INTRODUCTION
Diseases of the cardiovascular system remain the leading cause of death in many countries. About 18 million people die every year from cardiovascular system diseases worldwide. According to the Centers for Disease Control and Prevention, life expectancy would be 10 years longer if there were not such a high prevalence of cardiovascular disease (CVD), covering all countries of the world (Alzahrani et al., 2019; Mirzadeh et al., 2022; Mozaffarian et al., 2019; Scherer and Schafer, 2022; Weir et al., 2016; Wright et al., 2018). CVD leads to long-term disability in the adult population and has enormous economic costs. The World Health Organization forecasts are not optimistic: by 2025, about 23 million people will die from CVD, mainly from heart disease and strokes, which are projected to remain the major leading causes of death (Callow, 2006; Mendis et al., 2015; Varadarajan et al., 2022; Wright et al., 2018).
Platelets play a key role in arterial thrombosis, which is a recent event complicating CVD and both peripheral vascular disease and antiplatelet therapy improves the survival of patients with these disorders (Burnouf and Goubran, 2022; Koupenova et al., 2017; Paniccia et al., 2015).
An antiplatelet drug (antiaggregant) in general, or antiplatelet agent, reduces the ability of platelets to stick together (also known as platelet aggregation) and inhibits the formation of blood clots in blood vessels. Platelet agglutination inhibitors can be synthetic drugs or may be isolated from natural sources that belong to the pharmaceutical drug class, which reduce platelet aggregation and inhibit the formation of blood clots, thereby preventing interruption of blood supply to the brain and, ultimately, a stroke. Such drugs are effective and widely used in primary and secondary prevention of thrombotic cerebrovascular disease (Carvalhal et al., 2019; Hirsch et al., 2017; Passacquale et al., 2022; Roskoski, 2018; Xiang et al., 2019).
Antiplatelet therapy is one of the methods which helps reduce the ability of platelets to form blood clots during primary hemostasis. Various antiplatelet drugs help to reversibly or irreversibly inhibit platelet activation, which reduces the tendency of platelets to adhere to each other and to damage the human blood vessel endothelium (Bouasquevisque et al., 2019; Chen et al., 2020; Ferlini et al., 2019; Hackam and Spence, 2019; Xu et al., 2021).
The purpose of this review is to carry out a comparative analysis of known and potential biological activities of synthetic and natural drugs that are currently used in clinical medicine as antiplatelet agents with natural sulfur-containing hydrocarbons that are isolated from natural sources. For detailed characterization of the analyzed compounds’ pharmacological potential, we used the computer program PASS (Dembitsky, 2021, 2022; Filimonov et al., 2018; Pounina et al., 2021).
PASS predicts several thousand biological activities based on analyzing the known structure–activity relationships with an average accuracy of about 96%. Thus, in addition to the previously detected activities in the experiment, the PASS application allows us to identify the most promising but previously unknown activities for further studies (PASS, 2022).
BRIEF DESCRIPTION OF DRUGS CURRENTLY USED IN MEDICINE
According to Clarivate Analytics Integrity, 164 synthetic and natural antiplatelet agents are currently included, 25 of which are presented in this work. These drugs were selected given that they have been regularly used in medical practice for the past 20 years. It is known that many drugs that were used before 2000 are not currently used and their use is also prohibited for various reasons. In addition, they must contain sulfur atom(s). All presented antiplatelet agents are divided into two groups. The first group includes only synthetic chemical molecules, while the second group contains metabolites isolated from natural sources. Table 1–3 present 25 antiplatelet agents that have passed clinical trials and are widely used in medical practice. For each active pharmaceutical ingredient, numerous scientific papers have been published that describe their synthesis and broad aspects of their biological activity.
SYNTHETIC ANTIPLATELET DRUGS
Aspirin
Acetylsalicylic acid (1), which is known under the brand name Aspirin, is a medicinal substance that is used in medical practice to reduce pain; treat inflammation, Kawasaki disease (mucocutaneous lymph node syndrome), pericarditis, and rheumatism; and treat or prevent myocardial infarction, strokes, and angina pectoris. The antithrombotic effect of aspirin has long been known and is due to inhibition of platelet function by acetylation of platelet cyclooxygenase on a functionally important amino acid serine. Aspirin is an about 150–200 times more potent inhibitor of the platelet enzyme isoform. In addition, aspirin is the gold standard of antiplatelet agents for the prevention of arterial thrombosis. A complete historical background can be obtained from several review articles and books (Desborough and Keeling, 2017; Miner and Hoffhines, 2007; Schrör, 2016; Vane, 2000; Weissmann, 1991; Wick, 2012). The biological activity demonstrated with aspirin is also widely covered in the scientific literature (Drew and Chan, 2021; Kuzniatsova et al., 2012; Montinari et al., 2019; Sandler, 1996; Szczeklik et al., 2005; Tao et al., 2021; Wu, 2003).
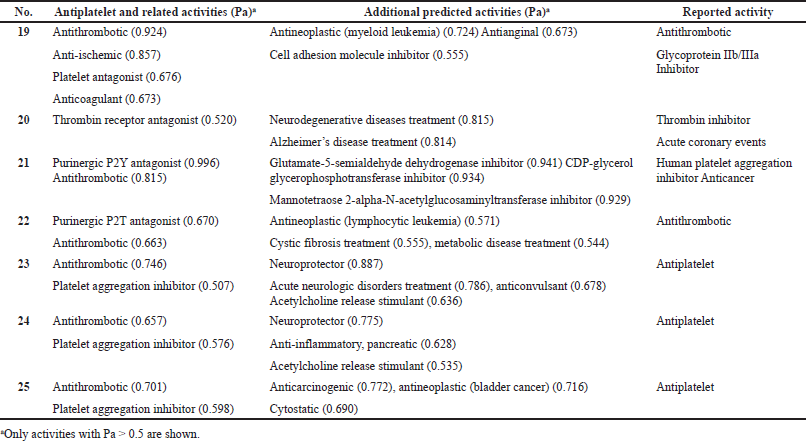
Aspirin is a miracle cure that has demonstrated surprising new biological activities for over 150 years. According to the data shown in Table 1, aspirin has antiplatelet, fibrinolytic, antiviral, antithrombotic, antiuremic, rheumatoid arthritis, anti-inflammatory, and anti-infective properties. The PASS predicted these pharmacological effects (Filimonov et al., 2018; Pounina et al., 2021) and revealed some other biological activities (see Table 1). Aspirin’s antiplatelet activity has been known for more than 120 years, and it is now widely used for this purpose worldwide (Dockray, 1905; Williamson, 1902). Table 1 shows what activities aspirin demonstrates (reported activity), which were published in the last 40 years (Desborough and Keeling, 2017; Schrör, 2016; Weissmann, 1991), as well as activities (confirmed activities) that were confirmed by PASS, as well as additional predicted activities. As we can see from Table 1, 41 activities are characteristic of aspirin, which are obtained when using both one drug or it in conjunction with other drugs.
Figure 1 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities. In addition, Figure 2 shows the predicted and calculated antiplatelet activity of aspirin with high confidence (96%). Figure 3 shows the antipyretic properties of aspirin with over 94% confidence. The structures of synthetic antiplatelet drugs are shown in Figure 4, and the biological activity is shown in Table 2.
Indobufen
Indobufen (2, also known as K-3920) was first synthesized more than 40 years ago and was used as a platelet anticoagulant (Fuccella et al., 1979; Tamassia et al., 1979; Vinazzer, 1979). Since then, it has been widely regarded as an inhibitor of platelet aggregation (Liu et al., 2018; Muruganantham et al., 2021).
Ticlopidine
Ticlopidine (3), known by the tradename Ticlid, was synthesized in 1973, and the platelet aggregation inhibitor was used in humans in the mid-1970s (Thebault et al., 1975). It is also currently being used effectively in the antiplatelet therapy associated with coronary disease and type 2 diabetes (Bates, 2019; Flores-Runk and Raasch, 1993; Mori and Geirsson, 2019; Natsuaki and Kimura 2021; Ziada and Moliterno, 2019).
Clopidogrel
Clopidogrel (4), an analog of ticlopidine (3) and known by the tradename Plavix, was patented in 1982 and approved for medical use in 1998 (Diener, 1998; Féliste et al., 1987; Gachet et al., 1990). It is an antiplatelet drug used to reduce the risk of CVD and strokes in high-risk individuals. In addition, it is used together with aspirin for myocardial infarctions and after the installation of a stent of the coronary artery (Akkaif et al., 2021a; Hankey, 1997; Schrör, 1993; Yao et al., 1993). It is included in the World Health Organization’s List of Essential Medicines as the safest and most effective drug and is widely used in the United States (Badjatiya and Rao, 2019; Florescu et al., 2019; Zeb et al., 2018).
 | Table 1. Reported and confirmed activities of aspirin currently used in medicine. [Click here to view] |
Prasugrel
Prasugrel (5), known by the brand name Effient, is used to prevent blood clots and is a platelet inhibitor and irreversible antagonist of the ADP P2Y12 receptor (Gurbel and Tantry, 2008; Niitsu et al., 2005; Ray et al., 2021; Scott et al., 2009). This drug was developed by the Japanese company Daiichi Sankyo Co. and was approved for use in Europe and the United States in 2009 (Dobesh et al., 2016; Laine et al., 2016; Siller-Matula et al., 2017; Siu et al., 2016).
 | Table 2. Pharmacological activities of synthetic antiplatelet drugs currently used in medicine. [Click here to view] |
 | Table 3. Pharmacological activities of the natural and seminatural antiplatelet drugs currently used in medicine. [Click here to view] |
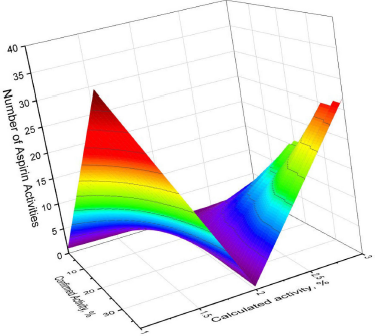 | Figure 1. 3D graph of the confirmed and calculated pharmacological activities of acetylsalicylic acid, also known by the trade name Aspirin, which is a medication used for inflammation, pain, and other dysfunctions in the human body. Aspirin as a synthetic drug has 41 different activities as shown by various authors over the past 30–40 years. Thirty-eight activities were confirmed using PASS, with the most prominent properties of aspirin being fibrinolytic (96.0%), antipyretic (94.8%), and antiseptic (92.3%) with more than 90% confidence. Other activities are summarized in Table 1 . [Click here to view] |
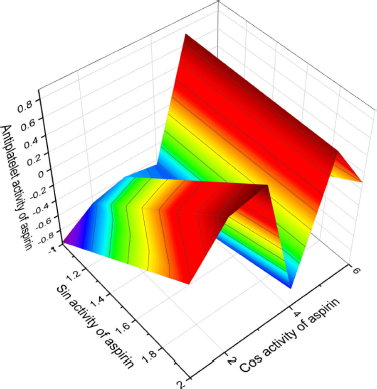 | Figure 2. 3D graph shows the predicted and calculated antiplatelet activity of aspirin showing the highest degree of confidence, 96%. [Click here to view] |
Dipyridamole
Dipyridamole (6), patented in 1957 by the name Persantine, inhibits the formation of blood clots, causes the expansion of blood vessels, inhibits the formation of proinflammatory cytokines (MCP-1, MMP-9) in vitro, and leads to a decrease in hsCRP in humans. In addition, it is a phosphodiesterase 5A inhibitor, which prevents platelet aggregation by increasing cGMP levels and blocking adenosine reuptake via red blood cells (Eisert, 2012; FitzGerald, 1987; Jialiken et al., 2021; Kapil et al., 2017; Zakeri and Nimjee, 2018). Originally, in the late 1950s and early 1960s, Persantine was used to treat coronary heart diseases that eventually lead to myocardial infarction (Degraff and Lyon, 1963; Greif, 1960; Junemann, 1959; Knabe, 1964; Tetzlaff and Tetzlaff, 1959).
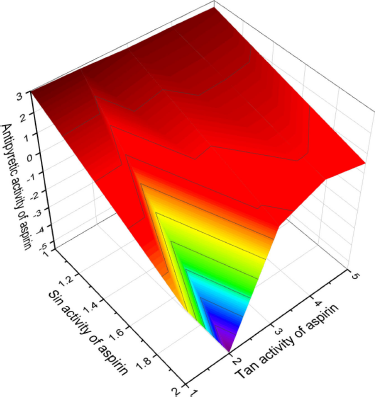 | Figure 3. 3D graph shows the predicted and calculated antipyretic activity of aspirin showing the highest degree of confidence, 94.8%. [Click here to view] |
Figure 5 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities.
