INTRODUCTION
In recent decades, therapeutic delivery systems have been extensively researched and analyzed with the aim of enhancing the effectiveness and safety of therapeutic substances for diverse biomedical use. The implementation of nanostructured systems has been proposed as an effective strategy to address the limitations associated with drug delivery, namely inadequate drug dispersion, insufficient penetration across biological barriers, and unintended off-target effects [1–3]. The incorporation of bioinspired nanomaterials into the surfaces of drug carriers presents a promising approach for modulating the drug carrier-biological systems interaction [4].
At the nanoscale level, diverse biological events take place, consequently rendering engineered nanomaterials as valuable tools capable of regulating bio-interfaces and bioprocesses to enhance therapeutic efficacy. The utilization of nanoparticle drug carriers has been shown to enhance the stability and prolong the serum half-lives of drugs, thereby ensuring sustained therapeutic drug concentrations. Nanostructured thin films, patches, and devices have emerged as wearable and implantable materials, wherein the bio interfaces are engineered to cater to the reduction of the foreign body response and inflammation, while simultaneously facilitating sustained drug release.
The extracellular membrane surrounds cells and holds significant importance in regenerative medicine and drug delivery, providing a scaffold for cell growth and regulating drug activity. Integrins bind to ligands and are crucial for cell-extracellular matrix (ECM) interactions, affecting both embryonic development and mature tissue function. ECM molecules bind to cell surface receptors, including integrins. Drug delivery system (DDS) can use ECM molecules as ligands for drug targeting.
Types of ECM molecules
The ECM consists of collagen, elastin, and glycoproteins such as laminin and fibronectin as shown in Figure 1. Collagen and fibronectin play key roles in tissue strength and cellular processes. Fibronectin governs a myriad of cellular processes, encompassing adhesion, movement, proliferation, and recuperation. This structure is made of connected homogenous parts with disulfide bridges. Subunit III in Figure 1 has 100 amino acids organized in β sheets, with domains for binding integrin and heparin [5]. Laminin improves attachment, movement, neurology, and blood vessel formation. It is in the basement membrane with type IV collagen, 19 forms are created by three subunits (α, β, and γ) of glycoproteins. Each isoform has unique roles related to cell differentiation, morphology, motion, and tissue viability. Isoforms attach to integrin receptors to influence cells and interact with other ECM proteins [6]. The ECM forms the structural framework with polysaccharides such as chondroitin sulfate, heparin sulfate, dermatan sulfate, keratin sulfate, and hyaluronic acid. Cell interaction is indirect through intermediary proteins. Heparin consists of GlcNAc and GlcA segments in a polysaccharide configuration, produced by connecting to cell proteins sodium deoxycholate and gel permeation chromatography. Syndecans and glypicans absorb ligands, while heparin interacts with proteins to enhance biological activities and receptor binding. Heparin and proteins interact to create useful DDSs. Heparin hydrogels often deliver growth factors for tissue regeneration. Incomplete cellular receptors such as CD44 cling to chondroitin sulfate and hyaluronic acid, which is formed by repetitive segments of glucoronate combined with β-1.3 (GalNAc) and D-glucuronic acid bonded to β-1,3. Chondroitin sulfate’s role differs based on sulfation and affects signal regulation, cell development, and central nervous system function. Hyaluronic acid has no sulfation and has a unique pattern of repeat units. Hyaluronic acid boosts precise and prolonged drug delivery by contributing to tissue structure, cellular function, and regeneration [7]. It is widely used as a pharmaceutical transporter due to its strong attraction to cellular receptors. Gene therapy in the liver, kidneys, lymphatic vessels, and tumors. Various types of ECM molecules along with their functions are listed in Table 1.
BIOINSPIRED NANOFIBER-BASED DDSS
The uses of bioinspired nanofiber-based therapeutic delivery systems have appeared as a highly promising approach in the field of nanomedicine. The aforementioned systems employ nanofibers, which are modeled after fibers present in organisms that are alive, such as collagen or spider silk, to convey medicinal substances to designated sites within the body [11]. Nanofibers exhibit distinct characteristics, rendering them potentially advantageous for drug delivery purposes, such as their expanded surface area and proportionality factor, as well as their capacity to imitate the ECM of tissues. Diverse methodologies including electrospinning, self-assembly, and templating can effectively facilitate the manufacturing of bioinspired nanofibers. The controlled drug administration through nanofibers may be achieved through the modulation of their physical and chemical characteristics, including but not limited to, fiber diameter, surface chemistry, and porosity [12]. Nanofibers possess the capability of being subjected to functionalization with targeting moieties, including antibodies or proteins, to selectively bind to selected cells or tissues of interest [13]. The utilization of bioinspired nanofiber-based DDSs has exhibited remarkable potential in sundry domains, including but not limited to tissue regeneration, cancer therapy, and regenerative medicine. Notably, the production of biocompatible nanofibers can be achieved through deliberate material selection and meticulous optimization of the production process. The careful deliberation of the physiochemical properties of the nanofibers and the selection of the drug and its accompanying release mechanism are imperative for the successful implementation of the drug-delivery strategy. Scalability and commercial viability are crucial factors to contemplate in the advancement of bioinspired nanofiber-mediated drug delivery mechanisms. For the purpose of facilitating sizable manufacturing of these systems, it is imperative that the production process exhibit scalability and cost-effectiveness. The utilization of these systems in human subjects necessitates regulatory sanction, thereby introducing an augmented degree of intricacy to their development.
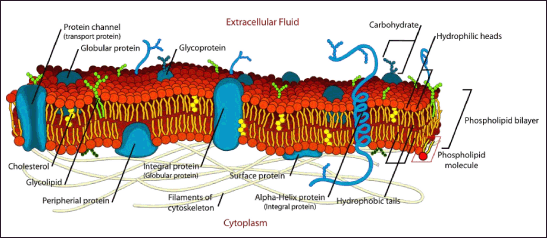 | Figure 1. Systematic representation of extracellular membrane. [Click here to view] |
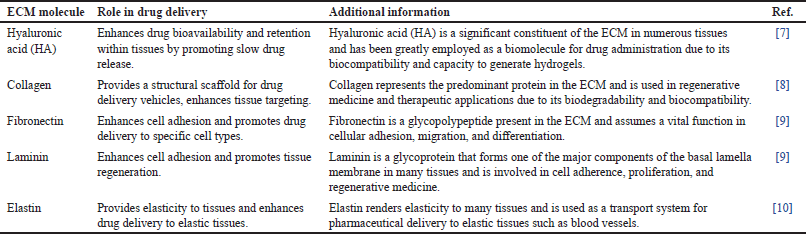 | Table 1. Different types of ECM molecules. [Click here to view] |
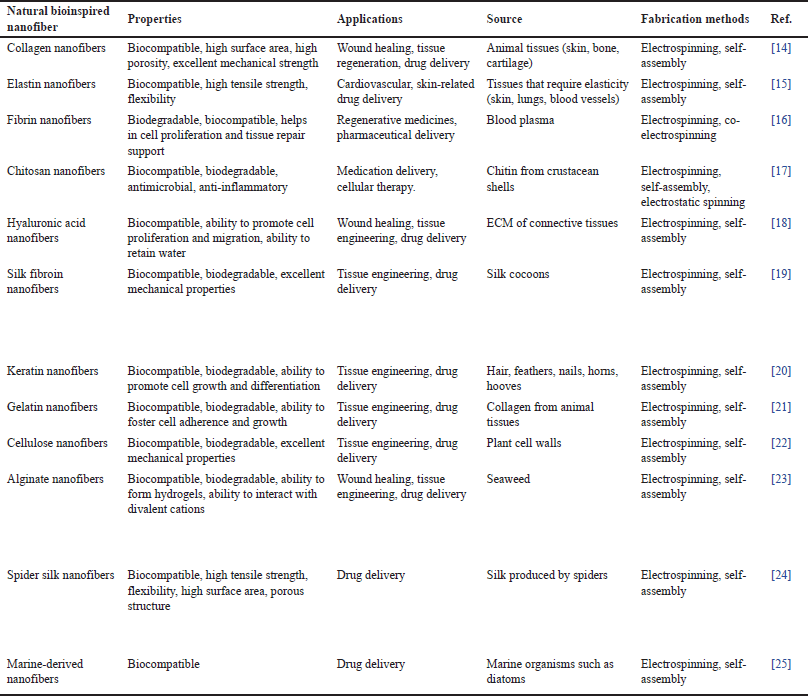 | Table 2. Naturally obtained bioinspired nanofibers. [Click here to view] |
Natural polymers used in nanofiber fabrication
Bioinspired nanofiber can be fabricated using both organic and artificial polymers. Organic polymers are often preferred due to their non-toxicity to biological systems and ability to reproduce ECM characteristics. Table 2 represents different types of natural nanofibers that mimic ECM.
