INTRODUCTION
Urinary tract infections (UTIs) are classified among the most common bacterial infections, especially in women (Negus et al., 2020). Annually, UTIs cause health, economic, and social burdens worldwide. With the continuous emergence of antibiotic resistance among uropathogens and the high rate of recurrence, controlling these infections becomes more challenging and the choices of antibiotheraby become less (Lodhia et al., 2020; Negus et al., 2020). Uropathogenic Escherichia coli (UPEC) is the most predominant cause of UTIs; it accounts for up to 80% of uncomplicated UTIs, 95% of community-acquired infections, and half of the infections acquired in hospitals (Kot, 2019). UPEC have developed a number of smart strategies that enable them to survive and colonize under the conditions of the urinary tract, especially the constant flow of urine. These strategies are achieved by a variety of virulence factors such as fimbrial and nonfimbrial adhesins, curli, Lipopolysaccharides (LPS), surface vesicles, polysaccharide capsules, flagella, secreted toxins, two-component signaling systems, and the iron acquisition system (Terlizzi et al., 2017).
Recently, multidrug resistance (MDR) has noticeably increased among UPEC because of the frequent and uncontrolled use of antibiotics, which resulted in a lack of treatment and prophylaxis choices (Chaudhary et al., 2021). UPEC resistance to a wide range of antibiotics has already been reported worldwide (Terlizzi et al., 2017). This resistance has led to complexity and high cost of treatment as well as prolonged hospitalization (Kot, 2019). Hence, antibiotic resistance in UPEC represents a heavy burden on individuals and governments, demonstrating the urgent need to develop alternative and effective approaches to counteract these highly prevalent infections. Indeed, several approaches are being worked on in this regard, such as vaccines, nanoparticles, probiotics, mannosides and galactosides, bacteriophages, and phytochemicals (Terlizzi et al., 2017; Zalewska-Pi?tek and Pi?tek, 2019).
From time immemorial, humans have been using plants as a natural source of therapeutic compounds and traditional medicine became a rich reference for developing medicines to treat many health conditions. Recently, medicinal plants have been intensively studied as they represent a low-cost, effective, and ecofriendly source of medicinal compounds. This attention to medicinal plants has significantly increased in microbiology as tremendous efficiency of many plants was proved against a wide range of pathogens (Khameneh et al., 2019; Shaheen et al., 2019; Zalewska-Pi?tek and Pi?tek, 2019).
Syrian sumac is a worldwide consumed plant, especially in the Mediterranean region (Dziki et al., 2021; Sakhr and El Khatib, 2020; Shabbir, 2012). The scientific name of sumac is Rhus coriaria Linn. (family Anacardiaceae) (Ravindran et al., 2012). The common name “Syrian sumac” or “sumac” is mainly used to refer to the fruit of the plant R. coriaria, and it is believed to have an Arabic origin meaning dark red (Sakhr and El Khatib, 2020). Sumac is well known for its great nutritional and therapeutic properties and has been used in traditional medicine in Persia, Turkey, and the Mediterranean countries (Reidel et al., 2017; Shabbir, 2012) where it grows abundantly in the form of small trees with a length of 1 to 3 m, giving small clustered fruits with a dark red color (Shabbir, 2012; Tohma et al., 2019). These fruits are usually collected and dried well to use as a condiment or souring agent in various dishes (Farag et al., 2018). In addition to its culinary use, sumac is increasingly gaining importance in the pharmaceutical and cosmetic industries (Sakhr and El Khatib, 2020). From a traditional perspective, sumac has been used to treat a variety of health conditions, such as liver diseases, diarrhea, ulcers, strokes, hemorrhoids, hemorrhage, animal bites, dysentery, diuresis, hematemesis, hemoptysis, ophthalmia, conjunctivitis, leucorrhea, sore throat, wounds, and high blood cholesterol, glucose, and uric acid levels, in addition to its use as a stomach tonic, abortifacient, and antimicrobial agent (Dziki et al., 2021; Farag et al., 2018; Shabbir, 2012). Due to its medical importance, researchers have paid great attention to this plant and indeed its effectiveness in treating and preventing many diseases has been proven. In several studies, sumac has been shown to exhibit antibacterial, antifungal, and antioxidant properties (Dziki et al., 2021). Furthermore, sumac has been shown to reduce blood sugar (Shidfar et al., 2014) and lipids (Akbari-Fakhrabadi et al., 2018), in addition to its analgesic (Mohammadi et al., 2016), anti-inflammatory, antihypertensive, and anticancer effect (Farag et al., 2018). The benefits of sumac extends as far as protecting the liver (Pourahmad et al., 2010) and cardiovascular system (Beretta et al., 2009).
From the phytochemical point of view, sumac mainly contains hydrolysable tannins, polyphenols, flavonoids, anthocyanins, isoflavonoids, terpenoids, monoterpenes, diterpenes, organic acids, fatty acids, amino acids, vitamins, minerals, xylose, glucose, and essential oils (Ardalani et al., 2016; Elagbar et al., 2020; Farag et al., 2018).
With regard to confronting bacterial pathogens, sumac has been shown to be a very effective antibacterial agent against a wide range of Gram-negative and Gram-positive bacteria, including MDR strains (Akrayi and Abdullrahman, 2013; Gabr and Alghadir, 2019; Mahdavi, 2018). To the authors’ knowledge, at the time of writing this article, we have not found any study that investigates the antibacterial activity of R. coriaria against UPEC. Therefore, we aimed to test the ability of this great plant to confront MDR UPEC.
MATERIALS AND METHODS
Bacteria, media, and growth conditions
This research was performed on eight clinical UPEC isolates. The reference strain E. coli ATCC 25922 was used as a quality control strain. All bacteria were provided by the Laboratory of Microbiology and Virology of the Peoples’ Friendship University of Russia in Moscow. The media used to grow bacteria consisted of brain heart infusion broth (BHIB) (HIMEDIA®, Ref. 173-500G) and Mueller–Hinton agar (MHA) (HIMEDIA®, Ref. 173-500G). For culturing bacteria, the following procedures were carried out, if not stated otherwise. Overnight cultures were prepared by growing bacteria in BHIB for 16 to 18 hours at 37°C in aerobic conditions. Bacterial inoculums were prepared by taking 1 ml of overnight cultures, centrifuging for 10 minutes at 3,000 rpm (in Eppendorf Centrifuge 5415 R), washing the centrifugate twice with phosphate buffer saline (PBS), and resuspending in physiological water (0.9% NaCl). The density of inoculums was adjusted photometrically to that of 0.5 McFarland standard so that they contained approximately 1.5 × 108 scolony forming units (CFUs)/ml. For antibiotic susceptibility tests, Petri dishes (90 mm) with 4 mm of MHA were used.
