INTRODUCTION
Panax notoginseng (P. notoginseng) is the root of P. notoginseng (Burk.) F. H. Chen, a traditional Chinese medicine, that has been widely exploited for the management of chronic diseases like cerebral, diabetic, and cardiovascular diseases (Ng, 2006). In Eastern countries, P. notoginseng has been developed in various dosage forms such as injections and capsules commercially. The active compounds from P. notoginseng including saponins, flavonoids, sterols, volatile oil, amino acids, and polysaccharides contributed in different ways to the application of P. notoginseng (Wang et al., 2008). Among them, saponins are considered the most popular and include ginsenoside Rh1, Rg1, and Re, notoginsenoside R1, ginsenoside Rb1, and Rb1 (Xu et al., 2019). In patients with coronary artery and cardiovascular diseases, P. notoginseng saponins (PNS) have positively affected the management of conditions with different functional mechanisms including anti-inflammation, regulation of lipid metabolism, regulation of coagulation system, antiapoptosis, proangiogenesis, antiatherosclerosis, and protection against myocardial ischemia (Duan et al., 2017). In addition, PNS were able to manage hyperglycemia and obesity via manipulating glucose production and absorption and inflammatory processes (Uzayisenga et al., 2014). Those potentiate the application of PNS in older adults who normally have chronic body issues.
Older adults and chronic diseases are a common pair in nature, due to which the function of the body is impaired inducing complex health disorders. The therapeutic selections for older adults are not just based on the safety and efficacy of drugs but also the acceptability of patients including the ease and safety of administration and palatable properties (Gnjidic et al., 2018). To address those concerns, soluble, oral dispersible, or chewable dosage forms were found promising, especially in patients who have dysphagia (Khan and Roberts, 2018). Among the modern options, oral films offer older adults several advantages such as no need to swallow, low risk of misdosing, good stability, and convenient transport and storage (Walsh et al., 2018). Therefore, the orodispersible film (ODF) could be an efficient drug delivery carrier for PNS in older adults.
On the other hand, despite the mentioned advantages, PNS, orally administrated, suffer from the acidic environment of the gastric fluid, resulting in degradation and reduced bioavailability (Jin et al., 2018). Several approaches have been investigated to encapsulate PNS such as mucoadhesive enteric-coating microparticles (Baek et al., 2015), colon-specific osmotic pump capsules (Jin et al., 2018), and coated sustained-release pellets (Zhao et al., 2021). This issue of acid on PNS could be solved when an ODF is applied to deliver PNS since most of the film components dissolve in the oral cavity leading to the absorption of the drug on-site.
Bearing those in mind, in this study, we try to develop a comprehensive solution for adults to use PNS conveniently as well as effectively. In detail, PNS were isolated and purified from the roots of P. notoginseng and then loaded onto an ODF that on one hand could induce quick absorption of PNS in the oral cavity to escape digestive tract traveling and on the other hand could support the ease of use without water needed, especially in adults with dysphagia. The films were prepared for quick disintegration (less than 30 seconds) and robust physical properties. The stability of PNS in a different pH medium was checked to track the impact. The physical properties of the formed films were characterized by scanning electron microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR) analysis. The release of PNS was also performed to confirm the concept of design.
MATERIALS AND METHODS
Materials
Standard substances including notoginsenoside R1 (98%), ginsenoside Rg1 (98%), ginsenoside Re (98%), ginsenoside Rb1 (98%), and ginsenoside Rd (99.7%) were purchased from ChemFaces Co., Ltd., China. The macroporous D101 adsorbent resin was supplied by Donghong Chemical Co., Ltd., China. The methanol and acetonitrile for High Performance Liquid Chromatography (HPLC) were provided by Merck, Germany. Other substances were at the pharmaceutical usage level.
Methods
Extraction of PNS
The roots of P. notoginseng (Burk.) F. H. Chen were collected in Simacai, Lao Cai Province, Vietnam, in September 2019. The samples were kept at the Department of Pharmacognosy and Traditional Pharmacy, University of Medicine and Pharmacy, Vietnam National University, Hanoi.
