):3636–44; doi:10.13040/IJPSR.0975-8232.11(8).3636-44. CrossRef
Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK, Lu L, Wang L, Debnath AK, Zheng BJ, Zhou Y, Jiang S. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol, 2013; 87(17):9939–42; doi:10.1128/JVI.01048-13. CrossRef
Dykman LA, Staroverov SA, Fomin AS, Khanadeev VA, Khlebtsov BN, Bogatyrev VA. Gold nanoparticles as an adjuvant: influence of size, shape, and technique of combination with CpG on antibody production. Int Immunopharmacol, 2018; 54:163–8; doi:10.1016/j.intimp.2017.11.008. CrossRef
Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, Panda S. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis, 2021; 21(5):637–46; doi:10.1016/S1473-3099(20)30942-7. CrossRef
Elmowafy EM, Tiboni M, Soliman ME. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Investig, 2019; 49:347–80; doi:10.1007/s40005-019-00439-x. CrossRef
European Medicines Agency Suvaxyn PCV Product Information. Accessed on 21 February 2022. EMEA/V/C/000149-R/0028. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/suvaxyn-pcv#product-information-section. CrossRef
Fanciullino R, Ciccolini J, Milano G. COVID-19 vaccine race: watch your step for cancer patients. Br J Cancer, 2021; 124(5):860–1; doi:10.1038/s41416-020-01219-3. CrossRef
Foged C. Thermostable subunit vaccines for pulmonary delivery: how close are we? Curr Pharm Des, 2016; 22(17):2561–76; doi:10.2174/1381612822666160202141603. CrossRef
Fratoddi I. Hydrophobic and hydrophilic Au and Ag nanoparticles. Breakthroughs and perspectives. Nanomaterials, 2017; 8(1):11; doi:10.3390/nano8010011. CrossRef
Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis, 2003; 37(2):241–50; doi:10.1086/375824 CrossRef
Ganda IS, Zhong Q, Hali M, Albuquerque RLC, Padilha FF, da Rocha SRP, Whittum-Hudson JA. Dendrimer-conjugated peptide vaccine enhances clearance of Chlamydia trachomatis genital infection. Int J Pharm, 2017; 527(1–2):79–91; doi:10.1016/j.ijpharm.2017.05.045. CrossRef
Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an adjuvant system containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines, 2012; 11(3):349–66; doi:10.1586/erv.11.192. CrossRef
Ge X, Wei M, He S, Yuan WE. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics, 2019; 11(2):55; doi:10.3390/pharmaceutics11020055 CrossRef
Gillani SS, Munawar MA, Khan KM, Chaudhary JA. Synthesis, characterization and applications of poly-aliphatic amine dendrimers and dendrons. J Iran Chem Soc, 2020; 17:2717–36; doi:10.1007/s13738-020-01973-4. CrossRef
Glück R, Mischler R, Brantschen S, Just M, Althaus B, Cryz SJ Jr. Immunopotentiating reconstituted influenza virus virosome vaccine delivery system for immunization against hepatitis A. J Clin Invest, 1992; 90(6):2491–5; doi:10.1172/JCI116141. CrossRef
Gogoi H, Mani R, Bhatnagar R. A niosome formulation modulates the Th1/Th2 bias immune response in mice and also provides protection against anthrax spore challenge. Int J Nanomed, 2018; 13:7427–40; doi:10.2147/IJN.S153150. CrossRef
Gregoriadis G, Allison AC. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett, 1974; 45(1):71–4; doi:10.1016/0014-5793(74)80813-6. CrossRef
Gurunathan S, Qasim M, Choi Y, Do JT, Park C, Hong K, Kim J-H, Song H. Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses? Nanomaterials, 2020; 10(9):1645; doi:10.3390/nano10091645. CrossRef
Hamilton BS, Whittaker GR, Daniel S. Influenza virus-mediated membrane fusion: determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses, 2012; 4(7):1144–68; doi:10.3390/v4071144. CrossRef
Hamouda T, Chepurnov A, Mank N, Knowlton J, Chepurnova T, Myc A, Sutcliffe J, Baker J. Efficacy, immunogenicity and stability of a novel intranasal nanoemulsion-adjuvanted influenza vaccine in a murine model. Hum Vaccines, 2010; 6(7):585–94; doi:10.4161/hv.6.7.11818. CrossRef
Han FY, Thurecht KJ, Whittaker AK, Smith MT. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol, 2016; 7:185; doi:10.3389/fphar.2016.00185. CrossRef
Han M, Watarai S, Kobayashi K, Yasuda T. Application of liposomes for development of oral vaccines: study of in vitro stability of liposomes and antibody response to antigen associated with liposomes after oral immunization. J Vet Med Sci, 1997; 59(12):1109–14; doi:10.1292/jvms.59.1109. CrossRef
Hasany SF, Ahmed I, Rajan J, Rehman A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci Nanotechnol, 2013; 2(6):148–58; doi:10.5923/j.nn.20120206.01. CrossRef
Hasnain MS, Nayak AK. Carbon nanotubes for targeted drug delivery. Springer Singapore (Springer Briefs in Applied Sciences and Technology), The Gateway, Singapore, 2019; doi:10.1007/978-981-15-0910-0. CrossRef
Hassan Y, Brewer JM, Alexander J, Jennings R. Immune responses in mice induced by HSV-1 glycoproteins presented with ISCOMs or NISV delivery systems. Vaccine, 1996; 14(17–18):1581–9; doi:10.1016/s0264-410x(96)00155-7. CrossRef
Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, Glück R. Eleven years of inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine, 2009; 27(33):4381–7; doi:10.1016/j.vaccine.2009.05.029. CrossRef
Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ, 2011; 89(3):221–6; doi:10.2471/BLT.10.079426. CrossRef
Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: cross-presentation and T cell activation. Vaccine, 2010; 28(50):7897–906; doi:10.1016/j.vaccine.2010.09.077. CrossRef
Hook S, Rades T. Immune stimulating complexes (ISCOMs) and Quil-A containing particulate formulations as vaccine delivery systems. In: Flower D, Perrie Y (eds.). Immunomic discovery of adjuvants and candidate subunit vaccines. Immunomics reviews: (An Official Publication of the International Immunomics Society), Springer, New York, NY, Vol. 5, 2013; doi:10.1007/978-1-4614-5070-2_12. CrossRef