Antibiotic susceptibility profile (antibiogram)
The Bauer–Kirby disk diffusion assay (Bauer et al., 1966) was performed to obtain the antibiograms of the bacterial strains against eight antibiotics (HIMEDIA®): ampicillin (AMP), 25 μg/disc; ceftriaxone (CTR), 30 μg/disc; ciprofloxacin (CIP), 30 μg/disc; trimethoprim (TR), 30 μg/disc; ceftazidime/clavulanic acid (CAC), 30/10 μg/disc; ceftazidime (CAZ), 30 μg/disc, imipenem (IPM), 10 μg/disc; and tetracyclines (TE), 30 μg/disc. The procedure was performed in accordance with CLSI (2021) guidelines. Briefly, 100 μl of fresh bacterial inoculums (prepared as previously described) was spread on MHA plates and allowed to dry for 5 minutes. After that, antibiotic discs were placed onto the agar surface and the plates were incubated for 18 hours at 37°C. After incubation, diameters of growth-free zones were measured and recorded in mm. Bacterial sensitivity to antibiotics was interpreted in accordance with CLSI (2021) interpretative values.
Multiple antibiotic resistance (MAR) Index and MDR identifying
The MAR index for each bacterial strain was calculated by dividing the number of antibiotics to which the bacterium is resistant by the total number of tested antibiotics (Ayandele et al., 2020). Nonsusceptibility of bacteria to at least one agent in three or more antimicrobial categories has been defined as MDR (Basak et al., 2016). Accordingly, MDR bacteria within the tested strains were further identified.
Plant material and extraction
Sumac fruits were purchased from a local market in Tartous, Syria, in sun-dried, ground form. The hydroalcoholic extract of sumac was prepared by the extraction at room temperature method (Kothari et al., 2012) with some modifications. Briefly, 80% ethanol was used as a solvent with a sample-to-solvent ratio of 1/10 (w/v). Fifty grams of ground sumac was placed in a 1,000 ml flask with the addition of 500 ml of 80% ethanol. The flask was then stoppered and covered with aluminum foil to prevent any evaporation and placed on a shaker (300 rpm) at room temperature (22°C) for 24 hours. Afterward, vacuum filtration was carried out to filter the mixture using Whatman filter paper ? 1. Filtration was repeated three times. The filtrate was then concentrated using a rotary evaporator (IKA Werke, Staufen, Germany) at 40°C in a previously weighed flask. The yield of extraction was calculated as the percentage of the initial sumac mass. The concentrated crude extract was stored in the dark at 4°C until use.
Preparation of working solution
A stock solution of the sumac crude extract was made in a concentration of 200 mg/ml in dimethyl sulfoxide (DMSO) (VWR International LLC, USA) (10% v/v in dH2O), sterilized via passing through a Millipore filter (0.22 μm), and stored in the dark at 4°C. The solution was always refiltered prior to each experiment.
Antibacterial screening of sumac extract against UPEC
Well diffusion method
To assess the antibacterial activity of the sumac extract against UPEC, the agar well diffusion method was carried out as described by Balouiri et al. (2016). Briefly, after seeding MHA plates with 100 μl of fresh bacterial inoculums, wells were aseptically made in the agar by a 6 mm cork borer, followed by loading the wells with 45 μl of the sumac extract in three concentrations: 50, 100, and 200 mg/ml. DMSO (10%) was included as the negative control. The plates were then incubated for 24 hours at 37°C, after which the diameters of growth-free zones were measured and recorded in mm.
Quantitative antibacterial assay by minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs)
The broth microdilution method was used to determine the MICs of the sumac extract as previously described (Wiegand et al., 2008). In brief, the assay was performed in sterile U-bottomed 96-well microplates. Serial two-fold dilutions were made from the stock solution of the sumac extract in BHIB. Then, 50 μl of each dilution and 50 μl of fresh bacterial inoculums were added to the respective wells. The final density of bacteria in all wells was approximately 5 × 105 CFUs/ml, and the final concentrations of the extract ranged from 100 to 0.0031 mg/ml. DMSO (10%) was used as a negative control. Wells with all used solutions, except bacteria, were included to check sterility. Microplates were then incubated for 16–20 hours at 37°C. After incubation, the lowest concentration of extract that inhibited the visible growth of bacteria was considered as MIC. To determine MBCs, a loopful of each clear well was streaked onto an MHA plate and incubated for 18 hours at 37°C (Lara et al., 2016). The lowest concentration that resulted in no bacterial growth on the agar plates was considered as MBC.
Yeast agglutination (YA)
The sumac extract in subinhibitory concentration was tested to observe any effect on the ability of bacteria to agglutinate with yeast cells (Saccharomyces cerevisiae). The previously described slide method (Stærk et al., 2016) was performed with some modifications. Standardized concentrations (OD492 = 0.05) of overnight cultures were added to BHIB containing the sumac extract in a final concentration that equals MIC/2 and incubated at 37°C for 24 hours. Control cultures were included with the substitution of the extract by 10% DMSO. Following the incubation period, cultures were centrifuged, washed twice, and resuspended in PBS. Agglutination tests were performed on sterile glass slides at room temperature by mixing 20 μl of bacterial suspension with an equal volume of yeast suspension (1% w/v in PBS). The visible appearance of aggregates was observed within 10 minutes and evaluated as follows: − (no agglutination), + (weak), ++ (moderate), or +++ (strong). To assess the effect of mannose on agglutination, the same procedure was performed with the addition of 20 μl of D-mannose (1% in PBS). Mannose-resistant (MR) indicates the same degree of agglutination with and without mannose, whereas mannose-sensitive (MS) indicates that the agglutination was completely inhibited or significantly reduced in the presence of mannose. Further, agglutination was confirmed by light microscopy at 100×.
Hemagglutination (HA)
The hHA assay was performed by the slide method previously described (Evans et al., 1980) with some modifications. Fresh human RBCs (B+) from a healthy man were obtained from the university clinic. After washing twice, a 5% suspension was made in PBS. Bacteria were incubated with and without sumac exactly as described for YA. On sterile glass slides, equal volumes of bacterial suspensions and RBCs were mixed, in the presence and absence of D-mannose. HA was observed within 10 minutes and evaluated as described for YA. Aggregates were further observed using light microscopy at 100×.
Adhesion to polystyrene
The capacity of UPEC to adhere to polystyrene surfaces was investigated in the presence and absence of sumac. First, bacteria were incubated with the sumac extract (at MIC/2) or DMSO (control), as described for the YA test. After incubation, 2 ml of each culture was poured onto polystyrene Petri dishes (4 cm) and allowed to stand for 4 hours at room temperature. Afterward, dishes were washed from planktonic bacteria with sterile distilled water and adhered bacteria were stained with 2 ml of 1% crystal violet for 10 minutes. The dishes were rinsed with sterile distilled water to remove the excess dye and then air-dried. Adhesion of bacteria to Petri surfaces was observed by light microscopy at 1,000×. In order to evaluate the difference in adhesion between treated and nontreated bacteria, adhered cells were counted in 10 random fields of view, the mean was calculated, and the p value was further identified for 3 trials. Inhibition of adhesion was expressed as a percentage of the control groups.
Morphology
Light microscopy was used to investigate any morphological changes in the general shape and dimension of UPEC after exposure to the sumac extract. Standardized concentrations (OD492 = 0.05) of overnight cultures were incubated with the extract at MIC/2 in BHIB for 24 hours at 37°C, after which cultures were washed twice and resuspended in PBS. Finally, bacteria were Gram-stained and observed under a light microscope at 1,000×. In each sample, 100 random cells were observed. Images and measurements were obtained by Levenhuk M300 Base Digital Camera and Levenhuk ToupView (3.7.6273) software. Control cultures consisted of bacteria incubated with 10% DMSO.
