Rapid high Performance liquid Chromatography- Tandem mass Spectrometry Method For Quantitation of Milnacepran in Human Plasma
Umesh Thorat
DOI: 10.7324/JAPS.2013.3427Pages: 146-151

Bioanalytical Method Development and Validation for the Determination of Levocetirizine in Pharmaceutical Dosage Form and Human Plasma by RP-HPLC
Nilesh Jain, Deepak Kumar Jain, Ruchi Jain, Vijay Kumar Patel, Preeti Patel, Surendra Kumar Jain
DOI: 10.7324/JAPS.2016.601008Pages: 063-067

A Liquid Chromatography/Tandem Mass Spectrometric Method for Determination of Captopril in Human Plasma: Application to a Bioequivalence Study
Eman S. Elzanfaly, Hanan A. Merey
DOI: 10.7324/JAPS.2017.70202Pages: 008-015

Validation of a simple isocratic HPLC-UV method for rifampicin and isoniazid quantification in human plasma
Laura Carolina Luciani-Giacobbe, María Laura Guzman, Rubén Hilario Manzo, María Eugenia Olivera
DOI: 10.7324/JAPS.2018.8715Pages: 093-099

Development and validation of LC-MS/MS method for the determination of amikacin in human plasma and its application in adult hospitalized patients in Yogyakarta Indonesia
Esti Dyah Utami, Ika Puspitasari, Rizka Humardewayanti Asdie, Endang Lukitaningsih, Eka Noviana, Ratna Budhi Pebriana, Dita Amalia Prihati
DOI: 10.7324/JAPS.2024.142046Pages: 108-118