Hong HC, Kim KS, Park SA, Chun MJ, Hong EY, Chung SW, Kim HJ, Shin BG, Braka A, Thanappan J, Jang S, Wu S, Cho YJ, Kim SH. An mRNA vaccine against SARS-CoV-2: Lyophilized, liposome-based vaccine candidate EG-COVID induces high levels of virus-neutralizing antibodies. bioRxiv, 2021 (Reprint article)doi:10.1101/2021.03.22.436375. CrossRef
Huang MH, Chou AH, Lien SP, Chen HW, Huang CY, Chen WW, Chong P, Liu SJ, Leng CH. Formulation and immunological evaluation of novel vaccine delivery systems based on bioresorbable poly(ethylene glycol)-block-poly(lactide-co-epsilon-caprolactone). J Biomed Mater Res B Appl Biomater, 2009; 90(2):832–41; doi:10.1002/jbm.b.31352. CrossRef
Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, Beermann ML, Simone-Roach C, Lindstrom-Vautrin J, Olejnik J, Suder EL, Bullitt E, Hinds A, Sharma A, Bosmann M, Wang R, Hawkins F, Burks EJ, Saeed M, Wilson AA, Mühlberger E, Kotton DN. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell, 2020 Dec 3;27(6):962-973.e7; doi: 10.1016/j.stem.2020.09.013. CrossRef
Ingolotti M, Kawalekar O, Shedlock DJ, Muthumani K, Weiner DB. DNA vaccines for targeting bacterial infections. Expert Rev Vaccines, 2010; 9(7):747–63; doi:10.1586/erv.10.57. CrossRef
Itani R, Tobaiqy M, Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics, 2020; 10(13):5932–42; doi:10.7150/thno.46691. CrossRef
Jain S, Harde H, Anura Indulkar A, Agrawal AK. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine NBM, 2014b; 10(2):431–40; doi:10.1016/j.nano.2013.08.012. CrossRef
Jain S, Indulkar A, Harde H, Agrawal AK. Oral mucosal immunization using glucomannosylated bilosomes. J Biomed Nanotechnol, 2014c; 10(6):932–47; doi:10.1166/jbn.2014.1800. CrossRef
Jain S, Jain V, Mahajan SC. Lipid based vesicular drug delivery systems. Adv Pharm, 2014a; 2014:1–12; doi:10.1155/2014/574673. CrossRef
Jain S, Vyas SP. Mannosylated niosomes as carrier adjuvant system for topical immunisation. J Pharm Pharmacol, 2005; 57(9):1177–1184; doi:10.1211/jpp.57.9.0012. CrossRef
Jain S, Vyas SP. Mannosylated niosomes as adjuvant-carrier system for oral mucosal immunisation. J Liposome Res, 2006; 16(4):331–345; doi:10.1080/08982100600992302.
Jiang T, Singh B, Li HS, Kim YK, Kang SK, Nah JW, Choi YJ, Cho CS. Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan. Biomaterials, 2014; 35(7):2365–73; doi:10.1016/j.biomaterials.2013.11.073.
Jeevana JB, Jyosna D. Multiparticulate drug delivery systems using natural polymers as release retardant materials. IJPPS, 2014; 6(10):61–5. CrossRef
Jones ST, Cagno V, Jane?ek M, Ortiz D, Gasilova N, Piret J, Gasbarri M, Constant DA, Han Y, Vukovi? L, Král P, Kaiser L, Huang S, Constant S, Kirkegaard K, Boivin G, Stellacci F, Tapparel C. Modified cyclodextrins as broad-spectrum antivirals. Sci Adv, 2020; 6(5):eaax9318; doi:10.1126/sciadv.aax9318.
Kalepu S, Sunilkumar KT, Betha S, Mohanvarma M Liposomal drug delivery system—a comprehensive review. Int J Drug Dev Res, 2013; 5(4):62–75.
Kammer A, Amacker M, Zurbriggen R, inventors. Multiepitope vaccine for her2/neu-associated cancers. French Patent WO2011020604A1. 2011-02-24. CrossRef
Kaminskas LM, Boyd BJ, Porter CJ. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties. Nanomedicine (Lond), 2011; 6(6):1063–84; doi:10.2217/nnm.11.67. CrossRef
Kandeel M, Al-Taher A, Park BK, Kwon H-J, Al-Nazawi M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J Med Virol, 2020; 92:1665–70; doi:10.1002/jmv.25928. CrossRef
Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, Nakamura H, Morishita R, Kotani H. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther, 2002; 6(2):219–26; doi:10.1006/mthe.2002.0647. CrossRef
Kantipakala R, Bonam SR, Vemireddy S, Miryala S, Halmuthur M SK. Squalane-based emulsion vaccine delivery system: composition with murabutide activate Th1 response. Pharm Dev Technol, 2019; 24(3):269–75; doi:10.1080/10837450.2018.1469150. CrossRef
Kapoor B, Gupta R, Gulati M, Singh SK, Khursheed R, Gupta M. The why, where, who, how, and what of the vesicular delivery systems. Adv Colloid Interface Sci, 2019; 271:101985; doi:10.1016/j.cis.2019.07.006. CrossRef
Kaurav M, Madan J, Sudheesh MS, Pandey RS. Combined adjuvant-delivery system for new generation vaccine antigens: alliance has its own advantage. Artif Cells Nanomed Biotechnol, 2018; 46(sup3):S818–31; doi:10.1080/21691401.2018.1513941. CrossRef
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity, 2011; 34(5):637–50; doi:10.1016/j.immuni.2011.05.006. CrossRef
Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol, 2014; 5:461; doi:10.3389/fimmu.2014.00461. CrossRef
Khan AK, Rashid R, Murtaza G, Zahra A. Gold nanoparticles: synthesis and applications in drug delivery. Trop J Pharm Res, 2014; 13(7):1169–77; doi:10.4314/tjpr.v13i7.23. CrossRef
Khatri K, Goyal AK, Gupta PN, Mishra N, Mehta A, Vyas SP. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine, 2008; 26(18):2225–33; doi:10.1016/j.vaccine.2008.02.058. CrossRef
Kim DK, Chang JH, Kang YJ. Efficient internalization of peptide-conjugated SPIONs in dendritic cells for tumor targeting. J Nanosci Nanotechnol, 2012; 12(7):5191–8; doi:10.1166/jnn.2012.6379. CrossRef
Kim SK, Yun CH, Han SH. Induction of dendritic cell maturation and activation by a potential adjuvant, 2-Hydroxypropyl-β-Cyclodextrin. Front Immunol, 2016; 7:435; doi:10.3389/fimmu.2016.00435. CrossRef
Klinguer C, Beck A, De-Lys P, Bussat MC, Blaecke A, Derouet F, Bonnefoy JY, Nguyen TN, Corvaïa N, Velin D. Lipophilic quaternary ammonium salt acts as a mucosal adjuvant when co-administered by the nasal route with vaccine antigens. Vaccine, 2001; 19(30):4236–44; doi:10.1016/s0264-410x(01)00156-6. CrossRef
Klucker MF, Dalençon F, Probeck P, Haensler J. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J Pharm Sci, 2012; 101(12):4490–500; doi:10.1002/jps.23311. CrossRef
Kojima C, Saito K, Kondo E. Design of peptide–dendrimer conjugates with tumor homing and antitumor effects. Res Chem Intermed, 2018; 44(8):4685–95; doi:10.1007/s11164-018-3280-9.