Synthetic polymers used in nanofiber fabrication
The versatility and capacity for the customization of synthetic polymers render them a highly prevalent option for the manufacturing of bioinspired nanofibers. A range of synthetic polymers appears to be commonly utilized as constituents in the fabrication of nanofibers. Table 3 represents different types of synthetic nanofibers that mimic ECM.
LITERATURE SURVEY
The utilization of nanofibers that are bioinspired has achieved greater attention recently as a result of their exceptional properties and prospective applications across diverse fields. Over time, many investigations have been conducted on bioinspired nanofibers, intricately examining their attributes, fabrication techniques, and usage. This literature review endeavors to furnish a comprehensive inventory of current studies pertaining to bioinspired nanofibers. Specifically, it seeks to elucidate their synthesis methodologies, properties, and applications, as well as to accentuate their potential within various fields.
In research conducted in 2022, a nanofibre magnet was created to load and deliver bovine serum albumin (BSA) using Iron (III) oxide magnetic nanoparticles coated with polyvinyl alcohol (PVA) and collagen which led to faster BSA release within a period of 3 hours [42]. Collagen from Rana chensinensis skin in China was used as a drug carrier in another study in two volatile compound mixtures systems–blended nanofibers and core-shell nanofibers by blending random copolymer star copolymers (RCSC) and poly-L-Lactic acid (PLLA) in HFIP [43]. Both scaffolds sustained control release for 80 hours, but coaxial RCSC/PLLA were better due to superior mechanical properties and sustained effect. Poly(ε-caprolactone) (PCL)/Col nanofibers loaded with artesunate were prepared to study ART’s anti-crystallization and release behaviors [44]. The sustained drug administration up to 48 hours follows the Fickian mechanism analyzed by the Korsmeyer-Peppas equation. The study conducted in 2019 hemostatic patches with a tranexamic acid (TXA) and PVA: chitosan nanofiber (1:1) showed CT of 167? ± ?6?s, while 3:2 showed CT of 210? ±? 10?s) [45]. Drug release via Fickian diffusion in TXA-chitosan nanofibers can produce hemostatic membranes for clinical and battlefield settings. In another study by Gouda M in 2022 ST-CH nanofibers were made by electrospinning with CH/PVP and characterized by scanning electron microscopy (SEM) containing chlorinated N-amido-cholestano-aziridine and acetylated N-amido-cholestano-aziridine showed strong efficacy against Staphylococcus aureus and E. Transport exponent estimated that solvent migration and polymer chain relaxation were involved in ST-CH nanofibers, which are promising DDSs [46].
Evaluative research where hybridizing hyaluronic acid blends with cumulative drug release (CDF) nanofiber mats were tested against bacteria and CDF/Cur against S. aureus DHFR enzyme receptor. Cur and CDF had similar anti-bacterial properties; their nanofiber mats released 25% and 37% of Cur and CDF in vitro, respectively [47]. CDF remains both antibacterial and effective against cancer, making it a promising choice for treatment. Dadras Chomachayi in his investigation prepared nanofiber comprising of silk fibroin (SF) and gelatin (GT) was further analyzed and loaded with triethyl orthoacetate (TEO) and dichloromethane (DCMH) as antibacterials [48]. TEO was released in 3 hours, while DCMH had a 48-hours sustained release. In 2021, keratin/poly (butylene succinate) blend. Rhodamine B-doped with keratin/poly (butylene succinate) electrospun mats were examined [49]. Keratin electrophoresis proved the solvent’s inability to degrade protein whereas else Keratin/phosphate-buffered saline blends depict higher polymer orientation with shear stress. RhB release increased with higher keratin content and drug diffusion combined. Nano scaffolds with drug-loaded Poly (lactic-co-glycolic acid) (PLGA) and PLGA/GT were tested for fasting blood glucose (FBF) release. More GT led to increased FBF release and aligned scaffolds released FBF slower than randomly oriented ones. Crosslinking reduces burst release of FBF in PLGA/GT nanofibrous scaffold. pH of the buffer solution can alter the polymer state and affect the FBF release rate [50]. Zeynep Aytac and his co-researchers combined Sprague-Dawley female rats (SFS) with HPβCD in hydroxypropyl cellulose (HPC) nanofibers by electrospinning, and the resulting SFS/HPβCD-IC complex was analyzed using differential scanning calorimetry (DSC) and Job’s plot for formation and stoichiometry [51]. More SFS released from HPC/SFS/HPβCD-IC-NF than HPC/SFS-NF due to increased solubility. PCL-HPC/SFS/HPβCD-IC-NF had slower SFS release compared to sandwiches of HPC/SFS/HPβCD-IC-NF. Another examination was carried out where Alginates were added to wound dressing PVA with Glutaraldehyde which reinforce nanofibers during electrospinning [52]. Dexpanthenol was added to polyvinyl alcohol and sodium alginate to speed up healing. Chitosan at 1% in the shell improved drug release. Dexpanthenol-added PVA/SA/Triton-Chitosan nanofibers improved fibroblast morphology and attachment, making them ideal for tissue engineering. BAP2 fiber reduces inflammation and promotes angiogenesis, proven effective in pharmacokinetic tests. To test the healing properties Huang X and his co-fellows proved that this biomaterial can improve diabetic wound healing by mimicking ECM and healing full-thickness epidermis and dermis wounds in murine models of diabetes [53].
In 2013, researchers showed the mouse cells on TCH/HNTs/PLGA nanofibers were cytocompatible and released antimicrobial TCH for 42 days. PCL/f-CNOs nanofibers were created via Forcespinning® by Narsimha Mamidi and fellow researchers in 2020 [54]. PCL/f-CNOs released doxorubicin in response to pH, reaching 87% at pH 6.5 and 99% at pH 5.0 in 15 days [55]. SA/Polyethylene oxide (PEO) nanofibers with VC were made in 2019 using co-electrospinning and coaxial electrospinning and the drug release was evaluated which showed a controlled release of the drug [54], [56]. In another research analysis, PET nanostructured membranes were electrospuned and silver nanoparticles were added for antimicrobial purposes. Silver fibers have potential as an antimicrobial with lower toxicity, reduced inflammation, and better antibiofilm activity [57]. In 2021, CH/PANI nanofibers are made by polymerizing aniline with CH, producing a suitable drug encapsulation network. Ketoprofen was added to the hybrid and subsequently tested for release in three different pH buffers resembling oral administration (2, 6.7, and 7.4) [58]. pH affected drug release rate; various models studied kinetics. Veronika Pavli?áková and associate researchers in 2018 made a nanofibrous elastic material from PCL, Gel, and HNTs using green chemistry principles which showed improved mechanical properties w/0.5?wt% and Safer halloysite nanotubes nanofibers for drugs [59]. PU/HPC nanofibers with donepezil hydrochloride were made in 2017 for transdermal drug delivery and characterized using SEM, DSC, and Pascal mercury porosimetry [60]. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay revealed skin tolerance to the PU/HPC nanofiber mat with no irritation. The work of Zhang X demonstrated Dexamethasone loaded into poly (lactic acid) fibers and integrated into Poly(trimethylene carbonate) (PTMC) resin to produce hybrid films [61]. These hybrids have superior mechanical and UV protection properties compared to PTMC-only films. A scaffold of PCL, Poly(glycerol sebacate) (PGS), Hydroxyapatite nanoparticles, and simvastatin was created in 2020 to mimic bone ECM and enhance bone cell regeneration [62]. SIM had sustained release via diffusion. In vitro tests showed enhanced cell proliferation and adhesion with PCL-PGS-HA for improved regeneration.
