UPLC method development and validation for Cefditoren Pivoxil in active pharmaceutical Ingredient
Ram Garg, Navneet Singh, Kona S. Srinivas, Binayak Deb, Ayaz Ahmed
Pages: 149-153

Development and validation of selective UV spectrophotometric analytical method for budesonide pure sample
Shakuntala, Pushp Bharti, Neetu Sachan, Phool Chandra, Kavita GahloMallikarjuna Gouda M, Ramakrishna Shabaraya. A, Shantakumar S.M, Somashekar Shyale. S, Putta Rajesh kumar
Pages: 158-161

Development and validation of a rapid liquid chromatographic method for the analysis of Ketorolac Tromethamine and its related production impurities
O' Connor N., Geary M., Wharton M., Curtin L
DOI: 10.7324/JAPS.2012.2524Pages: 15-21

A RP-HPLC Method Development and Validation for the Estimation of Gliclazide in bulk and Pharmaceutical Dosage Forms
B.V.V Ravi kumar, A.K. Patnaik, Saroj Kumar Raul, Nagireddy Neelakanta Rao
DOI: 10.7324/JAPS.2013.3410Pages: 059-062

Simultaneous estimation of Cefpodoxime proxetil and Ofloxacin In tablet dosage form using RP-HPLC
Annadi Chiranjeevi and Medidi Srinivas
DOI: 10.7324/JAPS.2014.40508Pages: 046-050

Development and Validation of a Stability-Indicating High Performance Thin Layer Chromatography (HPTLC) Method for estimation of Canagliflozin in bulk and Pharmaceutical Dosage Form
Ishpreet Kaur, Sharad Wakode, Harsharan Pal Singh
DOI: 10.7324/JAPS.2016.60508Pages: 051-057

Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Sumatriptan in Bulk and Pharmaceutical Dosage Form
M. Srinidhi, Md. Mushabbar Basha, V. Raj Kumar, J. Rajendra Kumar
DOI: 10.7324/JAPS.2016.60604Pages: 020-025

Development and validation of a new RP-HPLC method for the estimation of dutasteride in bulk and pharmaceutical formulations
Poonguzhali Subramanian, P. S. Rajinikanth
DOI: 10.7324/JAPS.2016.601207Pages: 047-055

Development and Validation of UV-Spectroscopic Method for Estimation of Niacin in Bulk and Pharmaceutical Dosage Form
Indranil Chanda, Ripunjoy Bordoloi, Debarupa D. Chakraborty, Prithviraj Chakraborty, Smriti Rekha Chanda Das
DOI: 10.7324/JAPS.2017.70911Pages: 081-084

Enantioseparation of Tedizolid phosphate by RP-HPLC, using ð›½-Cyclodextrin as a Chiral Mobile Phase Additive
Ajit Anerao, Vikram Dighe, Satish John, Nitin Pradhan
DOI: 10.7324/JAPS.2017.71005Pages: 030-036

Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107
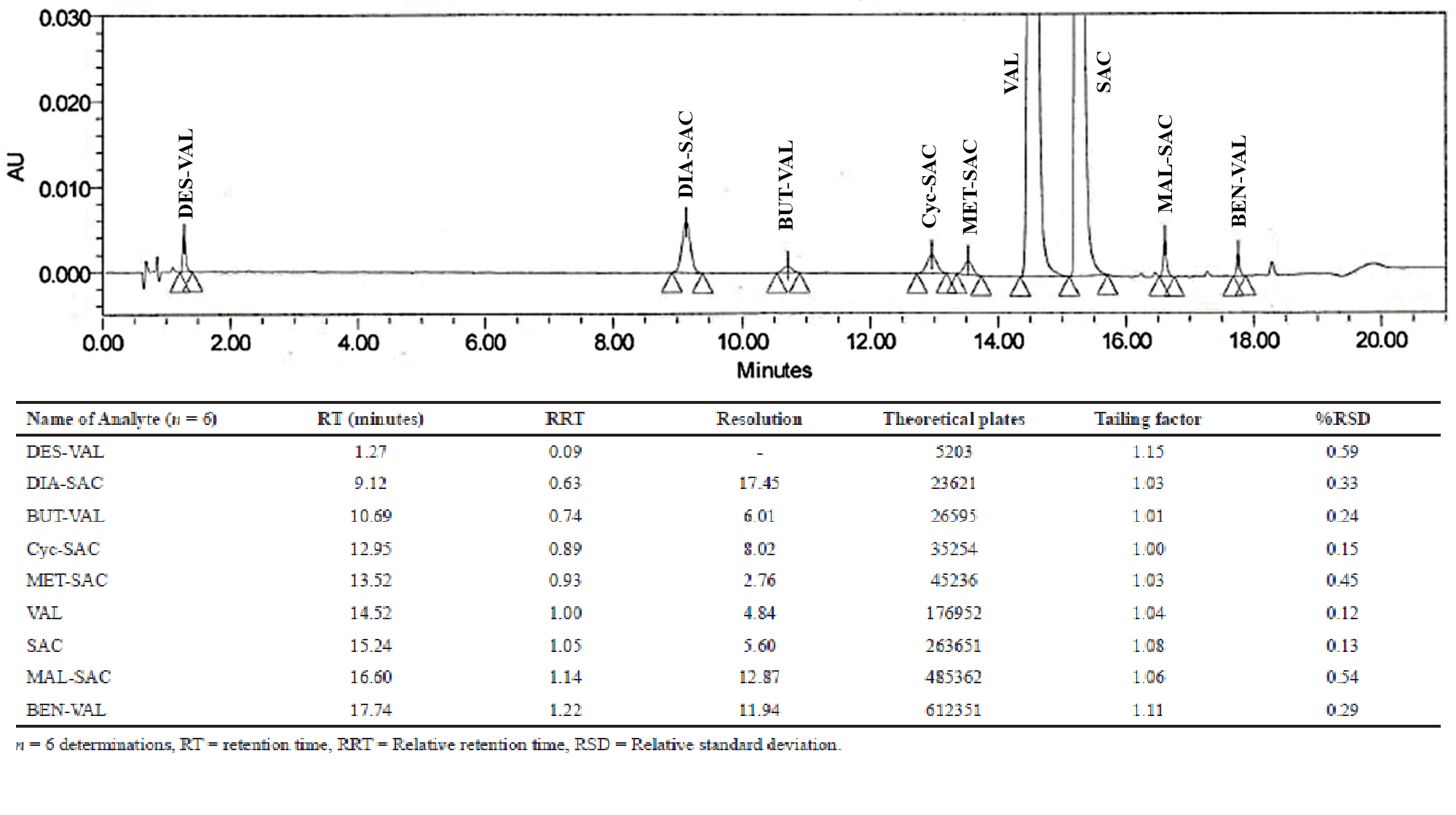
Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138

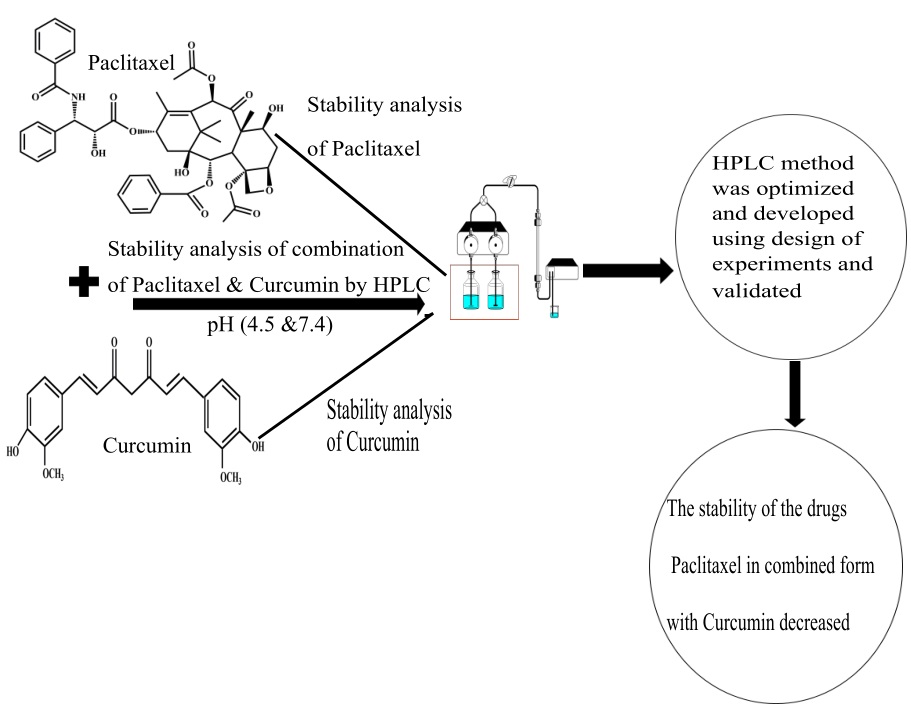
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
Simultaneous determination of Brivaracetam and its isomers in the Brivaracetam drug by RP-HPLC
Palaniappan Ilayaraja, Murugan Maniavannan, Paramasivam Parthiban
DOI: 10.7324/JAPS.2022.120915Pages: 127-138