Krukemeyer MG, Krenn V, Huebner F, Wagner W, Resch R. History and possible uses of nanomedicine based on nanoparticles and nanotechnological progress. J Nanomed Nanotechnol, 2015; 6:336; doi:10.4172/2157-7439.1000336. CrossRef
Kumar A, Jena PK, Behera S, Lockey RF, Mohapatra S, Mohapatra S. Multifunctional magnetic nanoparticles for targeted delivery. Nanomedicine NBM, 2010; 6(1):64–9; doi:10.1016/j.nano.2009.04.002. CrossRef
Kurup VM, Thomas J. Edible vaccines: promises and challenges. Mol Biotechnol, 2020; 62:79–90; doi:10.1007/s12033-019-00222-1.
Kwon HJ, inventor. Hallym University Industry-Academic Cooperation Foundation, assignee. Immunostimulatory compositions comprising liposome-encapsulated oligonucleotides and epitopes. US Patent US20140348906A1. 2014-11-27. CrossRef
Lamaisakul S, Tantituvanont A, Lipipun V, Ritthidej G. Development of novel cationic microemulsion as parenteral adjuvant for influenza vaccine. Asian J Pharm Sci, 2020; 15(5):591–604; doi:10.1016/j.ajps.2019.08.002. CrossRef
Lambricht L, Peres C, Florindo H, Véronique P, Gaëlle V. Polymer-based nanoparticles as modern vaccine delivery systems. In: Micro and nanotechnology in vaccine development, Elsevier Inc, pp 185–203, 2017; doi:10.1016/B978-0-323-39981-4.00010-5. CrossRef
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 2020; 581(7807):215–20; doi:10.1038/s41586-020-2180-5. CrossRef
Leclercq SY, dos Santos RM, Macedo LB, Campos PC, Ferreira TC, de Almeida JG, Seniuk JG, Serakides R, Silva-Cunha A, Fialho SL. Evaluation of water-in-oil-in-water multiple emulsion and microemulsion as potential adjuvants for immunization with rabies antigen. Eur J Pharm Sci, 2011; 43(5):378–85; doi:10.1016/j.ejps.2011.05.008. CrossRef
Lee C, Jeong J, Lee T, Zhang W, Xu L, Choi JE, Park JH, Song JK, Jang S, Eom CY, Shim K. Virus-mimetic polymer nanoparticles displaying hemagglutinin as an adjuvant-free influenza vaccine. Biomaterials, 2018; 183:234–42; doi:10.1016/j.biomaterials.2018.08.036. CrossRef
Lee JJ, Shim A, Lee SY, Kwon BE, Kim SR, Ko HJ, Cho HJ. Ready-to-use colloidal adjuvant systems for intranasal immunization. J Colloid Interface Sci, 2016; 467:121–8; doi:10.1016/j.jcis.2016.01.006. CrossRef
Lengyel M, Kállai-Szabó N, Antal V, Laki AJ, Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci Pharm, 2019; 87(3):20; doi:10.3390/scipharm87030020. CrossRef
Li H, Yang L, Cheng G, Wei HY, Zeng Q. Encapsulation, pharmacokinetics and tissue distribution of interferon α-2b liposomes after intramuscular injection to rats. Arch Pharm Res, 2011a; 34(6):941–8; doi:10.1007/s12272-011-0611-4. CrossRef
Li K, Zhao X, Xu S, Pang D, Yang C, Chen D. Application of Ulex europaeus agglutinin I-modified liposomes for oral vaccine: ex vivo bioadhesion and in vivo immunity. Chem Pharm Bull (Tokyo), 2011b; 59(5):618–23; doi:10.1248/cpb.59.618. CrossRef
Li X, Hufnagel S, Xu H, Valdes SA, Thakkar SG, Cui Z, Celio H. Aluminum (Oxy)hydroxide nanosticks synthesized in bicontinuous reverse microemulsion have potent vaccine adjuvant activity. ACS Appl Mater Interfaces, 2017; 9(27):22893–901; doi:10.1021/acsami.7b03965. CrossRef
Li Z, Xiong F, He J, Dai X, Wang G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: effect of surface modification with chitosan and mannan. Eur J Pharm Biopharm, 2016; 109:24–34; doi:10.1016/j.ejpb.2016.08.012. CrossRef
Liu B, Wu Z, Liu T, Qian R, Wu T, Liu Q, Shen A. Polymeric nanoparticles engineered as a vaccine adjuvant-delivery system. In: Wang N, Wang T (eds.). Immunisation-vaccine adjuvant delivery system and strategies, IntechOpen, 2018; doi:10.5772/intechopen.81084. Available via https://www.intechopen.com/books/immunization-vaccine-adjuvant-delivery-system-and-strategies/polymeric-nanoparticles-engineered-as-a-vaccine-adjuvant-delivery-system. CrossRef
Liu H, Tu Z, Feng F, Shi H, Chen K, Xu X. Virosome, a hybrid vehicle for efficient and safe drug delivery and its emerging application in cancer treatment, Acta Pharm, 2015; 65(2):105–16; doi:10.1515/acph-2015-0019. CrossRef
Liu J, Wu J, Wang B, Zeng S, Qi F, Lu C, Kimura Y, Liu B. Oral vaccination with a liposome-encapsulated influenza DNA vaccine protects mice against respiratory challenge infection. J Med Virol, 2014; 86:886–94; doi:10.1002/jmv.23768. CrossRef
Lemoine C, Thakur A, Krajišnik D, Guyon R, Longet S, Razim A, Górska S, Panteli? I, Ili? T, Nikoli? I, Lavelle EC, Gamian A, Savi? S, Milicic A. Technological approaches for improving vaccination compliance and coverage. Vaccines, 2020; 8(2):304; doi:10.3390/vaccines8020304. CrossRef
