INTRODUCTION
Diabetes mellitus (DM) is a noncontagious disease and a leading cause of death globally. It is characterized by a disorder in glucose metabolism, resulting in uncontrolled hyperglycemia [1]. Chronic uncontrolled, abnormal high glucose level is a metabolic disorder caused by either a compromised insulin action, an insufficiency of insulin secretion, or both [2]. The most common cases of DM are type 2 DM (T2DM), which reaches 90% of all DM cases [3]; continued T2DM raises the risk of mortality, diminishes the quality of life, and leads to higher treatment expenses [4]. Complications linked to T2DM include microvascular issues such as diabetes, kidney disease, retinopathy, and peripheral neuropathy, as well as macrovascular problems such as coronary heart disease, stroke, and peripheral arterial disease [5]. Cohort studies have shown that DM is associated with various cancers, functional cognitive disabilities, liver disease, affective disorders, and sleep disorders. These studies have also provided new insights into infection-related complications of DM [6]. According to the International Diabetes Federation, there were 10.5% of the global population, or 537 million people with diabetes, in 2021, which cost $966 billion for diabetes treatment. This cost is estimated to continue to increase in 2024 to $1054 billion. The global incidence of diabetes is estimated to increase alarmingly, reaching 643 million, or 11.3% of the worldwide population, in 2030 and 783 million (12.2% of the global population) in 2045 [1,7]. Various classes of T2DM therapy have demonstrated therapeutic benefits, but the global incidence continues to rise, necessitating the discovery of new therapeutic agents for type 2 diabetes [7,8].
α-Glucosidase inhibitors are commonly used as one of the initial therapies for T2DM. They are widely used to prevent T2DM in people at high risk, in pre-diabetic conditions, and in stage 1 and stage 2 diabetes; they are also combined with other T2DM therapies [9–11]. α-Glucosidase inhibitors can be given to individuals with kidney disease, and this makes them a suitable therapy for people living with diabetes complicated by nephropathy. Therefore, this class of therapy is more beneficial for people living with T2DM with kidney disorders compared to other therapy classes, such as sulfonylureas, metformin, SGLT2 inhibitors, and glinide, which are not recommended for individuals with kidney disorders [10,12]. α-Glucosidase inhibitors are vital enzymes that are important in converting polysaccharides into glucose, where these therapeutic agents can suppress the glucose absorption rate by delaying the digestion of polysaccharides. This delay in converting polysaccharides to glucose has proven very important in treating hyperglycemia, making it beneficial in treating T2DM [13,14].
Various studies have demonstrated that traditional medicinal plants used for treating diabetes show potential in both in vitro and in vivo studies and have also successfully reduced blood sugar levels in pre-clinical and clinical trials [15–18]. One genus of medicinal plants with potential antidiabetic properties is Uncaria, which is reported to contain phenols, flavonoids, terpenoids, and alkaloids [19,20]. Various studies on the antidiabetic activity of the Uncaria genus have shown the potential of this genus in treating diabetes, including Uncaria laevigata, Uncaria tomentosa, Uncaria cordata, Uncaria gambier, Uncaria longiflora, Uncaria acida, and Uncaria callophylla, both in vitro and in vivo [21–26].
Our previous study reported that Uncaria sclerophylla Roxb, a popular medicinal plant in Kalimantan, Indonesia, is used as a traditional anti-diabetic medicine, and studies have focused on the plant’s potential as an antidiabetic using its stems and twigs [27]. However, the anti-diabetic properties of the leaves as an α-Glucosidase inhibitor have never been reported. This study explores the potential of U. sclerophylla leaves as an antidiabetic agent with inhibitory effects against a-Glucosidase. The study used a four-grade maceration technique and column chromatography fractionation to obtain the most promising fraction for further development as an antidiabetic therapy. The compounds in the selected fraction will be identified using UPLC-QToF/MS-MS, and their inhibitory effects against a-Glucosidase will be assessed using in silico methods. Additionally, the identified compounds’ Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) profiles will be evaluated in silico.