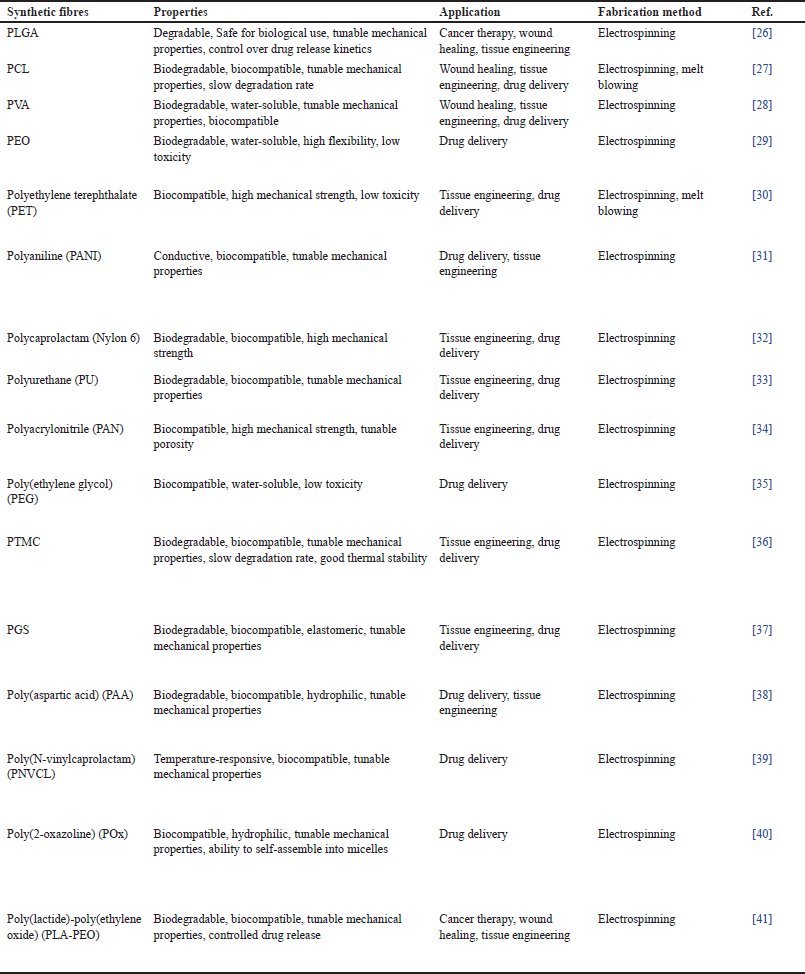 | Table 3. Synthetically obtained bioinspired nanofibers. [Click here to view] |
It can be seen that Nanofiber-based DDSs have been explored in recent years for their unconventional properties, including elevated surface/volume proportion, tunable pore size, and ability to control drug release. Various types of both organic and synthetic polymers are utilized to produce nanofibers for therapeutics, and fibers are then loaded with different types of drugs, including antimicrobial agents, chemotherapeutic drugs, and tissue regenerating agents. The drug release mechanism has been analyzed using mathematical models, and the diffusion rate has been shown to be affected by a variety of factors, such as polymer type, drug loading, and nanofiber morphology. These studies present revelations into the development of nanofiber used for pharmaceutical delivery and their probable use in medicine.
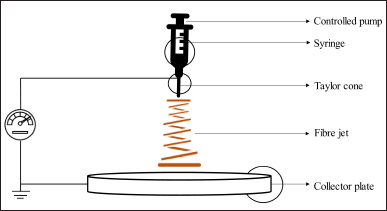 | Figure 2. Nanofiber production through electrospinning. [Click here to view] |
FABRICATION METHODS FOR BIOINSPIRED NANOFIBER
To accurately replicate the structural and functional properties of the ECM, the process of producing nanofibers must be appropriately adapted. The utilization of fabrication techniques that draw inspiration from biological processes has gained noteworthy popularity for the purpose of enhancing therapeutic delivery based on nanofibers. The employment of these techniques has enabled the production of nanofibers possessing regulated morphology, mechanical characteristics, and surface composition, all of which are pivotal in facilitating the effective administration of therapeutic agents.
Electrospinning
This is a widely used technique that involves the application of an electrostatic field to a polymer dispersion or melt to produce a charged jet that is collected on a fixed target depicted in Figure 2.
The resulting fibers exhibit diameters that span from tens of nanoscale to micrometer scale, and their characteristics can be customized for precise drug delivery purposes through meticulous regulation of process factors [63]. Electrospinning is a highly versatile method that offers precise control over fiber diameter, orientation, and surface morphology. It is compatible with an extensive variety of polymers, enabling the creation of fibers with diverse compositions and drug-loading capacities. This technique involves the stretching and formation of fibers using an electrified droplet [64]. The essential equipment comprises a high-voltage electrical source, a capillary tube fitted with a compact pipette or needle, and a metallic collecting screen. One electrode is positioned inside the polymer solution, while a second electrode is connected to the collector. At the tip of the capillary tube, an electric field is employed, which retains the polymer solution in position as a result of surface tension forces. This electric potential elicits a charge on the liquid. As the electric field strength increases, the previously curved fluid surface at the tip of the capillary stretches, eventually forming a cone-like structure called the Taylor cone. Once the field reaches a certain threshold, a threshold is reached where the repelling electrostatic force becomes more influential than the surface tension force in the system. Consequently, a charged fluid jet is forcefully ejected from the tip of the Taylor cone as a direct result of this phenomenon. This phenomenon has noteworthy implications for a wide range of practical applications and warrants further investigation from a scientific standpoint. The polymer solution jet, subsequent to being discharged, exhibits instability which leads to extention and consequent decrease in jet thickness [65]. This phenomenon allows the jet to attain considerable length and diminutive diameter. The polymeric fibers become charged due to the effects of high electric potential and subsequently solidify through the process of solvent evaporation. Upon completion of this process, a collection of randomly oriented nanofibers is obtained on the surface of a designated collector [66]. The generation of highly aligned nanofibers can be facilitated by employing specific collection methods, including the use of a rotating drum, metal frame, or a system with two parallel plates. To maintain consistency in nanofiber diameter and morphology, it is essential to control several parameters, such as the jet stream flow and the concentration of the polymer solution. The resulting electrospun nanofiber network exhibits a remarkable similarity to the ECM with a high level of accuracy.
 | Table 4. Different types of self-assembly techniques used in nanofabrication. [Click here to view] |
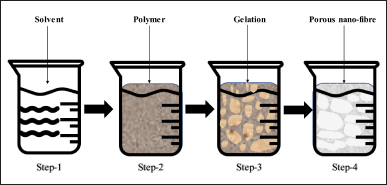 | Figure 3. Steps involved in thermal induced phase separation. [Click here to view] |
Self-assembly
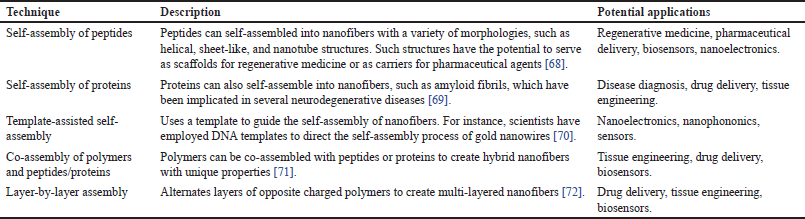
This approach involves the self-assembly of molecules or polymers into organized structures through non-covalent interactions. Self-assembling polypeptides, for example, can form nanofibers via intermolecular hydrogen bonding and van der Waals interactions. These fibers can be functionalized with active pharmaceutical ingredient or other excipients for drug delivery applications. Self-assembly can produce fibers with high order and regulated surface properties [67]. It is also a straightforward and adaptable technique that finds application with a diverse array of materials, encompassing natural peptides and proteins. The fiber morphology and drug release properties can be affected by the specific self-assembling system used as shown in Table 4, and the process can be challenging to scale up for industrial production.
