INTRODUCTION
Oroxylum indicum (L.) Kurz (Peka in Thai name) is a plant in the family Bignoniaceae and is a traditional medicinal herb found in many parts of Thailand. This plant is known by various local names depending on the region, such as Lin-fa (Loei), Ka-do-dong (Kanchanaburi), Du-kae (Maehongsorn), Be-do (Narathiwat), and Lin-mai (North Thailand). Thai people commonly consume the young, immature fruit of Peka [1–6]. It has been widely used in Ayurveda, where its bark, roots, seeds, leaves, and fruits are valued for their anti-inflammatory, antioxidant, and antimicrobial properties. Bark is one of the key ingredients in Dashamoola, an Ayurvedic formulation used to treat respiratory, digestive, and some inflammatory disorders [7–9].
Oroxylum indicum is rich in bioactive phytochemicals, which contribute to its therapeutic properties. The major classes of compounds were identified in various parts of the plant, including flavonoids, alkaloids, tannins, saponins, phenolic compounds, steroids, and glycosides [3,6,10–15]. Flavonoids such as baicalein, chrysin, and oroxylin A are abundant in the roots and bark [16,17]. Baicalein has demonstrated potent anti-inflammatory activity by inhibiting pro-inflammatory cytokines and enzymes such as COX-2, along with strong antioxidant and hepatoprotective effects by reducing oxidative stress and liver damage [18,19]. Chrysin shows anxiolytic effects by modulating GABA receptors, anti-inflammatory properties, and anti-cancer potential through apoptosis induction in tumor cells [20]. Oroxylin-A has been noted for its neuroprotective benefits, improving cognitive functions via the enhancement of brain-derived neurotrophic factors, while also exhibiting antibacterial and hepatoprotective properties [21,22]. Baicalin is a flavonoid glycoside found abundantly in the root bark of O. indicum, and exhibits a broad spectrum of pharmacological activities addressing inflammation, oxidative stress, cancer, microbial infections, and metabolic disorders [23]. Other phytochemicals include alkaloids, tannins, saponins, and phenolic compounds, each contributing uniquely. Alkaloids are recognized for their analgesic and antimicrobial effects, while tannins provide astringent, antioxidant, and wound-healing benefits. Saponins enhance respiratory health with their expectorant properties and display anti-cancer effects [24]. Many parts of this plant including root, leave, flower, fruit, and bark (called Phega 5) were mixed and used in a traditional Thai formula from Chao Phya Abhaibhubejhr Hospital in Thailand. The hospital has developed herbal capsules, tinctures, teas, and topical formulations derived from the Phega-5 formula. These products are commonly used in integrative care for patients with chronic conditions, pain, and inflammation, and in wellness programs for detoxification and general health enhancement. The root part of O. indicum contains many phytochemical compounds. Scientific studies have evaluated its traditional uses, confirming its biological activities, including anti-inflammatory, antioxidant, analgesic, hepatoprotective, and antimicrobial properties. Additionally, strong antioxidant properties help neutralize free radicals, protecting the body from oxidative stress and preventing chronic diseases like cardiovascular disease and cancer. Oroxylum indicum root has hepatoprotective effects, protecting the liver from damage caused by toxins and oxidative stress, and it is traditionally used to treat liver-related ailments such as jaundice and fatty liver disease [25].
The extraction method for the good quality and high content of extract from the herbal plant is key to the development of innovative herbal formulation. The use of advanced techniques to enhance the extraction of bioactive compounds from traditional Thai herbal formulations is necessary. This process is particularly significant within the context of Thai traditional medicine, which has historically relied on herbal remedies that play a crucial role in the healthcare system of many communities. With over half of the global population utilizing herbal drugs, optimizing extraction methods has become essential for ensuring the efficacy, safety, and quality of these products in a competitive market. Many extraction techniques have been used, including liquid-liquid extraction, solid-phase extraction, microwave-assisted extraction, and ultrasound-assisted extraction, among others [26–28]. Ultrasound-assisted extraction (UAE) has gained an advantage as an innovative technique that improves extraction efficiency by using ultrasonic waves to facilitate solvent penetration and disrupt plant cell walls [29]. This method not only enhances the yield and quality of bioactive compounds but also reduces extraction time compared to conventional methods [30]. Recent studies have shown that optimizing UAE conditions can lead to higher concentrations of vital phytochemicals such as phenolic compounds and flavonoids, which are known for their antioxidant properties and potential health benefits [30]. The merging of modern extraction techniques like UAE with traditional methods serves to bridge the gap between cultural and scientific standards, ensuring the therapeutic benefits of Thai herbal formulations are preserved. Other conventional methods include various solvent extraction techniques and some traditional techniques, which are guided by principles of solubility and phase change properties.
Experimental design or design of experiment (DOE) is a statistical methodology that focuses on systematically planning and analyzing experiments for the optimization of the response results. Optimization of the extraction method using the context of DOE can provide the conditions of extraction that can achieve a high content of active compounds. Box-Behnken Design (BBD) with response surface methodology is a tool that can provide the setting of factor conditions for the optimization of extraction and maximize the levels of active compounds [31–36].
From the perspective of Thai traditional medicine, integrating these advanced extraction techniques with traditional knowledge not only preserves local cultural practices but also ensures that the therapeutic benefits of herbal formulations remain intact in an up-to-date healthcare system. This holistic approach aims to harmonize the efficacy of herbal medicines with rigorous scientific standards, providing a pathway for the continued relevance of traditional remedies in modern health care. However, the variability of active ingredients in herbal plant materials poses challenges for quality control and standardization, prompting the need for quality control measurement and the application of experimental design or DOE in herbal production. Thus, optimizing the extraction method for O. indicum roots is the crucial part of this study to develop high-quality and content of phytochemical compounds.
