INTRODUCTION
Papilionaceae, Leguminosae, or Fabaceae, also named the pea, legume, or bean family, belongs to the Fabales order. With 19,400 species and 740 genera, Fabaceae is the third-largest plant family after Orchidaceae (orchid family) and Asteraceae (aster family), and it is one of the world’s estimated 12 flowering plants [1]. The Fabaceae family has over 490 species, making it the second-largest family of medicinal plants, the majority of which have been utilized as traditional medicines, making it one of the most interesting families in research [2]. The family features aquatic plants, woody lianas, climbing annuals, shrubs, subshrubs, trees, and herbs. Stems are twining, erect, or climbing. Leaves are rarely simple and mostly compound [3]. Flowers are rarely zygomorphic, actinomorphic, or unisexual. They are bisexual, mainly in corymbs, heads, racemes, panicles, or spikes [4].
The genus Ononis, commonly named restharrow, is part of the Fabaceae family. It is widely spread in West Asia, the Atlantic Islands, North Africa, and Europe, and more than 75 species in the genus have been identified worldwide, particularly near the coasts of the Mediterranean Sea [5]. The scientific interest in the genus Ononis has increased recently as it contains several active components that have therapeutic benefits and are cost-effective. Mainly, phenolic compounds, particularly flavonoids, have been extracted. Mainly, phenolic compounds, particularly flavonoids, have been extracted. Genus Ononis exhibits diverse pharmacological activities, including diuretic, antitussive, anti-inflammatory, analgesic, antioxidant [6,7], antimicrobial [6], and anticancer effects [8]. Additional uses include the treatment of urinary tract infections, rheumatism, wound healing, eczema, and other skin diseases [9]. In this work, we attempt to summarize and review the phenolic constituents of plants belonging to the genus Ononis, their pharmacological effects, structure–activity relationship, and reported mechanisms of action.
METHODS
The keywords Ononis, phytochemical constituents, pharmacological activities, and structure-activity relationship were searched. The search was obtained from journals and books in databases, such as Scopus, ScienceDirect, Web of Science, PubMed, Google Scholar, and The Cochrane Library, from 1990 to 2024.
RESULTS AND DISCUSSION
Phytochemical profile
Ononis species are the source of several pharmacologically important flavonoids and isoflavonoids. Flavonoids are classified as secondary metabolites. Their primary structural components are phenolic or polyphenolic groups found at different positions of a benzopyrone ring. They are divided into several categories based on the oxidation of the carbon ring, their degree of unsaturation, and their chemical structure. The several subclasses of flavonoids comprise isoflavonoids, flavanones, anthoxanthins (flavanol and flavanone), flavans , flavanones, flavanonols, chalcones, and anthocyanidins [10].
Flavonoids and isoflavonoids have been described from Ononis species so far, including the isoflavones and the less prevalent isoflavonones and pterocarpans. In addition to flavonoids and isoflavonoids, other types of constituents, including resorcinol derivatives [11], isocoumarins [12], phenolic lactones [13], and terpenoids [7,14], have been isolated from Ononis species. According to most data in the literature, flavonoids are very abundant in herbs, stems, fruits, flowers, vegetables, and seeds. It is highly suggested they are found in chloroplasts. Table 1 summarizes the phenolic profile of different Ononis species.