Changes in antibiogram and susceptibility to sumac after long exposure to the extract
To investigate if long exposure to sumac can affect bacterial susceptibility to antibiotics or to the extract, the following assay was performed. In a sterile U-bottomed 96-well microplate, 100 μl of the sumac extract at MIC was added to 100 μl of BHIB, giving a final concentration of MIC/2. Afterward, wells in the A-row were inoculated with 10 μl of fresh bacterial inoculums and the microplate was incubated at 37°C. The next day, wells in the A-row were homogenized and 10 μl of each was introduced to the corresponding well in the B-row and incubated again and so forth until the fourth day. At this point, 10 μl of the last generation was added to sumac-free BHIB and incubated overnight. Subsequently, antibiograms and MICs were determined as aforementioned. As a negative control, the same procedure was simultaneously performed with 10% DMSO instead of extract.
Statistical analysis
All experiments were performed separately in triplicate. Excel 2019 was used to analyze data and to calculate means and standard deviations (SD). Statistical significance was evaluated by Student’s t-test with p < 0.05 reflecting a statistically significant difference.
RESULTS AND DISCUSSION
Extraction
The final sumac crude extract was in the form of a dark red semisolid mass. Extraction yield comprised 42% of the initial raw mass. In general, extraction yield differs according to the used extraction method, type of solvent, and duration of extraction and drying, among other factors (Kothari et al., 2012).
Antibiotic susceptibility profile (antibiogram) before and after long exposure to sumac extract
The prevalence of antibiotic resistance among UPEC is becoming a worldwide threat that complicates UTIs treatment and prophylaxis (Ballesteros-Monrreal et al., 2020). The susceptibility of UPEC clinical isolates to eight antibiotics was determined and interpreted according to CLSI (2021). The results are shown in Table 1. In a total of eight isolates, resistance was highly observed to AMP (five isolates), whereas the most sensitivity was to IPM (seven isolates) followed by CIP (six isolates). High AMP resistance among UPEC has been reported in many studies (Greer et al., 2008; Gupta et al., 1999; Hart et al., 2001). This resistance is mainly due to β-lactamase production; thus, the combination of AMP with β-lactamase inhibitors became an alternative to overcome resistance (Sáez-Llorens and McCracken, 2006). IPM is still one of the best treatment options for UTIs, especially those caused by Extended-spectrum beta-lactamases (ESBL)-producing strains (Kot, 2019; Terlizzi et al., 2017). TE, CAC, CAZ, and TR were effective against five isolates. However, none of the tested antibiotics was effective against all isolates.
Furthermore, the capacity of the sumac extract to modulate bacterial susceptibility to antibiotics was investigated. The results showed no significant changes had been caused by long exposure to the sumac extract (p > 0.05) (Table 1). Control samples, which have been incubated with DMSO, showed the same susceptibility as for nontreated normal bacteria (p > 0.05) (data not shown). Interestingly, Samoilova et al. (2014) reported that plant extracts modulate E. coli responses to antibiotics. The modulation effect, whether enhancing or reducing susceptibility, depended on the antibiotic with which the bacteria were treated as the plants’ extracts exhibited a protective effect for bacteria from CIP and AMP, while the bactericidal effect of kanamycin was enhanced. The authors suggested that these modulation effects are more likely due to some properties of polyphenols such as antioxidant, iron-chelating, or prooxidant activity. Here, we did not observe any change in susceptibility to the tested antibiotics after 4 d of exposure to the sumac extract. We suppose that many factors may play a role in the modulation effect caused by plant extracts, such as duration of exposure, phytochemical content of the extract, extract concentration, and type of tested bacteria and antibiotics, among other possibilities.
MAR index and MDR identifying
The MAR index is a simple and cost-effective indicator of the level of multiresistance in bacteria, and it also presents an idea about the source of isolate in terms of frequency of using antibiotics (Ayandele et al., 2020). MAR values greater than 0.2 indicate MAR bacteria which exist in a high-risk contaminated source where antibiotics are frequently used (Adenaike et al., 2016; Osundiya et al., 2013). Here, we determined the MAR index of eight UPEC isolates (Table 2). Four isolates were identified as MAR, one of them exhibited resistance to all tested antibiotics (MAR index = 1), and three showed a MAR index greater than 0.2 (0.75, 0.625, and 0.375). However, three isolates were sensitive to all tested antibiotics (MAR index = 0). MAR E. coli (Adenaike et al., 2016; Ayandele et al., 2020; Titilawo et al., 2015) and MAR UPEC (Baldiris-Avila et al., 2020; De Souza et al., 2019) have been already reported. Generally, the high prevalence of MAR bacteria requires more clinical investigations and strong control of antibiotic use since misuse of antibiotics causes bacteria to accumulate resistance genes and increase multidrug efflux pumps and eventually become MDR (Nikaido, 2009). All MAR strains in this study have been shown to be also MDR which accounts for 50% of the total number of tested isolates. This correlates with recent studies (Hassuna et al., 2020; Vasudevan et al., 2020; Zhong et al., 2020) where MDR UPEC were identified at high rates.
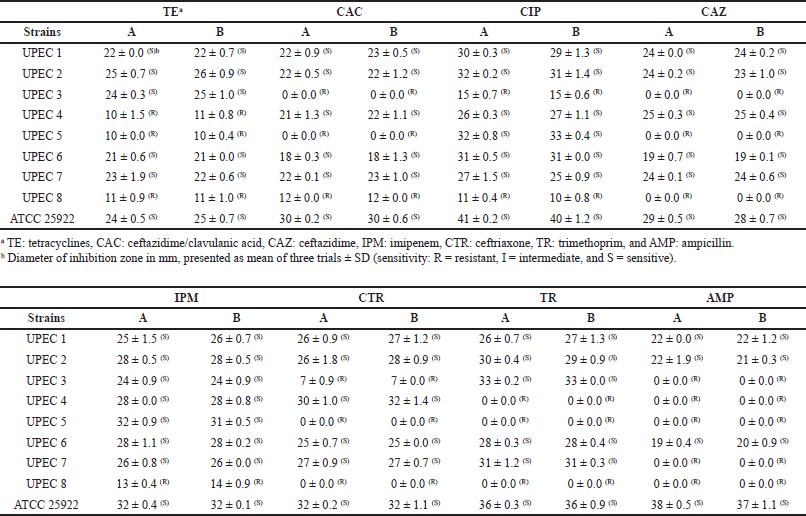 | Table 1. Antibiogram of E. coli strains. A: nontreated. B: after 4 days of incubation with the sumac extract. [Click here to view] |
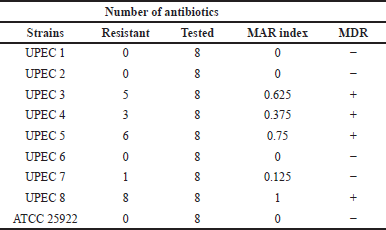 | Table 2. MAR index and MDR identifying. [Click here to view] |
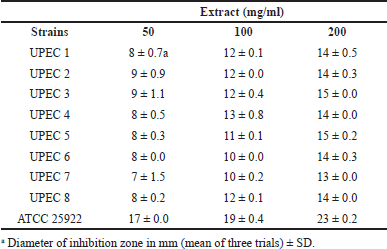 | Table 3. Well diffusion results for sumac extract against E. coli. [Click here to view] |
Well diffusion method
As a first screening, the antibacterial activity of the sumac extract was examined by the well diffusion method (Table 3, Fig. 1). All tested strains showed a considerable susceptibility to the extract in a concentration-dependent manner. There was not a significant difference in susceptibility between UPEC isolates (7–15 mm), while E. coli ATCC 25922 showed significantly higher susceptibility (17–23 mm). These findings correlate with other studies where the antibacterial activity of the sumac hydroalcoholic (Fazeli et al., 2007) and aqueous (Nasar-Abbas and Halkman, 2004; Vahid-Dastjerdi et al., 2014) extracts was significant against several Gram-negative and Gram-positive bacteria. Similarly, sumac essential oil was found to inhibit Pseudomonas aeruginosa, E. coli, Staphylococcus aureus, and Bacillus subtilis (Zhaleh et al., 2018). Additionally, the sumac aqueous extract strongly inhibited the growth of a MDR S. aureus isolate, in vitro and in vivo (Akrayi and Abdullrahman, 2013). Interestingly, the sumac ethanolic extract has been shown to exhibit a synergistic effect with antimicrobial drugs (oxytetracycline HCl, penicillin G, cephalexin, sulfadimethoxine as sodium, and enrofloxacin) against MDR P. aeruginosa (Adwan et al., 2010). In a similar manner, sumac has been reported to exert antibacterial activity against Gram-negative and Gram-positive strains, including S. aureus, P. aeruginosa, S. aureus Methicillin Resistant Staphylococcus Aureus (MRSA), and Salmonella enterica (Gabr and Alghadir, 2019; Mahdavi, 2018). Thus, our results support the reported antibacterial activity of sumac. This activity could be mainly attributed to sumac rich content of phytochemicals, especially phenolic compounds (Ardalani et al., 2016; Elagbar et al., 2020; Farag et al., 2018) which are known to interact with bacterial bioprocesses (Mandal et al., 2017; Takó et al., 2020).