Total PNS were prepared by a method described previously (Guo et al., 2013). Purified water was added to 100 g of P. notoginseng powder at a medicinal herb/solvent ratio of 1/7. The resulting mixture of water and medicinal herbs was extracted by hot reflux at 60°C for 2 hours. After that, the first extract was collected and filtered. The extraction process was repeated with pure water at the ratio of medicinal ingredients/solvents of 1/5 in 1.5 hours and the same condition as before. The combined extract was filtered and then evaporated at 60°C in a vacuum using the rotary evaporator R-300 (BÜCHI, Switzerland) to get P. notoginseng extractum.
Purification of PNS
The purification of total saponins from the extract was conducted by the column chromatography method using macroporous resin D101 particles. In detail, microporous resin D101 particles were soaked in 96% aqueous ethanol for 12 hours, then rinsed with water, and drained to dryness. Afterward, 300 g of resin was loaded onto the column with a volume of 500 ml. The adsorption process was performed as follows: 50 g of P. notoginseng extractum was diluted then adsorbed onto a column containing macroporous resin D101 at a rate of 10 ml/minute. At last, after all saponins had been adsorbed, the column was washed with 3,000 ml of purified water and 3,000 ml of 20% aqueous ethanol at the same rate of 30 ml/minute. The elution process was conducted with 3,000 ml of 70% aqueous ethanol at a rate of 20 ml/minute. The 70% aqueous ethanol fraction was concentrated at 60°C under reduced pressure to remove the solvent. Eventually, the extract was dried at 60°C in a vacuum to obtain the total PNS. The total PNS were analyzed to determine the content of five saponins, notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd, by HPLC-UV.
HPLC analysis
The saponins concentration is measured using HPLC equipment according to Chinese Pharmacopoeia 2015. In detail, the measurements were conducted on the HPLC Agilent 1260 Infinity Series System (Palo Alto, CA). The saponins were eluted on a C18 column (4.6 × 250 mm, 3.5 μm, Agilent Technologies) using a gradient elution mobile phase including acetonitrile (A) and purified water (B) at different ratios: 0–20 minutes (20% A), 20–45 minutes (25%–46% A), 45–55 minutes (46%–55% A), and 55–60 minutes (55% A). The signal was detected at a wavelength of 203 nm with a flow rate of 1.5 ml/minute and the system operated at 25°C.
Molecular weight determination of purified saponins
Approximately 10 mg of the purified saponins was weighed, dried, and then added to a 100 ml volumetric flask followed by adding of methanol. The obtained mixture was sonicated for 10 minutes. Saponins were determined by mass spectrometry, and retention times were compared with standard samples under the same chromatographic conditions by high-performance liquid chromatography coupled with a Diode Array Detector probe. Mass spectra were determined using a liquid chromatographic system coupled to an Liquid Chromatography-Tandem Mass Spectropmetry (LC-MS/MS) 6420 Triple Quad Mass Spectrometer Detector (Agilent Technologies) with the following parameters: Electrospray Ionization ion source of electric ray ionization, capillary voltage at 4,000 V, nebulizer pressure at 30 psi, nitrogen flow rate at 11 l/minute, temperature at 300°C, and scan mode from 100 to 1,500 m/z.
Preparation of ODFs
The ODFs contained a film-forming polymer, plastic agents, and PNS that were dissolved in water under constant stirring at 1,000 rpm using a magnetic bar. When the mixture was homogenous, it was divided into the wells of a 6-well plate. Subsequently, the film was dried in an oven at 40°C overnight (Lai et al., 2015). All ODFs were sealed in plastic and stored in the dark before further testing.
Characterization of ODFs
Film thickness
Film thickness was measured in three different films using the digital microscope MT5300L (Meiji, Japan). The film was placed under the microscope, and the images were captured and then analyzed using imaging software. The measurements were performed in triplicate.
Disintegration test
A disintegration test was conducted using a Petri disk. Briefly, 10 ml of a phosphate buffer of pH 6.8 in a Petri dish was warmed up to 37°C, and then each film (n = 3) was placed on the top of the water, and the dish was gently shaken. The disintegration time was calculated from the beginning until pieces of the film became invisible (Steiner et al., 2016).