Defibrotide
Defibrotide (7) is known by the brand name Defitelio, and its mechanism of action is poorly understood. However, it is used to treat vessel-occlusive liver disease in people who have undergone bone marrow transplantation. In combination with other drugs, it is used as an antiplatelet agent (Berge and Sandercock, 2002; Fareed et al., 2013; Onuora, 2022; Palmer et al., 2013).
Cangrelor
Cangrelor (8), known by the tradename Kengreal (in the USA) or Kengrexal (in Europe), is a P2Y12 FDA inhibitor and has been known as an antiplatelet agent since 2015. It is a high affinity, reversible P2Y12 receptor inhibitor, that causes almost complete inhibition of the ADP-induced platelet aggregate (De Luca et al., 2021; Gachet, 2008; Storey and Sinha, 2016; Tello-Montoliu et al., 2012; Ueno et al., 2010).
Naftazone
Naftazone (9, β-naphthoquinone monosemicarbazone) was first synthesized in France in the early 1970s and was used to prevent bleeding in prostate surgery (Charles and Coolsaet, 1972; Delwaide et al., 1974). It was later studied as an agent that prevents platelet coagulation and was used in the treatment of Parkinson’s disease and against MCF-7 human breast cancer cells (Chen et al., 2004; Corvol et al., 2019; Kitchens et al., 2020; McArdle and Erxleben 2021; McGregor et al., 1999).
Ticagrelor
Ticagrelor (10) is an inhibitor of platelet aggregation and is a P2Y12 receptor antagonist. It has been used in the European Union since 2010 and in the USA since 2011 (Akkaif et al., 2021b; Husted and van Giezen, 2009; Gurbel et al., 2010a,b; Marczewski et al., 2010; Nicolau et al., 2018).
Tirofiban
Tirofiban (11, trade name Aggrastat) is a synthetic nonpeptide platelet aggregation inhibitor that belongs to a class of antiplatelet agents called glycoprotein IIb/IIIa inhibitors. The GP IIb/IIIa receptors are fibrinogen attachment sites that promote platelet aggregation. Tirofiban was developed by the Merck chemical group of George Hartman, Melissa Egbertson, and Wasyl Halczenko in the early 1990s. Interestingly, tirofiban is a modified version of the molecule (mimics part of the natural protein) found in the venom of the scaly viper Echis carinatus, also known as “carpet” vipers, living in areas with sandy soil and rock outcrops in Africa and some countries of the Middle East (Coller, 2001; Dogne et al., 2002; Egbertson et al., 1994; Gong et al., 2020; Hartman et al., 1992; Menozzi et al., 2005; Tang et al., 2021).
Cilostazol
Cilostazol (12), known by the brand name Pletal, is a selective inhibitor of phosphodiesterase type 3 with a therapeutic focus on increasing cAMP. An increase in cAMP leads to an increase in the active form of protein kinase A, which is directly related to the inhibition of platelet aggregation, thereby exerting its vasodilating effect. This drug was approved for medical use in the United States in 1999 but is not yet available in Canada or the United Kingdom (Brown et al., 2021; Comerota, 2005; Goto, 2005; Hidaka et al., 1991; Hiatt, 2005; Kwon and Kim, 2016; Reilly and Mohler, 2001).
Elinogrel
Elinogrel (13) was developed by Portola Pharmaceuticals (an American clinical-stage biotechnology company) as an experimental antiplatelet drug that acts as a P2Y12 inhibitor. In addition, it has been used in antiplatelet therapy for acute coronary syndromes. Since 2012, its production has been discontinued (Bonadei et al., 2014; Gurbel et al., 2010; Lordan et al., 2021; Michelson, 2011; Müller et al., 2012).
Terutroban
Terutroban (14) is an antiplatelet agent that was developed by Servier Laboratories (Suresnes, France). It is a selective antagonist of thromboxane prostanoid and is an orally active drug in clinical development for the secondary prevention of acute thrombotic complications (Baidildinova et al., 2021; Coccheri, 2010; Franchini and Mannucci, 2009; Verbeuren, 2006).
Triflusal
Triflusal (15, known by the Disgren or Grendis trade name) was synthesized in Uriach laboratories in the late 1970s and has been commercially available in Spain and France since 1981 (Garcia-Rafanell and Morell, 1977; Garcia-Rafanell et al., 1979). Its salicylate is structurally like acetylsalicylic acid but is not a derivative of this acid (McNeely and Goa, 1998). It is an inhibitor of platelet aggregation and is available in more than 25 countries in Europe, Asia, Africa, and America. In combination with aspirin and other drugs, it has a good effect in inhibiting platelet aggregation (Huang et al., 2017; Kalantzi et al., 2019; Milionis et al., 2016; Yun et al., 2014) and has a neuroprotective effect (Kim et al., 2017).
 | Figure 4. Synthetic antiplatelet drugs currently used in medicine. The pharmacological profiles and biological activities are shown in Table 2. [Click here to view] |
Selexipag
Selexipag (16) was synthesized by Actelion Pharmaceuticals Ltd. and Nippon Shinyaku in the mid-2000s as a medicine for the treatment of pulmonary arterial hypertension (Kuwano et al., 2007; Nakamura et al., 2007). It is a highly selective nonprostanoid agonist of long-acting prostacyclin receptors and is used in antiplatelet therapy (Barnes et al., 2019; Coghlan et al., 2019; Genecand et al., 2021; Scott, 2016; Sitbon and Morrell, 2012; Skoro-Sajer and Lang, 2014 ).
Sulfinpyrazone
Initially, sulfinpyrazone (17, also known as anturan) was developed by Ciba-Geigy AG (Swiss Pharm Co. Ltd.) in the mid-1950s to treat gout, as well as an analog of phenylbutazone, but this drug does not have the properties of phenylbutazone (Brodie et al., 1954; Montgomery and Ogryzlo, 1960; Persellin, 1961; Wilhelmi and Currie, 1954). It shows a uricosuric and pronounced antiplatelet effect. It is suggested that the effect of sulfinpyrazone on platelet function may be associated with inhibition of prostaglandin synthesis. Sulfinpyrazone does not replace the well-known and traditional anticoagulant agents in the treatment of venous thrombosis, but it is an important drug for the treatment of conditions associated with arterial thrombosis and, possibly, for the prevention of recurrent venous thrombosis (Gallus, 1985; Jafri, 1991; Keyser, 1993; Margulies et al., 1980; Orhan and Deniz 2021).
 | Figure 5. 3D graph shows the predicted and calculated antiplatelet activity of the synthetic antiplatelet agent dipyridamole (6) showing the highest degree of confidence, 96%. [Click here to view] |
 | Figure 6. 3D graph shows the predicted and calculated antiplatelet activity of the synthetic antiplatelet agent beraprost (18) showing the highest degree of confidence, 96%. [Click here to view] |
Beraprost
Beraprost (18) contains a unique tricyclic core, which has four adjacent stereocenters, is an analog of prostacyclin PGI2, which demonstrated a strong antiplatelet effect measured by the Born (1962) method in the 1960s, and acts as an effective vasodilator. The effect was tested in platelet-rich plasma obtained from humans and some experimental animals, including rabbits, rats, guinea pigs, dogs, and cats. In addition, PGI2 has attracted much attention due to its ability to stimulate axonal remodeling of damaged neural networks after central nervous system disease (Callow, 2006; Wright and Wall, 2018). As a vasodilator and antiplatelet agent, it is widely used in some Asian countries in Japan, South Korea, and China (Nakura et al., 2020; Shen et al., 2019; Sharmin et al., 2021). Figure 6 shows the 3D graph of a wide range of confirmed and the calculated pharmacological activities.
NATURAL OR SEMINATURAL ANTIPLATELET DRUGS
Eptifibatide
Eptifibatide (19) is a cyclic heptapeptide derived from the disinterring protein (P22827) found in the venom of a southeast rattlesnake (Sistrurus miliarius barbouri) (Millennium Pharmaceuticals/Schering-Plow/Essex). It belongs to the class of arginine–glycine–aspartate mimetics and reversibly binds to platelets, has a short half-life, and is widely recognized. In addition, it is used to reduce the risk of acute ischemic events in the heart. The drug is usually used with aspirin or heparin (Dogne et al., 2002; Goa and Noble, 1999; Nana et al., 2021; O’Shea et al., 2002; Phillips and Scarborough, 1997; Tempelhof et al., 2012). The structures of natural or seminatural antiplatelet drugs are shown in Figure 7, and the biological activities are shown in Table 3. Figure 8 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities
Vorapaxar
Vorapaxar (20), known as SCH 530348, is a thrombin receptor antagonist, namely the protease-activated receptor, PAR-1, which has been synthesized from the natural himbacine product (Clasby et al., 2007; Chelliah et al., 2014). It is known that himbacine was isolated from the bark of rain forest trees Galbulimima belgraveana and G. baccata native to Papua New Guinea, Indonesia, and Northern Australia (Lan et al., 2018). This drug is part of the PAR-1 antagonist family, which inhibits platelet aggregation associated with thrombin. A study of the mechanism of action of vorapaxar showed that it works in a different way than other antiplatelet drugs, such as aspirin and P2Y12 inhibitors (Bhat et al., 1977; Gurbel et al., 2011; Poole and Elkinson, 2014; Tantry et al., 2015; Ten Cate et al., 2021; Wang, 2015; Ungar et al., 2016). Figure 9 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities.
Forskolin
Forskolin (21, also called coleonol) is a labdanic diterpenoid found in extracts from the roots of an Indian tropical plant (Coleus forskohlii) (Mohamed Saleem, 2013; Sapio et al., 2017). For centuries, it was used in traditional Indian and Chinese herbal medicine to treat various diseases such as asthma and urinary tract infections and has recently found application in the fight against cancerous tumors (Adnot et al., 1982; Bednarek et al., 2020). Forskolin has been shown to be a potent inhibitor of human platelet aggregation and is used in antiplatelet therapy (De Souza et al., 1983; Ju et al., 2021; Romero-Pérez et al., 1999).
 | Figure 7. Natural and seminatural antiplatelet drugs currently used in medicine. The pharmacological profiles and biological activities are shown in Table 3. [Click here to view] |
Piceid. Piceid (22) is a resveratrol glycoside that is present in the juice of red and white grapes (Lamuela-Raventos et al., 1995; Pezet et al., 2004). Many authors believe that, due to the content of resveratrol glycoside and other phenolic products, grape juice can have a beneficial effect on the health of those who cannot drink wine (Bonechi et al., 2017). Resveratrol is known to affect the inhibition of platelet adhesion and aggregation, as well as conformational changes in fibrinogen caused by adrenaline (Olas and Wachowicz, 2005). In addition, resveratrol reduces lipid peroxidation, oxidation, and nitration of platelet and plasma proteins and affects the function of blood platelets, which play an important role not only in the hemostatic process but also in the pathogenesis of CVD (Herrmann et al., 2017).
Indothiazinone
Indothiazinone (23) is an indolyl thiazolyl ketone that was discovered in cultures of novel myxobacteria strain 706 (family Sorangiineae) and was derived from compost (Jansen et al., 2014; Yang et al., 2017). Based on experimental data, indothiazinone was identified as a potential antiplatelet agent. It limits the thrombin and adenosine diphosphate-dependent distribution of human platelets on the fibrinogen matrix and can be used to develop antiplatelet agents with a new mode of action (Lee et al., 2016).
3-Hydroxyquinolin-8-yl hydrogen sulfate (24) and 3-[(4-carbamimidamido-butyl)-carbamoyl]-4-hydroxyphenyl hydrogen sulfate (25)
These low-molecular-weight anticoagulants, sulfated quinoline alkaloid (24) and acylated polyamine (25), were found in the extracts of Chinese red scolopendrium (Scolopendra subspinipes mutilans). These components were isolated, and their structures were established using chemical and physical methods (Block et al., 1984). Studies have shown that both bleached low-molecular-weight alkaloids (24 and 25) demonstrate antithrombotic and antiplatelet activities.