MICs and MBCs
A quantitative antibacterial assay of the sumac extract was performed by determining MICs and MBCs (Table 4). The extract showed a MIC of 3.125 mg/ml against all tested UPEC isolates whereas the reference strain required a far lower concentration (0.0244 mg/ml) to inhibit visible bacterial growth. The MICs of seven isolates have been shown to be also MBCs, whereas one isolate and ATCC 25922 had an MBC of 6.25 and 1.563 mg/ml, respectively. Similar low MICs of sumac were reported against E. coli strains: 2 mg/ml for hydroalcoholic extract (Fazeli et al., 2007), 3 mg/ml for hydrodistilled essential oil (Elagbar et al., 2020), and 6–6.3 mg/ml for aqueous extract (Nasar-Abbas and Halkman, 2004).
Bacteria are smart organisms that surprisingly evolve various mechanisms to survive in unsuitable environments and adapt to antibacterials. This fact can be seen obviously in the rapid prevalence of antibiotic resistance. Interestingly, bacteria are shown to develop less resistance to phytochemicals (Shaheen et al., 2019). To inspect this, adaptation of bacteria to the sumac extract after long exposure to it was investigated. The results showed no alteration in susceptibility to the extract as the MICs were the same as for normal nontreated bacteria (Table 4). On the contrary, a study has shown an increase in MIC for the sumac aqueous extract after three3 and seven7 days of exposure to it (Nasar-Abbas and Halkman, 2004). Moreover, loss of antibacterial activity with prolonged incubation was also reported for other herbs (Shelef, 1984), which raises an important question of whether the resistance against phytochemicals could also emerge and shrink the efficacy of medicinal plants against bacterial pathogens.
YA and HA
Adhesion to host cells is a critical stage for bacteria to colonize and form biofilms (Nizet et al., 2015). Bacterial adhesion is mainly mediated by lectins that bind to specific sugar moieties on other cell types (Mrázková et al., 2019). Agglutination is a simple and cost-effective test to investigate adhesive properties of bacteria. Here, adhesion of UPEC to yeast cells (S. cerevisiae) and human RBCs was investigated after exposure to the sumac extract (Table 5). The presence of aggregates in both assays was confirmed under a light microscope (Fig. 2).
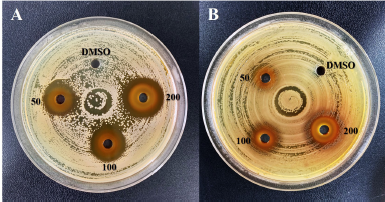 | Figure 1. Well diffusion method: inhibition zones of sumac extract at 50, 100, and 200 mg/ml. A: E. coli ATCC 25922. B: UPEC3. DMSO: negative control. [Click here to view] |
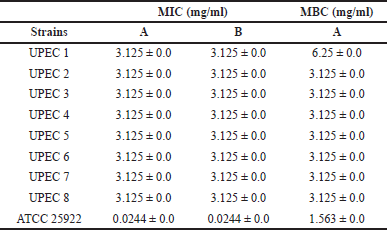 | Table 4. MICs and MBCs of the sumac extract. A: nontreated bacteria. B: bacteria after long exposure to sumac. [Click here to view] |
Out of eight isolates, seven were able to agglutinate with yeast cells in an MR manner; five of them showed strong agglutination whereas the other two were moderate. The sumac extract did not show any effect on the YA pattern of any isolate. Type 1 fimbriae (T1F) are widely expressed MS fimbriae in E. coli, and they are known to mediate binding of UPEC to mannosylated proteins (uroplakins) on the bladder epithelium (Kisiela et al., 2013; Stærk et al., 2016). T1F are encoded by the Fim operon which is a constitutive gene cluster located on the chromosome. The expression of this operon is a Phase variable which enables the bacterial cell to change between the Phase-ON (fimbriated cells) and the Phase-OFF (nonfimbriated cells) in response to environmental signals within the urinary tract (Terlizzi et al., 2017). Tamm–Horsfall proteins are mannosylated glycoproteins that normally exist in human urine as a part of the innate immune response. These proteins can bind to the TIF of UPEC and thus decrease their ability to bind to uroplakins (Scharf et al., 2019). From the aforementioned facts, it could be assumed that the Mannose-resistant yeast agglutination (MRYA) we observed in UPEC indicates a switched-off expression of T1F which is probably a defense response against Tamm-Horsfall proteins. A study conducted by Greene et al. (2015) supports this paradox by showing that when they are grown in human urine, expression and function of T1F in UPEC were inhibited and only restored by attachment to human bladder cells. Thus, it becomes predictable for planktonic UPEC in urine to lack effective T1F.