Loos C, Syrovets T, Musyanovych A, Mailänder V, Landfester K, Nienhaus GU, Simmet T. Functionalized polystyrene nanoparticles as a platform for studying bio-nano interactions. Beilstein J Nanotechnol, 2014; 5:2403–12; doi:10.3762/bjnano.5.250. CrossRef
Macedo LB, Lobato ZIP, Fialho SL, Viott AM, Guedes RMC, Silva-Cunha A. Evaluation of different adjuvants formulations for bluetongue vaccine. Braz arch Biol Technol, 2013; 56(6):932–41; doi:10.1590/S1516-89132013005000002. CrossRef
Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol, 2011; 9:55; doi:10.1186/1477-3155-9-55. CrossRef
Maheshwari C, Pandey RS, Chaurasiya A, Kumar A, Selvam DT, Prasad GB, Dixit VK. Non-ionic surfactant vesicles mediated transcutaneous immunization against hepatitis B. Int Immunopharmacol, 2011; 11(10):1516–22; doi:10.1016/j.intimp.2011.05.007. CrossRef
Mahor S, Rawat A, Dubey PK, Gupta PN, Khatri K, Goyal AK, Vyas SP. Cationic transfersomes based topical genetic vaccine against hepatitis B. Int J Pharm, 2007; 340(1–2):13–9; doi:10.1016/j.ijpharm.2007.03.006. CrossRef
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers, 2011; 3(3):1377–97; doi:10.3390/polym3031377. CrossRef
Manivannan S, Ponnuchamy K. Quantum dots as a promising agent to combat COVID-19. Appl Organometal Chem, 2020; 34(10):e5887; doi:10.1002/aoc.5887. CrossRef
Marasini N, Kaminskas LM. Subunit-based mucosal vaccine delivery systems for pulmonary delivery—are they feasible? Drug Dev Ind Pharm, 2019; 45(6):882–94; doi:10.1080/03639045.2019.1583758. CrossRef
Martínez-Donato G, Piniella B, Aguilar D, Olivera S, Pérez A, Castañedo Y, Alvarez-Lajonchere L, Dueñas-Carrera S, Lee JW, Burr N, Gonzalez-Miro M, Rehm BH. Protective T Cell and antibody immune responses against hepatitis C virus achieved using a biopolyester-bead-based vaccine delivery system. Clin Vaccine Immunol, 2016; 23(4):370–8; doi:10.1128/CVI.00687-15. CrossRef
Mata E, Igartua M, Patarroyo ME, Pedraz JL, Hernández RM. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur J Pharm Sci, 2011; 44(1–2):32–40; doi:10.1016/j.ejps.2011.05.015.
McEntee C, Lavelle EC, O’Hagan DT. Antigen delivery systems i: nonliving microparticles, liposomes, and immune-stimulating complexes (ISCOMs). In: Mestecky J, Strober W, Russell MW, Cheroutre H, Lambrecht BN, Kelsall BL (eds.). Mucosal immunology, 4th edition, Elsevier Inc, pp 1211–31, Chapter 63, 2015; doi:10.1016/B978-0-12-415847-4.00063-X.
McKee C, Bohannon K. Exploring the reasons behind parental refusal of vaccines. J Pediatr Pharmacol Ther, 2016; 21(2):104–9; doi:10.5863/1551-6776-21.2.104. CrossRef
Medhi R, Srinoi P, Ngo N, Tran HV, Lee TR. Nanoparticle-based strategies to combat COVID-19. ACS Appl Nano Mater, 2020; 25:acsanm.0c01978; doi:10.1021/acsanm.0c01978. CrossRef
GSK. Medicago and GSK start Phase 3 trial of adjuvanted COVID-19 vaccine candidate. 2021. Available via https://www.gsk.com/en-gb/media/press-releases/medicago-and-gsk-start-phase-3-trial-of-adjuvanted-covid-19-vaccine-candidate/ (Accessed 20 April 2021).
Minato S, Iwanaga K, Kakemi M, Yamashita S, Oku N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: effect of lipid dose on systemic and mucosal immunity. J Control Release, 2003; 89(2):189–97; doi:10.1016/s0168-3659(03)00093-2. CrossRef
Mishra D, Mishra PK, Dubey V, Nahar M, Dabadghao S, Jain NK. Systemic and mucosal immune response induced by transcutaneous immunization using Hepatitis B surface antigen-loaded modified liposomes. Eur J Pharm Sci, 2008; 33(4–5):424–33; doi:10.1016/j.ejps.2008.01.015. CrossRef
Moderna’s work on our COVID-19 vaccine. 2021. Available via https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19 (Accessed 20 April 2021).
Moran HBT, Turley JL, Andersson M, Lavelle EC. Immunomodulatory properties of chitosan polymers. Biomaterials, 2018; 184:1–9; doi:10.1016/j.biomaterials.2018.08.054. CrossRef
Morelli AB, Becher D, Koernig S, Silva A, Drane D, Maraskovsky E. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol, 2012; 61(Pt 7):935–43; doi:10.1099/jmm.0.040857-0. CrossRef
Mohanty S, Panda S, Aslesha Bhanja A, Pal A, Chandra S. Novel drug delivery systems for rheumatoid arthritis: an approach to better patient compliance. Biomed Pharmacol J, 2019; 12(1):12(1):157–70; doi:10.13005/bpj/1624. CrossRef
Nagata R, Haruta S, inventors. Shin Nippon Biomedical Laboratories Ltd KM Biologics Co Ltd, assignee. Method for generating dry vaccine powder formulation for intranasal delivery. European Patent 2689785A1. 2014-01-29.