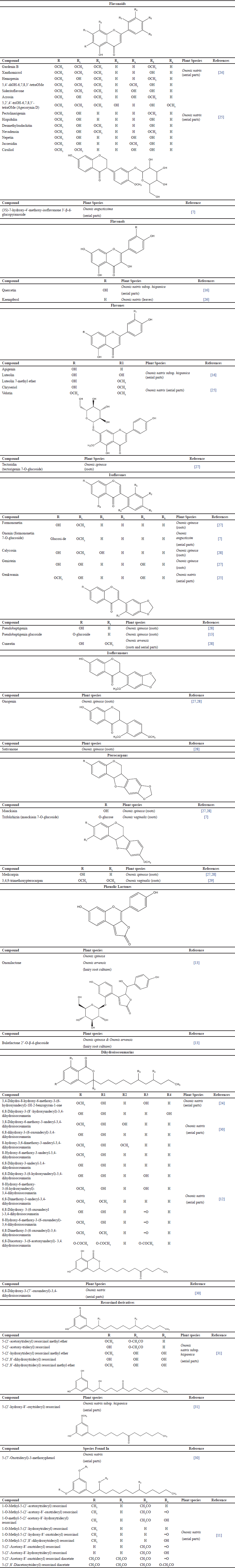 | Table 1. Phytochemistry of Ononis species and their chemical structures. [Click here to view] |
Pharmacological effects of some Ononis plants
Antioxidant and anti-inflammatory effects
Crude extracts (ethyl acetate, n-butanol, and petroleum ether) of Ononis mitissima plant showed moderate antioxidant activities. In addition, at 500 µg/ml, these extracts exhibited medium anti-inflammatory activity, with inhibition percentages of 33%, 11%, and 22% in comparison to diclofenac (86%) for ethyl acetate, n-butanol, and petroleum ether extracts, respectively [15]. Another study showed that ethyl acetate and methanolic root extracts of O. spinosa reduced inflammation by 40.4% and 35.4%, respectively [5]. Moreover, the leaf extract of Tunisian Ononis natrix demonstrated a high reducing power (ED50 = 100 μg/ml), with a low IC50 value (29 μg/ml) corresponding to an increased 2,2-diphenyl-1-picrylhydrazyl scavenging ability. With 60.94 mg of GAE/g DW, it also demonstrated a high level of overall antioxidant activity. [6].
Antimicrobial effects
Many studies reported on the antimicrobial effect of the Ononis species. Ononis natrix extract demonstrated efficacy against strains of gram-positive bacteria, particularly against Staphylococcus aureus ATC 25923 and Staphylococcus epidermidis strains [16]. Similarly, O. spinosa stopped Bacillus subtilis, S. aureus, Pseudomonas aeruginosa, and Escherichia coli growth. Moreover, potential antimicrobial activity against Candida albicans, MRSA, P. aeruginosa, Bacillus cereus, E. coli, Salmonella typhimurium, and Aspergillus niger has been reported from Oxalis hirta and Oxalis sicula ethanolic extracts [17]. The same study showed that chloroform extract of Oxalis arvensis leaves had significant inhibitory activity against E. coli and C. albicans, showing respective MIC values of 12.75 μg/ml and 51 μg/ml. [17].
Cytotoxic effect
By stopping the growth of MDA MB-231 breast cancer cells, Jordanian O. natrix revealed a potential antitumor effect with an IC50 of 29–41 µg/ml compared to that of tamoxifen (IC50 11 µg/ml) [18]. Turkish O. natrix extracts, on the other hand, exhibited an apoptotic effect against PC3 cancerous cell line at a very small concentration (0.1 μg/ml) [16].
Enzyme inhibitory effect
The dichloromethane extract of O. spinosa demonstrated a concentration-dependent suppression of TNF-α and IL-8 production from lipopolysaccharide-stimulated human neutrophils [19]. In addition, the ethyl acetate extract revealed high inhibitory activity of cholinesterase on both BChE (0.93 mg GALAE/g extract) and AChE (1.46 mg GALAE/g extract), unlike the water extract, which was ineffective on BChE. Conversely, the ethyl acetate extract had no inhibitory activity on tyrosinase, but the water extract had significant activity (52.81 mg KAE/g extract). Regarding glucosidase and amylase inhibition, the most effective extracts were the ethyl acetate (0.74 mmol ACAE/g and 17.52 mmol ACAE/g) and methanol extracts (0.59 mmol ACAE/g and 19.94 mmol ACAE/g) [16].