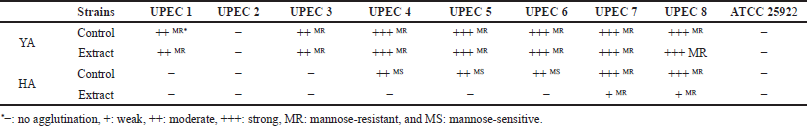 | Table 5. Agglutination patterns of E. coli with yeast (YA) and human RBCs (HA). [Click here to view] |
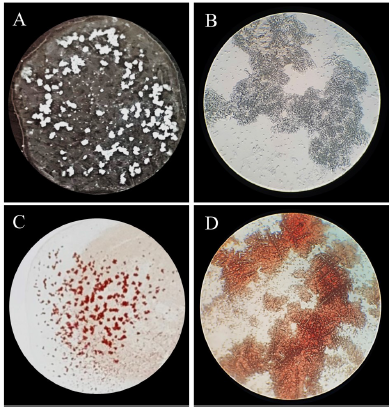 | Figure 2. Strong agglutination of UPEC 8 with yeast cells (A, B) and human RBCs (C, D). Aggregates on slides (A, C) and under a light microscope (100×) (B, D). [Click here to view] |
HA typing is commonly performed with human or animal RBCs to investigate hemagglutinating properties of bacterial cells (Evans et al., 1980) which have been associated with the adhesive capacity to epithelial cells (Alp et al., 2010; Hagberg et al., 1981). Here, five UPEC isolates were able to agglutinate with RBCs: three with a strong Mannose-resistant hemagglutination (MRHA) and two with a moderate MSHA. However, three isolates did not show any agglutination activity. The extract reduced HA in all positive cases by two degrees; i.e., strong HA became weak and moderate became negative. Escherichia coli have been reported to have various agglutination patterns with human RBCs depending on many factors such as culturing media and used blood group (Hrv et al., 2016; Shareef et al., 2010). MRHA with human RBCs is shown to be mediated mainly by P pili, one of the most common MR fimbriae in UPEC that mediate binding to the digalactoside epitope (Galα–1,4-Galβ) of glycolipids on epithelial kidney cells and P-blood group erythrocytes. P pili are evolved in kidneys colonization by UPEC and highly associated with pyelonephritis (Busch et al., 2015; Möllby et al., 1983). However, optimizing growth conditions (Mikcha et al., 2004) and selecting the appropriate blood group (Hrv et al., 2016) are essential to evaluate the HA activity of bacteria. Here, we mainly aimed to investigate the effect of the extract on bacterial HA patterns. Hence, we conclude that the extract was able to reduce the HA for all agglutinating bacteria suggesting downregulation of the mediating fimbriae. Therefore, sumac could be suspected to have an inhibiting effect on the adhesion capacity of UPEC in vivo.
Adhesion on polystyrene
Bacterial adhesion is not confined to living tissues but also widely found on abiotic surfaces with the subsequent formation of biofilms (Dunne, 2002). Biofilms represent a worrisome global issue as they are more resistant to antibacterial agents and harder to eliminate. This issue is highly associated with medical implants such as ventilators, catheters, contact lenses, and heart valves, complicating treatment and causing chronic inflammation (Simo, 2020). In this study, we investigated the effect of the sumac extract on the adhesion capacity of UPEC to polystyrene. The results revealed that the sumac extract significantly inhibited (p < 0.05) the adhesion of seven isolates at the following percentages: 95.1%, 94.3%, 92.1%, 91.8%, 85.4%, 82.7%, and 79.1% ± 0.4%–1.3%. Polystyrene surfaces are routinely used as in vitro platforms to investigate bacterial adhesion and biofilm formation (K?rmusao?lu, 2019). A correlation between bacterial adhesion to polystyrene and epithelial cells has been reported (Ruiz et al., 2011). Moreover, the adhesion ability of bacteria to polystyrene is considered a problem of significance for plastic-based surgical material and medical implants (Khatoon et al., 2018). Phytochemicals have been widely reported to exert antiadhesive and antibiofilm activity against UPEC (Amalaradjou et al., 2010; Lagha et al., 2019; Packiavathy et al., 2014; Rodríguez-Pérez et al., 2016). In particular, sumac has been shown to inhibit Streptococcus mutans biofilm formation and suppress the acidogenicity of the formed biofilm (Kacergius et al., 2017). This antibiofilm activity was mainly attributed to the presence of methyl gallate in the sumac methanolic extract. Similarly, sumac seems to protect against dental caries and plaque as its water extract inhibited five common oral bacteria (Streptococcus species and Enterococcus faecalis) and their ability to form biofilms (Vahid-Dastjerdi et al., 2014). Here, we showed that sumac could be an effective antiadhesive agent against UPEC as it effectively decreased their ability to adhere to polystyrene. This inhibition is more likely to be due to modification of bacterial cell surface hydrophobicity which is a crucial determinant in bacterial adhesion to biotic and abiotic surfaces (Krasowska and Sigler, 2014). These findings are promising in confronting biofilm formation on medical devices as they could have applications in catheter surface coating or lock solution, which in turn will greatly contribute to preventing UTIs.
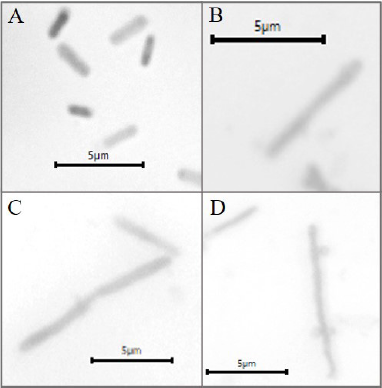 | Figure 3. Morphological changes in UPEC 3 after incubation with the sumac extract. A: normal cells. B, C, and D: short filaments (5–10 μm). Magnification 1,000×. [Click here to view] |
Morphological changes in UPEC after exposure to sumac extract
The capacity of the sumac extract to cause morphological changes in UPEC was investigated. For seven isolates, no differences were observed between the control and treated samples as they all contained rods of normal dimensions: length of 1–4 μm and diameter of 0.3–0.7 μm. On the contrary, a significant increase in length was observed in one isolate (UPEC 3) as the length of the control sample was of 1–3 μm, while 80% of treated cells were >4 μm and up to 8.4 μm (short filaments) (Fig. 3). SD for all the aforementioned values was of 0.4–1.3. Plant secondary metabolites have been shown to have different effects on bacterial cell morphology, probably depending on the tested bacteria and on the chemical nature of these compounds. For instance, in some studies, bacteria became visibly shorter after exposure to phytochemicals (Kurek et al., 2010; Szakiel et al., 2008), whereas in other cases the formation of filaments was highly induced by phytochemicals (Dorota et al., 2013). Bacterial filamentation is an abnormal growth in which cells grow in long threadlike strands composed of nondividing elongated cells. This phenomenon has been suggested to be a result of several factors such as DNA damage, defects in cell division by which bacteria lose the ability to separate at the end of the division, unfavorable environment, or antibiotics treatment (Buijs et al., 2008; Dorota et al., 2013; Justice et al., 2008). Here, we found that the sumac extract highly caused the bacterial cells of one UPEC strain to become significantly longer and to form short filaments. This observation may indicate a stress condition to bacteria caused by the sumac extract or penetration of active phytochemicals into bacterial cells, damaging DNA or interacting with the replication process.
CONCLUSION
Sumac appears as a promising medicinal plant for developing antibacterial pharmaceuticals against MDR UPEC. This study represents a basis for a variety of further investigations including identifying active compounds responsible for the antibacterial properties, investigating in vivo cytotoxicity, and determining the precise mechanisms of action.
ACKNOWLEDGMENTS
The authors are grateful to Joseph Mbarga, Alexander Senyagin, and Ibrahim Khelifi for their assistance in laboratory work.