Nagpal PS, Kesarwani A, Sahu P, Upadhyay P. Aerosol immunization by alginate coated mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain mycobacterium against M.tb. H37Rv infection in mice. BMC Infect Dis, 2019; 19(1):568; doi:10.1186/s12879-019-4157-2. CrossRef
Nair SC, Sreelekshmi AS, Abdul Rahiman CA, Krishnan K, Gopika G, Nair PT, Gauri U, Ajit UG. Virosomes: a groundbreaking revolution in novel drug delivery. Int J Pharm Sci Res, 2020; 11(4):5809–20; doi:10.26452/ijrps.v11i4.3230. CrossRef
Nanomedicine and the COVID-19 vaccines. Nat Nanotechnol, 2020; doi:10.1038/s41565-020-00820-0 (Accessed 20 April 2021). CrossRef
Neto LMM, Zufelato N, de Sousa-Júnior AA, Trentini MM, da Costa AC, Bakuzis AF, Kipnis A, Junqueira-Kipnis AP. Specific T cell induction using iron oxide based nanoparticles as subunit vaccine adjuvant. Hum Vaccin Immunother, 2018; 14(11):2786–801; doi:10.1080/21645515.2018.1489192. CrossRef
Nevagi RJ, Toth I, Skwarczynski M. Peptide-based vaccines. In: Peptide applications in biomedicine, biotechnology and bioengineering, Elsevier Inc, pp 327–58, 2018; doi:10.1016/B978-0-08-100736-5.00012-0. CrossRef
Nicol JR, Dixon D, Coulter JA. Gold nanoparticle surface functionalization: a necessary requirement in the development of novel nanotherapeutics. Nanomedicine, 2015; 10(8):1315–26; doi:10.2217/nnm.14.219. CrossRef
Nielsen HM, Hübschmann HB, Rades T. ISCOMs as a vaccine delivery system. In: Foged C, Rades T, Perrie Y, Hook S (eds.). Subunit vaccine delivery. Advances in delivery science and technology. Springer, New York, NY, 2015; doi:10.1007/978-1-4939-1417-3_8. CrossRef
Nijen Twilhaar MK, Czentner L, Grabowska J, Affandi AJ, Lau CYJ, Olesek K, Kalay H, van Nostrum CF, van Kooyk Y, Storm G, Haan JMMD. Optimization of liposomes for antigen targeting to splenic CD169+ macrophages. Pharmaceutics, 2020; 12(12):1138; doi:10.3390/pharmaceutics12121138. CrossRef
Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Larrea GZ, Sosa-Ferreyra CF, Mercado-Curiel RF, Manríquez J, Bustos E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater, 2014; 2014:507273; doi:10.1155/2014/507273. CrossRef
Novavax addresses urgent global public health needs with innovative technology. 2021. Available via https://www.novavax.com/our-unique-technology
Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. 2021. Available via https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3 (Accessed 20 April 2021). CrossRef
O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine, 2012; 30(29):4341–8; doi:10.1016/j.vaccine.2011.09.061. Erratum in: Vaccine, 2013 Apr 3; 31(14):1877. CrossRef
O’Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines, 2013; 12(1):13–30; doi:10.1586/erv.12.140. CrossRef
Oliveira C, Juliana M, José C, Isabella S, Barboza F. Is nanotechnology helping in the fight against COVID-19?’ Front Nanotechnol, 2020; 2:588915; doi:10.3389/fnano.2020.588915.
Okore VC, Attama AA, Ofokansi KC, Esimone CO, Onuigbo EB. Formulation and evaluation of niosomes. Indian J Pharm Sci, 2011; 73(3):323–8; doi:10.4103/0250-474X.93515. CrossRef
Onishi M, Ozasa K, Kobiyama K, Ohata K, Kitano M, Taniguchi K, Homma T, Kobayashi M, Sato A, Katakai Y, Yasutomi Y. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J Immunol, 2015; 194(6):2673–82; doi:10.4049/jimmunol.1402027. CrossRef
Osterhaus A, van Amerongen G, van Binnendijk R. Vaccine strategies to overcome maternal antibody mediated inhibition of measles vaccine. Vaccine, 1998; 16(14–15):1479–81; doi:10.1016/s0264-410x(98)00112-1. CrossRef
Parhiz H, Khoshnejad M, Myerson JW, Hood E, Patel PN, Brenner JS, Muzykantov VR. Unintended effects of drug carriers: big issues of small particles. Adv Drug Deliv Rev, 2018; 130:90–112; doi:10.1016/j.addr.2018.06.023. CrossRef
Park K. Controlled drug delivery systems: past forward and future back. J Control Release, 2014; 190:3–8; doi:10.1016/j.jconrel.2014.03.054. CrossRef
Park KS, Sun X, Aikins ME, Moon JJ. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev, 2021; 169:137–51; doi:10.1016/j.addr.2020.12.008. CrossRef
Pastor M, Esquisabel A, Marquínez I, Talavera A, Pedraz JL. Cellulose acetate phthalate microparticles containing Vibrio cholerae: steps toward an oral cholera vaccine. J Drug Target, 2014; 22(6):478–87; doi:10.3109/1061186X.2014.888071. CrossRef
Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol, 2018; 9:2224; doi:10.3389/fimmu.2018.02224. CrossRef
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol, 2018; 16(1):71; doi:10.1186/s12951-018-0392-8. CrossRef
Paull JRA, Heery GP, Bobardt MD, Castellarnau A, Luscombe CA, Fairley JK, Gallay PA. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. bioRxiv, 2021; doi:10.1101/2020.08.20.260190. CrossRef
Pawar D, Mangal S, Goswami R, Jaganathan KS. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm, 2013; 85(3 Pt A):550–9; doi:10.1016/j.ejpb.2013.06.017. CrossRef
Pedersen JS, Oliveira CL, Hübschmann HB, Arleth L, Manniche S, Kirkby N, Nielsen HM. Structure of immune stimulating complex matrices and immune stimulating complexes in suspension determined by small-angle x-ray scattering. Biophys J, 2012; 102(10):2372–80; doi:10.1016/j.bpj.2012.03.071. CrossRef
Pedziwiatr-Werbicka E, Milowska K, Dzmitruk V, Ionov M, Shcharbin D, Bryszewska M. Dendrimers and hyperbranched structures for biomedical applications. Eur Polym J, 2019; 119:61–73; doi:10.1016/j.eurpolymj.2019.07.013.
Pfizer and BioNTech achieve first authorization in the world for a vaccine to combat COVID-19. 2020. Available via https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-achieve-first-authorization-world (Accessed 12 May 2021). CrossRef
Piyush R, Rajarshi K, Chatterjee A, Khan R, Ray S. Nucleic acid-based therapy for coronavirus disease 2019. Heliyon, 2020; 6(9):e050072; doi:10.1016/j.heliyon.2020.e05007. CrossRef
Poovorawan Y, Theamboonlers A, Chumdermpadetsuk S, Glück R, Cryz SJ Jr. Safety, immunogenicity, and kinetics of the immune response to a single dose of virosome-formulated hepatitis A vaccine in Thais. Vaccine, 1995; 13(10):891–3; doi:10.1016/0264-410x(95)00007-n. CrossRef
Pramanik A, Ye Gao Y, Shamily Patibandla S, Dipanwita Mitra D, Martin G. McCandless MG, Fassero LA, Gates K, Tandon R, Ray PC. The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv, 2021; 3(6):1588–96; doi:10.1039/d0na01007c. CrossRef
Pratelli A, Colao V. Role of the lipid rafts in the life cycle of canine coronavirus. J Gen Virol, 2015; 96(Pt 2):331–7; doi:10.1099/vir.0.070870-0. CrossRef
Premanand B, Prabakaran M, Kiener TK, Kwang J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS One, 2013; 8(2):e55536; doi:10.1371/journal.pone.0055536. CrossRef
Puangpetch A, Anderson R, Huang YY, Sermswan RW, Chaicumpa W, Sirisinha S, Wongratanacheewin S. Cationic liposomes extend the immunostimulatory effect of CpG oligodeoxynucleotide against Burkholderia pseudomallei infection in BALB/c mice. Clin Vaccine Immunol, 2012; 19(5):675–83; doi:10.1128/CVI.05545-11. CrossRef
Pusic K, Aguilar Z, McLoughlin J, Kobuch S, Xu H, Tsang M, Wang A, Hui G. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J, 2013; 27:1153–66; doi:10.1096/fj.12-218362.