Thermal induced phase separation
Thermal-induced phase separation is a process that triggers the segregation of a homogeneous polymeric dispersion into multiple phases by making thermodynamic changes, thereby generating a multi-phase system. This method involves several sequential steps, as can be seen in Figure 3, including polymer dissolution, phase separation between liquid-liquid or liquid-solid phases, polymer gelation, solvent extraction utilizing water, and ultimately freeze-drying under vacuum conditions [73].
The initial step involving the homogenous polymer solution is characterized by thermodynamic instability, resulting in a tendency for segregation into separate phases comprised of polymer-rich and polymer-lean components, provided the appropriate temperature conditions are met. Upon solvent evaporation, the polymeric phase ultimately hardens to yield the matrix, while the polymeric phase depleted in content progresses into the formation of pores [73]. Thereon, two forms of phase separation, contingent on the intended configuration, may be conducted on the polymeric solution. The process of liquid-liquid separation is commonly employed to generate dual-phase architectures, whereas the technique of precipitation is typically employed to create crystalline structures. Recent research has demonstrated that the process of gelation is dependent on various factors, including temperature, polymer concentration, and solvent characteristics. The temperature plays a critical role in shaping the architecture of the fiber network. Lower gelation temperatures promote the growth of nanofiber network architectures, whereas higher gelation temperatures promote the development of platelet-like structures. The properties of the fibers are interconnected with the concentration of the polymer, as higher polymer concentrations are linked to reduced porosity and enhanced mechanical characteristics, such as increased tensile strength [74]. After the gelation process, the resulting gel is soaked in distilled water to enable solvent exchange. The subsequent step entails the separation of the gel from the aqueous solution, followed by subjecting it to the process of freezing and subsequent lyophilization. Subsequent to its preparation, the substance is conserved within a desiccator until subjected to characterization.
Template synthesis
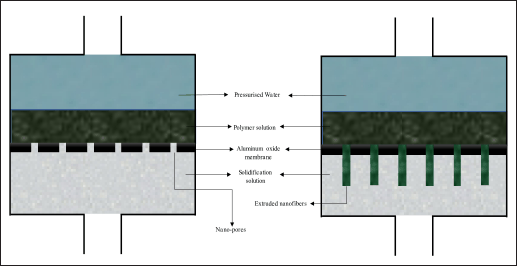
The template synthesis approach employs a nano sieve membrane scaffold, characterized by the presence of uniform cylindrical pores, to generate fibrils (i.e., solid nanofibers) and tubules (i.e., hollow nanofibers) as depicted in Figure 4. The proposed technique has the potential to generate fibrous and tubular structures of various materials, such as metals, semiconductors, and electrically conductive polymers.
 | Figure 4. Template synthesis approach for synthesis of nanofibers. [Click here to view] |
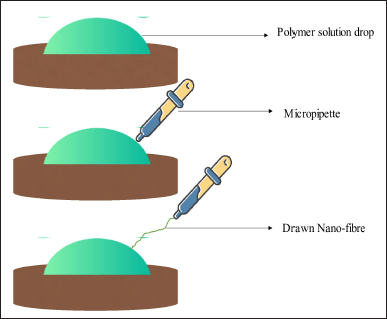 | Figure 5. Drawing process for nano-fibre synthesis. [Click here to view] |
The homogeneous pores in the structure facilitate the regulation of fiber size, thereby enabling the manufacturing of nanofibers with exceptionally minute dimensions using this method. One limitation of this approach is its incapacity to produce singular nanofibers consecutively [75].
Drawing
The drawing technique enables the production of elongated nanofibers individually, one strand at a time as shown in Figure 5. During the pulling process, the solubilized fibrous material undergoes solidification, transforming into a solidified fiber. For melt spinning, a cooling step is required, while for dry spinning, solvent evaporation is necessary. Besides this, a limitation of this method is that only viscoelastic materials capable of enduring significant deformity while maintaining adequate binding to withstand the strains generated during elongation can be transformed into nanofibers using this technique [76].
The initial step in the process of polymer preparation involves the generation of a solution or a melt of the polymer, which is capable of being drawn into nano-sized fibers. The polymer ought to possess commendable mechanical characteristics while having the ability to undergo dissolution or melting, allowing it to form a solution. The precise method of solution preparation for a given polymer is contingent upon the specific variety employed and may entail either complete dissolution of the polymer in a solvent or controlled melting of the polymer.
The drawing setup involves the loading of a polymer mix or melt into a syringe or spinneret, which is subsequently attached to a motorized stage. The stage serves the purpose of imposing a regulated mechanical stimulus onto the polymer solution or melt while it is being extruded from either a syringe or spinneret. The spinneret may take the form of a needle or capillary tube with a fine tip, serving to regulate the diameter of nanofibers being manufactured. The act of drawing in the production of polymers involves the controlled mechanical force applied to extract the polymer dispersion or melt from either the syringe or spinneret. Various determinants, such as the drawing rate, ambient temperature, and humidity levels, can exert influence over the nanofibers and properties of the resulting nanofibers [77]. The application of either uniaxial or biaxial stretching is determined by the desired orientation of the fibers. Subsequent to the fabrication process, the resultant nanofibers are gathered onto a substrate or collector, thereby rendering them liable for further processing that serves to enhance their mechanical and electrical characteristics. The application of annealing has the potential to induce fiber alignment and augment their level of crystallinity, while electrospinning may prove viable in generating fiber mats with a distinctive sense of orientation [78].
Upon examination of the aforementioned techniques, it becomes apparent that each possesses distinctive characteristics that may be leveraged to purposely generate nanofibers exhibiting desirable properties. Table 5 presented below offers a summary of the techniques, distinguished by their distinct advantages.
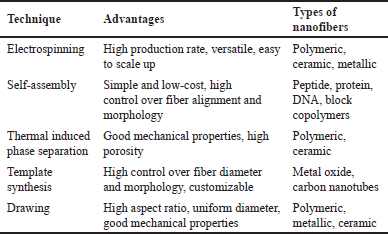 | Table 5. Summary of different techniques with distinct advantages. [Click here to view] |
Applications of bioinspired nanofiber in DDSs
With their distinctive properties and adaptable applications, bioinspired nanofibers in pharmaceutical deliveries have the capacity to bring about a paradigm shift in the field of drug delivery and regenerative medicine. The particular role of these systems in specific applications is contingent upon the specific characteristics of the nanofibers themselves and the drugs being transported. For example, in cancer therapy, nanofiber can be designed to selectively deliver therapeutic agents to cancer cells by incorporating targeting moieties such as antibodies or peptides into the nanofibers [79]. Nanofiber-based dressings designed for regenerative medicine purposes can be designed to facilitate the creation of a moist environment at the site of injury and mitigate exposure to infections. This can be achieved by incorporating antimicrobial agents directly into the nanofibers of the dressings [80]. In tissue regeneration, nanofiber-based scaffolds can be designed to imitate the ECM and promote the proliferation of stem cells by incorporating growth factors or other bioactive molecules into the nanofibers [81].