Surface pH
The surface pH was measured using a pH meter (Sension + pH 3, Hach, China). Three films were swelling in 1 ml of distilled water at room temperature for 1 hour. Afterward, the electrode was touched to the surface of the film and allowed to equilibrate for 1 minute for the pH record (Oh et al., 2020).
Loss on drying
The water content was measured using an infrared moisture analyzer (MB 45, Ohaus, Switzerland). Each film was weighted and subsequently heated at 105°C until unchanged weight. Loss on drying was the difference (in %) in mass between the initial weight and the final weight at equilibrium (Musazzi et al., 2018).
IR spectroscopy method
The test samples (about 5–10 mg) were placed directly on the face of the diamond spot of the Agilent Cary 630 machine (Agilent Technologies, Malaysia). The scanning was carried out with a wavelength range of 4,000–400 cm−1 and a resolution of 0.4 cm−1 (Tran et al., 2021).
Scanning electron microscopy (SEM)
Morphology of the samples was observed using the FE-SEM S-4800 electron microscope (Hitachi, Japan). The plain materials and film were placed on the SEM conductive adhesive tape and then gold-coated at 10 mA for 40 seconds in a vacuum sputter coater (SBC-12, KYKY Technology Co., Ltd., China) (Truong et al., 2016).
Uniformity of dosage
The uniformity of dosage units of the films was conducted in three pieces of films (Shen et al., 2013). Each saponin was evaluated by the HPLC method as described above.
Dissolution studies
Dissolution studies were performed in a beaker containing 20 ml of the dissolution medium Phosphate Buffered Saline (solution pH 6.8). The films were placed into the beaker, and then the media were stirred in the hotplate magnetic stirrer C-Mag HS10 S000 (IKA, Malaysia) at a speed of 100 rpm at 37°C ± 0.5°C. At 0.5, 1, 2, 3, and 5 minutes, 0.5 ml of the dissolution medium was withdrawn, and another 0.5 ml of fresh media was added to replace it. The test was repeated three times (Foo et al., 2018). The test sample was diluted with a phosphate buffer to the appropriate concentration and filtered through a 0.45 μm cellulose acetate filter and then evaluated by the HPLC method.
RESULTS
PNS contents and identification
Saponins are the major bioactive ingredient of P. notoginseng which were isolated as shown in Figure 1. In this study, five saponins of PNS, notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd, were analyzed. The chromatograms showed that notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd are completely separated in the chromatograms of the sample. The retention times of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd are similar to those of the standard substances. The chromatograms of the blank sample did not show any peaks at the same retention times as those of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd (Suppl. Fig. 1).
Saponins were structurally determined by mass spectrometry and compared with standards. The chromatogram results showed that the retention time of saponins is the same as the retention time of saponins R1, Rg1, Re, Rb1, and Rd, respectively, under the same chromatographic conditions. The mass spectrum depicted an m/z of 967.3, 835.3, 981.5, 1,143.5, and 981.6 corresponding to (R1 + Cl)-, (Rg1 + Cl)-, (Re + Cl)-, (Rb1 + Cl)-, and (Rd + Cl)-, respectively (Fig. 2).
According to HPLC analysis, PNS are about 8.06% of the raw material. After the purification process, the yield is 93.35%. Out of total saponins, notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd proportioned 6.34, 31.29, 3.21, 31.17, and 8.45%, respectively (Table 1).
Stability of PNS in different pH
To confirm the concept of the study, saponins were incubated in pH 1.2, 4.5, and 6.8 mimicking the environment of the digestive fluid (Fig. 3). At pH 4.5 and 6.8, after 24 hours exposure, the quantity of all saponins was maintained. The amount of R1 was slightly reduced but not significantly. Meanwhile, at pH 1.2, half of the saponins were degraded after 1 hour in the case of R1 and Rg1 and after 4 hours in the case of Re, Rb1, and Rd. This reduction may intensively impact the bioavailability of PNS when they are delivered orally without technical support.
