Enteric coated tablets of novel proton pump inhibitor with super disintegrants design, in-vitro evaluation and stability studies
Putta Rajesh Kumar, Hiremath Doddayya, S. Rajendra Reddy
Pages: 106-111

Stability Testing of Pharmaceutical Products
Sanjay Bajaj, Dinesh Singla, Neha Sakhuja
DOI: 10.7324/JAPS.2012.2322Pages: 129-138

Studies on directly compressed ondansetron hydrochloride mucoadhesive buccal tablets using gelatin, chitosan and xanthan gum along with HPMC K4M
Syed Amezuddin Azhar, Putta Rajesh Kumar, Vivek Sood, Somashekar Shyale
DOI: 10.7324/JAPS.2012.2517Pages: 100-105

Antibacterial, antitussive, antioxidant and toxicological evaluation of Joshanda lozenges
Monika Bansal, Monica Gulati, Sachin Kumar Singh, Sanjiv Duggal
DOI: 10.7324/JAPS.2015.50711Pages: 064-070

Formulation and Characterization of Ketoprofen Emulgels
Ramakanth Ambala, Sateesh Kumar Vemula
DOI: 10.7324/JAPS.2015.50717Pages: 112-117

Fabrication, Physicochemical Characterization and Evaluation of In vitro Anticancer Efficacy of a Novel pH Sensitive Polymeric Nanoparticles for Efficient Delivery of Hydrophobic Drug against Colon Cancer
Manikandan Mahalingam, Kannan Krishnamoorthy
DOI: 10.7324/JAPS.2015.501123Pages: 135-145

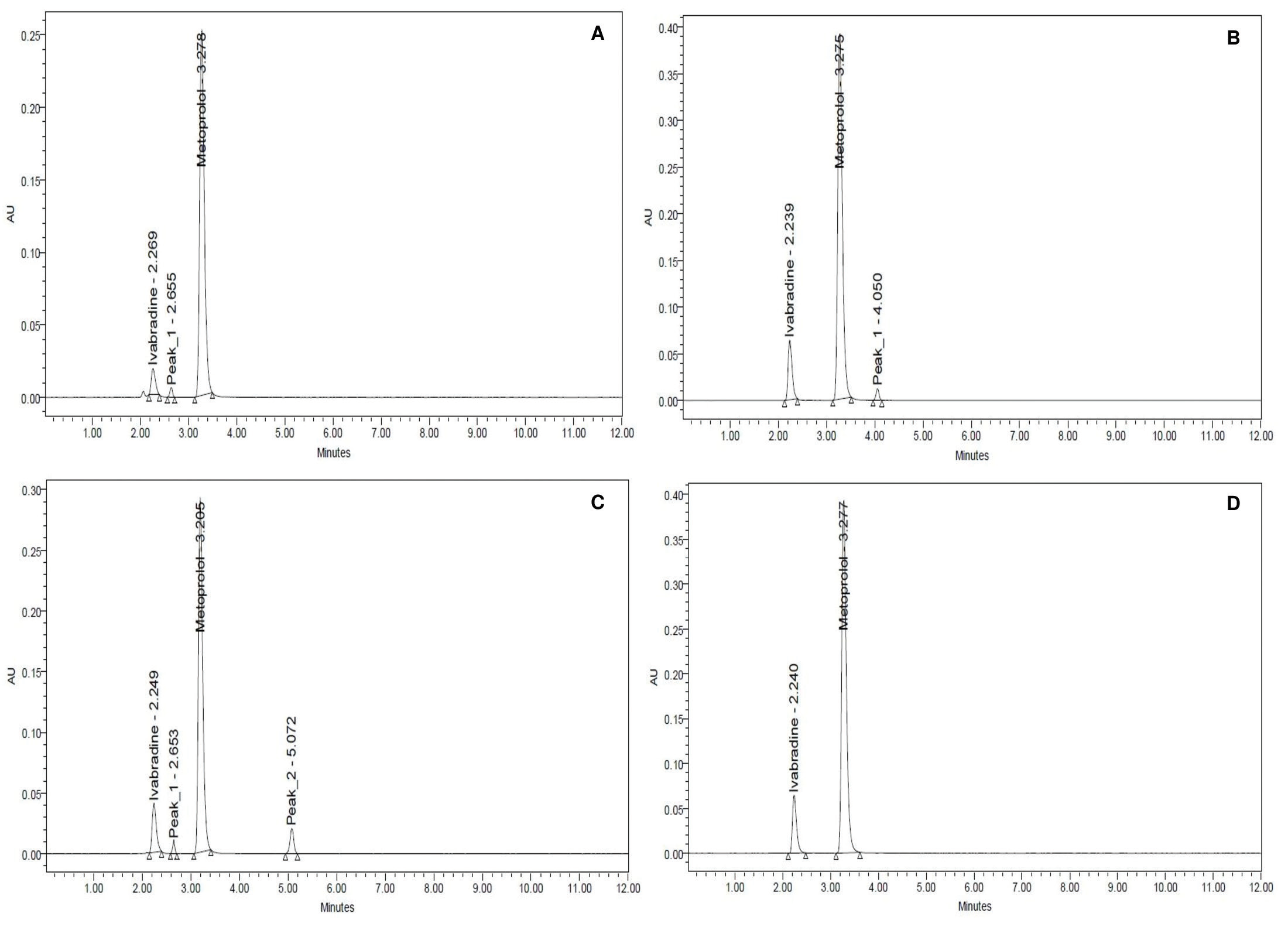
Stability indicating RP-HPLC method for the simultaneous estimation of ivabradine and metoprolol in bulk and tablet formulation
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90418Pages: 137-144
Development of validated stability indicating RP-HPLC method for the estimation of glecaprevir and pibrentasvir in bulk and pharmaceutical dosage form
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90607Pages: 052-060