NATURAL SULFUR-CONTAINING HYDROCARBONS AS POTENTIAL ANTIPLATELET DRUGS
Ajoene (26A) and deoxygenated ajoene (26) were isolated from garlic, and their structures were determined and synthesized in 1984 in the Block laboratory (Fenwick et al., 1985a). The structures of ajoene (26A) and the ajoene analog (26) are shown in Figure 10, and the biological activities are shown in Table 3. An ajoene is known to be an organosulfur compound contained in the extracts of garlic (Allium sativum). Interestingly, the name comes from ajo, the Spanish word for garlic (Fenwick et al., 1985b, 1985c; Yoshida et al., 1979). The antifungal activity of garlic was first studied more than 40 years ago, and in experiments ajoene showed the strongest activity against Aspergillus niger and Candida albicans at <20 μg/ml (Apitz-Castro et al., 1986a).
As far back as 1986, ajoene (26A) and/or the ajoene analog (26) were shown to demonstrate platelet aggregation inhibition, which was later proven by many investigators (Apitz-Castro et al., 1986b; 1992; Agarwal, 1996; Jamaluddin et al., 1988). MacDonald and Langler (2000) found that some simple organosulfur compounds isolated from garlic showed antithrombotic activity, and this activity is associated with disulfides (Ledezma et al., 2009). A wide range of activities including anticancer, hepatoprotective, gastroprotective, antidiabetic, antiobesity, neuroprotective, and renoprotective properties have also been shown for ajoene and other sulfur-containing metabolites (Clapperton et al., 1989; Patiño-Morales et al., 2021; Shang et al., 2019). The structures of natural sulfur-containing antiplatelet drugs are shown in Figure 10, and the biologic activities are shown in Table 4. Figure 11 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities of (26A).
It is known that mammals belonging to the Mustela family emit foul-smelling secretions when in danger (Brinck et al., 1983; Crump, 1978). An analysis of these odors was studied, and di-2-butenyl disulfide (27) was detected in the volatiles of Mustela altaica (Andersen and Bernstein, 1980; Hamilton et al., 1999). Therefore, the odors of low-molecular-weight compounds of divalent sulfur are usually very intense and irritate people, as well as other animals; in addition, many of these second secondary metabolites are found in plants and are also represented in mammalian secretions (Wei et al., 2007). Figure 12 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities.
It is known that thiadiamondoids were first found in crude oil in small quantities, but their quantitative content increases with the thermochemical reduction of sulfates using sodium sulfate as an oxidizing agent in the presence of elemental sulfur and deionized water. This procedure suggests that thiamondoids or diamondolioliols are formed during the thermochemical treatment of crude oil, and sulfur-containing compounds [4,8,10-trithia-adamantane (28) and 5,10,13-trithia-diadamantane (32)] were isolated from such oil (Abu-Awwad, 2010; Dahl et al., 1999; Wei et al., 2011). Figure 13 shows the 3D graph of a wide range of confirmed and calculated pharmacological activities of compound 28.
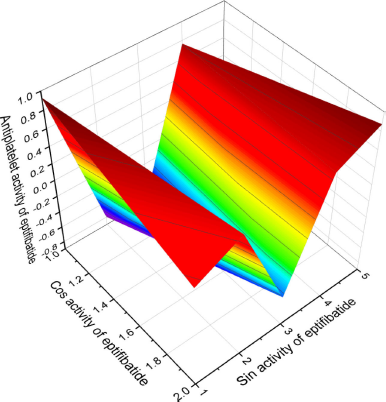 | Figure 8. 3D graph shows the predicted and calculated antiplatelet activity of the natural antiplatelet agent called eptifibatide (19), a cyclic heptapeptide from the disinterring protein (P22827), which was detected and isolated from the venom of a southeast rattlesnake (Sistrurus miliarius barbouri) with the highest degree of confidence, 92%. [Click here to view] |
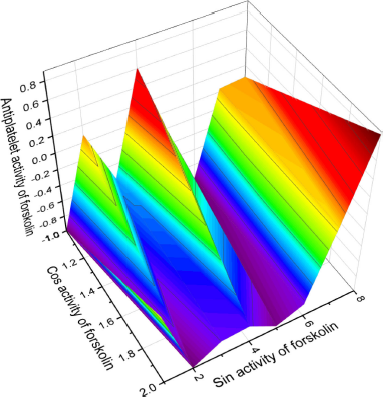 | Figure 9. 3D graph shows the predicted and calculated antiplatelet activity of the natural antiplatelet agent forskolin (21, also called coleonol), a labdanic diterpenoid isolated in the extracts from the roots of an Indian tropical plant (C. forskohlii), with the highest degree of confidence, 92.4%. [Click here to view] |
Four di- (29 and 30), tetra- (30, 34), and pentasulfide (36) derivatives were isolated from freshly cut garlic (A. sativum), onion (Allium cepa), and leek (Allium porrum), which exhibit antimicrobial and antibacterial activity (Abu-Lafi et al., 2004; Block, 1985; Block et al., 1996; Dembitsky et al., 2007a, 2007b; Kallio and Salorinne, 1990; Sommano et al., 2016; Vizer et al., 2015; Wijers et al., 1969). 1-Isopentyl-2-(prop-1-en-1-yl) disulfide (31) and compound (33) were found in three varieties of garlic (A. sativum) commonly used to produce essential oils in the northern Thai market, such as “Thai,” “Ping-Pong,” and “Chinese,” and in various sulfur-containing flavor volatiles in foods (Peppard, 1981; Shankaranarayana et al., 1974).
Sesquiterpene episulphide, 6,7-epithiogermacrene D (34) was isolated from hops and has shown anti-inflammatory and antineoplastic activity and demonstrated platelet aggregation properties (Hough et al., 1982; Lermusieau and Collin, 2003; Peppard et al., 1980; Poroikov et al. 2017; Xiong et al., 2018).
Diallyl disulfide (35, 4,5-dithia-1,7-octadiene) is a well-known metabolite that has a strong garlic smell and is produced by the decomposition of allicin, which is released by grinding garlic from garlic and some other plants of the Allium genus (Kallio and Salorinne, 1990). Diallyl disulfide has been shown to inhibit the growth and development of breast cancer, adenocarcinoma cells, and invasive ductal carcinoma (Bauer et al., 2014; Jo et al., 2008; Wratten et al., 1976; Yin et al., 2018).
It is known that cyclic natural polysulfides such as di-, tri-, and tetrathiacycloalkanes are found in marine green (chlorophytes), brown (phaeophytes), and red (rhodophytes) algae and some invertebrates (Dembitsky and Srebnik, 2002; Jiang et al., 2012; Gmelin et al., 1981; Rezanka and Dembitsky, 2002), plants (Da Silva et al., 2014; Huang et al., 2009; Lane et al., 2004; Sobik et al., 2007), and are also synthesized by some bacteria (Goeke, 2002; Ritzau et al., 1993; Schulz et al., 2010). Bioactive cyclic natural polysulfides (37, 38, 39, and 46; the structures are shown in Figure 14, and the biological activities are shown in Table 4) were identified from the extracts of two bacterial Cytophaga strains (CFB phylum) isolated from biofilms from the North Sea (Ritzau et al., 1993).
Volatile sulfur-containing hydrocarbons (41) and (45) were detected by GC-MS in grapefruit and orange juice (Cannon and Ho, 2018), and compound (47) was found among sulfur-containing metabolites in tropical fruits (Yoshida et al., 1987). Unusual sulfur-containing hydrocarbons α-mintlactone (43) and isomintlactone (44) and (50) were identified as minor and trace volatile components of peppermint oil Mitcham (Mentha piperita) (Takahashi et al., 1981; Tava et al., 2007). Mintsulfide has also been found in trace amounts in essential oils in various plant species such as Artemisia pallens, Bituminaria bituminosa, Cananga odorata, Cymbopogon winterianus, Hyssopus officinalis, Ocimum bacilicum, Matricaria chamomilla, Mentha spicata, Mentha piperita, Mentha arvensis, Stachys sylvatica, Solidago altissima, Rosa damascena, Piper nigrum, and Pelargonium graveolens (Murakami et al., 2006; Tava et al., 2007; Tirillini et al., 2004).
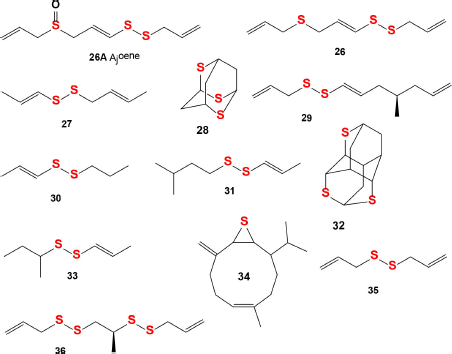 | Figure 10. Natural sulfur-containing compounds as potential antiplatelet agents. The pharmacological profiles and biological activities are shown in Table 4. [Click here to view] |
The Humulus lupulus plant is a species of flowering plant belonging to the family of hemp (Cannabaceae) and native to Europe, Asia, and North America. It is widely known throughout the world as a raw material for the brewing industry (Zanoli and Zavatti, 2008). Hop cones contain polyphenolic compounds and are widely used for canning beer and give it a characteristic aroma and taste. In addition, hop cones have long been used for medicinal purposes, especially for the treatment of sleep disorders, as a mild sedative (Orr and Sinninghe Damsté, 1990). Two bioactive compounds (48) and (49) were identified in hop (Humulus lupulus) which is cultivated all over the world for the brewery industry (Cannon and Ho, 2018).
Sulfur-containing compound (47) and triterpenoid (51) were found in fossil sour oil using high-resolution GC-MS (Orr and Sinninghe Damsté, 1990).
COMPARISON OF BIOLOGICAL ACTIVITIES OF NATURAL AND SYNTHETIC COMPOUNDS
Nowadays, it is generally accepted that drug-like compounds’ biological activities depend on their structural formula (Filimonov and Poroikov, 2008). Despite the activity cliffs (Stumpfe et al., 2019), which may be considered as violating this statement, structure–activity relationships (SAR) are widely used in medicinal chemistry for finding and optimization of novel pharmaceutical agents (Poroikov, 2020, Poroikov, 2020; Ermolenko et al., 2020; David 2002, Vil et al., 2019a,b,c,d).
Computer program PASS (Dembitsky et al., 2021; Filimonov et al., 2018; Pounina et al., 2021), whose development started over 30 years ago, currently predicts more than 5,000 pharmacological effects, molecular mechanisms of action, pharmacological effects, toxicity, and side effects, antitargets, transporters-related interactions, genes expression regulation, and metabolic terms (Druzhilovskiy et al., 2017). The average accuracy of PASS predictions achieved 96% due to the utilization of chemical descriptors and a robust mathematical approach for SAR analysis (Al Quntar et al., 2020; Dembitsky et al., 2021; Poroikov, 2020).
Freely available in the Internet web resource, PASS Online (PASS Online URL www.way2drug.com/passonline) is used by more than 20,000 users from 100 countries. Based on PASS predictions, the researchers identify the most promising assays for the synthesized compounds and select the virtually designed molecules with the required activities for synthesis (Al Quntar et al., 2020; Savidov et al., 2018).
In this study, we used PASS to estimate the analyzed natural and synthetic platelet aggregation inhibitors’ general pharmacological potential. Probabilities of belonging to the class of “actives” Pa were estimated for more than 4,200 pharmacological effects and molecular mechanisms of action. Pa values vary from zero to one; the higher the Pa value is, the higher the probability of confirming the predicted activity in the experiment is. On the other hand, estimated Pa values might be relatively small for some activities if the analyzed compound is less like the “actives” from the PASS training set. Thus, PASS prediction interpretation requires considering two contradictory issues: “high probability of activity” versus “high structural novelty.” The researcher decides which issue is more critical, depending on the task or the project (Dembitsky et al., 2020a, 2020b, 2020c; 2021a, 2021b; Poroikov, 2020).
Currently, tens of thousands of articles have been published that suggest synthesized or natural compounds as a preventative measure or treatment for dementia, but it turns out that more than 99% of the proposed compounds are not suitable for these purposes. The idea of using the PASS algorithm to test all drugs that are currently used in clinical medicine as antiplatelet agents was born long ago, but some time ago this idea was successfully implemented. By analyzing the data obtained using PASS for synthetic (1–18) and natural (19–25) antiplatelet drugs currently used in medicine, as well as natural sulfur-containing hydrocarbons (26–51), we can assume that sulfur-containing hydrocarbons have higher numerical values as platelet aggregation inhibitors than compounds (1–25).