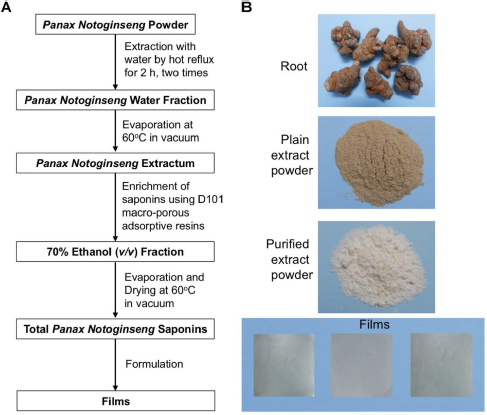 | Figure 1. The extraction of Panax Notoginseng Saponins and the formation of ODFs. (A) The schematic illustration of process, (B) The images of products. [Click here to view] |
Characterization of PNS-loaded films
The compositions of films were optimized to meet the criterion of physical properties (homogenous, robust) and short integrating time (less than 30 seconds). HPMC E6, maltodextrin, Sodium Carboxymethyl Cellulose, and Polyvinyl Alcohol (PVA) were used as the film-forming polymer whereas sorbitol, glycerin, and Polyethylene Glycol 400 are the plasticizer. The PNS-loaded films were prepared by dissolving a film-forming polymer, a plasticizer, and saponins into distilled water to become a homogenous mixture. After 24 hours of drying, the obtained films were collected to evaluate the surface, look, and integrating time (data not shown). PVA and sorbitol formed the best film with smooth surfaces and transparent look without any sign of ununiform drug distribution and hence were selected for further characterization. Different films were imaged using camera integrated microscopy, and then the thickness of films was measured and was 96.00 ± 3.47 μm (Table 2). During the optimization, we found that the thickness of the films is correlated with the disintegrated time. For the optimized films, the disintegrated time is 23.67 ± 1.53 seconds, less than 30 seconds that meets the requirement of Food and Drug Administration guidance for industry orally disintegrating tablets. Moreover, the loss on drying of the film is 7.25% ± 0.13% compared to the overall weight of films that is acceptable to avoid the stickiness of the ODFs. The increased moisture of materials could cause the growth of microorganisms.
Along with a quick dissolution, a robust texture is required to maintain physical integrity. The folding endurance of the optimized film was reported as more than 300 times indicating the robustness and toughness of the film.
The FTIR spectrum illustrated the featured peaks of the functional group of individual materials and films (Fig. 4). The main peaks of PVA were observed at 3,283, 2,937, 1,722, 1,424, 1,327, 1,088, and 842 cm−1 corresponding to the O–H stretching vibration of the hydroxy group, CH2 asymmetric stretching vibration, C=O carbonyl stretch, C–H bending vibration of CH2, C–H deformation vibration, C–O stretching of acetyl groups, and C–C stretching vibration, respectively. On the other hand, sorbitol showed bands at 3,321 cm−1 (-OH stretching vibrations), 2,926 cm−1 (C–H stretching in alkanes), and 1,640 cm−1 (C=O stretch of carbonyls) whereas the characteristic bands of PNS are as follows: 3,228 cm−1 (O–H stretching vibrations), 2,929 cm−1 (C–H stretching vibrations), and 1,252, 1,088, and 1,047 cm−1 (C–O stretching vibrations). The saponin film showed 3,284 cm−1 (O–H stretching vibration), 2,937 cm−1 (C–H stretching vibration), 1,655 cm−1 (C=O stretch of carbonyls), and 1,245 and 1,074 cm−1 (C–O stretching vibrations) suggesting that there might be no molecular interaction between the PNS and other ingredients.
The SEM analysis was conducted to observe the surface morphology of raw materials and formulated films. The PVA (Fig. 5A, D) and saponins (Fig. 5B, E) showed a sharp and robust surface whereas the filament pattern was observed in the film sample. This unique film morphology could fasten water absorption and then induce quick film disintegration (Fig. 5C, F).
The PNS were loaded at the strength of 50 mg into the film. The drug content was evaluated using the HPLC method. Figure 6A shows the included amount of each saponin with a homogenous distribution of amounts. The total saponins were 36 mg in which Rg1 and Rb1 were the most (more than 10 mg) and Re was the least as proportioned in the overall saponins. Of note, in all tested films (n = 5), the drug content is uniform suggesting the application as well as the reliability of the preparation methods for further investigation.