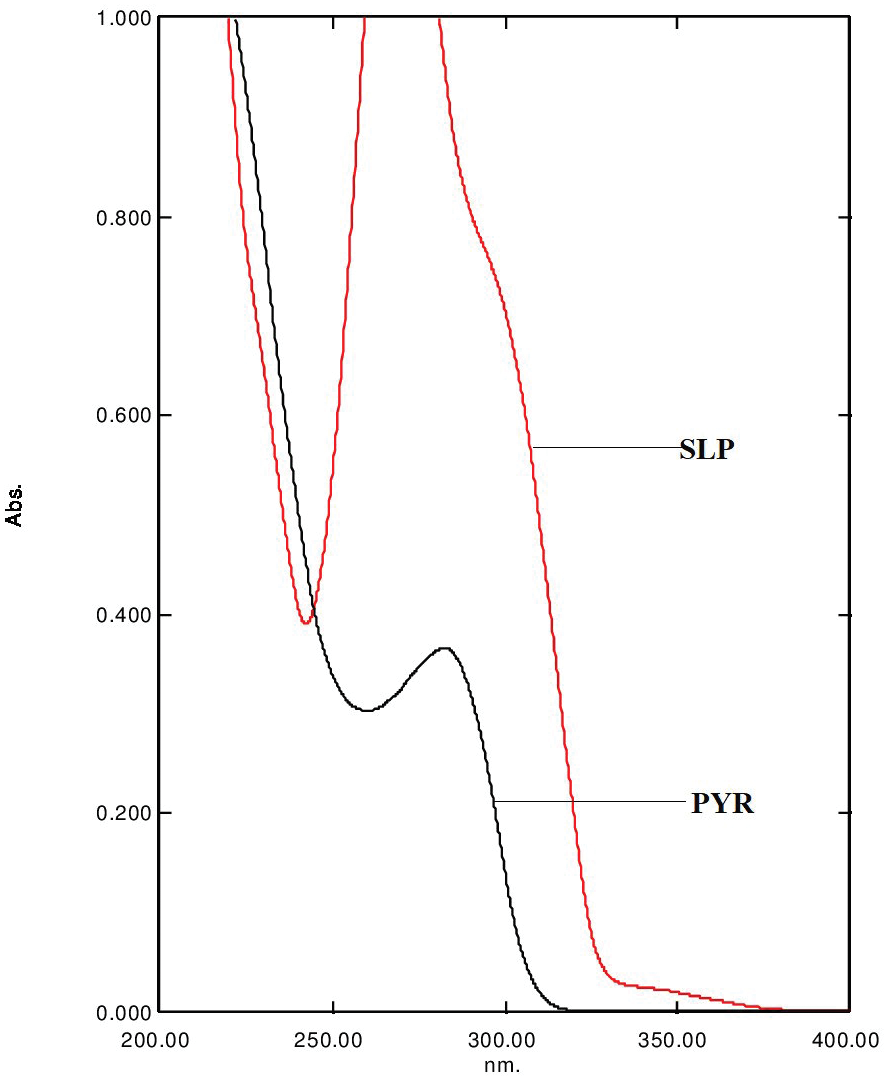
Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
Formulation and stability studies of metformin hydrochloride in a controlled porosity osmotic pump system
Hanan M. Hashem, Aya R. Abdou, Nesrin F. Taha, Nadia M. Mursi, Laila H. Emara
DOI: 10.7324/JAPS.2020.104013Pages: 100-112