Antidiabetic effect
Preclinical studies on O. natrix decoction have demonstrated its effectiveness in lowering blood glucose levels in rats [20]. Ononis extracts can improve glucose and starch tolerance by activating the glucose transporter type-4 (Glut-4) receptor in experimental rats [21]. Moreover, aqueous extracts of O. natrix had the ability to restore the islets of Langerhans suffering from alloxan-induced tissue damage in albino Swiss mice [22].
Structure–activity relationship of phenolic constituents from Ononis
The medicinal importance of Ononis plants is explained by their phenolic contents, mainly flavonoids. These compounds account for the anti-inflammatory, antioxidant, and anticancer characteristics of Ononis plants [23].
Anticancer
Formononetin, one of the most known isoflavones in the Ononis species, revealed anticancer activity against various types of cancers such as colon, breast, prostate, nasopharyngeal, bladder, cervical, lung, laryngeal, glioma, adrenal medulla, multiple myeloma, and osteosarcoma cancer. It arrests the G0/G1 phase of the cell cycle in ES2 and the G1 phase in human ovarian and lung cancerous cells and triggers apoptosis in breast cancerous cells [32]. Data about formononetin structure–activity relationships are extremely limited. One study suggested that the presence of a 2–3 double bond, a 4-carbonyl group, and ortho- as opposed to meta-hydroxylation in the B ring greatly increased the cytotoxic activity. Comparing 3-hydroxylated molecules to their non-hydroxylated counterparts, the latter showed noticeably greater cytotoxicity [33].
As for the antioxidant activity, the total number of hydroxyl groups has a significant influence. For example, apigenin, kaempferol, luteolin, and quercetin reduced nitric oxide and phagocytosis in a dose-dependent manner, and the phenolic hydroxyl groups number was proportional to their antioxidant effect [34]. Additionally, the presence of a 3’,4’-catechol structure in the B ring greatly increased the potential to inhibit lipid peroxidation. Thus, flavonoids are most efficient in scavenging peroxynitrite, superoxide, and peroxyl radicals. Due to oxidation on the flavonoid B ring with a catechol group, a relatively stable ortho-semiquinone radical that acts as a potent scavenger is produced. In contrast, flavones without a catechol system form unstable radicals when oxidized, showing less scavenging potential [35]. It is worth to mention that flavones and catechins appear to have the most potent activity against reactive oxygen species [35].
Concerning the antimicrobial activity, several structurally distinct flavonoids, such as isoflavones, flavones, isoflavanones, and flavanones, show that Streptococcus sobrinus and Streptococcus mutans growth was inhibited by 5-hydroxyisoflavanones and 5-hydroxyflavanones with one, two, or three additional hydroxyl groups at the 7, 2′, and 4′ positions, with position 2′ hydroxylation being crucial for the anti-staphylococcal activity. Additionally, hydroxyl groups at position 5 of flavones and flavanones are critical for their anti-MRSA action. It was also evident that chalcones work better than flavanones or flavones against MRSA. On the contrary, it has been observed that methoxy groups significantly reduce flavonoids’ antibacterial activity [36].
For type 2 diabetes mellitus, quercetin has the highest IC50 value to inhibit α-glucosidase and dipeptidyl peptidase IV. The existence of the C-2-C-3 double bond and the C-4 ketonic group is crucial to its antidiabetic properties. However, acetylation, methylation, and hydroxyl groups reduce the antidiabetic in-vitro effects of flavonoids [37].
Toxicity and safety assessments
Till this date, no studies assessing the toxicity and safety of the Ononis species have been reported, so the literature is lacking in this regard. However, there are several investigations that have evaluated the safety of Ononis-based compounds. For example, quercetin, one of the most common and known flavonoids in the Ononis species, has shown conflicting results. One study proved the safety of quercetin when taken orally for 98 days in male and female mice (doses ~ 12.5, 25, or 50 mg/kg of body weight) [38]. On the other hand, higher doses of quercetin >2,000 mg/kg were associated with hepatotoxic stress in mice liver [39], and a dose of 3807 mg/kg was lethal 22 hours after administration to mice [40]. Moreover, sodium formononetin-30-sulphonate, a water-soluble derivative of formononetin, was considered safe and showed no side effects at doses <100 mg/kg in dogs [41], and formononetin was safe at a dose of 1.5 mg/kg in mice [42]. The lethal dose of 50% was also considered to be 103.6 mg/kg, and no side effects were exhibited at doses <50 mg/kg [43].