MATERIALS AND METHODS
Chemicals and equipment
The standard substances baicalin, baicalein, chrysin, and oroxylin A were purchased from Axxo Chemicals and Services Co., Ltd. (Bangkok, Thailand). Folin–Ciocalteu phenol reagent, sodium acetate, and quercetin were obtained from Sigma-Aldrich (Germany), while gallic acid was sourced from Merck (Germany). Formic acid (99%) was acquired from Montecatini Edison S.p.A. (Electricité de, France). Sodium carbonate was purchased from Loba Chemie (India), aluminum chloride from Ajax Finechem (Australia), and methanol and ethanol of analytical reagent grade were obtained from Fisher Scientific UK Limited (United Kingdom). An ultrasound-assisted extraction device (Elma model: S30H, Germany) is used for the extraction process. A rotary evaporator (Buchi, Germany) is employed for solvent evaporation, and a console freeze dryer (Labconco Corporation, Missouri, USA) is used for freeze-drying. Analytical processes are conducted using HPLC equipment (Agilent Technologies Inc., Santa Clara, USA) with a C18 column (Thermo Fisher Scientific, 250x4 mm, 5 µm).
Plant materials
Oroxylum indicum root was obtained from Chaophraya Abhaibhubejhr Hospital. The plant material was identified by Dr. Prathan Leucha of the Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand. The voucher specimen was deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Khon Kaen University, Thailand.
Experimental design for Ultrasound-assisted extraction
A three-level factorial design using BBD for three factors (Table 1), the percentage of ethanol (70%, 80%, and 90%), extraction time (15, 30, and 45 minutes), and the ratio of material to solvent (1:3, 1:6, and 1:9), was studied. The design responses selected from this study were the percentage extraction yield, total phenolic content, total flavonoid content, and the content of active compounds (baicalin, baicalein, chrysin, and oroxylin-A). The experimental design for 17 sets was obtained, as shown in Table 2. The factors and responses were evaluated for their interaction and optimization by Design Expert software (version 13) via response surface methodology.
Table 1. The Box-Behnken design level for the optimal conditions of O. indicum extraction using the UAE method.
| Parameters | Level | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| Solvent concentration (%) | 70 | 80 | 90 |
| Extraction time (minutes) | 15 | 30 | 45 |
| Material to solvent ratio (g/ml) | 1:3 | 1:6 | 1:9 |
Ultrasound-assisted extraction for O. indicum root
The extraction method using ultrasound-assisted extraction was set and performed in 17 experimental runs, as shown in Table 2. Five grams of O. indicum root powder was weighed and added to 100 ml of extraction solvent and extracted in an ultrasonic bath using the conditions shown in Table 2. The extraction solution was filtered through filter paper (Whatman no.1), evaporated by rotary evaporator at 40°C, and freeze-dried for 24 hours. Three replications of extraction in each run were performed. The extract was kept at −20°C before analysis.
Table 2. Design of the experiment for extracting O. indicum roots by UAE method.
| Run order | Extraction parameter | ||
|---|---|---|---|
| Solvent concentration (%) | Extraction time (minutes) | Material to solvent ratio (g/ml) | |
| 1 | -1 | 0 | -1 |
| 2 | 0 | 0 | 0 |
| 3 | 0 | 1 | 1 |
| 4 | 0 | 1 | -1 |
| 5 | 0 | -1 | 1 |
| 6 | 0 | 0 | 0 |
| 7 | 0 | -1 | -1 |
| 8 | -1 | 1 | 0 |
| 9 | 1 | 1 | 0 |
| 10 | 1 | -1 | 0 |
| 11 | 1 | 0 | -1 |
| 12 | 1 | 0 | 1 |
| 13 | -1 | -1 | 0 |
| 14 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 |
| 17 | -1 | 0 | 1 |
Determination of extraction yield
The percentage extraction yield was evaluated and determined in this study. The calculation of the yield was from Equation 1.
Wc refers to the weight of the extract obtained from the O. indicum root powder.
Ws refers to the weight of the O. indicum root powder.
Evaluation of total phenolic and flavonoid content
Determination of total phenolic content was done using the Folin–Ciocalteu test as described by Ngamkhae et al. [31]. Twenty µl of ethanolic sample solution (1 mg/ml) was pipetted into a 96-well plate. Then, 10% Folin–Ciocalteu reagent (100 µl) and 7% sodium carbonate (80 µl) were added, and the solution was kept in a dark condition for 30 minutes. The absorbance was measured at 760 nm. Three replications were performed, and the content was expressed in terms of milligrams of gallic acid equivalents per gram extract (mg GAE/g extract).
Total flavonoid content was determined by aluminum chloride colorimetric methods as described by Ngamkhae et al. [31]. Twenty µl of the ethanolic sample solutions were added to a 96-well plate. Then, 15 µl of 2.5% aluminum chloride solution, 10% sodium acetate solution (20 µl), and 145 µl deionized water were added and kept dark for 15 minutes. The absorbance from three replications was measured at a wavelength of 430 nm. The flavonoid content was calculated and expressed as quercetin equivalents per gram extract (mg QE/g extract).