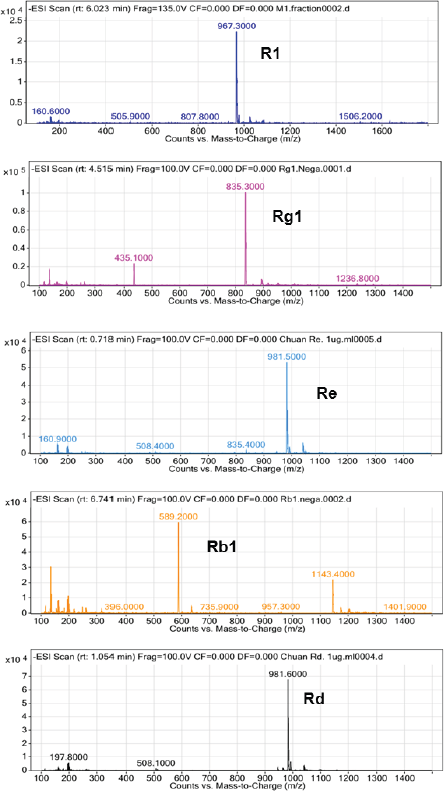 | Figure 2. The characterized LC-MS spectrum of PNS. [Click here to view] |
The release of saponins-loaded films was investigated in the pH 6.8 medium in which all saponins showed a quick dissolution (Fig. 6B–G). Within 5 minutes, almost 100% of the saponins were detected in the testing medium. As expected, this rapid release is attributed to the high solubility of saponins as well as film-forming agents.
DISCUSSION
Aging has been found as the promoter of chronic diseases due to its genetic, dietary, and pharmacologic influence. It is common to have vascular disease or diabetes in adults (Kennedy et al., 2014). Moreover, it is not preferable for older people who normally have malfunctioning organs like the kidney and liver to use the standard therapies. In this context, herbal medicine offers benefits as alternative therapies (Peltzer and Pengpid, 2019). Panax notoginseng is one of the most widely used traditional Chinese herbal medicines with multiple pharmacological activities (Yang et al., 2021). PNS are the main biologically active constituents of P. notoginseng, and notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd are considered to be the major components (Li et al., 2013). PNS positively contributed to the management of cardiocerebral vascular disease and diabetes (Xu et al., 2019).
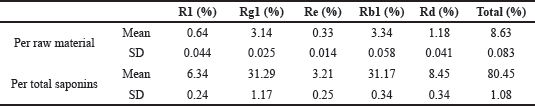 | Table 1. The proportion of purified saponins. [Click here to view] |
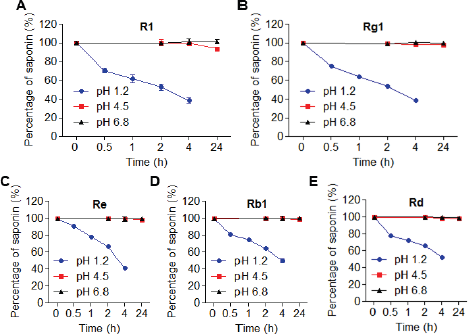 | Figure 3. The stability of PNS in different pH media 1.2, 4.5, and 6.8. (A) R1, (B) Rg1, (C) Re, (D) Rb1, and (E) Rd.s. [Click here to view] |
 | Table 2. Films properties. [Click here to view] |
However, the poor stability in an acidic environment limited the application of PNS. To address the issue, most of the studies try to escape the low pH condition of the gastric fluid by “by-passing” this site. Jin et al. (2018) used Eudragit® S100, a functional delayed-release polymer for colon delivery, in the semipermeable membrane to cover the PNS. This polymer was dissolved in a solution with pH above 7.0 inducing the delay of the release of PNS. Hence, the mean retention time was prolonged, and the Area under the Curve of the PNS was increased (Jin et al., 2018). In another study, a similar strategy was applied by using Eudragit NE30D, a polymer that dissolves at a pH of 5.5 to coat the PNS-loaded pellet that also delayed the release of PNS. There was no drug leakage in the 0.1 M hydrochloric acid buffer within 2 hours. Therefore, the drug was protected to be absorbed later with a higher oral bioavailability (Zhao et al., 2021). Previously, Baek et al. (2015) fabricated the microparticles containing Eudragit L100-55 that is dissolved in media at a pH > 5.5 to encapsulate PNS. This effort also limited the dissolution rate of PNS in the first 2 hours in an acidic medium.