LIST OF ABBREVIATIONS
UPEC: Uropathogenic Escherichia coli; UTIs: Urinary tract infections; MAR: Multiple antibiotic resistance; MDR: Multidrug resistance; YA: Yeast agglutination; HA: Hemagglutination; MS: Mannose-sensitive; MR: Mannose-resistant; T1F: Type 1 fimbriae.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
None.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
REFERENCES
Adenaike O, Olonitola OS, Ameh, Whong CMZ. Multidrug resistance and multiple antibiotic resistance index of Escherichia coli strains isolated from retailed smoked fish. J Nat Sci Res, 2016; 6:7–10.
Adwan G, Abu-Shanab B, Adwan K. Antibacterial activities of some plant extracts alone and in combination with different antimicrobials against multidrug-resistant Pseudomonas aeruginosa strains. Asian Pac J Trop Med, 2010; 3:266–9; doi:10.1016/S1995-7645(10)60064-8. CrossRef
Akbari-Fakhrabadi M, Heshmati J, Sepidarkish M, Shidfar F. Effect of sumac (Rhus Coriaria) on blood lipids: a systematic review and meta-analysis. Complement Ther Med, 2018; 40:8–12; doi:10.1016/j.ctim.2018.07.001. CrossRef
Akrayi HFS, Abdullrahman ZFA. Screening in vitro and in vivo the antibacterial activity of Rhus Coriaria extract against S. aureus. IJRRAS, 2013; 15:390–7.
Alp G, Aslim B, Suludere Z, Akca G. The role of hemagglutination and effect of exopolysaccharide production on bifidobacteria adhesion to Caco-2 cells in vitro. Microbiol Immunol, 2010; 54:658–65; doi:10.1111/j.1348-0421.2010.00227.x. CrossRef
Amalaradjou MAR, Narayanan A, Baskaran SA, Venkitanarayanan K. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J Urol, 2010; 184:358–63; doi:10.1016/j.juro.2010.03.006. CrossRef
Ardalani H, Hassanpour Moghadam M, Hadipanah A, Fotovat F, Azizi A, Soltani J. Identification and characterization of chemical composition of Rhus coriaria L. fruit from Hamadan, Western Iran. J Med Herbs, 2016; 6:195–8.
Ayandele A, Oladipo E, Oyebisi O, Kaka M. Prevalence of multi-antibiotic resistant Escherichia coli and Klebsiella species obtained from a Tertiary Medical Institution in Oyo State, Nigeria. Qatar Med J, 2020; 2020:9; doi:10.5339/qmj.2020.9. CrossRef
Baldiris-Avila R, Montes-Robledo A, Buelvas-Montes Y. Phylogenetic classification, biofilm-forming capacity, virulence factors, and antimicrobial resistance in uropathogenic Escherichia coli (UPEC). Curr Microbiol, 2020; 77:3361–70; doi:10.1007/s00284-020-02173-2. CrossRef
Ballesteros-Monrreal MG, Mp Arenas-Hernández M, Enciso-Martínez Y, Martínez-De La Peña CF, Del C Rocha-Gracia R, Lozano-Zaraín P, Navarro-Ocaña A, Martínez-Laguna Y, de la Rosa-López R. Virulence and resistance determinants of uropathogenic Escherichia coli strains isolated from pregnant and non-pregnant women from two states in Mexico. Infect Drug Resist, 2020; 13:295–310; doi:10.2147/IDR.S226215. CrossRef
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal, 2016; 6:71–9; doi:10.1016/j.jpha.2015.11.005. CrossRef
Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. J Pathog, 2016; 2016:1–5; doi:10.1155/2016/4065603. CrossRef
Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol, 1966; 45:493–6; doi:10.1093/ajcp/45.4_ts.493. CrossRef
Beretta G, Rossoni G, Santagati NA, Facino RM. Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta. Planta Med, 2009; 75:1482–8; doi:10.1055/s-0029-1185797. CrossRef
Buijs J, Dofferhoff ASM, Mouton JW, Wagenvoort JHT, Van Der Meer JWM. Concentration-dependency of β-lactam-induced filament formation in Gram-negative bacteria. Clin Microbiol Infect, 2008; 14:344–9; doi:10.1111/j.1469-0691.2007.01940.x. CrossRef
Busch A, Phan G, Waksman G. Molecular mechanism of bacterial type 1 and P pili assembly. Philos Trans R Soc A Math Phys Eng Sci, 2015; 373:20130153; doi:10.1098/rsta.2013.0153. CrossRef
Chaudhary N, Narayan C, Mohan B, Taneja N. Characterization and in vitro activity of a lytic phage RDN37 isolated from community sewage water active against MDR Uropathogenic E. coli. Indian J Med Microbiol, 2021; 39:343–8; doi:10.1016/j.ijmmb.2021.04.011. CrossRef
CLSI. Performance standards for antimicrobial susceptibility testing. 31st edition, CLSI supplement M100, United States, 2021.
Dorota W, Marta K, Dorota TG. Effect of asiatic and ursolic acids on morphology, hydrophobicity, and adhesion of UPECs to uroepithelial cells. Folia Microbiol (Praha), 2013; 58:245–52; doi:10.1007/s12223-012-0205-7. CrossRef
Dunne WM. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev, 2002; 15:155–66; doi:10.1128/CMR.15.2.155-166.2002. CrossRef
Dziki D, Cacak-Pietrzak G, Hassoon WH, Gawlik-Dziki U, Su?ek A, Ró?y?o R, Sugier D. The fruits of sumac (Rhus coriaria L.) as a functional additive and salt replacement to wheat bread. LWT, 2021; 136:110346; doi:10.1016/j.lwt.2020.110346. CrossRef
Elagbar ZA, Shakya AK, Barhoumi LM, Al-Jaber HI. Phytochemical diversity and pharmacological properties of Rhus coriaria. Chem Biodivers, 2020; 17:e1900561; doi:10.1002/cbdv.201900561. CrossRef
Evans DJ, Evans DG, Young LS, Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol, 1980; 12:235–42; doi:10.1128/jcm.12.2.235-242.1980. CrossRef
Farag MA, Fayek NM, Abou Reidah I. Volatile profiling in Rhus coriaria fruit (sumac) from three different geographical origins and upon roasting as analyzed via solid-phase microextraction. PeerJ, 2018; 6:e5121; doi:10.7717/peerj.5121. CrossRef
Fazeli MR, Amin G, Attari MMA, Ashtiani H, Jamalifar H, Samadi N. Antimicrobial activities of Iranian sumac and avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Control, 2007; 18:646–9; doi:10.1016/j.foodcont.2006.03.002. CrossRef
Gabr SA, Alghadir AH. Evaluation of the biological effects of lyophilized hydrophilic extract of Rhus coriaria on myeloperoxidase (MPO) activity, wound healing, and microbial infections of skin wound tissues. Evid Based Complement Altern Med, 2019; 2019:1–14; doi:10.1155/2019/5861537. CrossRef