The following section provides an overview of the different applications of nanofibers in therapeutic delivery and tissue engineering and a discussion of the role of nanofiber-based therapeutic systems in specific applications.
Cancer therapy
Nanofiber-based pharmaceutical delivery systems have exhibited significant potential in cancer treatment by means of their capacity to selectively administer drugs to cancer cells, while reducing the harm to healthy cells. The administration of nanofibers embedded with an anticancer agent offers the advantage of prolonged and localized drug release within specific targeted areas. This is due to their unique ability to be directly implanted within solid tumor cells, enabling them to serve therapeutic purposes. Within the context of this application, the utilization of nanoparticles may induce the accumulation of colloidal polymer carriers in the liver and spleen during systemic circulation, culminating in a reduction of the overall therapeutic efficacy. Mehnath et al. introduced a responsive polymeric nanofibrous patch for localized drug release to address the limitations associated with injection methods and reduce toxicity to healthy tissues in breast cancer treatment. The initial strategy involved encapsulating paclitaxel within micelles formed by linking chitosan acid (CA) with poly (bis (carboxyphenoxy) phosphazene). These micelles were additionally coated with a shell comprised of psyllium husk mucilage. The CA ligand, recognized for its strong attraction to the farnesoid X receptor, played a critical role in enhancing the uptake of the micelles by cancer cells. In an ex vivo study on skin permeation, it was observed that the formulation displayed increased penetration and retention in the skin. By positioning the drug delivery system adjacent to the tumor site, the medications could be concentrated on cancer cells, resulting in improved therapeutic effectiveness and reduced harm to other organs. In an alternative investigation, Li et al. fabricated a device composed of nanogel-in-microfiber architecture, featuring a temperature-responsive mechanism for drug release. The device comprises polymer fibers with a core/shell structure, wherein the drug is enclosed within a PEO core, while the shell contains temperature-responsive nanogels. The nanogels exhibit varying permeability in response to temperature changes, enabling precise control over drug release. In vitro experiments demonstrated efficient suppression of breast cancer cells at elevated temperatures, while preserving cell viability at lower temperatures. This approach shows potential for targeted treatment of tumors with minimized adverse effects on healthy tissues [82]. The nanofibers possess a notable feature of exhibiting a significantly elevated surface area to volume ratio, enabling them to hold substantial amounts of drug molecules. The diminutive dimensions of nanofibers potentially enable their infiltration into neoplastic tissues. Nanofiber-mediated drug release platforms have demonstrated efficacy in treating a diverse range of malignancies, such as breast carcinoma, lung carcinoma, and pancreatic carcinoma.
Wound healing
The utilization of nanofibers for the purpose of wound healing is due to their unique characteristics such as surface area, significant porosity, and an amplified surface-area-to-mass ratio. The utilization of wound dressings comprising nanofibers has demonstrated superior capacity in facilitating a conducive environment for tissue healing compared to conventional dressings. The augmented surface area of nanofibers enables a heightened rate of wound exudate absorption, thus mitigating the likelihood of infection and advancing the rate of wound healing. Nanofiber dressings exhibit superior permeability and breathability, fostering a conducive, moist milieu for regenerative medicine [83]. In the study carried out by Prarthana Mistry et al, nanofibrous bandages composed of starch-thermoplastic polyurethane (TPU) were manufactured using electrospinning. The bandages exhibited improved water stability, mechanical properties, water retention, and wound healing capabilities compared to traditional cotton gauze dressings, making them promising materials for rapid and effective wound healing [84]. The presence of moisture within the environment serves as a facilitator for cellular transport, regeneration, and differentiation, all of which are considered indispensable in the process of tissue generation.
Antibiotic delivery
The application of bioinspired nanofiber technology has demonstrated potential efficacy in the targeted delivery of antibiotics for antibacterial therapy. The enhanced surface area of nanofibers enables augmented drug encapsulation with continuous discharge, thereby increasing the effectiveness of the treatment and diminishing the possibility of antibiotic resistance evolution. These nanofibers enable the simultaneous administration of multiple drugs, are straightforward to produce, and are cost-effective [85]. Accordingly, considerable focus has been directed toward the development of electrospun nanofiber structures as a potentially efficacious vehicle for the delivery of antibacterial agents. Various drugs and substances beyond the previously mentioned examples have been successfully integrated into nanofiber frameworks using diverse techniques and approaches. These include antifungal agents such as fluconazole and ketoconazole, anti-inflammatory drugs such as dexamethasone and indomethacin, antioxidants such as vitamin C and resveratrol, antiviral agents such as remdesivir and ribavirin, growth factors including EGF and FGF, and pain-relieving drugs such as lidocaine and ibuprofen. By incorporating these diverse drugs into nanofibers, researchers are expanding the possibilities for targeted drug delivery, wound healing, infection control, and tissue engineering applications, leading to advancements in the field of nanofiber-based therapeutics.
Cardiovascular disease
Nanofibers possess the potential to serve as an effective carrier for delivering drugs intended for the treatment of cardiovascular ailments, including those that inhibit blood coagulation as well as those that mitigate inflammation [86]. The elevated surface area of nanofibers facilitates enhanced drug loading and sustained release, thereby potentially augmenting their efficacy while minimizing detrimental side effects. Fleischer, S et al research yielded findings that demonstrated the existence of three distinct fiber groups within the myocardium, distinguished by size and functionality [87]. The utilization of electrospinning fiber stent presents a potential tool to facilitate advancements in cardiac-vascular tissue remodeling [88]. Kumar et al employed the electrospinning technique in the creation of a patch composed of PCL and GT nanofibers [89], [90]. Cardiac patches have been observed to elicit synchronized contractions and prompt drug responsiveness in their constituent cells, thereby bestowing them with the potential to serve as a drug screening platform in cardiotoxicity investigations. Despite achieving certain levels of success, numerous scaffolds still exhibit restricted cell infiltration along with low survival rates. The author Seif-Naraghi and colleagues: A GAG mimetic peptide nanofiber gel was synthesized and subsequently administered as an injectable agent at the site of myocardial infarction. This intervention was performed with the aim of promoting neovascularization and facilitating myocardial tissue repair and was achieved without the involvement of biological factors or stem cells. The implementation of electrospinning technology utilizing nanofibrous hydrogels as a means of fabricating tissue engineering constructs appears to offer potential benefits in mitigating suturing-related damage to heart patches. The researchers also developed conductive nanofibrous membranes, taking inspiration from the adhesive properties of mussels, as a potential therapeutic strategy for mending myocardial infarction [91]. The findings of this study demonstrate a notable decrease of 50% in infarct size, a consequential increase of 20% in left ventricular fraction, and a substantial 9-fold escalation in neovascularization subsequent to 4 weeks of patch transplantation. In an effort to replicate the characteristics and attributes exhibited by the heart, Walker and colleagues (Walker et al.) endeavored to develop a model that could effectively mimic these features. Cardiac patches have been generated through the implementation of GelMA and bio-IL fibrous scaffolds, resulting in robust adhesion to rat myocardium, sans the need for sutures, courtesy of ionic bonding [92]. For the assessment of the functional efficacy of engineered cardiac tissue (ECT), a scaffold for 3D printing and electrospinning was fabricated using polylactic acid and polycaprolactone (PCL). Subsequent to the isolation of cardiomyocytes from SFS, they were cultivated atop to manufacture ECT. The robustness and compatibility of the scaffold were evaluated by scrutinizing the viability of the cells and their mechanical propensity to contract. A novel methodology for the assessment of electroconvulsive therapy that is applicable to pharmaceutical investigations has been developed.