Preparation of standard and sample solutions for HPLC analysis
Stock standard solutions (1000 µg/ml) of baicalin, baicalein, chrysin, and oroxylin-A were dissolved and prepared in methanol. Each stock standard solution was diluted to get the final solution of baicalin 1.0–7.0 µg/ml and 2.5–35.0 µg/ml for baicalein, chrysin, and oroxylin-A. The sample solution was prepared by dissolving the extract in methanol and diluted to 1000 µg/ml. Each standard and sample solution was injected into the HPLC system.
HPLC condition for the analysis of active compounds in O. indicum root
The analysis was conducted using the modified HPLC with a reversed-phase C18 column as described by Chalermwongkul et al. [37]. The mobile phase consisted of 0.2% formic acid in ultra-pure water and methanol, following a gradient elution system. The gradient system started with 70% formic acid and 30% methanol at 0.00 minutes, gradually changing to 65% formic acid and 35% methanol at 5.00 minutes, 60% formic acid and 40% methanol at 15.00 minutes, 50% formic acid and 50% methanol at 25.00 minutes, 45% formic acid and 55% methanol at 45.00 minutes, and 40% formic acid and 60% methanol at 50.00 minutes. The gradient returned to the initial composition of 70% formic acid and 30% methanol for starting the new injection. The flow rate was set at 1.2 ml/minute, the column temperature was maintained at 30ºC, and the injection volume was 20 µl. Detection was performed at a wavelength of 275 nm. Each sample solution from the extractions was injected into the HPLC system. This method allowed for the effective separation and quantification of four active compounds in the extract from each experimental extraction set. The quantification was performed by comparing the peak area with the concentration of the four standard solutions. The results were then reported in units of mg baicalin, mg baicalein, mg chrysin, and mg oroxylin-A per gram of extract. HPLC chromatograms of the mixed standard solution and sample extract solution are shown in the supplementary file (Fig. S1).
Statistical analysis
All data from each experiment were analyzed and evaluated using Design Expert (version 13), a statistical software developed by Stat-Ease Inc. based in Minneapolis, Minnesota, USA. This software was employed to design the experiment set by BBD with three levels at each parameter.
RESULTS AND DISCUSSION
Optimization of Ultrasound-assisted extraction for percentage extraction yield, total active contents, and active compound contents in O. indicum root
The results from the statistical analysis using ANOVA are shown in Table 3, and the comparison between the responses (%yield, TPC, TFC, baicalin, baicalein, chrysin, and oroxylin-A content) from experimental values and predicted value from ultrasound-assist extraction of O. indicum root in each run is shown in Table 4. It was found that all response model equations were performed in quadratic and cubic models. P-values less than 0.05 for all responses indicated that model terms were significant. The significant model terms for all responses were shown in equations 2–8. The model equation performed which relationship between each response and factors, as shown in equations 2–8, could be used for the estimation of each response. R2 and adjusted R2 were between 0.9430 and 0.9803 and 0.8800–0.9551, respectively, which indicated that the model equations could be used for the estimation of the responses with a high relationship to all factors. The experimental values and predicted values for each response were calculated in this study, and the graph illustrating the good relationship between these values is shown in Figure 1. The coefficient estimated from the model equation represented the expected change in response per unit change in factor value when all remaining factors are held constant.
Table 3. Statistical analysis and response surface model summary for percentage extraction yield and phytochemical content (TPC, TFC, baicalin, baicalein, chrysin, and oroxylin-A).
| Responses | Model | p-value | F-value | R2 | Adj. R2 | Mean (SD) | %CV | Adequate precision |
|---|---|---|---|---|---|---|---|---|
| Extraction yield (%) | Quadratic | 0.0087* | 19.47 | 0.9,615 | 0.9,121 | 9.92 (1.19) | 12.01 | 14.89 |
| TPC | Quadratic | 0.0029* | 38.78 | 0.9,803 | 0.9,551 | 42.67 (3.95) | 9.26 | 21.26 |
| TFC | Quadratic | 0.0416* | 17.46 | 0.9,574 | 0.9,027 | 12.98 (1.69) | 13.03 | 13.16 |
| Baicalin | Reduced cubic | 0.0009* | 19.05 | 0.9,694 | 0.9,186 | 10.73 (1.01) | 9.46 | 17.42 |
| Baicalein | Reduced cubic | 0.0011* | 14.03 | 0.9,475 | 0.8,800 | 20.85 (3.61) | 17.30 | 12.81 |
| Chrysin | Reduced quadratic | < 0.0001* | 26.91 | 0.9,642 | 0.9,283 | 4.86 (0.57) | 11.71 | 17.89 |
| Oroxylin-A | Reduced quadratic | < 0.0001* | 27.57 | 0.9,430 | 0.9,088 | 11.84 (1.73) | 14.62 | 19.37 |
*significant at p-value < 0.05.