The findings of the collected studies showed no discrepancies, contraindications, or conflicting viewpoints concerning pharmacological activities and associated structure–activity relationships. On the contrary, the main results of the different studies were consistent, enhancing the credibility of the available literature. However, it is important to mention that while most biological activities of Ononis flavonoids and their structure–activity relationship are well documented, there is a lack of studies investigating their cytotoxic activity. Accordingly, the cytotoxic activity of Ononis flavonoids needs to be thoroughly investigated to determine a detailed structure–activity relationship that could pave the way in the process of drug discovery. In addition, the available studies are mostly in vitro evaluations except for the antidiabetic activity, which has been studied in vivo. In addition, it is noteworthy to mention that the toxicity of some Ononis flavonoids has been assessed, but the Ononis plants or their extracts have not been studied in this aspect, thereby necessitating the need for further investigations. All in all, this is the first review article that summarizes the constituents of different Ononis species and their pharmacological activities, as well as highlighting their structure–activity relationship, thereby offering a collective review of these species. The use of several recent studies emphasizes the growing interest in the Ononis species and the importance of their therapeutic potential in various diseases and stimulates further investigations of these plants.
CONCLUSION
This review of genus Ononis highlights the presence of diverse bioactive compounds, mainly flavonoids and isoflavonoids, responsible for the pharmacological potential of these species. Based on the study findings, Ononis species could be considered important therapeutic remedies in curing oxidative stress, inflammation, infections, diabetes, and some cancers. It opens the gates for researchers to prepare more potent derivatives from the phenolic and flavonoid content of the Ononis plant. These derivatives could serve as novel molecule in the process of drug discovery. Furthermore, the study highlighted the therapeutic potential of the Ononis plant as preventive, treatment, and even adjuvant measures for various diseases. Preclinical and clinical studies will be recommended to support its clinical applications.
ACKNOWLEDGMENTS
The authors thank Beirut Arab University for granting the services throughout the manuscript preparation. The authors thank Acdlabs.com (ChemSketch free version) for making it possible to create all of the figures.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was not supported by funding sources in the public, private, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data supporting the results of this research are accessible in standard research databases such as PubMed, Scopus, ScienceDirect, Google Scholar, The Cochrane Library, Web of Science, and/or public domains that are accessible via keywords or DOI numbers.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors state that they have not utilized artificial intelligence (AI) tools for writing and editing of the manuscript, and no pictures were modified using AI.
REFERENCES
1. Tekdal D. Plant genes for abiotic stress in legumes. Abiotic Stress and Legumes. Singh VP, Singh S, Tripathi DK, Prasad SM, Bhardwaj R, Chauhan DK, editors. Academic Press; 2021. Pp 291–301.
2. Gao T, Yao H, Song J, Liu C, Zhu Y, Ma X, et al. Identification of medicinal plants in the family Fabaceae using a potential DNA barcode ITS2. J Ethnopharmacol. 2010;130(1):116–21.
3. Xu Z, Deng M, Xu Z, Deng M. Fabaceae or Leguminosae. Identification and Control of Common Weeds. Vol. 2. 2017. Pp 547–615.
4. Maroyi A. Medicinal uses of the Fabaceae family in Zimbabwe: a review. Plants. 2023;12(6):1255.
5. Öz BE, ??can GS, Akkol EK, Süntar ?, Kele? H, Ac?kara ÖB. Wound healing and anti-inflammatory activity of some Ononis taxons. Biomedicine Pharmacother. 2017;91:1096–105.
6. Mhamdi B, Abbassi F, Abdelly C. Chemical composition, antioxidant and antimicrobial activities of the edible medicinal Ononis natrix growing wild in Tunisia. Nat Prod Res. 2015;29(12):1157–60.