Skin care
Bioinspired nanofibrous materials possess the potential to efficiently deliver active ingredients, such as vitamins, antioxidants, and moisturizers, in various skincare formulations. The magnified surface area of nanofibers facilitates augmented absorption of active constituents within the skin, thereby enhancing effectiveness and minimizing wastage. Transdermal DDSs (TDDSs) have emerged as a well-received and widely accepted technique for providing drug administration via the skin. “Nanofiber-based TDDSs have gained popularity in the pharmaceutical industry owing to their low toxicity, high efficiency, and ability to prevent metabolization.” The utilization of natural nanofibers for transdermal delivery presents a plethora of advantageous features such as targeted delivery, sustained release, and responsive mechanisms. Recent research efforts have placed emphasis on developing nanofibers and nano-emulsions with significantly enhanced skin penetration properties [93]. The utilization of natural polymeric scaffolds in TDDSs has potential therapeutic implications in the management of diverse pathological conditions. The implementation of tailored scaffolds has the potential to enhance vaccination efficacy and streamline the process of self-administering medication.
Challenges and future prospects
Nanofiber in DDSs has shown immense potential in improving therapeutic delivery efficiency, bioavailability, and targeted delivery to specific cells or tissues. However, there are several impediments associated with these systems that need to be overcome for their successful translation into clinical practice. Scalability is one of the major challenges associated with nanofibers in DDSs. Currently, the production of nanofibers is limited to small-scale laboratory processes, which may not be cost-effective for large-scale production. The reproducibility of these systems can be influenced by a variety of factors such as ecological circumstances, polymer properties, and processing parameters, making it difficult to achieve consistent results. Another major challenge is the cost-effectiveness of nanofibers. The high cost of raw materials, manufacturing processes, and equipment can make these systems prohibitively expensive, especially for developing countries where affordable healthcare is a major concern.
Despite these challenges, there are several future prospects for bioinspired nanofiber. One potential approach is to combine nanofiber-based DDSs with other technologies such as microfluidics and 3D printing to enhance their scalability and reproducibility. These technologies can enable the production of large quantities of nanofibers with consistent properties and high precision. Many preclinical studies have shown promising results, and several nanofibers in DDSs are currently undergoing clinical trials. These systems have the potential to revolutionize drug delivery by enabling targeted and sustained drug release, reducing side effects, and improving patient compliance.
CONCLUSION
In this review article, we discussed the challenges and future prospects of bioinspired nanofiber in drug delivery. In terms of the potential impact of bioinspired nanofiber on drug delivery and tissue engineering, these systems have the potential to revolutionize healthcare by enabling targeted and sustained drug release, reducing side effects, and improving patient compliance. These systems can be used for regenerative medicine applications, including regeneration of impaired tissues and organs. Nanofiber-based scaffolds can mimic the natural ECM and provide an environment for cellular proliferation and differentiation.
In conclusion, bioinspired nanofiber in pharmaceutical delivery has immense potential to improve drug delivery efficiency, bioavailability, and targeted delivery to specific cells or tissues. These systems also have the potential to advance tissue engineering applications. Continued research and development of these systems can lead to significant improvements in healthcare outcomes and patient quality of life.
AUTHOR CONTRIBUTIONS
Conceptualization, A.S.R, B.B and S.N.S; methodology, A.S.R, B.B and S.N.S, validation, A.S.R, B.B and S.N.S, formal analysis,B.B and S.N.S, data curation, A.S.R, writing—original draft preparation, A.S.R, writing—review and editing, A.S.R, B.B and S.N.S visualization, A.S.R, B.B and S.N.S.; supervision, B.B and S.N.S.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors have no conflicts of interest regarding this investigation.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Verreck G, Chun I, Peeters J, Rosenblatt J, Brewster ME. Preparation and characterization of nanofibers containing amorphous drug dispersions generated by electrostatic spinning. Pharm Res. 2003;20:810–7. CrossRef
2. Yang S, Dong H. Modular design and self-assembly of multidomain peptides towards cytocompatible supramolecular cell penetrating nanofibers. RSC Adv. 2020;10(49):29469–74. CrossRef
3. Williams GR, Chatterton NP, Nazir T, Yu DG, Zhu LM, Branford-White CJ. Electrospun nanofibers in drug delivery: recent developments and perspectives. Ther Deliv. 2012;3(4):515–33. CrossRef
4. Sunoqrot S, Al-Shalabi E, Messersmith PB. Facile synthesis and surface modification of bioinspired nanoparticles from quercetin for drug delivery. Biomater Sci. 2018;6(10), 2656–66. CrossRef
5. Hay ED. Extracellular matrix. J Cell Nano. 1981;91(3 Pt 2):205s–223s. CrossRef
6. Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol J Pathol Soc Great Britain Ireland. 2003;200(4):423–8. CrossRef
7. Vasvani S, Kulkarni P, Rawtani D. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromole. 2020;151:1012–29. CrossRef
8. Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8(1):34–50. CrossRef
9. Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Progress Heritable Soft Connective Tissue Diseases, 2014;802:31–47. CrossRef
10. Audelo MLDP, Mendoza-Muñoz N, Escutia-Guadarrama L, Giraldo-Gomez D, González-Torres M, Florán B, et al. Recent advances in elastin-based biomaterial. J Pharm Pharm Sci. 2020;23:314–32. CrossRef
11. Labat-Robert J, Bihari-Varga M, Robert L. Extracellular matrix. FEBS Lett. 1990;268(2):386–93. CrossRef
12. Villalba-Rodriguez AM, Parra-Saldivar R, Ahmed I, Karthik K, Malik YS, Dhama K, et al. Bio-inspired biomaterials and their drug delivery perspectives-a review. Current Drug Metab. 2017;18(10):893–904. CrossRef
13. Yang D, Li Y, Nie J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydrate Polym. 2007;69(3):538–43. CrossRef
14. Law JX, Liau LL, Saim A, Yang Y, Idrus R. Electrospun collagen nanofibers and their applications in skin tissue engineering. Tissue Eng Regen Med. 2017;14:699–718. CrossRef
15. Aguirre-Chagala YE, Altuzar V, León-Sarabia E, Tinoco-Magaña JC, Yañez-Limón JM, Mendoza-Barrera C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater Sci Eng C. 2017;76:897–907. CrossRef
16. Du J, Liu J, Yao S, Mao H, Peng J, Sun X, et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309. CrossRef
17. Jayakumar R, Prabaharan M, Nair SV, Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv. 2010;28(1):142–50. CrossRef
18. Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y. Hyaluronic acid nanofiber wound dressing—production, characterization, and in vivo behavior. J Biomed Mater Res Part B Appl Biomater. 2011;97(1):20–29. CrossRef