Table 4. The comparison between the responses (Percentage yield, TPC, TFC, baicalin, baicalein, chrysin, and oroxylin-A content) from experimental values and predicted value from ultrasound-assist extraction of O. indicum root in each run.
| Run order | Results | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage yield (%) | TPC (mg GAE/g extract) | TFC (mg QE/g extract) | Baicalin (mg/g extract) | Baicalein (mg/g extract) | Chrysin (mg/g extract) | Oroxylin-A (mg/g extract) | ||||||||
| Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | |
| 1 | 9.87 | 9.19 | 36.58 | 36.82 | 7.27 | 8.21 | 4.34 | 4.40 | 11.78 | 11.57 | 2.78 | 2.90 | 5.79 | 6.69 |
| 2 | 11.37 | 10.89 | 38.04 | 33.88 | 12.92 | 11.07 | 11.86 | 12.60 | 21.49 | 17.74 | 3.87 | 4.04 | 9.21 | 10.24 |
| 3 | 15.49 | 14.97 | 76.63 | 74.81 | 15.88 | 17.82 | 7.99 | 7.78 | 23.50 | 23.48 | 4.65 | 5.23 | 11.32 | 12.63 |
| 4 | 10.53 | 11.31 | 31.91 | 33.88 | 10.38 | 10.16 | 9.24 | 9.03 | 19.17 | 19.62 | 4.09 | 4.33 | 9.81 | 9.95 |
| 5 | 15.33 | 14.56 | 14.68 | 12.71 | 6.55 | 6.77 | 9.23 | 9.02 | 9.74 | 9.72 | 2.22 | 2.05 | 4.84 | 3.47 |
| 6 | 10.97 | 10.89 | 31.20 | 33.88 | 10.36 | 11.07 | 11.06 | 12.60 | 24.44 | 17.74 | 4.76 | 4.04 | 11.90 | 10.24 |
| 7 | 5.66 | 6.19 | 75.29 | 77.11 | 16.58 | 14.64 | 18.84 | 18.63 | 30.88 | 31.33 | 6.37 | 5.86 | 17.46 | 14.92 |
| 8 | 13.87 | 13.78 | 36.67 | 34.45 | 10.26 | 9.54 | 6.04 | 5.98 | 20.14 | 20.12 | 3.99 | 3.67 | 9.64 | 8.40 |
| 9 | 5.06 | 4.91 | 60.85 | 62.91 | 24.85 | 23.85 | 13.25 | 13.73 | 41.69 | 41.28 | 9.22 | 8.72 | 23.64 | 21.00 |
| 10 | 3.32 | 3.42 | 51.09 | 53.30 | 18.25 | 18.97 | 7.73 | 8.21 | 30.59 | 30.18 | 7.40 | 7.65 | 18.02 | 18.90 |
| 11 | 3.12 | 2.50 | 69.76 | 65.73 | 18.54 | 19.76 | 13.53 | 13.05 | 39.23 | 39.41 | 9.16 | 9.46 | 23.53 | 25.00 |
| 12 | 6.93 | 7.61 | 53.61 | 53.37 | 21.77 | 20.83 | 12.36 | 11.88 | 24.81 | 25.45 | 6.31 | 6.26 | 14.61 | 14.90 |
| 13 | 9.59 | 9.74 | 27.24 | 25.19 | 6.84 | 7.84 | 6.67 | 6.61 | 5.86 | 5.84 | 2.64 | 3.07 | 5.70 | 6.30 |
| 14 | 9.05 | 10.89 | 31.01 | 33.88 | 10.98 | 11.07 | 12.96 | 12.60 | 14.48 | 17.74 | 3.61 | 4.04 | 9.00 | 10.24 |
| 15 | 10.53 | 10.89 | 32.31 | 33.88 | 10.29 | 11.07 | 12.29 | 12.60 | 14.24 | 17.74 | 3.61 | 4.04 | 9.16 | 10.24 |
| 16 | 12.53 | 10.89 | 36.83 | 33.88 | 10.84 | 11.07 | 13.98 | 12.60 | 14.92 | 17.74 | 4.51 | 4.04 | 9.45 | 10.24 |
| 17 | 15.49 | 16.12 | 21.67 | 25.70 | 8.16 | 6.94 | 11.10 | 11.16 | 7.53 | 7.79 | 3.41 | 3.19 | 8.28 | 8.01 |
Exp.: experimental value; Pred.: predicted value.
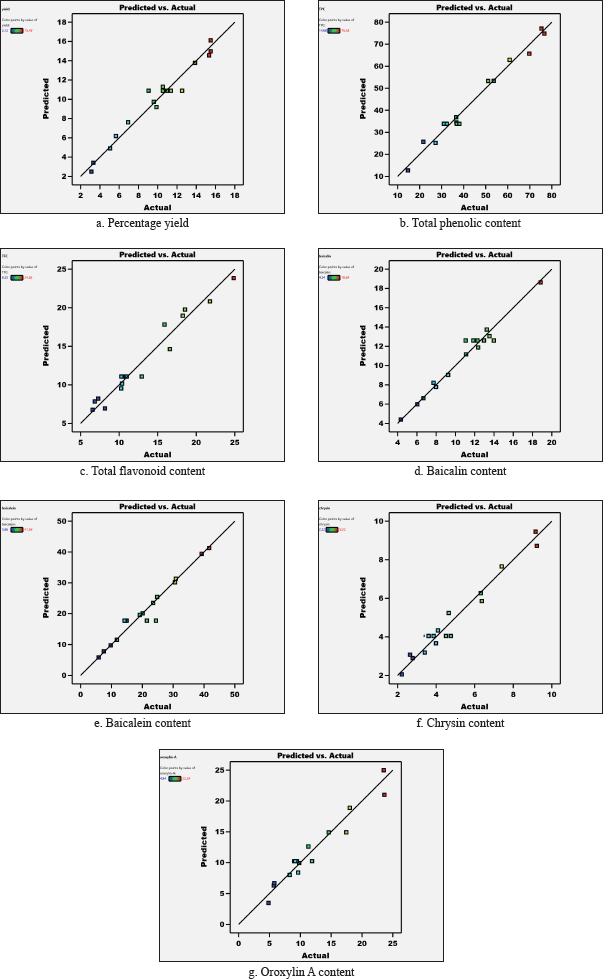 | Figure 1. Relationship between the predicted value from each model equation and experimental values of the contents using UAE extraction (a: Percentage yield, b: Total phenolic content, c: Total flavonoid content, d: Baicalin content, e: Baicalein content, f: Chrysin content, and g: Oroxylin A content). [Click here to view] |
Percentage extraction yield (Y1)
Y1= 10.89 − 3.80x1 + 1.38x2 + 3.01x3 − 0.63x1x2 − 0.45x1x3 −1.18x2x3 − 2.91x12 − 0.01x22 + 0.88x32 Equation 2
Total phenolic content (Y2)
Y2 = 33.88 + 14.14x1 + 4.72x2 − 5.87x3 + 0.08x1x2 − 0.31x1x3 + 26.33x2x3 + 2.93x12 + 7.15x2 2 + 8.60x32 Equation 3
Total flavonoid content (Y3)
Y3 = 11.08 + 6.36x1 + 1.64x2 − 0.05x3 + 0.80x1x2 + 0.59x1x3 + 3.88x2x3 + 2.78x12 +1.19x22 + 0.08x32 Equation 4
where Yn represents the responses (Y1: %yield, Y2: TPC, Y3: TFC, Y4: baicalin, Y5: baicalein, Y6: chrysin, and Y7: oroxylin-A content); xi represents factors or parameters (x1: solvent concentration (%), x2: extraction time, and x3: material to solvent ratio); xixj represents the interaction term between two factors; xi2 represents the polynomial terms of factors.