Greene SE, Hibbing ME, Janetka J, Chen SL, Hultgren SJ. Human urine decreases function and expression of type 1 pili in uropathogenic Escherichia coli. MBio, 2015; 6:e00820; doi:10.1128/mBio.00820-15. CrossRef
Greer LG, Roberts SW, Sheffield JS, Rogers VL, Hill JB, Mcintire DD, Wendel GD Jr. Ampicillin resistance and outcome differences in acute antepartum pyelonephritis. Infect Dis Obstet Gynecol, 2008; 2008:1–5; doi:10.1155/2008/891426. CrossRef
Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. J Am Med Assoc, 1999; 281:736–8; doi:10.1001/jama.281.8.736. CrossRef
Hagberg L, Jodal U, Korhonen TK, Janson GL, Lindberg U, Edan CS. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun, 1981; 31:564–70; doi:10.1128/iai.31.2.564-570.1981. CrossRef
Hart A, Nowicki BJ, Reisner B, Pawelczyk E, Goluszko P, Urvil P, Anderson G, Nowicki S. Ampicillin-resistant Escherichia coli in gestational pyelonephritis: increased occurrence and association with the colonization factor Dr adhesin. J Infect Dis, 2001; 183:1526–9; doi:10.1086/320196. CrossRef
Hassuna NA, Khairalla AS, Farahat EM, Hammad AM, Abdel-Fattah M. Molecular characterization of extended-spectrum β lactamase- producing E. coli recovered from community-acquired urinary tract infections in Upper Egypt. Sci Rep, 2020; 10:1–8; doi:10.1038/s41598-020-59772-z. CrossRef
Hrv R, Devaki R, Kandi V. Comparison of hemagglutination and hemolytic activity of various bacterial clinical isolates against different human blood groups. Cureus, 2016; 8:e489; doi:10.7759/cureus.489. CrossRef
Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol, 2008; 6:162–8; doi:10.1038/nrmicro1820. CrossRef
Kacergius T, Abu-Lafi S, Kirkliauskiene A, et al. Inhibitory capacity of Rhus coriaria L. extract and its major component methyl gallate on Streptococcus mutans biofilm formation by optical profilometry: Potential applications for oral health. Mol Med Rep, 2017; 16(1):949–56. doi:10.3892/mmr.2017.6674 CrossRef
Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control, 2019; 8:118; doi:10.1186/s13756-019-0559-6. CrossRef
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon, 2018; 4:1067; doi:10.1016/j.heliyon.2018.e01067. CrossRef
Kisiela DI, Chattopadhyay S, Tchesnokova V, Paul S, Weissman SJ, Medenica I, Clegg S, Sokurenko EV. Evolutionary analysis points to divergent physiological roles of Type 1 Fimbriae in Salmonella and Escherichia coli. MBio, 2013; 4:e00625–12; doi:10.1128/mBio.00625-12. CrossRef
K?rmusao?lu S. The methods for detection of biofilm and screening antibiofilm activity of agents. Antimicrobials, antibiotic resistance, antibiofilm strategies and activity methods. IntechOpen, 2019; doi:10.5772/intechopen.84411. CrossRef
Kot B. Antibiotic resistance among uropathogenic Escherichia coli. Polish J Microbiol, 2019; 68:403–15; doi:10.33073/pjm-2019-048. CrossRef
Kothari V, Gupta A, Naraniwal M. Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat Remedies, 2012; 12/2:162–73; doi:10.18311/jnr/2012/271.
Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol, 2014; 4:112; doi:10.3389/fcimb.2014.00112. CrossRef
Kurek A, Grudniak AM, Szwed M, Klicka A, Samluk L, Wolska KI, Janiszowska W, Popowska M. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie van Leeuwenhoek, 2010; 97:61–8; doi:10.1007/s10482-009-9388-6. CrossRef
Lagha R, Ben Abdallah F, AL-Sarhan B, Al-Sodany Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules, 2019; 24:1161; doi:10.3390/molecules24061161. CrossRef
Lara VM, Carregaro AB, Santurio DF, de Sá MF, Santurio JM, Alves SH. Antimicrobial susceptibility of Escherichia coli strains isolated from Alouatta spp. feces to essential oils. Evid Based Complement Alternat Med, 2016; 2016:1643762; doi:10.1155/2016/1643762. CrossRef
Lodhia S, Sharaf A, Foley C. Management of recurrent urinary tract infections in adults. Surg, 2020; 38:197–203; doi:10.1016/j.mpsur.2020.01.012. CrossRef
Mahdavi S. Antimicrobial and antioxidant activities of Iranian sumac (Rhus coriaria L.) fruit ethanolic extract. J Appl Microbiol Biochem, 2018; 02:5; doi:10.21767/2576-1412.100021. CrossRef
Mandal SM, Dias RO, Franco OL. Phenolic compounds in antimicrobial therapy. J Med Food, 2017; 20:1031–8; doi:10.1089/JMF.2017.0017. CrossRef
Mikcha JMG, Piantino Ferreira AJ, Astolfi Ferreira CS, Yano T. Hemagglutinating properties of Salmonella enterica serovar enteritidis isolated from different sources. Brazilian J Microbiol, 2004; 35:54–8; doi:10.1590/S1517-83822004000100008. CrossRef
Mohammadi S, Zarei M, Zarei MM, Salehi I. Effect of hydroalcoholic leaves extract of rhus coriaria on pain in male rats. Anesthesiol Pain Med, 2016; 6:e32128; doi:10.5812/aapm.32128. CrossRef
Möllby R, Källenius G, Svenson SB, Korhonen TK, Winberg J. P-fimbriae of pyelonephritogenic Escherichia coli: detection in clinical material by a rapid receptor-specific agglutination test. Infection, 1983; 11:68–72; doi:10.1007/BF01651363. CrossRef
Mrázková J, Malinovská L, Wimmerová M. Microscopy examination of red blood and yeast cell agglutination induced by bacterial lectins. PLoS One, 2019; 14:e0220318; doi:10.1371/journal.pone.0220318. CrossRef
Nasar-Abbas SM, Halkman AK. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int J Food Microbiol, 2004; 97:63–9; doi:10.1016/j.ijfoodmicro.2004.04.009. CrossRef
Negus M, Phillips C, Hindley R. Recurrent urinary tract infections: a critical review of the currently available treatment options. Obstet Gynaecol, 2020; 22:115–21; doi:10.1111/tog.12644. CrossRef
Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem, 2009; 78:119–46; doi:10.1146/annurev.biochem.78.082907.145923. CrossRef
Nizet V, Varki A, Aebi M. Microbial Lectins: Hemagglutinins, Adhesins, and Toxins. In: Varki A, Cummings RD, Esko JD, et al., eds. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, 2017; 481–91.