Raa J, Berstad AKH, Bakke HSW, Haneberg B, Haugen IH, Holst J, Janakova L, Korsvold GE, Oftung F, inventors. Biotec Pharmacon ASA, assignee. Novel, non-antigenic, mucosal adjuvant formulation which modulates the effects of substances, including vaccine antigens, in contact with mucosal body surfaces. United States Patents 20120014991A1. 2012-01-19. CrossRef
Ragelle H, Vandermeulen G, Préat V. Chitosan-based siRNA delivery systems. J Control Release, 2013; 172(1):207–18; doi:10.1016/j.jconrel.2013.08.005. CrossRef
Rajput T, Chauhan M. Bilosome: a bile salt based novel carrier system gaining interest in pharmaceutical research. JDDT, 2017; 7(5):4–16; doi:10.22270/jddt.v7i5.1479. CrossRef
Raska M, Turanek J. DNA vaccines for the induction of immune responses in mucosal tissues. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN (eds.). Mucosal immunology, 4th edition, Academic Press, pp 1307–35, Chapter 67, 2015; doi:10.1016/B978-0-12-415847-4.00067-7. CrossRef
Rattanapak T, Young K, Rades T, Hook S. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: characterisation and in vitro skin penetration. J Pharm Pharmacol, 2012; 64:1560–9; doi:10.1111/j.2042-7158.2012.01535.x. CrossRef
Ren W, Sun H, Gao GF, Chen J, Sun S, Zhao R, Gao G, Hu Y, Zhao G, Chen Y, Jin X. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates. Vaccine, 2020; 38(35):5653–8; doi:10.1016/j.vaccine.2020.06.066.
Renukaradhya GR, Dhakal S, Hiremath J, Lee CW, inventors. Ohio State Innovation Foundation, assignee. Nanoparticle based vaccine strategy against swine influenza virus. French Patent WO2016191553A1. 2016-12-01. CrossRef
Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. Modified mRNA vaccines protect against Zika virus infection. Cell, 2017; 168(6):1114–25.e10; doi:10.1016/j.cell.2017.02.017. CrossRef
Roberts A, Lamirande EW, Vogel L, Baras B, Goossens G, Knott I, Chen J, Ward JM, Vassilev V, Subbarao K. Immunogenicity and protective efficacy in mice and hamsters of a β-propiolactone inactivated whole virus SARS-CoV vaccine. Viral Immunol, 2010; 23(5):509–19; doi:10.1089/vim.2010.0028. CrossRef
Roberts CW, Brewer JM, Alexander J. Congenital toxoplasmosis in the Balb/c mouse: prevention of vertical disease transmission and fetal death by vaccination. Vaccine, 1994; 12(15), 1389–94; doi:10.1016/0264-410X(94)90147-3. CrossRef
Rodig SJ. Fixing attached cells for staining. Cold Spring Harb Protoc, 2020; 2020(8):099689; doi:10.1101/pdb.prot099689. CrossRef
Rodriguez B, Asmuth DM, Matining RM, Spritzler J, Jacobson J, Mailliard R, Li XD, Martinez AI, Tenorio A, Lori F, Lisziewicz J. Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J Acquir Immune Defic Syndr, 2013; 64(4):351–9; doi:10.1097/QAI.0b013e3182a99590. CrossRef
Sadoff J, Gars ML, Shukarev G, Heerwegh D, Truyers C, de Groot AM, Stoop J, Tete S, Van Damme W, Leroux-Roels I, Berghmans PJ. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomised, placebo-controlled trial. medRxiv (Reprint), 2020; doi:10.1101/2020.09.23.20199604 CrossRef
Saifuddin N, Raziah AZ, Junizah AR. Carbon nanotubes: a review on structure and their interaction with proteins. J Chem, 2013; 2013:676815; doi:10.1155/2013/676815 CrossRef
Salatin S, Barar J, Barzegar-Jalali M, Adibkia K, Kiafar F, Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res Pharm Sci, 2017; 12(1):1–14; doi:10.4103/1735-5362.199041 CrossRef
Sanders MT, Brown LE, Deliyannis G, Pearse MJ. ISCOMTM-based vaccines: the second decade. Immunol Cell Biol, 2005; 83:119–28; doi:10.1111/j.1440-1711.2005.01319.x CrossRef
Saroja C, Lakshmi P, Bhaskaran S. Recent trends in vaccine delivery systems: a review. Int J Pharm Investig, 2011; 1(2):64–74; doi:10.4103/2230-973X.82384 CrossRef
Scheinberg DA, Villa CH, Escorcia FE, McDevitt MR. Conscripts of the infinite armada: systemic cancer therapy using nanomaterials. Nat Rev Clin Oncol, 2010; 7(5):266–76; doi:10.1038/nrclinonc.2010.38 CrossRef
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol, 2015; 6:286; doi:10.3389/fphar.2015.00286
Sgouros G, Song H, Lingappa M, inventors. Hopkins University, assignee. Antitumor immunization by liposomal delivery of vaccine to the spleen. United States Patent US20110165223A1. 2011-07-07. CrossRef
Shah RR, Brito LA, O’Hagan DT, Amiji MM. Emulsions as vaccine adjuvants. In: Foged C, Rades T, Perrie Y, Hook S. (eds.). Subunit vaccine delivery. Advances in delivery science and technology, Springer, New York, NY, 2015; doi:10.1007/978-1-4939-1417-3_4 CrossRef
Shastri PN, Kim MC, Quan FS, D’Souza MJ, Kang SM. Immunogenicity and protection of oral influenza vaccines formulated into microparticles. J Pharm Sci, 2012; 101(10):3623–35; doi:10.1002/jps.23220 CrossRef
Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, Wirth DM, Chen A, Sack M, Pokorski JK, Steinmetz NF. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol, 2020; 15:646–55; doi:10.1038/s41565-020-0737-y CrossRef
Shukla A, Katare OP, Singh B, Vyas SP. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int J Pharm, 2010; 385(1-2):47–52; doi:10.1016/j.ijpharm.2009.10.027 CrossRef
Shukla A, Singh B, Katare OP. Significant systemic and mucosal immune response induced on oral delivery of diphtheria toxoid using nano-bilosomes. Br J Pharmacol, 2011; 164(2b):820–7; doi:10.1111/j.1476-5381.2011.01452.x CrossRef
Siler-Marinkovic S. Liposomes as drug delivery systems in dermal and transdermal drug delivery. In: Dragicevic N, Maibach H. (eds.). Percutaneous penetration enhancers chemical methods in penetration enhancement, Springer, Berlin, Heidelberg, 2016; doi:10.1007/978-3-662-47862-2_2 CrossRef
Silva AL, Soema PC, Slütter B, Ossendorp F, Jiskoot W. PLGA particulate delivery systems for subunit vaccines: linking particle properties to immunogenicity. Hum Vaccin Immunother, 2016; 12(4):1056–69; doi:10.1080/21645515.2015.1117714 CrossRef
Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv, 2009; 6(8):813–25; doi:10.1517/17425240903071029 CrossRef
Siraj SN, Raza S, Ansari MA, Khan GJ, Athar S. Overview on virosomes as a novel carrier for drug delivery. JDDT, 2019; 8(6-s):429–34; doi:10.22270/jddt.v8i6-s.2163