The information from Table 3 and all equations (equations 2–8) also showed the interaction effect of the factors on the responses (xixj) and affected the model terms in the equations. These might confirm the influence of factors affecting each other and consequently affect the final responses. The coefficient, both positive and negative values, could express the impact that occurred.
For percentage extraction yield, solvent concentration showed the strongest influence on this response, as shown in equation 2, which had the highest coefficient and revealed the most effect on percentage extraction yield. Figure 2 shows the perturbation graph from Design Expert Software which express the effect of all factors (A: solvent concentration (%), B: extraction time (minutes), and C: material to solvent ratio (g/ml)) on each response (a: percentage yield, b: total phenolic content, c: total flavonoid content, d: baicalin content, e: baicalein content, f: chrysin content, and g: oroxylin-A content). The perturbation graph of percentage extraction yield (Fig. 2a) showed that factor A (solvent concentration) performed the steepest curve when compared with the other factors, which confirmed the influence of this factor. Similar results for total phenolic content, total flavonoid content, baicalin content, baicalein content, chrysin content, and oroxylin-A content were demonstrated in the same way as percentage extraction, in which solvent concentration (factor A) performed the strongest effect on these responses, as shown in the perturbation graph in Figure 2.
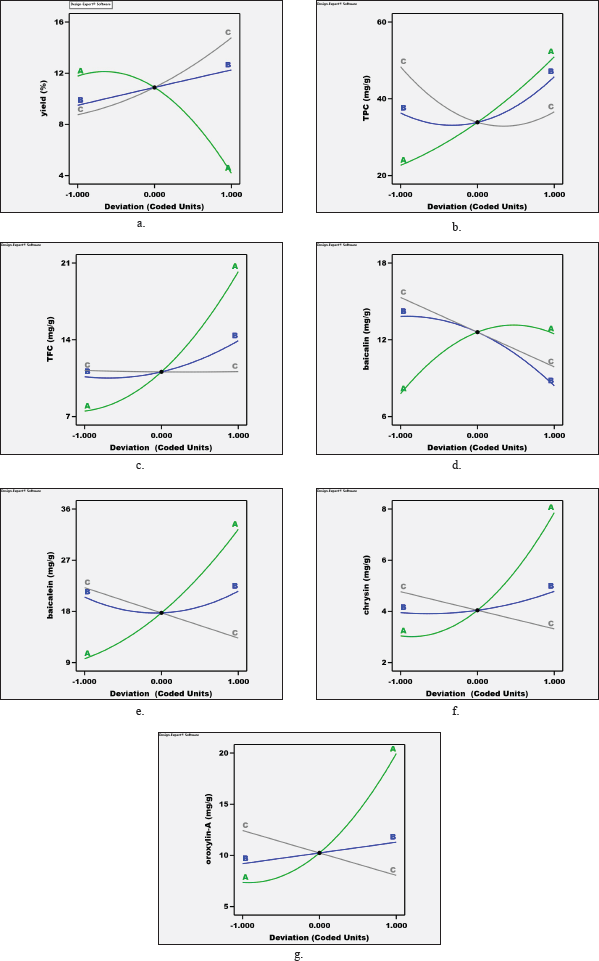 | Figure 2. The perturbation graph from Design Expert Software showed the effect of factors (A: solvent concentration (%), B: extraction time (minutes), and C: material to solvent ratio (g/ml)) on each response (a: Percentage yield, b: Total phenolic content, c: Total flavonoid content, d: Baicalin content, e: Baicalein content, f: Chrysin content, and g: Oroxylin A content). [Click here to view] |
The highest percentage extraction yield (15.49%) was performed in experimental sets No. 3 (the condition of solvent concentration at 80%, extraction time for 45 minutes, and the ratio of material to solvent at 1:9) and no.17 (the condition of solvent concentration at 70%, extraction time for 30 minutes, and the ratio of material to solvent at 1:9). The highest content of total phenolic and flavonoid compound was found in the experimental set no. 3 (the condition of solvent concentration at 80%, extraction time for 45 minutes, and the ratio of material to solvent at 1:9) and no. 9 (the condition of solvent concentration at 90%, extraction time for 45 minutes, and the ratio of material to solvent at 1:6) in, respectively. The highest baicalein, chrysin, and oroxylin-A content was found in experimental set no. 9 (the condition of solvent concentration at 90%, extraction time for 45 minutes, and the ratio of material to solvent at 1:6), while baicalin was found in experimental set no. 7 (the condition of solvent concentration at 80%, extraction time for 15 minutes, and the ratio of material to solvent at 1:3). The results from the content of baicalein, chrysin, and oroxylin-A seemed to relate to total flavonoid content in the experiment set no. 9. This might come from the high ratio of flavonoid compounds found in O. indicum was mainly from baicalein, chrysin, and oroxylin-A, which was the major content in O. indicum [10,11]. A higher percentage of ethanol and a longer time used for the extraction could provide better flavonoid content. The percentage of ethanol in a solvent affects the efficiency of flavonoid extraction because flavonoids have varying solubility depending on their polarity. Ethanol is a polar organic solvent, and its effectiveness depends on its concentration, as low ethanol concentration shows better for extracting more polar flavonoids like flavone glycosides (e.g., baicalin), while higher ethanol concentration provides more effective for less polar flavonoids like flavone aglycones (e.g., baicalein and chrysin) [38,39].