MATERIALS AND METHODS
Plant material
Uncaria sclerophylla leaves were collected from Meratus Forest in Kalimantan, Indonesia, during the dry season. The cleaned fresh leaves (8 kg) were dried in a blower oven at 35°C pulverized with a grinder, and sieved through a 40-mesh sieve, yielding 1.92 kg powdered leaves (24% yield), then refrigerated at 8°C while pending analysis. Plant authenticity was determined and deposited in the Pharmacognosy-Phytochemistry Laboratory, Faculty of Pharmacy, Universitas Indonesia (voucher specimen number 237/LB/XI/2021).
Chemicals and instrumentation
Chemicals: methanol, ethyl acetate, dichloromethane, n-hexane, silica Gel 70-230 mesh, TLC plate 254GF (Merck, Germany). Acarbose, α-Glucosidase enzyme, para-nitrophenyl-α-D-Glucopyranoside, bovine serum albumin, potassium dihydrogen phosphate, sodium carbonate, dimethylsulfoxide (Sigma-Aldrich, USA). Instrumentations: Microplate reader (Glomax Promega, UK), UPLC-QToF-MS/MS from Acquity UPLC H-Class System; Xevo G2-S QToF (Waters, USA).
Extraction
Uncaria sclerophylla leaf simplicia (1 kg) was extracted by four-level maceration using n-hexane, dichloromethane, ethyl acetate, and methanol as extraction solvents (1:20 ratio). The extraction process begins with n-hexane solvent, and the unextracted part is then extracted using dichloromethane. The same extraction process was continued with increasing polarity solvents such as ethyl acetate and methanol. Extract finishing is processed using a rotary evaporator and a blower oven at a temperature of 35°C. The resulting extracts are n-hexane extract, dichloromethane extract, ethyl acetate extract, and methanol extract.
Fractionation
Column chromatography was carried out in the selected extract fractionation with a combination of n-hexane, ethyl acetate, and methanol as solvent using a column with a length of 50 cm and a diameter of 3.5 cm. Silica gel (70–230 mesh) in a ratio of 1:15 was chosen as the stationary phase during the fractionation process. The extracts (40 g of methanol extract and 15 g of ethyl acetate extract) were fractionated using a gradient mobile phase system starting with a combination of n-hexane and ethyl acetate at a ratio of 9:1, 8:2, 7:3, up to 0:10, further with a combination of ethyl acetate and methanol as a solvent in the ratio 9:1, 8:2, 7:3, up to 0:10. Thin-layer chromatography is used to analyze the chromatogram pattern for every 100 ml of elution results. The same or similar chromatogram patterns are combined into the same fraction.
α-Glucosidase inhibition activity
The α-Glucosidase inhibitory potential of extracts and fractions was measured by spectrophotometric principles using a microplate reader, according to the adopted method [27]. The procedure involved mixing 30 µl of samples (positive control, extract, and fraction) with 36 µl of phosphate buffer pH 6.8 and 17 µl of pNP-G (5 mM) in 96 wells, followed by an incubation at 37°C for 5 minutes. Subsequently, 17 μl of α-Glucosidase enzyme was added to the mixture, and the incubation continued at 37°C for 15 minutes. The enzyme reaction was terminated by adding 100 μl of Na2CO3 (267 mM). The p-nitrophenol compound, generated during the enzyme reaction, was measured at 405 nm with a microplate reader. Each test was performed in triplicate, and the standard deviation for each test was calculated.
Compound profiling using UPLC-ESI-QToF-MS/MS
The compound profile in the most active fraction was identified using a combination of UPLC with mass spectrometry and ESI as an ionizer. The liquid chromatography separation utilized a reverse phase technique with a C18 column (Acquity UPLC®, Waters, USA) at 40°C and autosampler temperature of 15°C. The mobile phase was a gradient system consisting of 0.1% formic acid in water and 0.1% formic acid in acetonitrile, with a 0.6 ml/minute flow rate. After separation via UPLC, the compounds were converted into ions using electrospray ionization in positive mode. The compound ions were then analyzed using Quadrupole Time-of-Flight mass spectrometry with a mass analysis range of 50–1,200 m/z. Mass spectrometry condition: the capillary voltage was 3 KV, cone voltage was 100 V, low collision energy of 6 eV, and high collision energy of 15–40 eV, source temperature was 120°C, desolvation temperature was 500°C, cone gas flow was 30 l/hour, desolvation gas flow was 1,000 l/hour, acquisition time was 20 minutes. Data processing and analysis were performed using Masslynx software (Waters) and the Unifi database.