Smith G, Dinesh B. Shenoy DB, Robert W. Lee RW, inventors. Novavax Inc, assignee. Adjuvant and vaccine compositions. United States Patent 20100226932A1. 2010-09-09. CrossRef
Smith SM, Iwenofu OH. NY-ESO-1: a promising cancer testis antigen for sarcoma immunotherapy and diagnosis. Chin Clin Oncol, 2018; 7(4):44; doi:10.21037/cco.2018.08.11 CrossRef
Sun P, Lu X, Xu C, Wang Y, Sun W, Xi J. CD-sACE2 inclusion compounds: an effective treatment for coronavirus disease 2019 (COVID-19). J Med Virol, 2020; 92(10):1721–3; doi:10.1002/jmv.25804 CrossRef
Sung YK, Kim SW. Recent advances in polymeric drug delivery systems. Biomater Res, 2020; 24:12; doi:10.1186/s40824-020-00190-7 CrossRef
Surquin M, Tielemans C, Nortier J, Jadoul M, Peeters P, Ryba M, Roznovsky L, Domán J, Barthelemy X, Crasta PD, Messier M, Houard S. Anti-HBs antibody persistence following primary vaccination with an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency. Hum Vaccin, 2011; 7(9):913–8; doi:10.4161/hv.7.9.16225 CrossRef
Szyma?ski P, Markowicz M, Mikiciuk-Olasik E. Nanotechnology in pharmaceutical and biomedical applications. Dendrimers. Nano, 2011; 6(6):509–39; doi:10.1142/S1793292011002871 CrossRef
Tan L, Zheng T, Li M, Zhong X, Tang Y, Qin M, Sun X. Optimization of an mRNA vaccine assisted with cyclodextrin-polyethyleneimine conjugates. Drug Deliv Transl Res, 2020; 10(3):678–89; doi:10.1007/s13346-020-00725-4. CrossRef
Tan S, Ladewig K, Fu Q, Blencowe A, Qiao GG. Cyclodextrin-based supramolecular assemblies and hydrogels: recent advances and future perspectives. Macromol Rapid Commun, 2014; 35(13):1166–84; doi:10.1002/marc.201400080 CrossRef
Thakor AS, Jokerst JV, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically approved nanoparticle imaging agents. J Nucl Med, 2016; 57(12):1833–7; doi:10.2967/jnumed.116.181362 CrossRef
Thakur A, Foged C. Nanoparticles for mucosal vaccine delivery. Nanoeng Biomater Adv Drug Deliv, 2020; 2020: 603–46; doi:10.1016/B978-0- 08-102985-5.00025-5. CrossRef
Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Logue J, Pornoff A, Norton J, Guebre-Xabier M, Zhou B. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 elicits immunogenicity in baboons and protection in mice. bioRxiv, Cold Spring Harbor, NY, 2020; doi:10.1101/2020.06.29.178509
Ting J. Report: a vaccine against COVID-19 that strongly induces three branches of immunity, 2021. Available via https://collaboratory.unc.edu/wpcontent/uploads/sites/476/2021/02/a-vaccine-against-covid-19-that-strongly-induces-three-branches-of-immunity-report.pdf (Accessed 18 May 2021). CrossRef
Tiwari B, Agarwal A, Kharya AK, Lariya N, Saraogi G, Agrawal H, Agrawal GP. Immunoglobulin immobilized liposomal constructs for transmucosal vaccination through nasal route. J Liposome Res, 2011; 21(3):181–93; doi:10.3109/08982104.2010.498003 CrossRef
Turner CT, McInnes SJP, Voelcker NH, Cowin AJ. Therapeutic potential of inorganic nanoparticles for the delivery of monoclonal antibodies. J Nanomater, 2015; 2015:309602; doi:10.1155/2015/309602 CrossRef
Tzeng SY, McHugh KJ, Behrens AM, Rose S, Sugarman JL, Ferber S, Langer R, Jaklenec A. Stabilized single-injection inactivated polio vaccine elicits a strong neutralizing immune response. Proc Natl Acad Sci USA, 2018; 115(23):E5269–78; doi:10.1073/pnas.1720970115 CrossRef
Vangala A, Kirby D, Rosenkrands I, Agger EM, Andersen P, Perrie Y. A comparative study of cationic liposome and niosome-based adjuvant systems for protein subunit vaccines: characterisation, environmental scanning electron microscopy and immunisation studies in mice. J Pharm Pharmacol, 2006; 58(6):787–99; doi.org:10.1211/jpp.58.6.0009 CrossRef
Van Giau V, An SSA, Hulme J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des Devel Ther, 2019; 13:327–43; doi:10.2147/DDDT.S190577 CrossRef
Varsanyi TM, Morein B, Löve A, Norrby E. Protection against lethal measles virus infection in mice by immune-stimulating complexes containing the hemagglutinin or fusion protein. J Virol, 1987; 61(12):3896–901; doi:10.1128/JVI.61.12.3896-3901.1987. CrossRef
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O’Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet, 2021; 397(10269):99–111; doi:10.1016/S0140-6736(20)32661-1 CrossRef
Wallis J, Shenton DP, Carlisle RC. Novel approaches for the design, delivery and administration of vaccine technologies. Clin Exp Immunol, 2019; 196:189–204; doi:10.1111/cei.13287 CrossRef
Wallyn J, Anton N, Vandamme TF. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications-a review. Pharmaceutics, 2019; 11(11):601; doi:10.3390/pharmaceutics11110601 CrossRef
Wang J, Hu JH, Li FQ, Liu GZ, Zhu QG, Liu JY, Ma HJ, Peng C, Si FG. Strong cellular and humoral immune responses induced by transcutaneous immunization with HBsAg DNA-cationic deformable liposome complex. Exp Dermatol, 2007; 16(9):724–9; doi:10.1111/j.1600-0625.2007.00584.x CrossRef
Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunisation. J Control Release, 2019; 303:130–50; doi:10.1016/j.jconrel.2019.04.025 CrossRef
Wang N, Wu T, Wang T. Liposomes used as a vaccine adjuvant-delivery system. In: Catala A (ed.). Liposomes, InTechOpen, 2017; doi:10.5772/intechopen.68521. Available via https://www.intechopen.com/books/liposomes/liposomes-used-as-a-vaccine-adjuvant-delivery-system CrossRef
Wang X, Dai Y, Zhao S, Tang J, Li H, Xing Y, Qu G, Li X, Dai J, Zhu Y, Zhang X. PAMAM-Lys, a novel vaccine delivery vector, enhances the protective effects of the SjC23 DNA vaccine against Schistosoma japonicum infection. PLoS One, 2014; 9(1):e86578; doi:10.1371/journal.pone.0086578 CrossRef
Watkins HC, Pagan CL, Childs HR, Posada S, Chau A, Rios J, Guarino C, DeLisa MP, Whittaker GR, Putnam D. A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles. Vaccine, 2017; 35(40):5373–80; doi:10.1016/j.vaccine.2017.08.013
WHO. WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. WHO, 2020. Available via https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. (Accessed 18 May 2021).