The relationship of active compound content and experimental set for ultrasound-assisted extraction of O. indicum root
Four active compounds analyzed in each experimental set in this study could be efficiently extracted. The results shown in Figure 3 were useful for choosing the best extraction conditions for each compound. These four compounds were important biomarkers of this herbal plant. The optimal extraction could benefit from the high contents of these compounds and further be used as extract materials enriched with highly active compounds for high-quality herbal formulas.
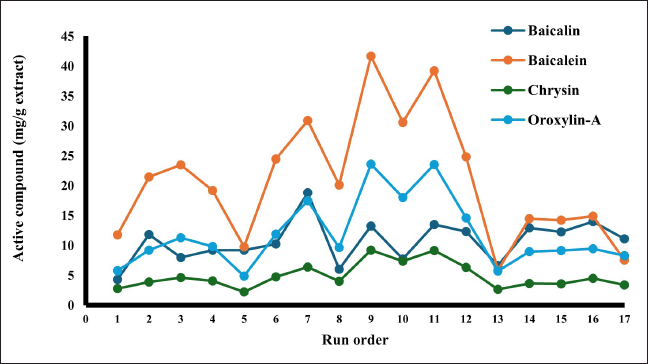 | Figure 3. Relationship between the experimental set and the content of baicalin, baicalein, chrysin, and oroxylin-A in O. indicum root. [Click here to view] |
Optimization of all factors to get the best-predicted responses using response surface methodology from Design Expert software was found and shown in Table 5. Desirability is a mathematical method that finds the optimum conditions of factors for the desirable responses. Numerical optimization was set to maximize all the responses, including percentage yield, total phenolic content, total flavonoid content, baicalin content, baicalein content, chrysin content, and oroxylin-A content in this study. Desirability was calculated from the optimization of these three factors and the highest score for desirability value from this study was 0.771 revealing the optimized condition of each factor at coded 1, 1, and 1 (solvent concentration at 90%, extraction time at 45 minutes, and the ratio of material to solvent at 1:9) as shown in Figure 4.
Optimizing extraction using experimental design or the design of experiments to achieve a high yield of active compounds has been reported and demonstrated in some herbal plants. Prommajak and coworkers reported using UAE with DOE for the extraction of phenolic and antioxidative compounds from lizard tail (Houttuynia cordata Thunb.), which resulted in optimal extraction conditions [40]. Moreover, experimental design for UAE was also used for optimizing the extraction conditions of mulberry leaves, resulting in a high yield of polysaccharides [41]. The results from this study and the other studies could confirm the usefulness and validity of UAE for the extraction of many active compounds from herbal medicine.
Table 5. Prediction factors and responses from desirability determination using numerical optimization by Design-Expert software (V13).
| Prediction responses | Prediction factors | ||
|---|---|---|---|
| Percentage of ethanol (%) | Extraction time (minutes) | The ratio of material to solvent | |
| 90 | 45 | 1:9 | |
| Percentage yields (%) | 7.162 | ||
| Total phenolic content (mg GAE/g extract) | 91.658 | ||
| Total flavonoid content (mg QE/g extract) | 28.342 | ||
| Baicalin content (mg/g extract) | 15.281 | ||
| Baicalein content (mg/g extract) | 40.669 | ||
| Chrysin content (mg/g extract) | 8.299 | ||
| Oroxylin-A content (mg/g extract) | 20.463 | ||
| Desirability value | 0.771 | ||
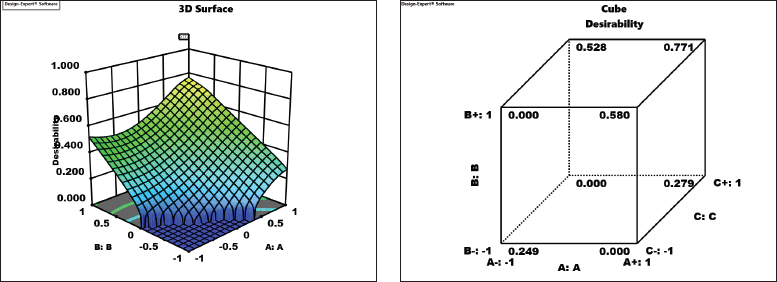 | Figure 4. Three-dimensional and cube desirability plot for the optimization of all responses. [Click here to view] |
CONCLUSION
Experimental design with the application of the BBD served as a robust and systematic approach to optimizing extraction processes. By applying this statistical methodology, the effects of multiple variables and their interactions can be efficiently assessed, enabling the identification of optimal conditions for maximum extraction efficiency. This approach not only reduces the need for extensive trial-and-error experimentation but also saves time and resources. Moreover, numerical optimization from the design studies can be applied to refine extraction protocols, ensuring reproducibility, high efficiency, and further development for industrial applications. As a result, the integration of experimental design into the development of extraction techniques holds significant potential for advancing research and production methodologies in both scientific and commercial domains. The trends in the concentration of these active compounds in O. indicum to use as the raw materials of novel herbal products largely correlated with the total flavonoid content and could be developed for the herbal formulation enriched with a high content of active compounds.