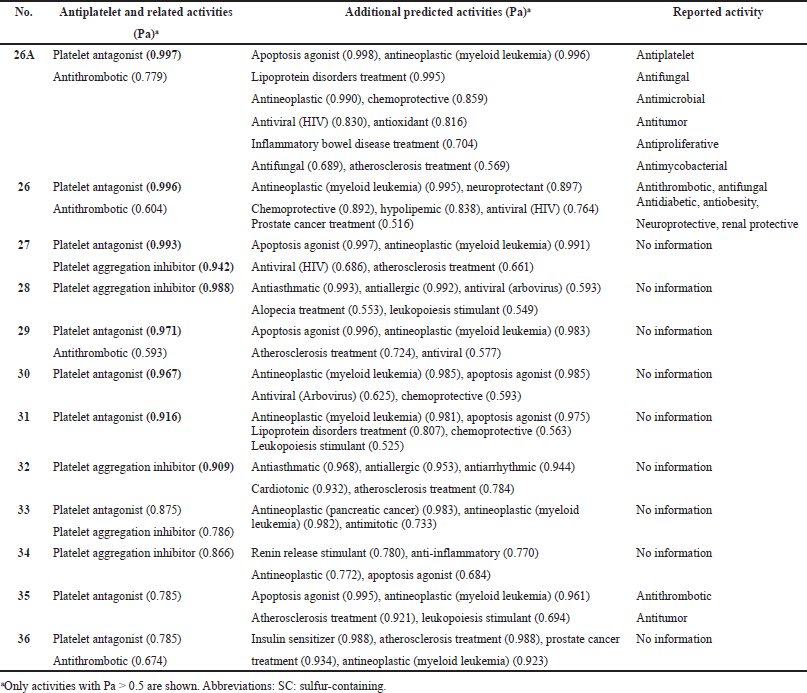 | Table 4. Pharmacological activities of natural SC hydrocarbons as potential antiplatelet drugs with high reliability from 78% to 99%. [Click here to view] |
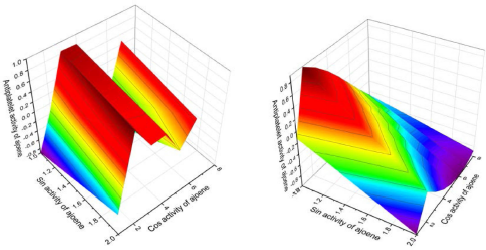 | Figure 11. 3D graph shows the predicted and calculated antiplatelet activity of ajoene (26A, left-hand side) or (E)-1-allyl-2-(3-(allylsulfinyl)-prop-1-en-1-yl)-disulfane, with 99.7% confidence, and deoxygenated ajoene or (E)-1-allyl-2-(3-(allylthio)-prop-1-en-1-yl)-disulfane (26, right side) showing the highest degree of confidence, 99.6%. [Click here to view] |
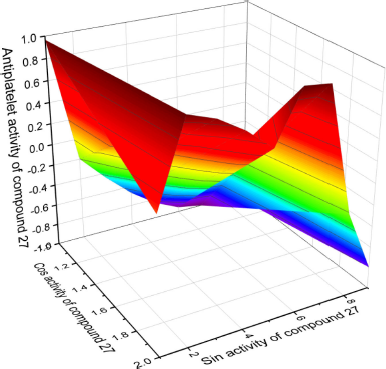 | Figure 12. 3D graph shows the predicted and calculated antiplatelet activity of sulfur-containing hydrocarbon (1-((E)-but-2-en-1-yl)-2-((E)-prop-1-en-1-yl)-disulfane, 27) showing the highest degree of confidence, 99.3%. [Click here to view] |
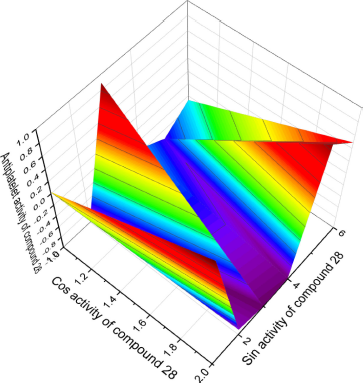 | Figure 13. 3D graph shows the predicted and calculated antiplatelet activity of sulfur-containing hydrocarbon (1S,3S,5S,7S)-2,4,6-trithiaadamantane, 28) showing the highest degree of confidence, 98.8%. [Click here to view] |
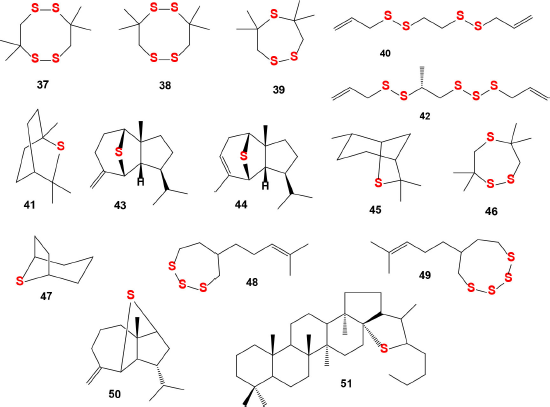 | Figure 14. Natural sulfur-containing compounds as potential antiplatelet agents. The pharmacological profiles and biological activities are shown in Table 5 . [Click here to view] |
In addition, sulfur-containing hydrocarbons have a more pronounced anticoagulant effect than many of the antiplatelet agents (7–25), ticlopidine (3), and clopidogrel (4), which help prevent the formation of thrombosis. By preventing these clots, platelet antagonists help prevent heart attacks, strokes, and other heart muscle complications.
As an example, we use beraprost (18) as an analog of prostacyclin PGI2, which according to experimental data is a strong antiplatelet inhibitor, and this is confirmed in data obtained by the PASS program. Given the reliable probability that beraprost demonstrates properties as an inhibitor of platelet aggregation is 96%, the antithrombotic effect is confirmed by 92.8% (Table 2). According to PASS, sulfur-containing hydrocarbons (26-32; for structures, see Figure 4 and biological activities are shown in Table 4) demonstrate properties as an inhibitor of platelet aggregation [compound 28 (probable confidence expressed as a percentage) = 98.8%, 27 = 94%, and 32 = 90%]. The effect as an antagonist of platelets is 26 = 99.6%, 27 = 99.3%, 29 = 97.1%, 30 = 96.7%, and 31 = 91.6%.
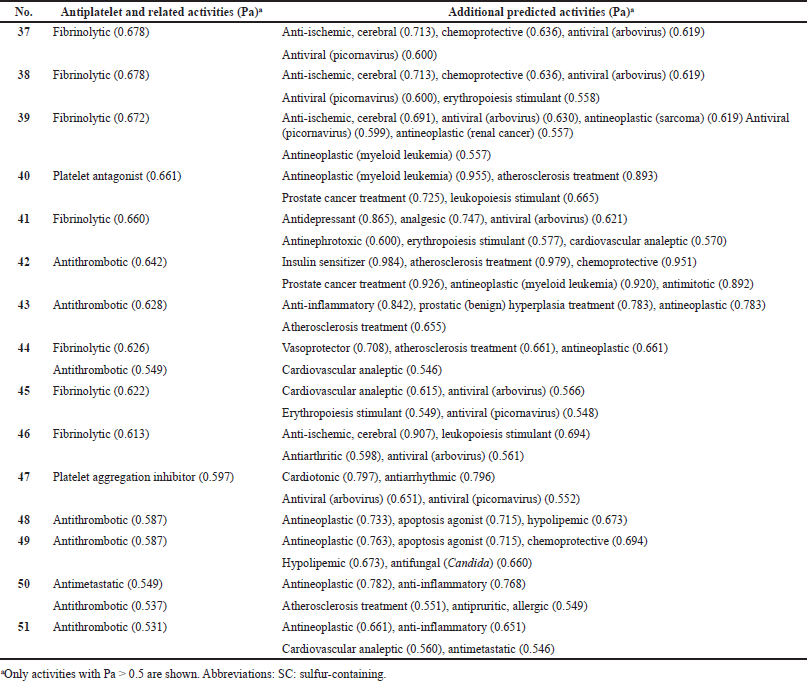 | Table 5. Pharmacological activities of natural SC hydrocarbons as potential antiplatelet drugs with low reliability from 53% to 80%. [Click here to view] |
Thus, the data presented using the unified PASS program of synthetic (1–18), natural (19–25), and sulfur-containing hydrocarbons (26–51) that are currently widely used show that sulfur-containing hydrocarbons, according to criteria determined by PASS algorithms, demonstrate different selective efficacy for the prevention or treatment of platelet dysfunction or aggregation.
CONCLUSION
Based on the data presented in this article, it was shown that well-known synthetic drugs such as aspirin, elinogrel, and beraprost, as well as natural drugs such as eptifibatide and vorapaxar, are widely used as platelet aggregation inhibitors. These drugs also show other activities such as antineoplastic, antiasthmatic, antiallergic, and antiarrhythmic activities with a high degree of confidence of over 90%. In addition, some show properties as an insulin sensitizer or can be used to treat atherosclerosis. Using the algorithms of quantitative structure–activity relationship, we conducted a detailed pharmacological analysis of the biological activities of these drugs for the treatment of platelet aggregation. This work was carried out with the aim of finding new drugs from natural sources that could supplement the arsenal of platelet aggregation inhibitors. This review is about the comparative pharmacological analysis of synthetic and natural drugs that are currently used in clinical medicine as antiplatelet agents in comparison with natural sulfur-containing hydrocarbons that are isolated from bacteria, plant, mammalian oils, and mineral oil. The data presented may have an optimistic forecast for the sulfur-containing hydrocarbons presented in this article, since they show a high antiplatelet effect and can be promising as medicines. It can be assumed that these agents may have other mechanisms of action and fewer side effects since these compounds are relatively simple chemical structures containing only sulfur heteroatom(s).
ACKNOWLEDGMENTS
The study (GTA and PVV) was carried out in the framework of the Program for Basic Research of State Academies of Sciences for 2021–2030, (No.122030100170-5).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abu-Awwad FM. QSAR study of the biologically active organosulfurs in natural products. J Chem, 2010; 7:S335–43. CrossRef
Abu-Lafi S, Dembicki JW, Goldshlag P, Hanuš L, Dembitsky VM. The use of the ‘Cryogenic’ GC/MS and on-column injection for study of organosulfur compounds of the Allium sativum. J Food Compos Anal, 2004; 17:235–45. CrossRef
Adnot S, Desmier M, Ferry N, Hanoune J, Sevenet T. Forskolin (a powerful inhibitor of human platelet aggregation). Biochem Pharmacol, 1982; 31:4071–4. CrossRef
Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev, 1996; 16:111–24. CrossRef
Akkaif MA, Daud NAA, Sha’aban A, Ng ML, Abdul Kader MAS, Noor DAM, Ibrahim B. The role of genetic polymorphism and other factors on clopidogrel resistance (CR) in an Asian population with coronary heart disease (CHD). Molecules, 2021a; 26:1987. CrossRef
Akkaif MA, Ng ML, SK Abdul Kader MA. A review of the effects of ticagrelor on adenosine concentration and its clinical significance. Pharmacol Rep, 2021b; 73:1551–64. CrossRef
Al Quntar AAA, Dweik H, Jabareen A, Gloriozova TA, Dembitsky VM. An Aminopyrrolidinyl phosphonates—a new class of antibiotics: Facile synthesis and predicted biological activity. Int J Org Chem, 2020; 10:170–81. CrossRef
Alzahrani T, Nguyen T, Ryan A, Dwairy A, McCaffrey J, Yunus R. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circ Cardiovasc Qual Outcomes, 2019; 12:e005597. CrossRef
Andersen KK, Bernstein DT. Sulfur compounds in Mustelids. In: Cavallini D, Gaull GE, Zappia V (eds.). Natural sulfur compounds, Springer, Boston, MA, pp 399–406, 1980. CrossRef
Apitz-Castro R, Badimon JJ, Badimon L. Effect of ajoene, the major antiplatelet compound from garlic, on platelet thrombus formation. Thromb Res, 1992; 68:145–55. CrossRef
Apitz-Castro R, Escalante J, Vargas R, Jain MK. Ajoene, the antiplatelet principle of garlic, synergistically potentiates the antiaggregatory action of prostacyclin, forskolin, indomethacin and dypiridamole on human platelets. Thromb Res, 1986a; 42:303–11. CrossRef
Apitz-Castro R, Ledezma E, Escalante J, Jain MK. The molecular basis of the antiplatelet action of ajoene: direct interaction with the fibrinogen receptor. Biochem Biophys Res Commun, 1986b; 141:145–50. CrossRef
Badjatiya A, Rao SV. Advances in antiplatelet and anticoagulant therapies for NSTE- ACS. Curr Cardiol Rep, 2019; 21:3–12. CrossRef
Baidildinova G, Nagy M, Jurk K, Wild PS, Cate HT, van der Meijden PEJ. Soluble platelet release factors as biomarkers for cardiovascular disease. Front Cardiovasc Med, 2021; 8:684920. CrossRef
Barnes H, Yeoh HL, Fothergill T, Burns A, Humbert M, Williams T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev, 2019; 5:CD012785. CrossRef
Bates ER. Antiplatelet therapy in patients with coronary disease and type 2 diabetes. N Engl J Med, 2019; 381:1373–5. CrossRef
Bauer D, Mazzio E, Soliman KF, Taka E, Oriaku E, Womble T, Darling-Reed S. Diallyl disulfide inhibits TNFα-induced CCL2 release by MDA-MB-231 cells. Anticancer Res, 2014; 34:2763–70.