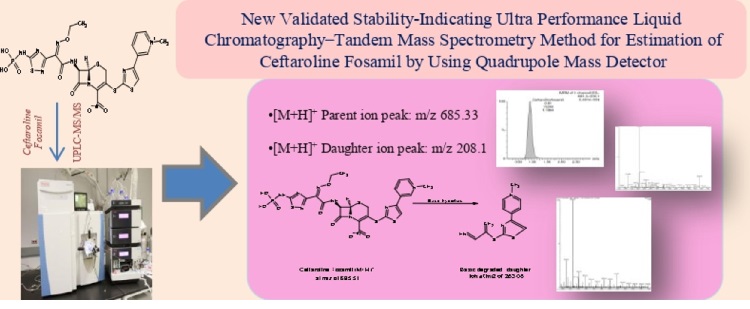
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
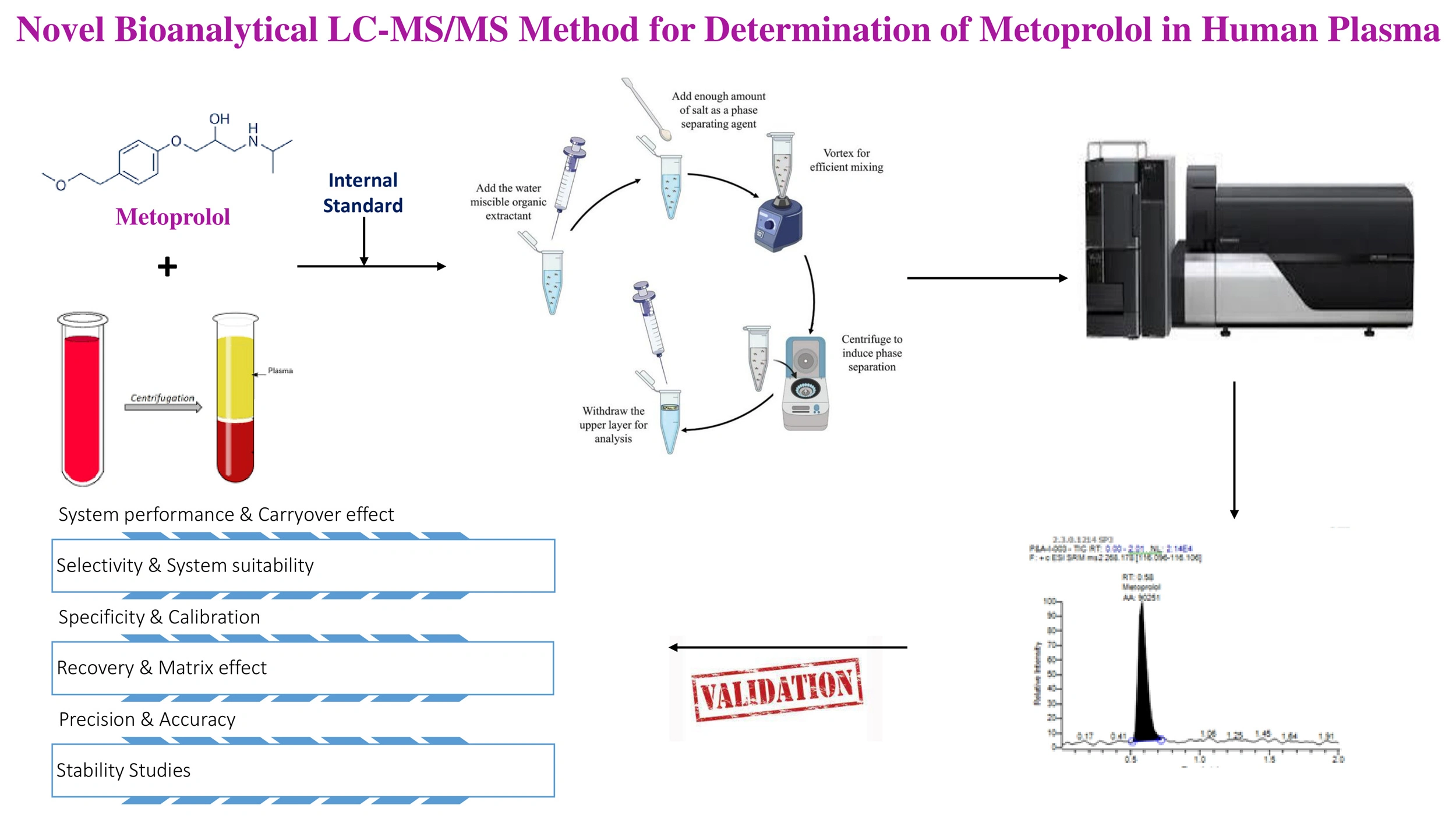
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138