Osundiya O, Oladele R, Oduyebo O. Multiple antibiotic resistance (MAR) indices of Pseudomonas and Klebsiella species isolates in Lagos University Teaching Hospital. African J Clin Exp Microbiol, 2013; 14:164–8; doi:10.4314/ajcem.v14i3.8. CrossRef
Packiavathy IASV, Priya S, Pandian SK, Ravi AV. Inhibition of biofilm development of uropathogens by curcumin—an anti-quorum sensing agent from Curcuma longa. Food Chem, 2014; 148:453–60; doi:10.1016/j.foodchem.2012.08.002. CrossRef
Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food Chem Toxicol, 2010; 48:854–8; doi:10.1016/j.fct.2009.12.021. CrossRef
Ravindran PN, Pillai GS, Divakaran M. Other herbs and spices: mango ginger to wasabi. Handbook of herbs and spices. Elsevier, Vol. 2, pp 557–82, 2012; doi:10.1533/9780857095688.557. CrossRef
Reidel RVB, Cioni PL, Majo L, Pistelli L. Evolution of volatile emission in Rhus coriaria organs during different stages of growth and evaluation of the essential oil composition. Chem Biodivers, 2017; 14:e1700270; doi:10.1002/cbdv.201700270. CrossRef
Rodríguez-Pérez C, Quirantes-Piné R, Uberos J, Jiménez-Sánchez C, Peña A, Segura-Carretero A. Antibacterial activity of isolated phenolic compounds from cranberry (Vaccinium macrocarpon) against Escherichia coli. Food Funct, 2016; 7:1564–73; doi:10.1039/c5fo01441g. CrossRef
Ruiz V, Rodríguez-Cerrato V, Huelves L, Del Prado G, Naves P, Ponte C, Soriano F. Adherence of Streptococcus pneumoniae to polystyrene plates and epithelial cells and the antiadhesive potential of Albumin and Xylitol. Pediatr Res, 2011; 69:23–7; doi:10.1203/PDR.0b013e3181fed2b0. CrossRef
Sáez-Llorens X, McCracken GH. Clinical pharmacology of antibacterial agents. Infectious diseases of the fetus and newborn infant. Elsevier, pp 1223–67, 2006; doi:10.1016/B0-72-160537-0/50039-6. CrossRef
Sakhr K, El Khatib S. Physiochemical properties and medicinal, nutritional and industrial applications of Lebanese Sumac (Syrian Sumac - Rhus coriaria): a review. Heliyon, 2020; 6:e03207; doi:10.1016/j.heliyon.2020.e03207. CrossRef
Samoilova Z, Smirnova G, Muzyka N, Oktyabrsky O. Medicinal plant extracts variously modulate susceptibility of Escherichia coli to different antibiotics. Microbiol Res, 2014; 169:307–13; doi:10.1016/j.micres.2013.06.013. CrossRef
Scharf B, Sendker J, Dobrindt U, Hensel A. Influence of cranberry extract on Tamm-Horsfall protein in human urine and its antiadhesive activity against uropathogenic Escherichia coli. Planta Med, 2019; 85:126–38; doi:10.1055/a-0755-7801. CrossRef
Shabbir A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance: a review. J Anim Plant Sci, 2012; 22:505–12.
Shaheen G, Akram M, Jabeen F, Ali Shah SM, Munir N, Daniyal M, Riaz M, Tahir IM, Ghauri AO, Sultana S, Zainab R, Khan M. Therapeutic potential of medicinal plants for the management of urinary tract infection: a systematic review. Clin Exp Pharmacol Physiol, 2019; 46:613–24; doi:10.1111/1440-1681.13092. CrossRef
Shareef HA, Abdulla ET, Mostafa ZN. Hemagglutination properties of some intestinal bacterial pathogens isolated from clinical samples. Tikrit J Pure Sci, 2010; 15:5–10.
Shelef LA. Antimicrobial effects of spices. J Food Saf, 1984; 6:29–44; doi:10.1111/j.1745-4565.1984.tb00477.x. CrossRef
Shidfar F, Rahideh ST, Rajab A, Khandozi N, Hosseini S, Shidfar S, Mojab F. The effect of Sumac Rhus coriaria L. powder on serum glycemic status, ApoB, ApoA-I and total antioxidant capacity in type 2 diabetic patients. Iran J Pharm Res, 2014; 13:1249–55; doi:10.22037/ijpr.2014.1585.
Simo M. Recent trends in biofilm science and technology. Elsevier, 2020; doi:10.1016/C2018-0-05115-4.
De Souza GM, Neto ERDS, da Silva AM, Iacia MVM de S, Rodrigues MVP, Pereira VC, Winkelstroter LK. Comparative study of genetic diversity, virulence genotype, biofilm formation and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from nosocomial and community acquired urinary tract infections. Infect Drug Resist, 2019; 12:3595–606; doi:10.2147/IDR.S228612. CrossRef
Stærk K, Khandige S, Kolmos HJ, Møller-Jensen J, Andersen TE. Uropathogenic Escherichia coli express type 1 Fimbriae only in surface adherent populations under physiological growth conditions. J Infect Dis, 2016; 213:386–94; doi:10.1093/infdis/jiv422. CrossRef
Szakiel A, Ruszkowski D, Grudniak A, Kurek A, Wolska KI, Doligalska M, Janiszowska W. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis). Planta Med, 2008; 74:1709–15; doi:10.1055/s-0028-1088315. CrossRef
Takó M, Kerekes EB, Zambrano C, Kotogán A, Papp T, Krisch J, Vágvölgyi C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants (Basel), 2020; 9:165; doi:10.3390/antiox9020165. CrossRef
Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol, 2017; 8:1566; doi:10.3389/fmicb.2017.01566. CrossRef
Titilawo Y, Sibanda T, Obi L, Okoh A. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of faecal contamination of water. Environ Sci Pollut Res, 2015; 22:10969–80; doi:10.1007/s11356-014-3887-3. CrossRef
Tohma H, Altay A, Köksal E, Gören AC, Gülçin ?. Measurement of anticancer, antidiabetic and anticholinergic properties of sumac (Rhus coriaria): analysis of its phenolic compounds by LC–MS/MS. J Food Meas Charact, 2019; 13:1607–19; doi:10.1007/s11694-019-00077-9. CrossRef
Vahid-Dastjerdi E, Sarmast Z, Abdolazimi Z, Mahboubi A, Amdjadi P, Kamalinejad M. Effect of Rhus coriaria L. water extract on five common oral bacteria and bacterial biofilm formation on orthodontic wire. Iran J Microbiol, 2014; 6:269–75.
Vasudevan S, Thamil Selvan G, Bhaskaran S, Hari N, Solomon AP. Reciprocal cooperation of type A procyanidin and nitrofurantoin against multi-drug resistant (MDR) UPEC: a pH-dependent study. Front Cell Infect Microbiol, 2020; 10:421; doi:10.3389/fcimb.2020.00421. CrossRef
Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc, 2008; 3:163–75; doi:10.1038/nprot.2007.521. CrossRef
Zalewska-Pi?tek BM, Pi?tek RJ. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains. Acta Biochim Pol, 2019; 66:129–38; doi:10.18388/abp.2018_2787. CrossRef
Zhaleh M, Sohrabi N, Zangeneh MM, Zangeneh A, Moradi R, Zhaleh H. Chemical composition and antibacterial effects of essential oil of Rhus coriaria fruits in the West of Iran (Kermanshah). J Essent Oil Bearing Plants, 2018; 21:493–501; doi:10.1080/0972060X.2018.1462739. CrossRef
Zhong ZX, Cui ZH, Li XJ, Tang T, Zheng ZJ, Ni WN, Fang LX, Zhou YF, Yu Y, Liu YH, Liao XP, Sun J. Nitrofurantoin combined with amikacin: a promising alternative strategy for combating mdr uropathogenic Escherichia coli. Front Cell Infect Microbiol, 2020; 10:811; doi:10.3389/fcimb.2020.608547. CrossRef