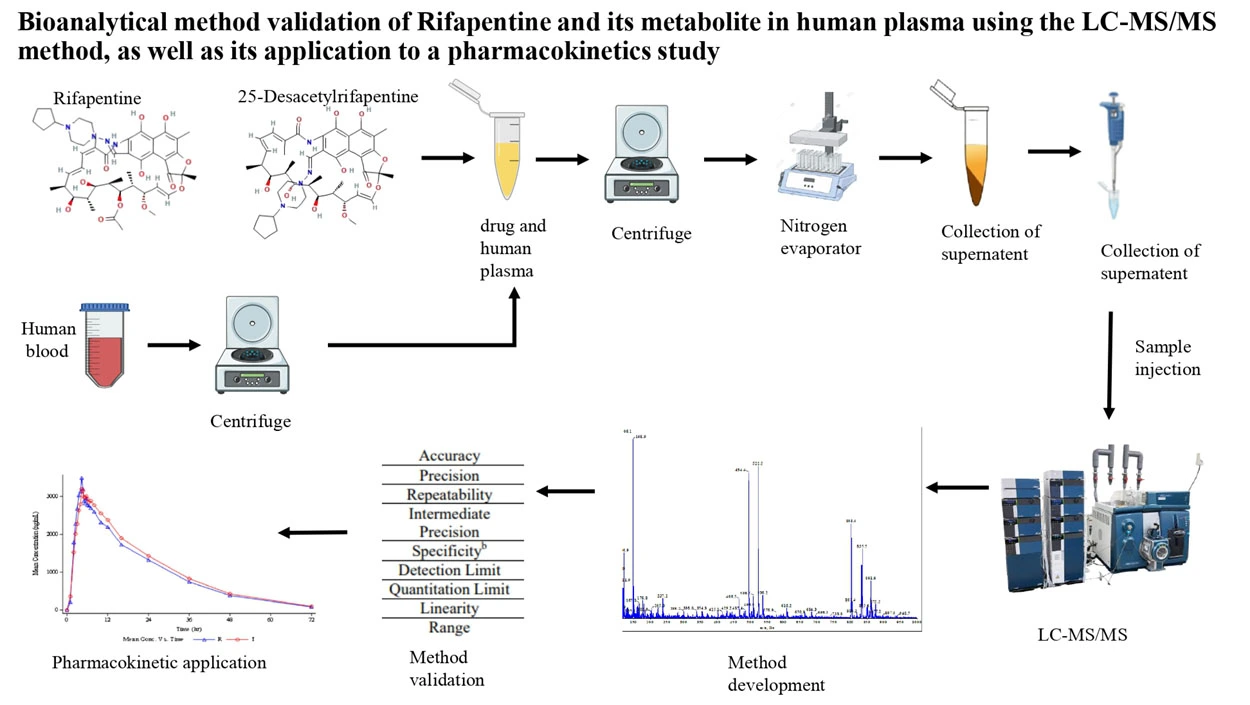
Quantification of asciminib by LC-MS/MS method in human plasma: Validation and stability studies
Hema, Naresh Panigrahi
DOI: 10.7324/JAPS.2024.163326Pages:
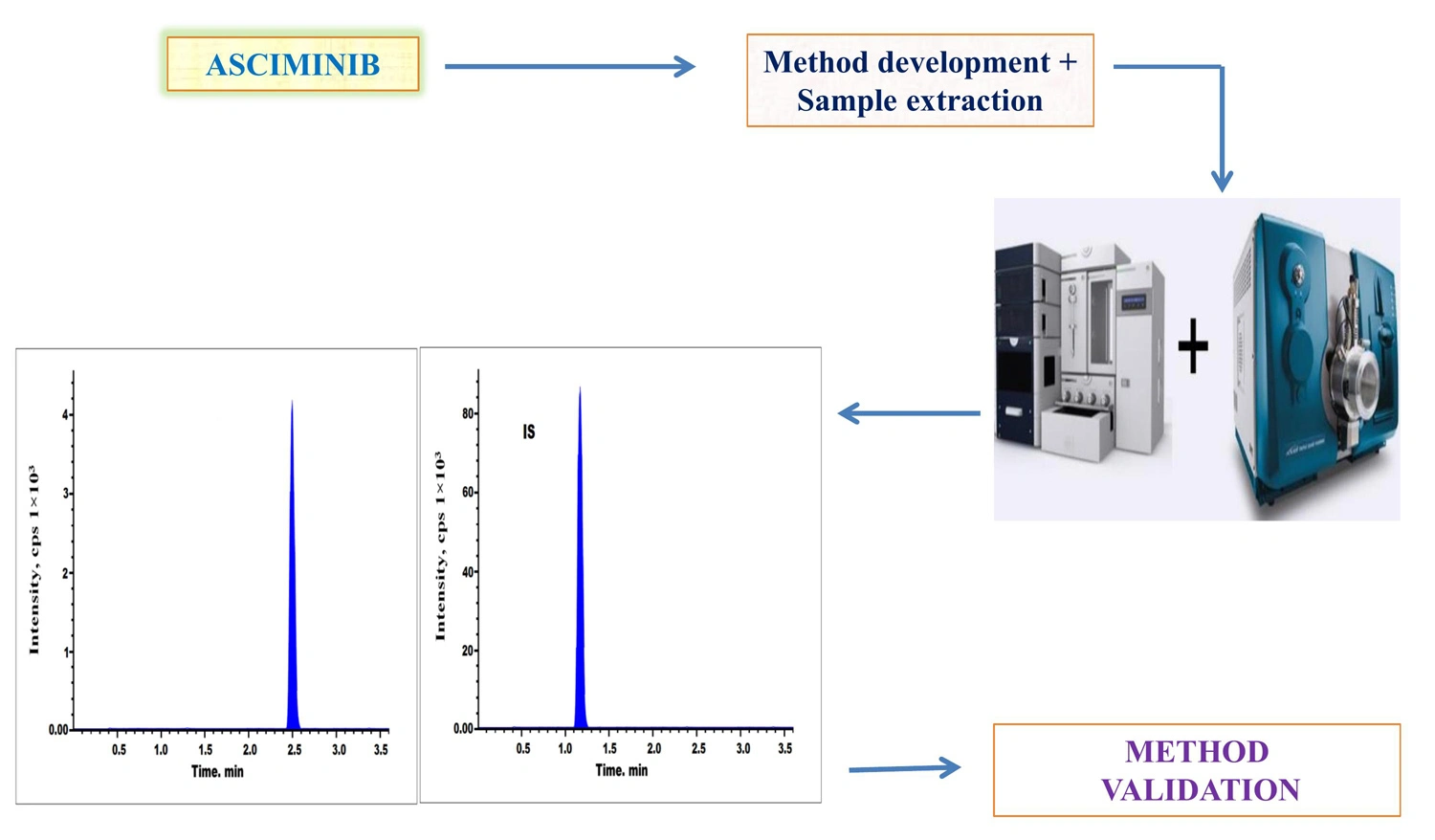
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138
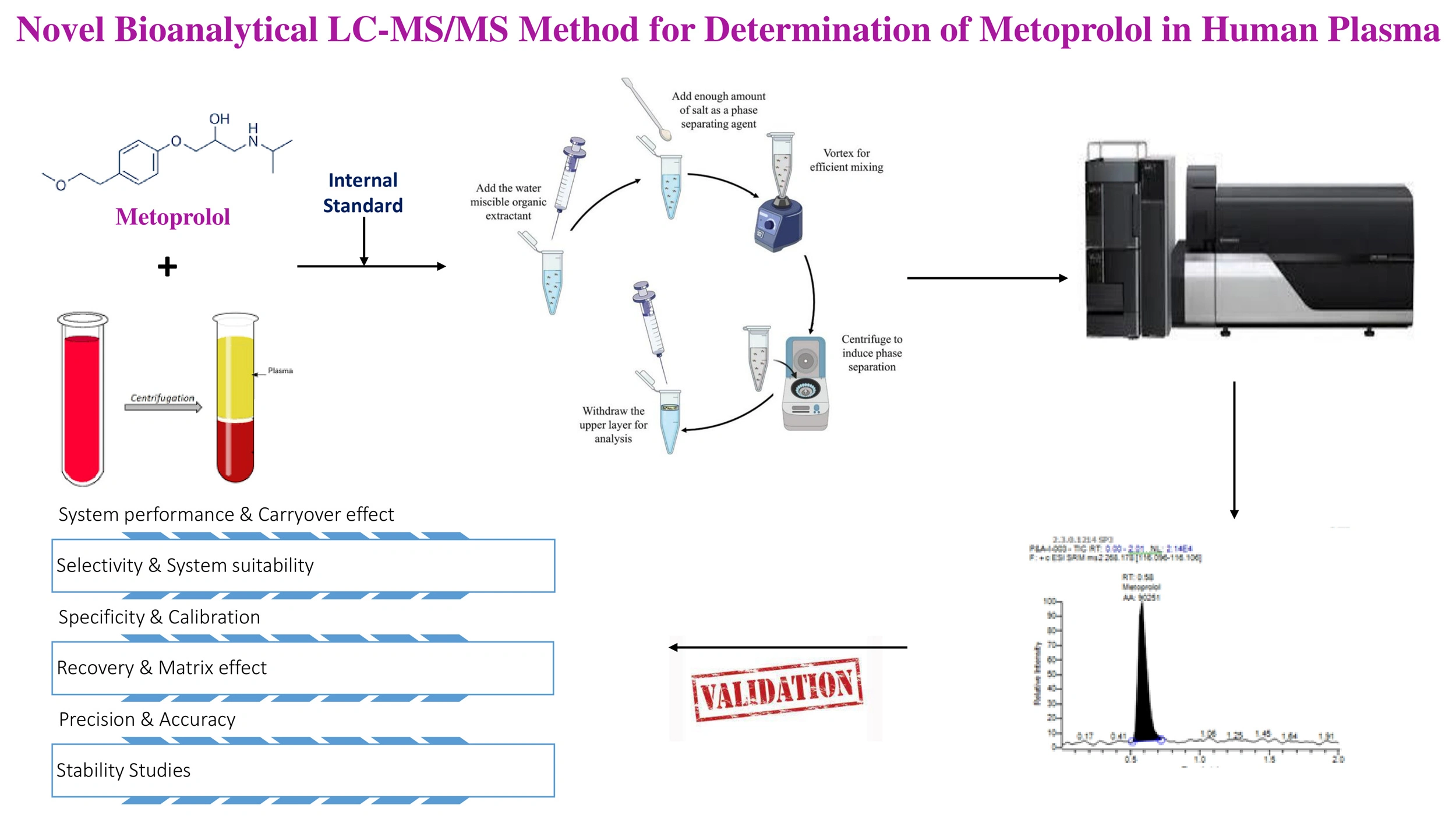
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201