19. Farokhi M, Mottaghitalab F, Reis RL, Ramakrishna S, Kundu SC. Functionalized silk fibroin nanofibers as drug carriers: advantages and challenges. J Control Release. 2020;321:324–47. CrossRef
20. Edwards A, Jarvis D, Hopkins T, Pixley S, Bhattarai N. Poly (ε-caprolactone)/keratin-based composite nanofibers for biomedical applications. J Biomed Mater Res Part B Appl Biomater. 2015;103(1):21–30. CrossRef
21. Huang ZM, Zhang YZ, Ramakrishna S, Lim CT. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer. 2004;45(15):5361–68. CrossRef
22. Shaghaleh H, Xu X, Wang S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018;8(2):825–42. CrossRef
23. Taemeh MA, Shiravandi A, Korayem MA, Daemi H. abrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr Polym. 2020;228:115419. CrossRef
24. Rammensee S, Hümmerich D, Hermanson KD, Scheibel T, Bausch AR. Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl Phys A. 2006;82:261–64. CrossRef
25. Lin X, Wang J, Wu X, Luo Y, Wang Y, Zhao Y. Marine-derived hydrogels for biomedical applications. Adv Funct Mater. 2023;33(6):2211323. CrossRef
26. Zhao W, Li J, Jin K, Liu W, Qiu X, Li C. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater Sci Eng C, 2016;59:1181–94. CrossRef
27. Saini P, Arora M, Kumar MR. Poly (lactic acid) blends in biomedical applications. Adv Drug Delivery Rev. 2016;107:47–59. CrossRef
28. Teixeira MA, Amorim MTP, Felgueiras HP. Poly (vinyl alcohol)-based nanofibrous electrospun scaffolds for tissue engineering applications. Polymers. 2019;12(1):7. CrossRef
29. Lu JW, Zhu YL, Guo ZX, Hu P, Yu J. Electrospinning of sodium alginate with poly (ethylene oxide). Polymer. 2006;47(23):8026–31. CrossRef
30. Ma Z, Kotaki M, Yong T, He W, Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26(15):2527–36. CrossRef
31. Bertuoli PT, Ordono J, Armelin E, Perez-Amodio S, Baldissera AF, Ferreira CA, et al. Electrospun conducting and biocompatible uniaxial and Core–Shell fibers having poly (lactic acid), poly (ethylene glycol), and polyaniline for cardiac tissue engineering. ACS Omega. 2019;4(2):3660–72. CrossRef
32. Liu Y, Cui L, Guan F, Gao Y, Hedin NE, Zhu L, et al. Crystalline morphology and polymorphic phase transitions in electrospun nylon-6 nanofibers. Macromolecules. 2007;40(17):6283–90. CrossRef
33. Zhuo H, Hu J, Chen S, Yeung L. Preparation of polyurethane nanofibers by electrospinning. J Appl Polym Sci. 2008;109(1):406–11. CrossRef
34. He JH, Wan YQ, Yu JY. ffect of concentration on electrospun polyacrylonitrile (PAN) nanofibers. Fibers Polym. 2008;9(2):140–42. CrossRef
35. Van Do C, Nguyen TTT, Park JS. Fabrication of polyethylene glycol/polyvinylidene fluoride core/shell nanofibers via melt electrospinning and their characteristics. Solar Energy Mater Solar Cells. 2012;104:131–39. CrossRef
36. Han J, Branford-White CJ, Zhu LM. Preparation of poly (ε-caprolactone)/poly (trimethylene carbonate) blend nanofibers by electrospinning. Carbohydr Polym. 2010;79(1):214–18. CrossRef
37. Hu J, Kai D, Ye H, Tian L, Ding X, Ramakrishna S, et al. Electrospinning of poly (glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater Sci Eng: C. 2017;70:1089–94. CrossRef
38. Liu Y, Miao YL, Qin F, Cao C, Yu XL, Wu YH, et al. Electrospun poly (aspartic acid)-modified zein nanofibers for promoting bone regeneration. Int J Nanomed. 2019;9497–12. CrossRef
39. Liu L, Bai S, Yang H, Li S, Quan J, Zhu L, et al. Controlled release from anofib-sensitive PNVCL-co-MAA electrospun nanofibers: the effects of hydrophilicity/hydrophobicity of a drug. Mater Sci Eng C. 2016;67:581–9. CrossRef
40. Kitasono S, Yamamoto K, Kadokawa JI. Preparation and gelation behaviors of poly (2-oxazoline)-grafted chitin nanofibers. Carbohydr Polym. 2021;259:117709. CrossRef
41. Oliveira JE, Moraes EA, Marconcini JM, Mattoso LHC, Glenn GM, Medeiros ES. Properties of poly (lactic acid) and poly (ethylene oxide) solvent polymer mixtures and nanofibers made by solution blow spinning. J Appl Polym Sci. 2013;129(6):3672–81. CrossRef
42. Yingying M, Xiu-Xia L, Luyun C, Jianrong L. pH-sensitive ε-polylysine/polyaspartic acid/zein nanofiber membranes for the targeted release of polyphenols. Food Funct. 2022;13(12),6792–801. CrossRef
43. Rahim Labbafzadeh M, Vakili MH. Application of magnetic electrospun polyvinyl alcohol/collagen anofibers for drug delivery systems. Mole Simul. 2022;48(1):1–7. doi: 10.1080/08927022.2020.1783462 CrossRef
44. Zhang M, Li Z, Liu L, Sun Z, Ma W, Zhang Z, et al. Preparation and characterization of vancomycin-loaded electrospun rana chensinensis skin collagen/Poly(L-lactide) nanofibers for drug delivery. Khatri Z, editor. Journal of Nanomaterials. 2016 Aug 18;2016:9159364. CrossRef
45. Huo P, Han X, Zhang W, Zhang J, Kumar P, Liu B. Electrospun nanofibers of polycaprolactone/collagen as a sustained-release drug delivery system for artemisinin. Pharmaceutics. 2021;13(8):1228. CrossRef
46. Sasmal P, Datta P. Tranexamic acid-loaded chitosan electrospun nanofibers as drug delivery system for hemorrhage control applications. J Drug Delivery Sci Technol. 2019;52:559–67. ISSN 1773 2247. CrossRef
47. Gouda M, Khalaf MM, Shaaban S, El-Lateef HMA. Fabrication of chitosan nanofibers containing some steroidal compounds as a drug delivery system. Polymers. 2022;14(10):2094. CrossRef
48. Patel PR, Singam A, Iyer AK, Gundloori RVN, Bioinspired hyaluronic acid based nanofibers immobilized with 3, 4- difluorobenzylidene curcumin for treating bacterial infections. J Drug Delivery Sci Technol, 2022;74:103480. ISSN 1773-2247. CrossRef
49. Dadras Chomachayi M, Solouk A, Akbari S, Sadeghi D, Mirahmadi F, Mirzadeh H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: thyme essential oil and doxycycline monohydrate release study. J Biomed Mater Res Part A 2018;106A:1092–103. CrossRef
50. Guidotti G, Soccio M, Bondi E, Posati T, Sotgiu G, Zamboni R, et al. Effects of the blending ratio on the design of keratin/poly(butylene succinate) nanofibers for drug delivery applications. Biomolecules. 2021;11(8):1194. CrossRef
51. Meng ZX, Xu XX, Zheng W, Zhou HM, Li L, Zheng YF, et al. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surf B Biointerfaces. 2011;84(1):97–102. ISSN 0927-7765. CrossRef
52. Aytac Z, Sen HS, Durgun E, Uyar T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf B Biointerf. 2015;128:331–38. ISSN 0927-7765. CrossRef
53. Najafiasl M, Osfouri S, Azin R, Zaeri S. Alginate-based electrospun core/shell nanofibers containing dexpanthenol: a good candidate for wound dressing, J Drug Deliv Sci Technol, 2020;57:101708, ISSN 1773-2247. CrossRef
54. Qi R, Guo R, Zheng F, Liu H, Yu J, Shi X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Colloids Surf B Biointerf. 2013;110:148–55. ISSN 0927-7765. CrossRef
55. Huang X, Guan N, Li Q. A marine-derived anti-inflammatory scaffold for accelerating skin repair in diabetic mice. Marine Drugs. 2021;19(9):496. CrossRef
56. Mamidi N, Zuníga AE, Villela-Castrejón J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater Sci Eng C 112, 2020, 110928, ISSN 0928-4931. CrossRef
57. Rezaei S, Valipouri A, Hosseini Ravandi SA, Kouhi M, Ghasemi Mobarakeh L. Fabrication, characterization, and drug release study of vitamin C–loaded alginate/polyethylene oxide nanofibers for the treatment of a skin disorder. Polym Adv Technol. 2019;30:2447–57. CrossRef