World Health Organization. Immunisation. World Health Organization, 2019. Available via https://www.who.int/news-room/facts-in-pictures/detail/immunization (Accessed 17 April 2021)
Wibowo D, Jorritsma SHT, Gonzaga ZJ, Evert B, Chen S, Rehm BHA. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials, 2021; 268:120597; doi:10.1016/j.biomaterials.2020.120597 CrossRef
Wilkhu JS, McNeil SE, Anderson DE, Perrie Y. Characterization and optimization of bilosomes for oral vaccine delivery. J Drug Target, 2013; 21(3):291–9; doi:10.3109/1061186X.2012.747528 CrossRef
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science, 2020; 367(6483):1260–3; doi:10.1126/science.abb2507 CrossRef
Wu M, Zhao H, Li M, Yue Y, Xiong S, Xu W. Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front Cell Infect Microbiol, 2017; 7:445; doi:10.3389/fcimb.2017.00445 CrossRef
Wu W, Wu Z, Yu T, Jiang C, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater, 2015; 16(2):023501; doi:10.1088/1468-6996/16/2/023501 CrossRef
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Huang W, Xu W, Huang B, Wang W, Zhang W, Li N, Xie Z, Zhu X, Ding L, You W, Zhao Y, Zhao J, Huang L, Shi X, Yang Y, Xu G, Wang W, Liu P, Ma M, Qiao Y, Zhao S, Chai J, Li Q, Fu H, Xu Y, Zheng X, Guo W, Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis, 2021; 21(1):39–51; doi:10.1016/S1473-3099(20)30831-8 CrossRef
Yadav HKS, Dibi M, Mohammad A, Srouji AE. Nanovaccines formulation and applications-a review. J Drug Deliv Sci Technol, 2018; 44:380–7; doi:10.1016/j.jddst.2018.01.015 CrossRef
Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science, 2020; 367(6485):1444–8; doi:10.1126/science.abb2762 CrossRef
Yang L, Li W, Kirberger M, Liao W, Ren J. Design of nanomaterial based systems for novel vaccine development. Biomater Sci, 2016; 4(5):785–802; doi:10.1039/c5bm00507h CrossRef
Yang M, Yang T, Jia J, Lu T, Wang H, Yan X, Wang L, Yuc L, Zhaod Y. Fabrication and characterisation of DDAB/PLA-alginate composite microcapsules as single-shot vaccine. RSC Adv, 2018; 8(24):13612–24; doi:10.1039/c8ra00013a CrossRef
Yang X, Wang X, Hong H, Elfawal G, Lin S, Wu J, Jiang Y, He C, Mo X, Kai G, Wang H. Galactosylated chitosan-modified ethosomes combined with silk fibroin nanofibers is useful in transcutaneous immunization. J Control Release, 2020; 327:88–99; doi:10.1016/j.jconrel.2020.07.047 CrossRef
Yu Q, Wang Y, Huang S, Liu S, Zhou Z, Zhang S, Zhao Z, Yu Y, Yang Y, Ju S. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics, 2020; 10(12):5641–8; doi:10.7150/thno.46465 CrossRef
Yuan M, Wu NC, Zhu X, Lee CD, So RTY, Lv H, Mok CKP, Wilson IA. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science, 2020; 368(6491):630–3; doi:10.1126/science.abb7269 CrossRef
Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials, 2009; 30(10):1928–36; doi:10.1016/j.biomaterials.2008.12.038 CrossRef
Zhang N, Channappanavar R, Ma C, Wang L, Tang J, Garron T, Tao X, Tasneem S, Lu L, Tseng CT, Zhou Y, Perlman S, Jiang S, Du L. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol, 2016; 13(2):180–90; doi:10.1038/cmi.2015.03 CrossRef
Zhang N, Zheng BJ, Lu L, Zhou Y, Jiang S, Du L. Advancements in the development of subunit influenza vaccines. Microbes Infect, 2015a; 17(2):123–34; doi:10.1016/j.micinf.2014.12.006 CrossRef
Zhang W, Wang L, Yang T, Liu Y, Chen X, Liu Q, Jia J, Ma G. Immunopotentiator-loaded polymeric microparticles as robust adjuvant to improve vaccine efficacy. Pharm Res, 2015b; 32(9):2837–50; doi:10.1007/s11095-015-1666-6 CrossRef
Zhang Y, Ng W, Feng X, Cao F, Xu H. Lipid vesicular nanocarrier: quick encapsulation efficiency determination and transcutaneous application. Int J Pharm, 2017; 516(1-2):225–30; doi:10.1016/j.ijpharm.2016.11.011 CrossRef
Zhang Y, Ng W, Hu J, Mussa SS, Ge Y, Xu H. Formulation and in vitro stability evaluation of ethosomal carbomer hydrogel for transdermal vaccine delivery. Coll Surf B Biointer, 2018; 163:184–91; doi:10.1016/j.colsurfb.2017.12.031 CrossRef
Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis, 2021; 21(2):181–92; doi:10.1016/S1473-3099(20)30843-4 CrossRef
Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. Iron oxide nanoparticles-based vaccine delivery for cancer treatment. Mol Pharm, 2018; 15(5):1791–9; doi:10.1021/acs.molpharmaceut.7b01103 CrossRef
Zieli?ska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules, 2020; 25(16):3731; doi:10.3390/molecules25163731 CrossRef
Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv, 2016; 23(9):3319–29; doi:10.1080/10717544.2016.1177136 CrossRef