ACKNOWLEDGMENTS
The authors would like to acknowledge and express gratitude to the faculty of Pharmaceutical Sciences at Khon Kaen University for providing all facilities.
LIST OF ABBREVIATIONS
ANOVA, Analysis of variance; DOE, Design of experiment; HPLC, High-performance liquid chromatography; mgGAE/g, milligram gallic acid equivalents per gram extract; mgQE, milligram quercetin equivalent per gram extract; O. indicum, Oroxylum indicum; TPC, Total phenolic content; TFC, Total flavonoid content.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising the content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was supported by the Fundamental Fund of Khon Kaen University, administered through the National Science, Research and Innovation Fund (NSRF), Thailand (2024).
CONFLICTS OF INTEREST
The authors report no financial or other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in the research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Faculty of Pharmacy, Ubon Ratchathani University. Peka [Internet]. Ubon Ratchathani, Thailand: Herbal Database; [cited 2022 Sep 12]. Available from: http://www.phargarden.com/main.php?action=viewpage&pid=84
2. Dev LR, Anurag M, Rajiv G. Oroxylum indicum: a review. Pharmacogn J. 2010;2(9):304–10. CrossRef
3. Jagetia GC. A review on the medicinal and pharmacological properties of traditional ethnomedicinal plant Sonapatha, Oroxylum indicum. Sinusitis. 2021;5(1):71–89. CrossRef
4. Sharma KG, Devi TL, Singh OM, Singh TP. Oroxylum indicum Vent.: a review on its phytochemical and pharmacological profile. Asian J Chem. 2022;34(3):459–72. CrossRef
5. Singh V, Chaudhary AK. A review on the taxonomy, ethnobotany, chemistry, and pharmacology of Oroxylum indicum Vent. Indian J Pharm Sci. 2011;73(5):483–90. CrossRef
6. Dinda B, SilSarma I, Dinda M, Rudrapaul P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: from traditional uses to scientific data for its commercial exploitation. J Ethnopharmacol. 2015;161:255–78. CrossRef
7. Tripathi YB, Singh VP. Anti-inflammatory properties of Dashamoola. J Ethnopharmacol. 1996;54(3):151–6.
8. Gupta M, Mitra P. Therapeutic applications of Dashamoola: a review. Int J Ayurvedic Med. 2014;5(1):49–58.
9. Kulkarni PH, Patil RS. Dashamoola: a traditional Ayurvedic formulation for pain and inflammation. J Ayurveda Integr Med. 2011;2(2):72–80.
10. Sithisarn P, Rojsanga P, Sithisarn P. Flavone-rich fractions and extracts from Oroxylum indicum and their antibacterial activities against clinically isolated zoonotic bacteria and free radical scavenging effects. Molecules. 2021;26(6):1773. CrossRef
11. Sithisarn P, Rojsanga P, Sithisarn P. Inhibitory effects on clinically isolated bacteria and simultaneous HPLC quantitative analysis of flavone contents in extracts from Oroxylum indicum. Molecules. 2019;24:1937. CrossRef
12. Krüger A, Ganzera M. Oroxylum indicum seeds—analysis of flavonoids by HPLC-MS. J Pharm Biomed Anal. 2012;70:553–6. CrossRef
13. Rojsanga P, Bunsupa S, Sithisarn P. Flavone contents in extracts from Oroxylum indicum seeds and plant tissue cultures. Molecules. 2020;25(7):1545. CrossRef
14. Yan RY, Cao YY, Chen CY, Dai HQ, Yu SX, Wei JL, et al. Antioxidant flavonoids from the seed of Oroxylum indicum. Fitoterapia. 2011;82(6):841–8. CrossRef
15. Wei XN, Lin BB, Xie GY, Li JW, Qin MJ. Chemical constituents of seeds of Oroxylum indicum. Zhongguo Zhong Yao Za Zhi. 2013;38:204–7.
16. Mohanta BC, Arima S, Sato N, Harigaya Y. Flavonoids from the stem-bark of Oroxylum indicum. Nat Prod Sci. 2007;13:190–4.
17. Raghu AV, George S, Krishna RK, Sindhu KK. Bioactive properties of phenolics present in Oroxylum indicum—a review. J Pharmacogn Phytochem. 2013;2:23–7.