Bednarek R, Selmi A, Wojkowska D, Karolczak K. Functional inhibition of F11 receptor (F11R/junctional adhesion molecule-A/JAM-A) activity by a F11R-derived peptide in breast cancer and its microenvironment. Breast Cancer Res Treat, 2020; 179:325–35. CrossRef
Berge E, Sandercock P. Anticoagulants versus antiplatelet agents for acute ischaemic stroke. Cochrane Database Syst Rev, 2002; 4:CD003242; doi: 10.1002/14651858.CD003242. CrossRef
Bhat SV, Bajqwa BS, Dornauer H, Souza NJ, Fehlhaber HW. Structures and stereochemistry of new labdane diterpiniods from Coleus forskohlii briq. Tetrahedron Lett, 1977; 18:1669–72. CrossRef
Block E. The chemistry of garlic and onions. Sci Amer, 1985; 252:114–9. CrossRef
Block E, Ahmad S, Jain MK, Crecely R, Apitz-Castro R, Cruz MR. (E,Z)-Ajoene: a potent antithrombotic agent from garlic. J Am Chem Soc, 1984; 106:8295–6. CrossRef
Block E, Bayer T, Naganathan S, Zhao S-H. Allium chemistry:? synthesis and sigmatropic rearrangements of alk(en)yl 1-propenyl disulfide S-oxides from cut onion and garlic. J Am Chem Soc, 1996; 118:2799–810. CrossRef
Bonadei I, Sciatti E, Vizzardi E, D’Aloia A, Raddino R, Metra M. New frontiers in the management of acute coronary syndromes: cangrelor and elinogrel. Recent Pat Cardiovasc Drug Discov, 2014; 9:22–7. CrossRef
Bonechi C, Lamponi S, Donati A, Tamasi G, Magnani A. Effect of resveratrol on platelet aggregation by fibrinogen protection. Biophys. Chem. 2017; 222:41–8. CrossRef
Born GVR. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature, 1962; 194:927–29. CrossRef
Bouasquevisque DS, Benavente OR, Shoamanesh A. Antiplatelet therapy in cerebral small vessel disease. Curr Neurol Neurosci Rep, 2019; 19:61. CrossRef
Brinck C, Erlinge S, Sandell M. Anal sac secretion in mustelids a comparison. J Chem Ecol, 1983; 9:727–45. CrossRef
Brodie BB, Yu TF, Burns JJ, Chenkin T, Paton BC, Steele JM, Gutman AB. Observations on G-25671, a phenylbutazone analogue (4-phenylthioethyl)-1,2-diphenyl 3,5- pyrazolidinedione). Proc Soc Exp Biol Med, 1954; 86:884–7. CrossRef
Brown T, Forster RB, Cleanthis M, Mikhailidis DP, Stansby G, Stewart M. Cilostazol for intermittent claudication. Cochrane Database Syst Rev, 2021; 6:CD003748. CrossRef
Burnouf T, Goubran HA. Regenerative effect of expired platelet concentrates in human therapy: an update. Transfusion Apheresis Sci, 2022; 103363 (in press). CrossRef
Callow AD. Cardiovascular disease 2005, the global picture. Vascul Pharm, 2006; 45:302–7. CrossRef
Cannon RJ, Ho CT. Volatile sulfur compounds in tropical fruits. J Food Drug Anal, 2018; 26:445–68. CrossRef
Carvalhal F, Cristelo RR, Resende DISP, Pinto MMM, Sousa E, Correia-da-Silva M. Antithrombotics from the Sea: Polysaccharides and beyond, Mar Drugs 2019; 17:E170. CrossRef
Charles O, Coolsaet B. Prevention of hemorrhage in prostatic surgery. Apropos of the study of the hemostatic activity in prostatectomy of a new molecule: beta-naphthoquinone monosemicarbazone (Naftazone). Ann Urol (Paris), 1972; 6:209–12.
Chelliah MV, Eagen K, Guo Z, Chackalamannil S, Xia Y, Tsai H. Himbacine-derived thrombin receptor antagonists: C7-spirocyclic analogues of vorapaxar. ACS Med Chem Lett, 2014; 5:561–5. CrossRef
Chen CM, Lu HC, Tung YT, Chen W. Antiplatelet therapy for acute respiratory distress syndrome. Biomedicines, 2020; 8:230. CrossRef
Chen J, Huang YW, Liu G, Afrasiabi Z, Sinn E, Padhye S, Ma Y. The cytotoxicity and mechanisms of 1,2-naphthoquinone thiosemicarbazone and its metal derivatives against MCF-7 human breast cancer cells. Toxicol Appl Pharmacol, 2004; 197:40–8. CrossRef
Clapperton BK, Minot EO, Crump DR. Scent lures from anal sac secretions of the ferret Mustela furo L. J Chem Ecol, 1989; 15:291–308. CrossRef
Clasby MC, Chackalamannil S, Czarniecki M, Doller D, Eagen K. Himbacine derived thrombin receptor antagonists: discovery of a new tricyclic core. Bioorg Med Chem Lett, 2007; 17:3647–51. CrossRef
Coccheri S. Antiplatelet drugs - do we need new options? With a reappraisal of direct thromboxane inhibitors. Drugs 2010; 70:887–908. CrossRef
Coghlan JG, Picken C, Clapp LH. Selexipag in the management of pulmonary arterial hypertension: an update. Drug Healthc Patient Saf, 2019; 11: 55-64. CrossRef
Coller BS. Anti-GPIIb/IIIa drugs: current strategies and future directions. Thromb Haemost, 2001; 86:427–43. CrossRef
Comerota AJ. Effect on platelet function of cilostazol, clopidogrel, and aspirin, each alone or in combination. Atheroscler Suppl, 2005; 6:13–9. CrossRef
Corvol JC, Durif F, Meissner WG, Azulay JP, Haddad R. Naftazone in advanced Parkinson’s disease: An acute L-DOPA challenge randomized controlled trial. Parkinsonism Relat Disord, 2019; 60:51–6. CrossRef
Crump DR. The major malodorous substance from the anal gland of the stoat (Mustela erminea). Tetrahedron Lett, 1978; 22:5233. CrossRef
Dahl J, Moldowan J, Peters K, Claypool GE, Rooney MA, Michael GE, Mello MR, Kohnen ML. Diamondoid hydrocarbons as indicators of natural oil cracking. Nature, 1999; 399:54–7. CrossRef
Da Silva CB, Simionatto E, Gebara SS, Re N, Rech K, Peres MTLP, Santos GO, Dias JFG, Zanin S, Miguel OG, Miguel MD. Sensitivity of Brachiaria decumbens and Ipomoea cordifolia to cyclic polysulfides from leaves of Microlobius foetidus. Allelopathy J, 2014; 33:213–26.
David JL. Recent advances in antiplatelet agents. Curr Med Chem, 2002; 9:577–89. CrossRef
Degraff AC, Lyon AF. Evaluation of dipyridamole (Persantin). Am Heart J, 1963; 65:423–4. CrossRef
De Luca L, Steg PG, Bhatt DL, Capodanno D, Angiolillo DJ. Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc, 2021; 10:e022125. CrossRef
Delwaide PA, Derouaux G, Heusghem C. The pharmacokinetics of naftazone-3H. Arch. Int Pharmacodyn Ther, 1974; 208(2):357–61.
Dembitsky VM. Hydrobiological aspects of saturated, methyl-branched, and cyclic fatty acids derived from aquatic ecosystems: Origin, distribution, and biological activity. Hydrobiology, 2022; 1:89–110. CrossRef
Dembitsky VM. In silico prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar Drugs, 2021; 19:650. CrossRef
Dembitsky VM, Abu-Lafi S, Hanuš LO. Occurrence of sulfur-containing fatty acids in Allium sativum. Nat Prod Commun, 2007a; 2(7):771–4. CrossRef
Dembitsky VM, Abu-Lafi S, Hanus LO. Separation of sulfur-containing fatty acids from garlic, Allium sativum, using serially coupled capillary columns with consecutive nonpolar, semipolar, and polar stationary phases. Acta Chromatograph, 2007b; 19:206–16.
Dembitsky VM, Dzhemileva L, Gloriozova T, D’yakonov V. Natural and synthetic drugs used for the treatment of the dementia. Biochem Biophys Res Commun, 2020a; 524(3):772–83. CrossRef
Dembitsky VM, Ermolenko E, Savidov N, Gloriozova TA, Proroikov VV. Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: origin, structures and biological activity. Molecules, 2021b; 26(3):68. CrossRef
Dembitsky VM, Gloriozova TA, Poroikov VV. Antitumor profile of carbon-bridged steroids (CBS) and triterpenoids. Mar Drugs, 2021a; 19(6):324. CrossRef
Dembitsky VM, Gloriozova TA, Poroikov VV. Pharmacological profile of natural and synthetic compounds with rigid adamantane-based scaffolds as potential agents for the treatment of neurodegenerative diseases. Biochem Biophys Res Commun, 2020b; 529(4):1225–41. CrossRef
Dembitsky VM, Srebnik M. Use of serially coupled capillary columns with different polarity of stationary phases for the separation of the natural complex volatile mixture of the marine red alga Corallina elongata. Biochemistry (Moscow), 2002; 67:1068–74. https://doi.org/10.1023/A:1020594507571. CrossRef
Desborough MJR, Keeling DM. The aspirin story—from willow to wonder drug. Br J Haematol, 2017; 177:674–83. CrossRef
De Souza NJ, Dohadwalla AN, Reden Ü. Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev, 1983; 3:201–19. CrossRef
Diener HC. Antiplatelet drugs in secondary prevention of stroke. Int J Clin Pract, 1998; 52:91–7.
