RP HPLC Method for the determination of Tamsulosin in bulk and Pharmaceutical Formulations
Manish Kumar Thimmaraju, Venkat Rao, Hemanth .K, P. Siddartha Kumar
Pages: 177-180

Evaluation of Transdermal Formulations: A Technical Note
Mudasir Mohamad, Roheena Jan
Pages: 37-40

Method Development and Validation ofLevosalbutamol in Pure and Tablet Dosage Formby RP-HPLC
Narendra Nyola, Govinda Samy Jeyabalan, Garima Yadav, Rajesh Yadav , Subash Gupta, Habibullah Khalilullah
DOI: 10.7324/JAPS.2012.2628Pages: 155-158

Simultaneous estimation of Cefpodoxime proxetil and Ofloxacin In tablet dosage form using RP-HPLC
Annadi Chiranjeevi and Medidi Srinivas
DOI: 10.7324/JAPS.2014.40508Pages: 046-050

Development and validation of high-performance liquid chromatography method for simultaneous determination of acyclovir and curcumin in polymeric microparticles
Jéssica Brandão Reolon, Maicon Brustolin, Sandra Elisa Haas, Eduardo André Bender, Marcelo Donadel Malesuik, Letícia Marques Colomé
DOI: 10.7324/JAPS.2018.8120Pages: 136-141

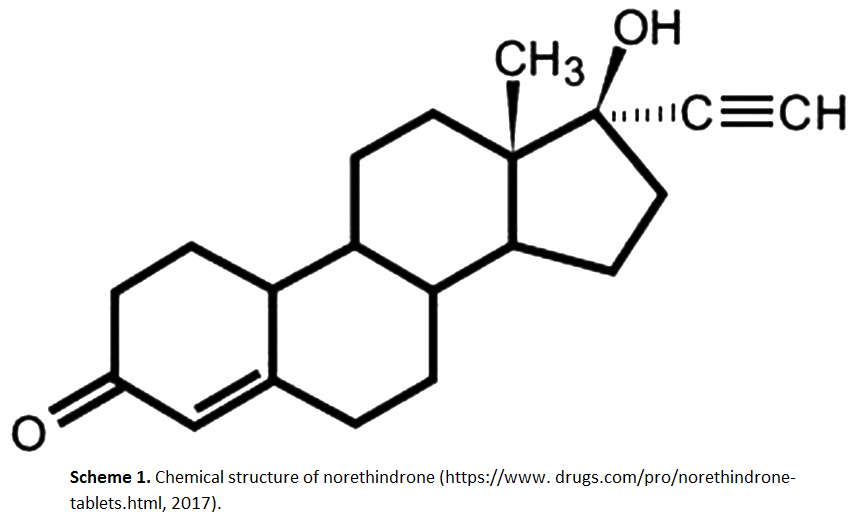
RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation
Suhair S. Al-Nimry, Bashar M. Altaani, Razan H. Haddad
DOI: 10.7324/JAPS.2019.90211Pages: 079-086
Box–Behnken design-based HPLC optimization for quantitative analysis of chloramphenicol and hydrocortisone acetate in cream
Kusnul Khotimah, Sudibyo Martono, Abdul Rohman
DOI: 10.7324/JAPS.2020.10916Pages: 134-139

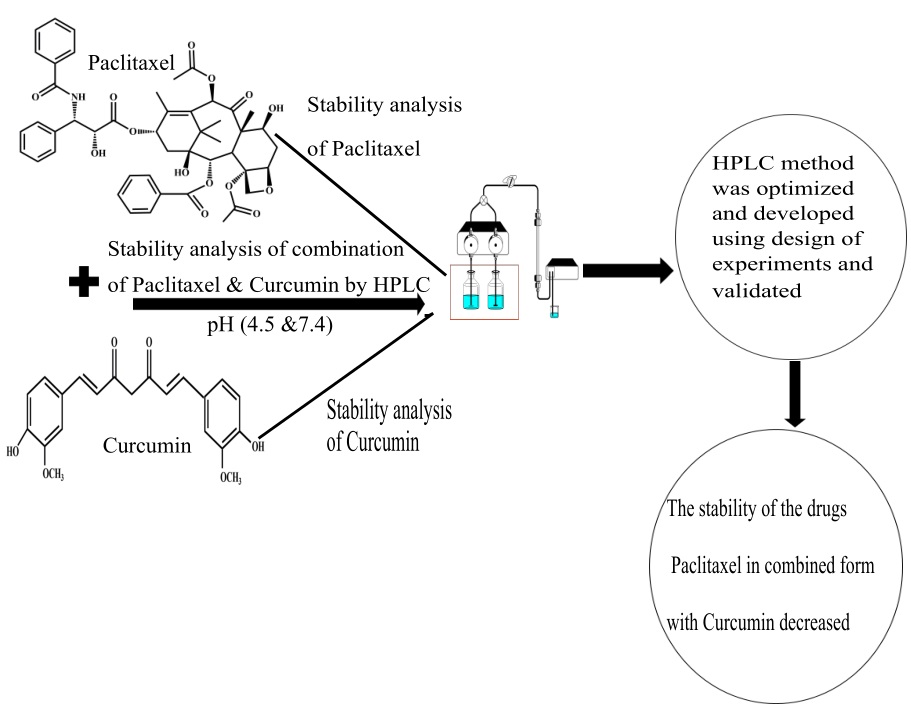
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
Anticancer activity of liposomal formulation co-encapsulated with coumarin and phenyl butyric acid
Ali Allateef, Naeem Shalan, Zainab Lafi
DOI: 10.7324/JAPS.2024.181335Pages: 208-215
_.jpg)
Supercritical fluid extraction, LC-MS profiling, and QbD-guided green HPLC method for standardization of Careya arborea Roxb. nanoemulsion
Abhijit S. Salokhe, Archana S. Patil, Yadishma Gaude, Pooja Rayanade, Rahul Koli, Namdeo S. Jadhav
DOI: 10.7324/JAPS.2025.266427Pages:
_.jpg)