7. Mezrag A, Malafronte N, Bouheroum M, Travaglino C, Russo D, Milella L, et al. Phytochemical and antioxidant activity studies on Ononis angustissima L. aerial parts: isolation of two new flavonoids. Nat Prod Res. 2017;31(5):507–14.
8. Ghribi L, Waffo-Téguo P, Cluzet S, Marchal A, Marques J, Mérillon JM, et al. Isolation and structure elucidation of bioactive compounds from the roots of the Tunisian Ononis angustissima L. Bioorgan Med Chem Lett. 2015;25(18):3825–30.
9. Al-Snafi AE. The traditional uses, constituents and pharmacological effects of Ononis spinosa. IOSR J Pharm. 2020;10(2):53–9.
10. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243.
11. Cañedo LM, del Corral JM, San Feliciano A. 5-Alkylresorcinols from Ononis natrix. Phytochemistry. 1997;44(8):1559–63.
12. San Feliciano A, del Corral JM, Cañedo LM, Medarde M. 3, 4-Dihydroisocoumarins from Ononis natrix. Phytochemistry. 1990;29(3):945–8.
13. Gampe N, Szakács Z, Darcsi A, Boldizsár I, Sz?ke É, Kuzovkina I, et al. Qualitative and quantitative phytochemical analysis of Ononis hairy root cultures. Front Plant Sci. 2021;11:622585.
14. Abu Zarga MH, Al-Jaber HI, Al-Qudah MA, Al-Aboudi AM. A new cyclic polyketide and other constituents from Ononis spinosa growing wildly in Jordan and their antioxidant activity. J Asian Nat Prod Res. 2022;24(3):290–5.
15. Besbas S, Mouffouk S, Haba H, Marcourt L, Wolfender JL, Benkhaled M. Chemical composition, antioxidant, antihemolytic and anti-inflammatory activities of Ononis mitissima L. Phytochem Lett. 2020;37:63–9.
16. Yerlikaya S, Zengin G, Mollica A, Baloglu MC, Celik Altunoglu Y, Aktumsek A. A multidirectional perspective for novel functional products: in vitro pharmacological activities and in silico studies on Ononis natrix subsp. hispanica. Front Pharmacol. 2017;8:600.
17. Dénes T, Bartha SG, Kerényi M, Varga E, Balázs VL, Csepregi R, et al. Histological and antimicrobial study of Ononis arvensis L. Acta Biol Hung. 2017;68:321–33.
18. Al-Zereini WA. Ononis natrix and Salvia verbenaca: two Jordanian medicinal plants with cytotoxic and antibacterial activities. J Herbs Spices Med Plants. 2017;23(1):18–25.
19. Spiegler V, Gierlikowska B, Saenger T, Addotey JN, Sendker J, Jose J, et al. Root extracts from Ononis spinosa inhibit IL-8 release via interactions with toll-like receptor 4 and lipopolysaccharide. Front Pharmacol. 2020;11:889.
20. Al-Mubideen BF, Al-Serhan AA, Amarin JZ, Al-Dweikat A, Al-Muhaisen RA, Shreikh YA, et al. Ononis natrix L. lowers the blood glucose concentration in wistar rats with streptozotocin-induced diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2021;21(5):854–8.
21. Al-Mterin MA, Aboalhaija N, Zihlif MA, Afifi FU. Effects of Ononis natrix on glucose and lipid metabolism: an in vivo study. J Res Pharm. 2024;28(1):278–88.
22. El Khiat A, Bouftini K, Lafhal K, Tastift MA, El-Mansoury B, Ali DA, et al. Evaluation of the Anti-hyperglycemic activity of Ononis natrix aqueous extract against alloxan-induced experimental model of insulinopenic diabetes in albino Swiss mice. Academic J. 2024;39(4):57–64.
23. Kanso MA, Hijazi MA, El-Lakany A, Aboul-Ela M. Review on phytochemical constituents and pharmacological activities of genus Galium. J Appl Pharm Sci. 2024;14(9):046–56.