In this study, we exploited the advantage of ODFs that could deliver PNS into the oral cavity where the pH of saliva is neutral (6.2–7.6). As shown in the results section, at above pH 4.5, all of the saponins are maintained over 24 hours suggesting that the issue of stability of PNS no longer exists in that condition. “Moreover, the quick release of saponins could trigger immediate absorption via the sublingual and buccal vascular , that possibly induces an early onset of PNS in blood circulation and improved bioavailability.” Of note, the application of ODFs is widely accepted with several marketed products (Visser et al., 2020). Last but not least, as mentioned above, the engagement of older adults with the therapeutic course could be enhanced by simplifying the administration route/method without swallowing or water needed.
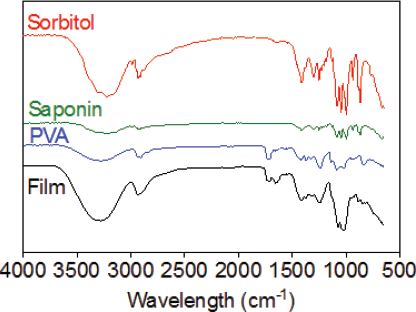 | Figure 4. FTIR analysis of sorbitol, PVA, saponins, and saponins-loaded ODFs. [Click here to view] |
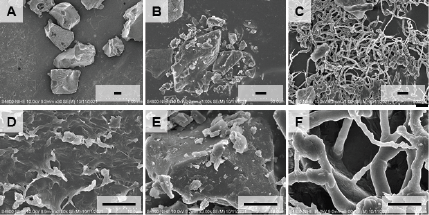 | Figure 5. Scanning electron microscopic images of (A, D) PVA, (B, E) saponins, and (C, F) saponins-loaded films. [Click here to view] |
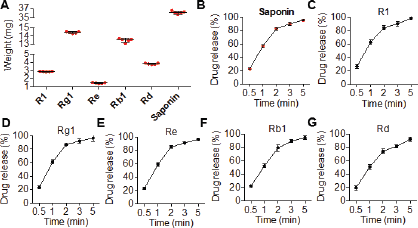 | Figure 6. (A) The contents of PNS in ODFs. (B–G) The dissolution profile of total saponins and individual saponins in release medium. [Click here to view] |
The advantage of this dosage form is clear. However, some points should be considered and improved for better delivery of PNS. Firstly, PNS are known for their bitter taste; thereby, taste masking might support the comfort of people who use them. Moreover, in vivo investigation should be conducted on the scale-up products that will provide insight into the user’s feeling as well as the kinetic profile of PNS in the blood circulation system.
CONCLUSIONS
In this study, for the first time, PNS were successfully loaded into ODFs composed of PVA and sorbitol. In the preparation process, the saponins fraction of P. notoginseng was extracted and purified with a yield of 91.33%. The PNS-loaded films could disintegrate within 30 seconds, and the dissolution results showed that all five saponins, R1, Rg1, Re, Rb1, and Rd, were released nearly 100% after 5 minutes allowing quick absorption. Most importantly, the pH induction studies confirmed the stability of PNS in pH above 4.5 and the ease of film use without water needed suggesting that P. notoginseng-loaded ODFs are potential dosage forms for adults in managing chronic diseases.
ACKNOWLEDGMENTS
This work was supported by Research Project QG.20.61, “Study of Effect of Saponin in the Genus Panax on Steroid Hormone Levels in Experimental Animals,” from Vietnam National University, Hanoi.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
FUNDING
This work was supported by Research Project QG.20.61, Study of Effect of Saponin in the Genus Panax on Steroid Hormone Levels in Experimental Animals, from Vietnam National University, Hanoi.