Dobesh PP, Varnado S, Doyle M. Antiplatelet agents in cardiology: a report on aspirin, clopidogrel, prasugrel, and ticagrelor. Curr Pharm Des, 2016; 22:1918–32. CrossRef
Dockray JS. Some toxic effects of aspirin. Br Med J, 1905; 2:1692–3. CrossRef
Dogne JM, de Leval X, Benoit P, Delarge J, Dogne JM, de Leval X, Benoit P, Delarge J, Masereel B, Masereel B, David JL. Recent advances in antiplatelet agents. Curr Med Chem, 2002; 9:577–89. CrossRef
Drew DA, Chan AT. Aspirin in the prevention of colorectal neoplasia. Ann Rev Med, 2021; 72:415–30. CrossRef
Druzhilovskiy DS, Rudik AV, Filimonov DA, Gloriozova TA, Lagunin AA, Dmitriev AV, Pogodin PV, Dubovskaya VI, Ivanov SM, Tarasova OA, Bezhentsev VM, Murtazalieva KA, Semin MI, Maiorov IS, Gaur AS, Sastry GN, Poroikov VV. Computational platform Way2Drug: from the prediction of biological activity to drug repurposing. Russ Chem Bull, 2017; 66:1832–41. CrossRef
Egbertson MS, Chang CT, Duggan ME, Gould RJ. Non-peptide fibrinogen receptor antagonists. 2. Optimization of a tyrosine template as a mimic for Arg-Gly-Asp. J Med Chem, 1994; 37:2537–51. CrossRef
Eisert WG. Dipyridamole in antithrombotic treatment. Adv Cardiol, 2012; 47:78–86. CrossRef
Ermolenko EV, Imbs AB, Gloriozova TA, Poroikov VV, Sikorskaya TV, Dembitsky VM. Chemical diversity of soft coral steroids and their pharmacological activities. Marine Drugs 2020; 18:613. CrossRef
Fareed J, Moorman MJ, III, Jeske W, Hoppensteadt D. Defibrotide interaction with newer oral anticoagulant and antiplatelet drugs. Blood, 2013; 122:4804. CrossRef
Féliste R, Delebassée D, Simon MF, Chap H, Defreyn G, Vallée E, Douste-Blazy L, Maffrand JP. Broad-spectrum anti-platelet activity of ticlopidine and PCR 4099 involves the suppression of the effects of released ADP. Thromb Res, 1987; 48:403–15. CrossRef
Fenwick GR, Hanley AB, Whitaker JR. The genus allium—Part 1. CRC Crit Rev Food Sci Nutr, 1985a; 22:199–271. CrossRef
Fenwick GR, Hanley AB, Whitaker JR. The genus allium—Part 2. CRC Crit Rev Food Sci Nutr, 1985b; 22:273–377. CrossRef
Fenwick GR, Hanley AB, Whitaker JR. The genus allium—Part 3. CRC Crit Rev Food Sci Nutr, 1985c; 22: 1–73. CrossRef
Ferlini M, Mauri S, Rossini R. Dual antiplatelet therapy after TAVR: a drop in the bucket? Int J Cardiol, 2019; 280:46–8. CrossRef
Filimonov DA, Druzhilovskiy DS, Lagunin AA, Gloriozova TA, Rudik AV, Dmitriev AV, Pogodin PV, Poroikov VV. Computer-aided prediction of biological activity spectra for chemical compounds: opportunities and limitations. Biomed Chem Res Meth, 2018; 1:e00004. CrossRef
Filimonov DA, Poroikov VV. Probabilistic approach in activity prediction. In: Varnek A, Tropsha A (eds.). Chemoinformatics approaches to virtual screening. RSC Publishing, Cambridge, UK, pp 182–216, 2008. CrossRef
FitzGerald GA. Dipyridamole. New Engl J Med, 1987; 316:1247–57. CrossRef
Florescu C, Mustafa ER, Târtea EA, Florescu DR, Albu VC. Antiplatelet therapy in secondary ischemic stroke prevention—a short review. Rom J Morphol Embryol, 2019; 60:383–7.
Flores-Runk P, Raasch RH. Ticlopidine and antiplatelet therapy. Ann Pharmacother, 1993; 27:1090–8. CrossRef
Franchini M, Mannucci PM. New antiplatelet agents: why they are needed. Eur J Intern Med, 2009; 20:733–8. CrossRef
Fuccella LM, Corvi G, Moro E, Pogliani E, Tamassia V, Tosolini G. Pharmacokinetic, bioavailability and pharmacodynamic study of indobufen (K 3920), an inhibitor of platelet aggregation, after a single dose in man. Eur J Clin Pharmacol, 1979; 12:323–7. CrossRef
Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost, 2008; 99:466–72. CrossRef
Gachet C, Stierlé A, Cazenave JP, Ohlmann P, Lanza F, Bouloux C, Maffrand JP. The thienopyridine PCR 4099 selectively inhibits ADP-induced platelet aggregation and fibrinogen binding without modifying the membrane glycoprotein IIb-IIIa complex in rat and in man. Biochem Pharmacol, 1990; 40:229–38. CrossRef
Gallus AS. Aspirin and other platelet-aggregation inhibiting drugs. Med J Aust, 1985; 142:41–7. CrossRef
Garcia-Rafanell J, Morell J. Inhibitory effect of 2-acetoxy-4-trifluoromethylbenzoic acid (triflusal) and aspirin on platelet aggregation in man and rat: in vitro study. Therapie 1977; 32: 337-344.
Garcia-Rafanell J, Planas JM, Puig-Parellada P. Comparison of the inhibitory effects of acetylsalicylic acid and trifusal on enzymes related to thrombosis. Arch Int Pharmacodyn Ther, 1979; 237:343–50.
Genecand L, Wacker J, Beghetti M. Selexipag for the treatment of pulmonary arterial hypertension. Expert Rev Respirat Med, 2021; 15:583–95. CrossRef
Gmelin R, Susilo R, Fenwick GR. Cyclic polysulphides from Parkia speciose. Phytochemistry, 1981; 20:2521–3. CrossRef
Goa KL, Noble S. Eptifibatide: a review of its use in patients with acute coronary syndromes and/or undergoing percutaneous coronary intervention. Drugs, 1999; 57:439–62. CrossRef
Goeke A. Sulfur-containing odorants in fragrance chemistry. Sulfur Rep, 2002; 23:243–78. CrossRef
Gong J, Shang J, Yu H, Wan Q, Su D, Sun Z, Liu G. Tirofiban for acute ischemic stroke: systematic review and meta-analysis. Eur J Clin Pharmacol, 2020; doi: 10.1007/s00228-019-02817-8. CrossRef
Goto S. Cilostazol: potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl, 2005; 6:3–11. CrossRef
Greif S. Clinical and experimental studies on persantin in coronary diseases. Wien Med Wochenschr, 1960; 110:463–6.
Gurbel PA, Bliden KP, Antonino MJ, Stephens G, Gretler DD. The effect of elinogrel on high platelet reactivity during dual antiplatelet therapy and the relation to CYP2C19*2 genotype: first experience in patients. J Thromb Haemost, 2010a; 8:43–53. CrossRef
Gurbel PA, Jeong YH, Tantry US. Vorapaxar: a novel protease-activated receptor-1 inhibitor. Expert Opin Investig Drugs, 2011; 20:1445–53. CrossRef
Gurbel PA, Kereiakes DJ, Tantry US. Ticagrelor for the treatment of arterial thrombosis. Expert Opin Pharmacother, 2010b; 11:2251–9. CrossRef
Gurbel PA, Tantry US. Prasugrel, a third generation thienopyridine and potent platelet inhibitor. Curr Opin Investig Drugs, 2008; 9:324–36.
Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke, 2019; 50:773–8. CrossRef
Hamilton DW, Arogo J, Brake JT, Freeman DW. Understanding farmstead odors: an annotated review. Prof Anim Sci, 1999; 15:203–10. CrossRef
Hankey GJ. Clopidogrel: a new safe and effective antiplatelet agent. But unanswered questions remain. Med J Aust, 1997; 167:120–1. CrossRef
Hartman GD, Egbertson MS, Halczenko W, Laswell WL, Duggan ME. Non-peptide fibrinogen receptor antagonists. 1. Discovery and design of exosite inhibitors. J Med Chem, 1992; 35:4640–2. CrossRef
Herrmann J, Fayad Abou A, Müller R. Natural products from myxobacteria: novel metabolites and bioactivities. Nat Prod Rep, 2017; 34:135–60. CrossRef
Hiatt WR. The US experience with cilostazol in treating intermittent claudication. Atheroscler Suppl, 2005; 6:21–31. CrossRef
Hidaka H, Hagiwara M, Watanabe Y. Trend in the development of new inhibitors of cyclic nucleotide metabolism and their clinical application. Nihon Rinsho, 1991; 49:961–7.
Hirsch GE, Viecili PRN, de Almeida AS, Nascimento S, Porto FG, Otero J. Natural products with antiplatelet action. Curr Pharm Des, 2017; 23:1228–46. CrossRef
Hough JS, Briggs DE, Stevens R, Young TW. The chemistry of hop constituents. Malting Brewing Sci, 1982; 2:422–55. CrossRef
Huang HP, Lin WH, Chen SG, Chen LZ. Comparative efficacy and safety of nine anti-platelet therapies for patients with ischemic stroke or transient ischemic attack: a mixed treatment comparison. Mol Neurobiol, 2017; 54:1456–66. CrossRef
Huang XY, Wang Q, Liu HL, Zhang Y, Xin GR. Diastereoisomeric macrocyclic polydisulfides from the mangrove Bruguiera gymnorrhiza. Phytochemistry, 2009; 70:2096–100. CrossRef
Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther, 2009; 27:259–74. CrossRef
Jafri SM. Antiplatelet and anticoagulant therapy in acute myocardial infarction. Henry Ford Hosp Med J, 1991; 39:195–9.
Jamaluddin MP, Krishnan LK, Thomas A. Ajoene inhibition of platelet aggregation: possible mediation by a hemoprotein. Biochem Biophys Res Commun, 1988; 153:479–86. CrossRef
Jansen R, Mohr KI, Bernecker S, Stadler M, Müller R. Indothiazinone, an indolyl thiazolyl ketone from a novel myxobacterium belonging to the Sorangiineae. J Nat Prod, 2014; 77:1054–60. CrossRef
Jialiken D, Qian L, Ren S, Wu L, Xu J, Zou C. Combined therapy of hypertensive nephropathy with ginkgo leaf extract and dipyridamole injection and antihypertensive drugs. a systematic review and meta-analysis. Medicine (Baltimore), 2021; 100(19):e25852. CrossRef
Jiang C-S, Müller WEG, Schröder HC, Guo Y-W. Disulfide- and multisulfide- containing metabolites from marine organisms. Chem Rev, 2012; 112:2179–07. CrossRef
Jo HJ, Song JD, Kim KM, Cho YH, Kim KH, Park YC. Diallyl disulfide induces reversible G2/M phase arrest on a p53-independent mechanism in human colon cancer HCT-116 cells. Oncol Rep, 2008; 19:275–80. CrossRef
Ju H, Zhang C, Lu W. Progress in heterologous biosynthesis of forskolin. J Ind Microbiol Biotechnol, 2021; 48:kuab009. CrossRef
Junemann P. Persantin, a new substance for therapy of coronary insufficiency. Munch Med Wochenschr, 1959; 101:340–1.
Kalantzi K, Ntalas IV, Chantzichristos VG, Tsoumani ME. Comparison of triflusal with aspirin in the secondary prevention of atherothrombotic events; a randomised clinical trial. Curr Vasc Pharmacol, 2019; 17(6):635–43. CrossRef
Kallio H, Salorinne L. Comparison of onion varieties by headspace gas chromatography- mass spectrometry. J Agric Food Chem, 1990; 38:1560–4. CrossRef
Kapil N, Datta YH, Alakbarova N, Bershad E, Selim M. Antiplatelet and anticoagulant therapies for prevention of ischemic stroke. Clin Appl Thromb Hemost, 2017; 23:301–18. CrossRef
Keyser A, Platelet aggregation inhibitors in neurology. Pharm World Sci, 1993; 15:243–51. CrossRef
Kim SW, Choi KJ, Park JY, Yoon SH, Lee JK. Neuroprotective effect of triflusal and its main metabolite, 2-hydroxy-4-trifluoromethylbenzoic acid (HTB), in the postischemic brain. Neurosci Lett, 2017; 643:59–64. CrossRef
Kitchens BP, Snyder RJ, Cuffy CA. A literature review of pharmacological agents to improve venous leg ulcer healing. Compendium Clin Res Pract, 2020; 32(7):195–207.
Knabe J. Chemistry of cardiac drugs. Med Monatsschr, 1964; 18:54–8.
Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J, 2017; 38:785–91. CrossRef
Kuwano K, Hashino A, Asaki T, Hamamoto T. 2-[4-[(5,6-diphenylpyrazin-2-yl)- (isopropyl)amino]-butoxy]-N-(methylsulfonyl)-acetamide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther, 2007; 322:1181–8. CrossRef
Kuzniatsova N, Shantsila E, Blann A, Lip GY. A contemporary viewpoint on aspirin resistance. Ann Med, 2012; 44:773–83. CrossRef
Kwon SU, Kim JS. Antithrombotic therapy. Front Neurol Neurosci, 2016; 40:141–51. CrossRef
Laine M, Paganelli F, Bonello L. P2Y12-ADP receptor antagonists: Days of future and past. World J Cardiol, 2016; 26:327–32. CrossRef
Lamuela-Raventós RM, Romero-Pérez AI, Waterhouse AL, de La Torre-Boronat MC. Direct HPLC analysis of cis- and trans-resveratrol and piceid isomers in Spanish red Vitis vinifera wines. J Agric Food Chem, 1995; 43:281–3. CrossRef
Lan P, Herlt AJ, Willis AC, Taylor WC, Mander LN. Structures of New Alkaloids from Rain Forest Trees Galbulimima belgraveana and Galbulimima baccata in Papua New Guinea, Indonesia, and Northern Australia. ACS Omega, 2018; 3:1912−21. CrossRef
Lane N, Weidenhamer JD, Romeo JT. Zapoteca formosa: Sulfur chemistry and phytotoxicity. J Chem Ecol, 2004; 30:425–37. CrossRef
Ledezma E, Wittig O, Alonso J, Cardier JE. Potentiated cytotoxic effects of statins and ajoene in murine melanoma cells. Melanoma Res, 2009; 19:69–74. CrossRef
Lee W, Lee J, Kulkarni R. Antithrombotic and antiplatelet activities of small-molecule alkaloids from Scolopendra subspinipes mutilans. Sci Rep, 2016; 6:21956. CrossRef
Lermusieau G, Collin S. Volatile sulfur compounds in hops and residual concentrations in beer—a review. J Am Soc Brew Chem, 2003; 61:109–13. CrossRef
Liu J, Xu D, Xia N, Hou K, Chen S, Wang Y, Li Y. Anticoagulant activities of indobufen, an antiplatelet drug. Molecules, 2018; 23:E1452. CrossRef
Lordan R, Tsoupras A, Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: potential role of antiplatelet agents. Blood Rev, 2021; 45:100694. CrossRef
MacDonald JA, Langler RF. Structure-activity relationships for selected sulfur-rich antithrombotic compounds. Biochem Biophys Res Commun, 2000; 273:421–4. CrossRef
Marczewski MM, Postula M, Kosior D. Novel antiplatelet agents in the prevention of cardiovascular complications—focus on ticagrelor. Vasc Health Risk Manag, 2010; 6:419–29. CrossRef
Margulies EH, White AM, Sherry S. Sulfinpyrazone: a review of its pharmacological properties and therapeutic use. Drugs, 1980; 20:179–97. CrossRef
McArdle P, Erxleben A. Sublimation–a green route to new solid-state forms. CrystEngComm, 2021; 23:5965–75. CrossRef
McGregor L, Chignier E, Bloy C, Rousselle C, Peltier-Pujol F, McGregor JL. Effect of naftazone on in vivo platelet function in the rat. Platelets, 1999; 10(1):66–70. CrossRef
McNeely W, Goa KL. Triflusal. Drugs, 1998; 55(6):823–45. CrossRef
Mendis S, Davis S, Norrving B. The World Health Organization global status report on noncommunicable diseases 2014; One more Landmark step in the cmbat against stroke and vascular disease. Stroke, 2015; 46:e121–2.
Menozzi A, Merlini PA, Ardissino D. Tirofiban in acute coronary syndromes. Expert Rev Cardiovasc Ther, 2005; 3:193–206. CrossRef
Michelson AD. Advances in antiplatelet therapy. Hematol Am Soc Hematol Educ Program, 2011; 1:62–9. CrossRef
Milionis H, Liontos A, Vemmos K, Spengos K. Antiplatelet treatment in stroke: new insights. Curr. Pharm. Des. 2016; 22(29): 4617-4626. CrossRef
Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex. Heart Inst. J. 2007; 34:179–86.
Mirzadeh Z. Faber CL, Schwartz MW. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Ann Rev Pharmacol Toxicol, 2022; 62:55–84. CrossRef
Mohamed Saleem A. Methods of isolation and analysis of forskolin from Coleus forskohlii. In: Ramawat K, Mérillon JM (eds.). Natural products, Springer, Berlin, Heidelberg, pp 3325–43, 2013. CrossRef
Montgomery DB, Ogryzlo MA. Comparison of the uricosuric effect of sulfinpyrazone (anturan) and zoxazolamine (flexin). Can Med Assoc J, 1960; 83:885–8.
Montinari MR, Minelli S, De Caterina R. The first 3500 years of aspirin history from its roots—a concise summary. Vascul Pharmacol, 2019; 113:1–8. CrossRef
Mori M, Geirsson A. Antiplatelet therapy after coronary artery bypass grafting. JAMA, 2018; 320:1035–6. CrossRef
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation, 2016; 133:e38–48.
Müller KA, Geisler T, Gawaz M. Elinogrel, an orally and intravenously available ADP- receptor antagonist. How does elinogrel affect a personalized antiplatelet therapy? Hamostaseologie, 2012; 32:191–4. CrossRef
Murakami A, Darby P, Javornik B, Pais MSS, Seigner E, Lutz A, Svoboda P. Molecular phylogeny of wild Hops, Humulus lupulus L. Heredity, 2006; 97:66–74. CrossRef
Muruganantham S, Krishnaswami V, Alagarsamy S, Kandasamy R. Anti-platelet drug-loaded targeted technologies for the effective treatment of atherothrombosis. Current Drug Targets, 2021; 22:399–419. CrossRef
Nakamura A, Yamada T, Asaki T. Synthesis and evaluation of N-acylsulfonamide and N-acylsulfonylurea prodrugs of a prostacyclin receptor agonist. Bioorg Med Chem, 2007; 15:7720–5. CrossRef
Nakura M, Miyashita T, Yamamoto Y, Takada S, Kanou S, Tajima H. Inhibitory effects of beraprost sodium in murine hepatic sinusoidal obstruction syndrome. Anticancer Res, 2020; 40:5171–80. CrossRef
Nana M, Morgan H, Moore S, Lee ZX, Ang E, Nelson-Piercy C. Antiplatelet therapy in pregnancy: a systematic review. Pharmacol Res, 2021; 168:105547. CrossRef
Natsuaki M, Kimura T. Antiplatelet therapy after percutaneous coronary intervention—past, current and future perspectives. Circulation J, 2021; doi:10.1253/circj.CJ-21-0751 CrossRef
Nicolau JC, Baracioli LM, Giugliano RP. Ticagrelor for the prevention of ischemic events in patients with prior myocardial infarction and peripheral artery disease. Expert Opin Pharmacother, 2018; 19:1013–9. CrossRef
Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost, 2005; 31:184–94. CrossRef
Olas B, Wachowicz B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets, 2005; 16:251–60. CrossRef
Onuora, S. Defibrotide inhibits NET-mediated thrombosis in APS models. Nat Rev Rheumatol, 2022; 18:63. CrossRef
Orhan IE, Deniz FSS. Natural products and extracts as xantine oxidase inhibitors—a hope for gout disease? Curr Pharm Design, 2021; 27:143–58. CrossRef
Orr WL, Sinninghe Damsté JS. Geochemistry of sulfur in petroleum systems. In: Geochemistry of sulfur in fossil fuels. Chapter 1, pp 2–29, 1990; doi: 10.1021/bk-1990-0429.ch001. CrossRef
O’Shea JC, Tcheng JE. Eptifibatide: a potent inhibitor of the platelet receptor integrin glycoprotein IIb/IIIa. Expert Opin Pharmacother, 2002; 3:1199–210. CrossRef
Patiño-Morales CC, Jaime-Cruz R, Sánchez-Gómez C, Corona JC, Hernández-Cruz EY, Kalinova-Jelezova I, Pedraza-Chaverri J, Maldonado PD, Silva-Islas CA, Salazar-García M. Antitumor effects of natural compounds derived from Allium sativum on neuroblastoma: an overview. Antioxidants, 2021; 11:48. CrossRef
Passacquale G, Sharma P, Perera D, Ferro A. Antiplatelet therapy in cardiovascular disease: current status and future directions. Br J Clinic Pharmacol, 2022; doi:10.1111/bcp.15221. CrossRef
Pezet R, Gindro K, Viret O, Spring J-L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol Mol Plant Pathol, 2004; 65:297–303. CrossRef
Palmer SC, Di Micco L, Razavian M, Craig JC, Perkovic V. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev, 2013; 4:CD008834. CrossRef
Paniccia R, Priora R, Liotta AA, Abbate R. Platelet function tests: a comparative review. Vasc Health Risk Manag, 2015; 11:133–48. CrossRef
PASS Online URL. http://www.way2drug.com/passonline/ (Accessed 22 January 2022).
Peppard TL. Volatile organosulfur compounds in hops and hop oils: a review. J Inst Brewing, 1981; 87:376–85. CrossRef
Peppard TL, Sharpe FR, Elvidge JA. Chemistry of hop constituents. Part 42. The formation and characterisation of sesquiterpene episulphides in the essential oil of hops. J Chem Soc., Perkin Trans 1, 1980; 2:311–3. CrossRef
Persellin RH, Schmid FR. The use of sulfinpyrazone in the treatment of gout. JAMA, 1961; 175:971–5.
Poole RM, Elkinson S. Vorapaxar: first global approval. Drugs, 2014; 74:1153–63. CrossRef
Poroikov VV. Computer-aided drug design: from discovery of novel pharmaceutical agents to systems pharmacology. Biochemistry (Moscow), 2020; 14:216–27. CrossRef
Poroikov VV, Gloriozova TA, Dembitsky VM. Natural occurring thiirane containing compounds: Origin, chemistry, and their pharmacological activities. Pharmaceut Chem J, 2017; 4:107–20.
Pounina TA, Gloriozova TA, Savidov N, Dembitsky VM. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar Drugs, 2021; 19:240. CrossRef
Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol, 1997; 80:11B–20B. CrossRef
Ray A, Najmi A, Khandelwal G. Prasugrel versus ticagrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a systematic review and meta-analysis of randomized trials. Cardiovasc Drugs Ther, 2021; 35:561–74. CrossRef
Reilly MP, Mohler ER, 3rd. Cilostazol: treatment of intermittent claudication. Ann Pharmacother, 2001; 35:48–56. CrossRef
Rezanka T, Dembitsky VM. Eight-membered cyclic 1,2,3-trithiocane derivatives from Perophora viridis, an Atlantic tunicate. Eur J Org Chem, 2002; 22:2400–4. CrossRef
Ritzau M, Keller M, Wessel P, Stetter KO, Zeeck A. Secondary metabolites by chemical screening, 25. New cyclic polysulfides from hyperthermophilic archaea of the genus Thermococcus. Liebigs Annalen der Chem, 1993; 12: 871-876. CrossRef
Romero-Pérez AI, Ibern-Gómez M, Lamuela-Raventós RM, de La Torre-Boronat MC. Piceid, the major resveratrol derivative in grape juices. J Agric Food Chem, 1999; 47(4):1533–6. CrossRef
Roskoski R, Jr. The role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disorders. Pharmacol Res, 2018; 129:65–83. CrossRef
Sandler RS. Aspirin and other nonsteroidal anti-inflammatory agents in the prevention of colorectal cancer. Important Adv Oncol, 1996; 1:123–37.
Sapio L, Gallo M, Illiano M, Chiosi E, Naviglio D, Spina A, Naviglio S. The natural cAMP elevating compound forskolin in cancer therapy: is it time? J Cell Physiol, 2017; 232:922–7. CrossRef
Savidov N, Gloriozova TA, Poroikov VV, Dembitsky VM. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: origin and biological activities. Steroids, 2018; 140:114–24. CrossRef
Scherer LD, Schafer ES. Prophylaxis and treatment of central nervous system (CNS) acute lymphoblastic leukemia. In Litzow MR, Raetz EA (eds.). Clinical management of acute lymphoblastic leukemia, Springer, Cham, Switzerland, 2022; doi:10.1007/978-3-030-85147-7_11 CrossRef
Schrör K. The basic pharmacology of ticlopidine and clopidogrel. Platelets, 1993; 4:252–61. CrossRef
Schrör K. Acetylsalicylic acid. 2nd edition, Wiley-VCH Verlag GmbH & Co. K GaA, Weinheim, Germany, 2016.