18. Ganguly R, Gupta A, Pandey AK. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: a review. World J Gastroenterol. 2022;28(26):3047–62. CrossRef
19. Roy MK, Nakahara K, Na Thalang V, Trakoontivakorn G, Takenaka M, Isobe S, et al. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum, inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. Pharmazie. 2007;62(2):149–53. CrossRef
20. Rodríguez-Landa JF, Hernández-López F, Cueto-Escobedo J, Herrera-Huerta EV, Rivadeneyra-Domínguez E, Bernal-Morales B, et al. Chrysin (5,7-dihydroxyflavone) exerts anxiolytic-like effects through GABAA_AA receptors in a surgical menopause model in rats. Biomed Pharmacother. 2019;109:2387–95. CrossRef
21. Ji Y, Han J, Lee N, Yoon JH, Youn K, Ha HJ, et al. Neuroprotective effects of baicalein, wogonin, and oroxylin A on amyloid beta-induced toxicity via NF-κB/MAPK pathway modulation. Molecules. 2020;25(21):5087. CrossRef
22. Kim DH, Lee Y, Lee HE, Park SJ, Jeon SJ, Jeon SJ, et al. Oroxylin A enhances memory consolidation through brain-derived neurotrophic factor in mice. Brain Res Bull. 2014;108:67–73. CrossRef
23. Bhusari S, Morey S, Nikam K, Wakte P. Comparative evaluation of baicalein from Oroxylum indicum using conventional and non-conventional extraction methodology. Res J Pharm Technol. 2019;12(4):1817–22. CrossRef
24. Rathod K, Jaliya R, Jha S, Ram M, Dhaka R, Desai B. Oroxylum indicum: ethnobotany, phytochemistry, and therapeutic uses. Int J Pharm Sci Drug Res. 2021;11:200–3.
25. Chaudhary R, Kumar S, Saini V, Bhati A, Kumar S. Evaluation of hepatoprotective activity of Oroxylum indicum root ethanolic extract. Int J Pharm Sci Drug Res. 2020;5:206–11.
26. Bitwell C, Indra SS, Luke C, Kakoma MK. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Afr. 2023;19:e01585.
27. Abubakar AR, Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci. 2020;12(1):1–10.
28. Murokore BJ, California PV, Wacoo AP, Wangalwa R, Ajayi CO, Gumisiriza H, et al. Effect of extraction period on total phenolics, total flavonoids, and antioxidant capacity of Ugandan Camellia sinensis (L) Kuntze, black primary grades and green tea. J Food Qual. 2023;2023:3504280.
29. Kumar K, Srivastav S, Sharanagat VS. Ultrasound-assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem. 2021;70:105325. CrossRef
30. Demesa AG, Saavala S, Pöysä M, Koiranen T. Overview and toxicity assessment of ultrasound-assisted extraction of natural ingredients from plants. Foods. 2024;13:3066. CrossRef
31. Ngamkhae N, Monthakantirat O, Chulikhit Y, Boonyarat C, Khamphukdee C, Maneenat J, et al. Optimized extraction method for Kleeb Bua Daeng formula with the aid of the experimental design. J Chem. 2021:1457729.
32. Limsakul S, Monthakantirat O, Chulikhit Y, Maneenet J, Khamphukdee C, Chotritthirong Y, et al. Optimizing extraction, evaluating antioxidant activity, and analyzing bioactive compounds in Trikaysornmas formula. Adv Pharmacol Pharm Sci. 2024;2024:8335536. CrossRef
33. Ngamkhae N, Monthakantirat O, Chulikhit Y, Boonyarat C, Maneenet J, Khamphukdee C, et al. Optimization of extraction method for Kleeb Bua Daeng formula and comparison between ultrasound-assisted and microwave-assisted extraction. J Appl Res Med Aromat Plants. 2022;28:100369. CrossRef
34. Ferreira N, Viana T, Henriques B, Tavares DS, Jacinto J, Colónia J, et al. Application of response surface methodology and Box–Behnken design for the optimization of mercury removal by Ulva sp. J Hazard Mater. 2023;445:130405.
35. Leyva-Jiménez FJ, Fernández-Ochoa Á, Cádiz-Gurrea ML, Lozano-Sánchez J, Oliver-Simancas R, Alañón ME, et al. Application of response surface methodologies to optimize high-added value products developments: cosmetic formulations as an example. Antioxidants. 2022;11(8):1552. CrossRef
36. Silva EO, Borges LL, Conceição EC, Bara MTF. Box–Behnken experimental design for extraction of artemisinin from Artemisia annua and validation of the assay method. Rev Bras Farmacogn. 2017;27(4):519–24.
37. Chalermwongkul C, Khamphukdee C, Maneenet J, Daodee S, Monthakantirat O, Boonyarat C, et al. Antidepressant-like effect of Oroxylum indicum seed extract in mice model of unpredictable chronic mild stress. Nutrients. 2023;15(22):4742. CrossRef
38. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013:162750. CrossRef
39. Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26(17):5377. CrossRef
40. Prommajak T, Surawang S, Rattanapanone N. Ultrasonic-assisted extraction of phenolic and antioxidative compounds from lizard tail (Houttuynia cordata Thunb.). Songklanakarin J Sci Technol. 2014;36(1):65–72.
41. Mengdi Z, Hong Z, Heng W, Zhao W, Kim HJ, Li L. Design of experiment approach for the process optimization of ultrasonic-assisted extraction of polysaccharide from mulberry leaves by response surface methodology. Afr J Biotechnol. 2015;14(20):1724–30. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4643_pdf.pdf]