REFERENCES
Baek, J.-S., Yeon, W.-G., Lee, C.-A., Hwang, S.-J., Park, J.-S., Kim, D.-C., Cho, C.-W., 2015. Preparation and characterization of mucoadhesive enteric-coating ginsenoside-loaded microparticles. Arch. Pharm. Res. 38, 761–768. https://doi.org/10.1007/s12272-014-0395-4 CrossRef
Duan L, Xiong X, Hu J, Liu Y, Li J, Wang J. Panax notoginseng saponins for treating coronary artery disease: a functional and mechanistic overview. Front Pharmacol, 2017; 8:702; doi:10.3389/fphar.2017.00702 CrossRef
Foo WC, Khong YM, Gokhale R, Chan SY. A novel unit-dose approach for the pharmaceutical compounding of an orodispersible film. Int J Pharm, 2018; 539:165–74; doi:10.1016/j.ijpharm.2018.01.047 CrossRef
Gnjidic D, Husband A, Todd A. Challenges and innovations of delivering medicines to older adults. Adv. Adv Drug Deliv Rev, 2018; 135:97–105; doi:10.1016/j.addr.2018.08.003 CrossRef
Guo X, Zhang X, Feng J, Guo Z, Xiao Y, Liang X. Purification of saponins from leaves of Panax notoginseng using preparative two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography. Anal Bioanal Chem, 2013; 405:3413–21; doi:10.1007/s00216-013-6721-8 CrossRef
Jin D, Wang B, Hu R, Su D, Chen J, Zhou H, Lu W, Guo Y, Fang W, Gao S. A novel colon-specific osmotic pump capsule of Panax notoginseng saponins (PNS): formulation, optimization, and in vitro-in vivo evaluation. AAPS PharmSciTech, 2018; 19:2322–9; doi:10.1208/s12249-018-1068-2 CrossRef
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell, 2014; 159:709–13; doi:10.1016/j.cell.2014.10.039 CrossRef
Khan MS, Roberts MS. Challenges and innovations of drug delivery in older age. Adv Drug Deliv Rev, 2018; 135:3–38; doi:10.1016/j.addr.2018.09.003 CrossRef
Lai F, Franceschini I, Corrias F, Sala MC, Cilurzo F, Sinico C, Pini E. Maltodextrin fast dissolving films for quercetin nanocrystal delivery. A feasibility study. Carbohydr Polym, 2015; 121:217–23; doi:10.1016/j.carbpol.2014.11.070 CrossRef
Li SP, Qiao CF, Chen YW, Zhao J, Cui XM, Zhang QW, Liu XM, Hu DJ. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J Chromatogr A, 2013; 1313:302–7; doi:10.1016/j.chroma.2013.07.025 CrossRef
Musazzi UM, Selmin F, Ortenzi MA, Mohammed GK, Franzé S, Minghetti P, Cilurzo F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int J Pharm, 2018; 551:52–9; doi:10.1016/j.ijpharm.2018.09.013 CrossRef
Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol, 2006; 58:1007–19; doi:10.1211/jpp.58.8.0001 CrossRef
Oh BC, Jin G, Park C, Park JB, Lee BJ. Preparation and evaluation of identifiable quick response (QR)-coded orodispersible films using 3D printer with directly feeding nozzle. Int J Pharm, 2020; 584:119405; doi:10.1016/j.ijpharm.2020.119405 CrossRef
Peltzer K, Pengpid S. The use of herbal medicines among chronic disease patients in Thailand: a cross-sectional survey. J Multidiscip Healthc, 2019; 12:573–82; doi:10.2147/JMDH.S212953 CrossRef