Schulz S, Dickschat JS, Kunze B, Wagner-Dobler I, Diestel R, Sasse F. Biological activity of volatiles from marine and terrestrial bacteria. Mar Drugs, 2010; 8:2976–87. CrossRef
Scott DM, Norwood RM, Parra D. P2Y12 inhibitors in cardiovascular disease: focus on prasugrel. Ann Pharmacother, 2009; 43:64–76. CrossRef
Scott LJ. Selexipag: First global approval. Drugs, 2016; 76:413–8. CrossRef
Shang A, Cao SY, Xu XY, Gan RY, Tang GY, Corke H, Mavumengwana V, Li HB. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods, 2019; 8:E246. CrossRef
Shankaranarayana ML, Raghavan B, Abraham KO, Natarajan CP, Brodnitz HH. Volatile sulfur compounds in food flavors. CRC Crit Rev Food Technol, 1974; 4:3395–435. CrossRef
Sharmin N, Nganwuchu CC, Nasim MT. Targeting the TGF-β signaling pathway for resolution of pulmonary arterial hypertension. Trends Pharmacol Sci, 2021; 42:510–3. CrossRef
Shen L, Patel JA, Norel X, Moledina S, Whittle BJ, von Kessler K, Sista P, Clapp LH. Pharmacology of the single isomer, esuberaprost (beraprost-314d) on pulmonary vascular tone, IP receptors and human smooth muscle proliferation in pulmonary hypertension. Biochem Pharmacol, 2019; 166:242–52. CrossRef
Siller-Matula JM, Petre A, Delle-Karth G, Huber K, Ay C, Lordkipanidzé M, De Caterina R, Kolh P, Mahla E, Gersh BJ. Impact of preoperative use of P2Y12 receptor inhibitors on clinical outcomes in cardiac and non- cardiac surgery: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care, 2017; 6:753–70. CrossRef
Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev, 2012; 21:321–7. CrossRef
Siu H, Kaliyadan A, Fischman DL, Nardone E, Poll D, Savage MP. Use of prasugrel in the setting of clopidogrel hypersensitivity: case report and systematic review of the literature. Platelets, 2016; 27:824–7. CrossRef
Skoro-Sajer N, Lang IM. Selexipag for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother, 2014; 15:429–36. CrossRef
Sobik P, Grunenberg J, Böröczky K, Laatsch H, Wagner-Döbler I, Schulz S. Identification, synthesis, and conformation of tri- and tetrathiacycloalkanes from marine bacteria. J Org Chem, 2007; 72:3776–82. CrossRef
Sommano S, Saratan N, Suksathan R, Pusadee T. Chemical composition and comparison of genetic variation of commonly available Thai garlic used as food supplement. J Appl Bot Food Qual, 2016; 89:235–42.
Storey RF, Sinha A. Cangrelor for the management and prevention of arterial thrombosis. Expert Rev Cardiovasc Ther, 2016; 14:991–9. CrossRef
Stumpfe D, Hu H, Bajorath J. Evolving concept of activity cliffs. ACS Omega, 2019; 4:14360–8. CrossRef
Szczeklik A, Musia? J, Undas A, Sanak M. Aspirin resistance. J Thromb Haemost, 2005; 3:1655–62. CrossRef
Takahashi K, Muraki S, Yoshida T. Synthesis and distribution of (?)-mintsulfide, a novel sulfur-containing sesquiterpene. Agric Biol Chem, 1981; 45:129–32. CrossRef
Tamassia V, Corvi G, Fuccella LM, Moro E, Tosolini G, Tremoli E. Indobufen (K 3920), a new inhibitor of platelet aggregation: effect of food on bioavailability, pharmacokinetic and pharmacodynamic study during repeated oral administration to man. Eur J Clin Pharmacol, 1979; 12:329–33. CrossRef
Tang L, Tang X, Yang Q. The application of tirofiban in the endovascular treatment of acute ischemic stroke: a meta-analysis. Cerebrovasc Dis, 2021; 50:121–31. CrossRef
Ten Cate H, Guzik TJ, Eikelboom J. Pleiotropic actions of factor Xa inhibition in cardiovascular prevention: mechanistic insights and implications for anti-thrombotic treatment. Cardiovasc Res, 2021; 117:2030–44. CrossRef
Tantry US, Liu F, Chen G, Gurbel PA. Vorapaxar in the secondary prevention of atherothrombosis. Expert Rev Cardiovasc Ther, 2015; 13:1293–305. CrossRef
Tao DL, Yunga ST, Williams CD, Owen J, McCarty T. Aspirin and antiplatelet treatments in cancer. Blood, 2021; 137(23):3201–11. CrossRef
Tava A, Pecetti L, Ricci M, Pagnotta MA, Russi L. Volatile compounds from leaves and flowers of Bituminaria bituminosa (L.) Stirt. (Fabaceae) from Italy. Flavour Frag J, 2007; 22:363–70. CrossRef
Tello-Montoliu A, Jover E, Rivera J, Valdés M, Angiolillo DL, Marín F. New perspectives in antiplatelet therapy. Curr Med Chem, 2012; 19:406–27. CrossRef
Tempelhof MW, Benzuly KH, Fintel D, Krichavsky MZ. Eptifibatide-induced thrombocytopenia: with thrombosis and disseminated intravascular coagulation immediately after left main coronary artery percutaneous coronary angioplasty. Tex Heart Inst J, 2012; 39:86–91.
Tetzlaff H, Tetzlaff D. Treatment of coronary insufficiency with persantin. Med Monatsschr, 1959; 13:229–31.
Thebault JJ, Blatrix CE, Blanchard JF, Panak EA. Effects of ticlopidine, a new platelet aggregation inhibitor in man. Clin Pharmacol Ther, 1975; 18:485–90. CrossRef
Tirillini B, Pellegrino R, Bini LM. Essential oil composition of Stachys sylvatica L. from Italy. Flavour Frag J, 2004; 19:330–2. CrossRef
Ueno M, Ferreiro JL, Angiolillo DJ. Update on the clinical development of cangrelor. Expert Rev Cardiovasc Ther, 2010; 8:1069. CrossRef
Ungar L, Rodriguez F, Mahaffey KW. Vorapaxar: emerging evidence and clinical questions in a new era of PAR-1 inhibition. Coron Artery Dis, 2016; 27:604–15. CrossRef
Vane SJ. Aspirin and other anti-inflammatory drugs. Thorax, 2000; 55:S3–9.
Varadarajan SG, Hunyara JL, Hamilton NR, Kolodkin AL, Huberman AD. Central nervous system regeneration. Cell, 2022; 185:77–94. CrossRef
Verbeuren TJ. Terutroban and endothelial TP receptors in atherogenesis. Med Sci (Paris), 2006; 22:437–43. CrossRef
Vil V, Gloriozova TA, Terentev A, Savidov N, Dembitsky VM. Hydroperoxides derived from marine sources: Origin and biological activities. Appl Microbiol Biotechnol, 2019a; 103(1):1627–42. CrossRef
Vil V, Gloriozova TA, Poroikov VV, Terentev A, Savidov N, Dembitsky VM. Naturally occurring of α,β-diepoxy-containing compounds: origin, structures, and biological activities. Appl Microbiol Biotechnol, 2019b; 103(8):3249–64. CrossRef
Vil V, Terentev A, Al Quntar AAA, Gloriozova TA, Savidov N, Dembitsky VM. Oxetane-containing metabolites: origin, structures and biological activities. Appl Microbiol Biotechnol, 2019c; 103(6):2449–67. CrossRef
Vil V, Terentev A, Savidov N, Gloriozova TA, Poroikov VV, Pounina TA, Dembitsky VM. Hydroperoxy steroids and triterpenoids derived from plant and fungi: origin, structures and biological activities. J Steroid Biochem Mol Biol, 2019d; 190:76–87. CrossRef
Vinazzer H. Clinical-experimental studies on the inhibition of thrombocyte function with acetylsalicylic acid and with indobufen. Folia Haematol Int Mag Klin Morphol Blutforsch 1979; 106:783–96.
Vizer SA, Sycheva ES, Al Quntar AAA, Kurmankulov NB, Yerzhanov KB, Dembitsky VM. Propargylic sulfides: synthesis, properties, and application. Chem Rev, 2015; 115(3):1475–1502. CrossRef
Wang A. Review of vorapaxar for the prevention of atherothrombotic events. Expert Opin Pharmacother, 2015; 16:2509–22. CrossRef
Wei Z, Mankiewicz P, Walters C, Qian K, Phan NT, Madincea ME, Nguyena PTH. Natural occurrence of higher thiadiamondoids and diamondoidthiols in a deep petroleum reservoir in the Mobile Bay gas field. Org Geochem, 2011; 42:121–33. CrossRef
Wei Z, Moldowan JM, Fago F, Dahl JE, Cai C, Peters KE. Origins of thiadiamondoids and diamondoidthiols in petroleum. Energy Fuels, 2007; 21:3431–6. CrossRef
Wijers HE, Boelens H, van der Gen A, Brandsma L. Synthesis and some properties of 1-alkenyl alkyl disulfides and di(1-alkenyl) disulfides. Recueil des Travaux Chim, 1969; 88: 519–29. CrossRef
Wermuth CG, Aldous D, Raboisson R, Rognan D (Eds.). The practice of medicinal chemistry, 4th edition, Academic Press, Amsterdam, The Netherlands, p 902, 2015.
Weir HK, Anderson RN, S.M. King C, Soman A, Thompson TD. Heart disease and cancer deaths—trends and projections in the United States, 1969–2020. Prev Chronic Dis, 2016; 13: E157. CrossRef
Weissmann G. Aspirin Sci Amer, 1991; 264:84–91. CrossRef
Wick JY. Aspirin: a history, a love story. Consult Pharm, 2012; 27:322–9. CrossRef
Wilhelmi G, Currie JP. Pharmacological and clinical characteristics of G 25671 (Geigy Basel), a new preparation of the pyrazolidine series. Schweiz Med Wochenschr, 1954; 84:1315–8.
Williamson RT. On the treatment of glycosuria and diabetes mellitus with aspirin. Br Med J, 1902; 2:1946–8. CrossRef
Wratten SJ, Faulkner DJ. Cyclic polysulfides from the red alga Chondria californica. J Org Chem, 1976; 41:2465–7. CrossRef
Wright J., Wall HK, Ritchey MD. Million hearts 2022 small steps are needed for cardiovascular disease prevention. JAMA, 2018; 320:1857–8. CrossRef
Wu KK. Aspirin and other cyclooxygenase inhibitors: new therapeutic insights. Semin Vasc Med, 2003; 3:107–12. CrossRef
Xiang Q, Pang X, Liu Z, Yang G, Tao W, Pei Q, Cui Y. Progress in the development of antiplatelet agents: Focus on the targeted molecular pathway from bench to clinic. Pharmacol Ther, 2019; 203:107393. CrossRef
Xiong T, Liu XW, Huang XL, Xu XF, Xie WQ, Zhang SJ, Tu J. Tristetraprolin: A novel target of diallyl disulfide that inhibits the progression of breast cancer. Oncol Lett, 2018; 15:7817–27. CrossRef
Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev, 2021; 73:924–67. CrossRef
Yang C, Kwon S, Kim S-J, Jeong M, Park J-Y, Park D, Hong SJ, Jung JW, Kim C. Identification of indothiazinone as a natural antiplatelet agent. Chem Biol Drug Des, 2017; 90:873–82. CrossRef
Yao SK, Ober JC, Ferguson JJ, Maffrand JP, Anderson HV, Buja LM, Willerson JT . Clopidogrel is more effective than aspirin in preventing coronary artery reocclusion after thrombolysis. Trans Assoc Am. Physicians, 1993; 106:110–9.
Yin X, Feng C, Han L, Ma Y, Jiao Y, Wang J. Diallyl disulfide inhibits the metastasis of type II esophageal?gastric junction adenocarcinoma cells via NF-κB and PI3K/AKT signaling pathways in vitro. Oncol Rep, 2018; 39:784–94. CrossRef
Yoshida S, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S. Antifungal activity of ajoene derived from garlic. Appl. Environ. Microbiol. 1987; 53: 615-617. CrossRef
Yoshida T, Muraki S, Takahashi K, Kato T, Kabuto C, Suzuki T, Uyehara T, Ohnuma T. Isolation and X-ray crystal structure of mintsulphide, a novel sulphur-containing sesquiterpene. J Chem Soc Chem Commun, 1979; 3:512–4. CrossRef
Yun HY, Kang W, Lee BY, Park S, Yoon YR, Yeul Ma J, Kwon KI. Semi-mechanistic modelling and simulation of inhibition of platelet aggregation by antiplatelet agents. Basic Clin Pharmacol Toxicol, 2014; 115:352–9. CrossRef
Zakeri AS, Nimjee SM. Use of antiplatelet agents in the neurosurgical patient. Neurosurg Clin N Am, 2018; 29:517–27. CrossRef
Zanoli P, Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J Ethnopharmacol, 2008; 116:383–96. CrossRef
Zeb I, Krim N, Bella J. Role of CYP2C19 genotype testing in clinical use of clopidogrel: is it really useful? Expert Rev Cardiovasc Ther, 2018; 16:369–77. CrossRef
Ziada KM, Moliterno DJ. Dual antiplatelet therapy: is it time to cut the cord with aspirin? JAMA, 2019; 321:2409–411. CrossRef