58. Grumezescu AM, Stoica AE, Dima-B?lcescu M-?, Chircov C, Gharbia S, Balt? C, et al. Electrospun polyethylene terephthalate nanofibers loaded with silver nanoparticles: novel approach in anti-infective therapy. J Clin Med. 2019;8(7):1039. CrossRef
59. Minisy IM, Salahuddin NA, Ayad MM. In vitro release study of ketoprofen-loaded chitosan/polyaniline nanofibers. Polym. Bull. 2021;78:5609–22. CrossRef
60. Pavli?áková V, Fohlerová Z, Pavli?ák D, Khunová V, Vojtová L. Effect of halloysite nanotube structure on physical, chemical, structural and biological properties of elastic polycaprolactone/gelatin nanofibers for wound healing applications. Mater Sci Eng C. 2018;91:94–102. ISSN 0928-4931. CrossRef
61. Gencturk A, Kahraman E, Güngör S, Özhan G, Özsoy Y, Sarac AS. Polyurethane/hydroxypropyl cellulose electrospun nanofiber mats as potential transdermal drug delivery system: characterization studies and in vitro assays. Artif CellsNanomed Biotechnol. 2017;45(3):655–64. doi: 10.3109/21691401.2016.1173047 CrossRef
62. Zhang X, Geven MA, Wang X, Qin L, Grijpma DW, Peijs T, et al. A drug eluting poly(trimethylene carbonate)/poly(lactic acid)-reinforced nanocomposite for the functional delivery of osteogenic molecules. Int J Nanomed. 2018;24(13):5701–18. doi: 10.2147/IJN.S163219. PMID: 30288042; PMCID: PMC6161751. CrossRef
63. Rezk AI, Kim K-S, Kim CS. Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) composite nanofibers incorporating hydroxyapatite nanoparticles and simvastatin for bone tissue regeneration and drug delivery applications. Polymers. 2020;12(11):2667. CrossRef
64. Teo WE, Inai R, Ramakrishna S. Technological advances in electrospinning of nanofibers. Sci Technol Adv Mater. 2011 Feb 16;12(1):013002. CrossRef
65. Li Z, Wang C. One-dimensional nanostructures: electrospinning technique and unique nanofibers. New York Dordrecht London: Springer Berlin Heidelberg. 2013. pp. 15–29. CrossRef
66. Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. CrossRef
67. Greiner A, Wendorff JH. Functional self-assembled nanofibers by electrospinning. Self-assembled nanomaterials I: Nanofibers. 2008;168:107–71. CrossRef
68. Stendahl JC, Rao MS, Guler MO, Stupp SI. Intermolecular forces in the self-assembly of peptide amphiphile nanofibers. Adv Funct Mater. 2006;16(4):499–508. CrossRef
69. Jiao Q, Liu Z, Li B, Tian B, Zhang N, Liu C, et al. Development of antioxidant and stable conjugated linoleic acid Pickering emulsion with protein nanofibers by microwave-assisted self-assembly. Foods. 2021;10(8):1892. CrossRef
70. Calahorra Y, Datta A, Famelton J, Kam D, Shoseyov O, Kar-Narayan S. Nanoscale electromechanical properties of template-assisted hierarchical self-assembled cellulose nanofibers. Nanoscale. 2018;10(35):16812–21. CrossRef
71. Okesola BO, Mata A. Multicomponent self-assembly as a tool to harness new properties from peptides and proteins in material design. Chem Soc Rev. 2018;47(10), 3721–36. CrossRef
72. Li D, Dai F, Li H, Wang C, Shi X, Cheng Y, et al. Chitosan and collagen layer-by-layer assembly modified oriented nanofibers and their biological properties. Carbohydr Polym. 2021;254:117438. CrossRef
73. Shao J, Chen C, Wang Y, Chen X, Du C. Early stage evolution of structure and nanoscale property of nanofibers in thermally induced phase separation process. React Funct Polym. 2012:72(10):765–72. CrossRef
74. Xie F, Wang Y, Zhuo L, Jia F, Ning D, Lu Z. Electrospun wrinkled porous polyimide nanofiber-based filter via thermally induced phase separation for efficient high-temperature PMs capture. ACS Appl Mater Interf. 2020;12(50):56499–508. CrossRef
75. Tao SL, Desai TA. Aligned arrays of biodegradable poly (?-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007;7(6):1463–8. CrossRef
76. Morie A, Garg T, Goyal AK, Rath G. Nanofibers as novel drug carrier–an overview. Artif Cells Nanomed Biotechnol. 2016;44(1):135–43. CrossRef
77. Karim Haidar M, Eroglu H. Nanofibers: new insights for drug delivery and tissue engineering. Current Topics Med Chem. 2017;17(13):1564–79. CrossRef
78. Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. J Control Release. 2014;185:12–21. CrossRef
79. Chen Z, Chen Z, Zhang A, Hu J, Wang X, Yang Z. Electrospun nanofibers for cancer diagnosis and therapy. Biomater Sci. 2016;4(6):922–32. CrossRef
80. Alavi M, Nokhodchi A. Antimicrobial and wound healing activities of electrospun nanofibers based on functionalized carbohydrates and proteins. Cellulose. 2022;29(3):1331–47. CrossRef
81. Sahoo S, Ang LT, Goh JCH, Toh SL. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res Part A Official J Soc Biomater Japanese Soc Biomater Austr Soc Biomater Korean Soc Biomater. 2010;93(4):1539–50. CrossRef
82. Li J, Liu Y, Abdelhakim HE. Drug delivery applications of coaxial electrospun anofibers in cancer therapy. Molecules. 2022;27(6):1803. CrossRef
83. Liu X, Xu H, Zhang M, Yu DG. Electrospun medicated nanofibers for wound healing. Membranes. 2021;11(10):770. CrossRef
84. Mistry P, Chhabra R, Muke S, Narvekar A, Sathaye S, Jain R, et al. Fabrication and characterization of starch-TPU based nanofibers for wound healing applications. Mater Sci Eng C. 2021;119:111316. CrossRef
85. Wang Y, Wang B, Qiao W, Yin T. A novel controlled release drug delivery system for multiple drugs based on electrospun nanofibers containing nanoparticles. J Pharm Sci. 2010;99(12):4805–11. CrossRef
86. Lee CH, Liu KS, Roth JG, Hung KC, Liu YW, Wang SH, et al. Telmisartan loaded nanofibers enhance re-endothelialization and inhibit neointimal hyperplasia. Pharm., 2021;13(11):1756. CrossRef
87. Fleischer S, Tavakol DN, Vunjak-Novakovic G. From arteries to capillaries: approaches to engineering human vasculature. Adv Funct Mater. 2020;30(37):1910811. CrossRef
88. Kuraishi K, Iwata H, Nakano S, Kubota S, Tonami H, Toda M, et al. Development of nanofiber-covered stents using electrospinning: in vitro and acute phase in vivo experiments. J Biomed Mater Res Part B Appl Biomater Official J Soc Biomater, Japn Soc Biomater Austr Soc Biomater Korean Soc Biomater. 2009;88(1):230–39. CrossRef
89. Kumar N, Sridharan D, Palaniappan A, Dougherty JA, Czirok A, Isai DG. et al. Scalable biomimetic coaxial aligned nanofiber cardiac patch: a potential model for “Clinical Trials in a Dish”. Front Bioeng Biotechnol. 2020;8:567842. CrossRef
90. Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman, KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Engineering Part A, 2010;16(6):2017–27. CrossRef
91. Ye G, Wen Z, Wen F, Song X, Wang L, Li C, et al. Mussel-inspired conductive Ti2C-cryogel promotes functional maturation of cardiomyocytes and enhances repair of myocardial infarction. Theranostics 2020;10(5):2047. CrossRef
92. Walker BW, Lara RP, Yu CH, Sani ES, Kimball W, Joyce S, et al. Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials. 2019;207:89–101. CrossRef
93. Coelho D, Veleirinho B, Mazzarino L, Alberti T, Buzanello E, Oliveira RE, et al. Polyvinyl alcohol-based electrospun matrix as a delivery system for nanoemulsion containing chalcone against Leishmania (Leishmania) amazonensis. Colloids Surf B Biointerf. 2021;198:111390. CrossRef