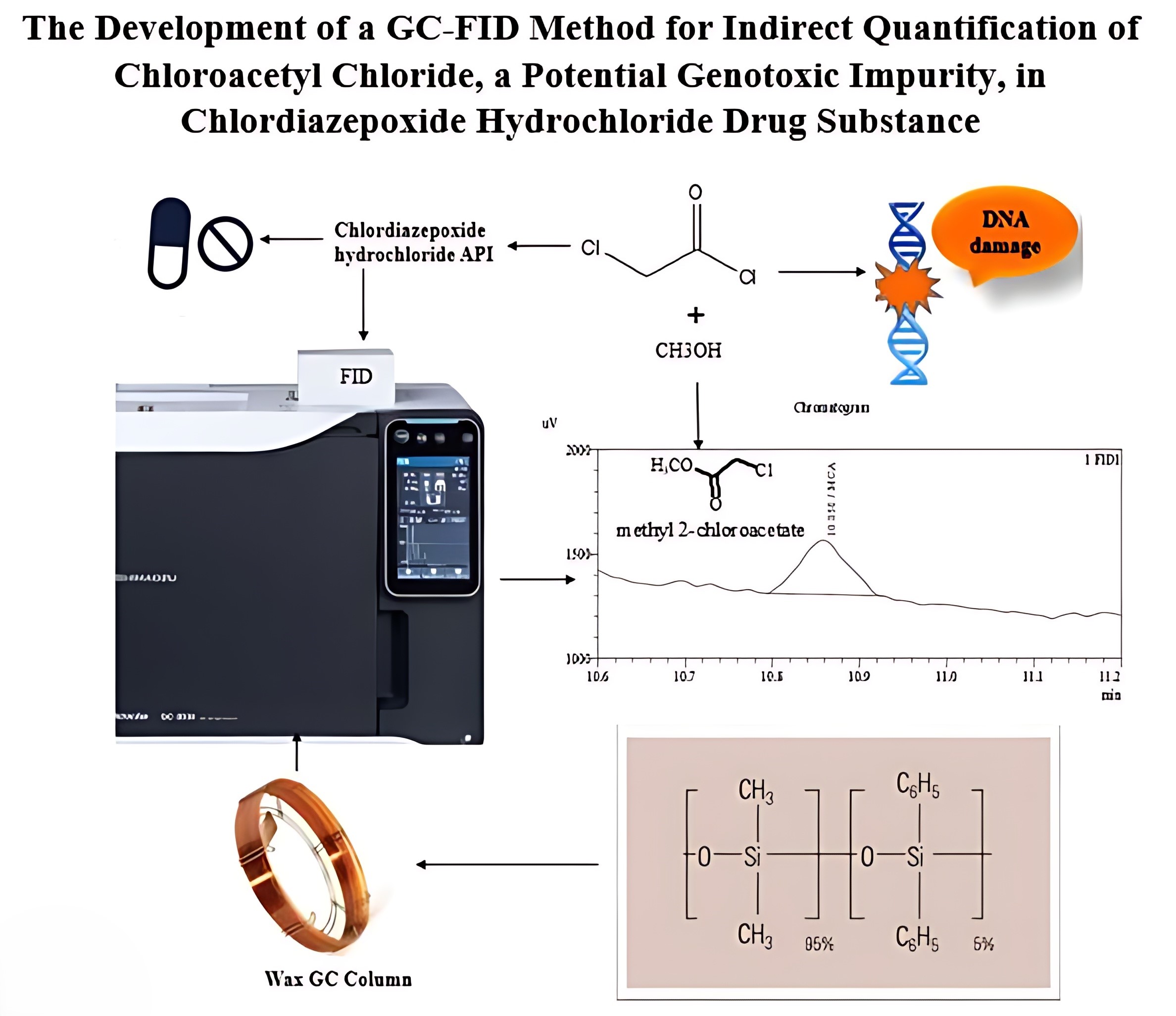
The development of a GC-FID method for indirect quantification of chloroacetyl chloride, a potential genotoxic impurity, in chlordiazepoxide hydrochloride drug substance
Srinivas Birudukota, Bhaskar Mangalapu, Ramesha Andagar Ramakrishna, Swagata Halder, Venkata Narayana Palakollu
DOI: 10.7324/JAPS.2024.182017Pages: 196-207
Advancing relugolix analysis: A comparative study and AQbD-driven method optimization with stability testing
Priyanka Nagar, Arvind Kumar Sharma, Robin Kumar, Chhaya Chauhan, Rini Singhal, Minakshi Garg
DOI: 10.7324/JAPS.2025.239585Pages: 055-070
_.jpg)