Shen BD, Shen CY, Yuan XD, Bai JX, Lv QY, Xu H, Dai L, Yu C, Han J, Yuan HL. Development and characterization of an orodispersible film containing drug nanoparticles. Eur J Pharm Biopharm, 2013; 85:1348–56; doi:10.1016/j.ejpb.2013.09.019 CrossRef
Steiner D, Finke JH, Kwade A. Efficient production of nanoparticle-loaded orodispersible films by process integration in a stirred media mill. Int J Pharm, 2016; 511:804–13; doi:10.1016/j.ijpharm.2016.07.058 CrossRef
Tran TB, Tran TH, Vu YH, Le TG, Do TT, Nguyen NT, Nguyen TT, Pham TB, Ngo TQ, Luong QA, Nguyen CN. pH-responsive nanocarriers for combined chemotherapies: a new approach with old materials. Cellulose, 2021; 28:3423–33; doi:10.1007/s10570-021-03769-y CrossRef
Truong DH, Tran TH, Ramasamy T, Choi JY, Lee HH, Moon C, Choi HG, Yong CS, Kim JO. Development of solid self-emulsifying formulation for improving the oral bioavailability of erlotinib. AAPS PharmSciTech, 2016; 17:466–73; doi:10.1208/s12249-015-0370-5 CrossRef
Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res, 2014; 28:510–6; doi:10.1002/ptr.5026 CrossRef
Visser JC, Wibier L, Mekhaeil M, Woerdenbag HJ, Taxis K. Orodispersible films as a personalized dosage form for nursing home residents, an exploratory study. Int J Clin Pharm, 2020; 42:436–44; doi:10.1007/s11096-020-00990-w CrossRef
Walsh J, Ranmal SR, Ernest TB, Liu F. Patient acceptability, safety and access: A balancing act for selecting age-appropriate oral dosage forms for paediatric and geriatric populations. Int J Pharm, 2018; 536:547–62; doi:10.1016/j.ijpharm.2017.07.017 CrossRef
Wang D, Hong D, Koh HL, Zhang YJ, Yang CR, Hong Y. Biodiversity in cultivated Panax notoginseng populations1. Acta Pharmacol Sin, 2008; 29:1137–40; doi:10.1111/j.1745-7254.2008.00875.x CrossRef
Xu C, Wang W, Wang B, Zhang T, Cui X, Pu Y, Li N. Analytical methods and biological activities of Panax notoginseng saponins: recent trends. J Ethnopharmacol, 2019; 236:443–65; doi:10.1016/j.jep.2019.02.035 CrossRef
Yang Y, Ju Z, Yang Y, Zhang Y, Yang L, Wang Z. Phytochemical analysis of Panax species: a review. J Ginseng Res, 2021; 45:1–21; doi:10.1016/j.jgr.2019.12.009 CrossRef
Zhao YL, Zhang SQ, Lu WX, Shen SZ, Wei L. Preparation of Panax notoginseng flower saponins enteric-coated sustained-release pellets and its pharmacokinetics and in vitro-in vivo correlation. J Drug Deliv Sci Technol, 2021; 62:102321; doi:10.1016/j.jddst.2021.102321 CrossRef
SUPPLEMENTARY MATERIALS
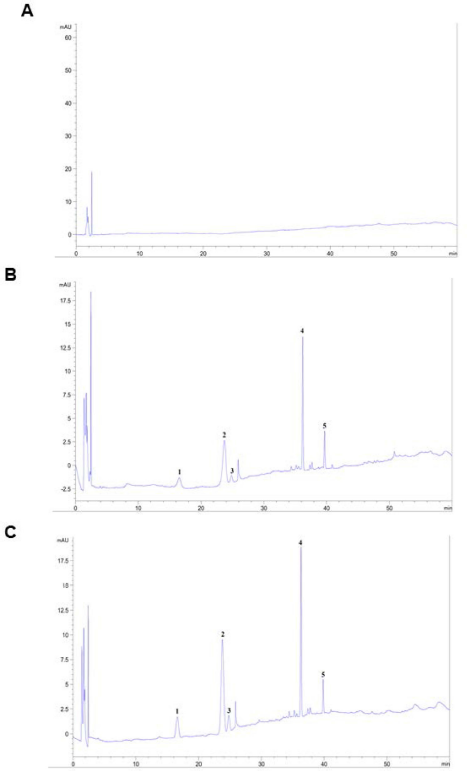 | Suppl. Fig. 1. Chromatography of (A) blank sample, (B) test sample, (C) samples and added mixture of 5 saponin standards. 1, notoginsenoside R1; 2, ginsenoside Rg1; 3, ginsenoside Re; 4, ginsenoside Rb1; 5, ginsenoside Rd. [Click here to view] |