24. Al-Khalil S, Masalmeh A, Abdalla S, Tosa H, Iinuma M. N-arachidylanthranilic acid, a new derivative from Ononis natrix. J Nat Prod. 1995;58(5):760–3.
25. Wollenweber E, Dörr M, Rivera D, Roitman JN. Externally accumulated flavonoids in three Mediterranean Ononis species. Zeitschrift fuer Naturforschung C. 2003;58(11-12):771–5.
26. Gampe N, Darcsi A, Lohner S, Béni S, Kursinszki L. Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC–MS/MS and NMR. J Pharm Biomed Anal. 2016;123:74–81.
27. Gampe N, Dávid DN, Takács-Novák K, Backlund A, Béni S. In vitro and in silico evaluation of Ononis isoflavonoids as molecules targeting the central nervous system. PLoS One. 2022;17(3):e0265639.
28. Gampe N, Darcsi A, Nagyné Nedves A, Boldizsár I, Kursinszki L, Béni S. Phytochemical analysis of Ononis arvensis L. by liquid chromatography coupled with mass spectrometry. J Mass Spectrometry. 2019;54(2):121–33.
29. Abdel-Kader MS. Phenolic constituents of Ononis vaginalis roots. Planta Medica. 2001;67(04):388–90.
30. Yousaf M, Al-Rehaily AJ, Ahmad MS, Mustafa J, Al-Yahya MA, Al-Said MS, et al. A 5-alkylresorcinol and three3, 4-dihydroisocoumarins derived from Ononis natrix. Phytochem Lett. 2015;13:1–5.
31. Barrero AF, Sánchez JF, Rodríguez I. N-Δ13-Docosenoylanthranilic acid and alkylresorcinols from Ononis natrix subsp. hispanica. Phytochemistry. 1990;29(6):1967–9.
32. Jiang D, Rasul A, Batool R, Sarfraz I, Hussain G, Mateen Tahir M, et al. Potential anticancer properties and mechanisms of action of formononetin. BioMed Res Int. 2019;2019(1):5854315.
33. Plochmann K, Korte G, Koutsilieri E, Richling E, Riederer P, Rethwilm A, et al. Structure–activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch Biochem Biophy. 2007;460(1):1–9.
34. Tian C, Liu X, Chang Y, Wang R, Lv T, Cui C, et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South African J Bot. 2021;137:257–64.
35. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;2013(1):162750.
36. Cushnie TT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26(5):343–56.
37. Sarian MN, Ahmed QU, Mat So’ad SZ, Alhassan AM, Murugesu S, Perumal V, et al. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study. BioMed Res Int. 2017;2017(1):8386065.
38. Cunningham P, Patton E, VanderVeen BN, Unger C, Aladhami A, Enos RT, et al. Sub-chronic oral toxicity screening of quercetin in mice. BMC Complementary Med Ther. 2022;22(1):279.
39. Singh P, Sharma S, Rath SK. A versatile flavonoid Quercetin: study of its toxicity and differential gene expression in the liver of mice. Phytomed Plus. 2022;2(1):100148.
40. Dibal NI, Garba SH, Jacks TW. Acute toxicity of quercetin from onion skin in mice. Pharm Biomed Res. 2020;6(4):269–76.
41. Li C, Li G, Gao Y, Sun C, Wang X. A 90-day subchronic toxicity study with sodium formononetin-3′-sulphonate (Sul-F) delivered to dogs via intravenous administration. Regul Toxicol Pharmacol. 2016;77:87–92.
42. Singh KB, Dixit M, Dev K, Maurya R, Singh D. Formononetin, a methoxy isoflavone, enhances bone regeneration in a mouse model of cortical bone defect. Br J Nutr. 2017;117(11):1511–22.
43. Pingale TD, Gupta GL. Acute and sub-acute toxicity study reveals no dentrimental effect of formononetin in mice upon repeated ip dosing. Toxicol Mech Methods. 2023;33(8):688–97.