Molecular docking
A total of 22 phytochemical compounds identified through compound profiling were analyzed. The 3D structures of 20 compounds were downloaded from the PubChem database, while the 3D structures of the remaining two compounds were generated using OpenBabel. The structure of the α-Glucosidase protein was downloaded from the research collaboratory for structural bioinformatics protein data bank (PDB)with the PDB ID: 3A4A [28]. The protein was processed by separating the native ligand from the protein and removing water molecules. Molecular docking was performed using AutoDock Vina through PyRx [29,30]. The center was set at x 21.595, y −7.436, and z 24.042, and the grid box for docking was configured to dimensions of 30 × 30 × 30 Å with a spacing of 0.375 Å. The exhaustiveness parameter was set to 200. Docking validation was performed by observing alpha-D-glucopyranose (native ligand) through redocking, and the results showed a root mean square deviation of less than 2 Å. The docking results were visualized using Discovery Studio 2021.
ADMET analysis
Twenty-two phytochemical compounds from U. sclerophylla were analyzed for their ADMET profiles. This analysis utilized the Deep-Pk prediction tool (https://biosig.lab.uq.edu.au/deeppk/prediction) to evaluate pharmacokinetic predictions and drug-likeness based on Lipinski’s rule of five [31,32].
RESULTS
α-Glucosidase inhibition activity of extracts
The antidiabetic potential of U. sclerophylla leaves was investigated in vitro, focusing on their ability to inhibit α-Glucosidase. The study found that all extracts exhibited varying inhibitory activities. However, the ethyl acetate and methanol extract of U. sclerophylla demonstrated superior activity compared to the other extracts; details refer to Figure 1. The ethyl acetate and methanol extracts of U. sclerophylla leaves were then analyzed for IC50 values and compared with acarbose when the methanol extract of the leaves exhibited superior activity compared to acarbose (Table 1).
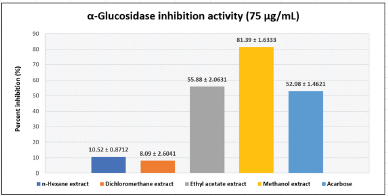 | Figure 1. α-Glucosidase inhibition activity of extracts and acarbose. [Click here to view] |
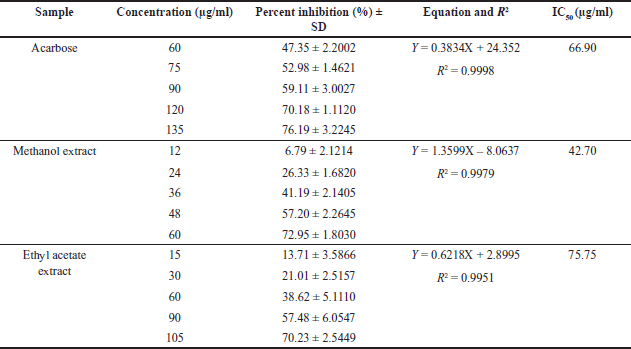 | Table 1. IC50 of α-Glucosidase inhibition for selected extracts and acarbose. [Click here to view] |
Fractionation and α-glucosidase inhibition activity of fractions
Ethyl acetate and methanol extracts of U. sclerophylla leaves have shown potential in α-Glucosidase inhibitory activity and were further explored for fractionation. These extracts were fractionated using column chromatography, producing 11 methanol and 12 ethyl acetate fractions. All fractions exhibited inhibitory activity against α-Glucosidase (Figs. 2 and 3), and several fractions with inhibition percentages above 60% were further analyzed for their IC50 values. The fraction with the highest activity (USMeth5) was found to have an IC50 value of 22.85 µg/ml, outperforming acarbose as a positive comparator (Table 2).
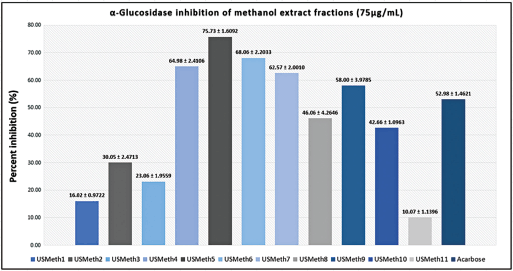 | Figure 2. α-Glucosidase inhibition activity of methanol extract fractions and acarbose. [Click here to view] |
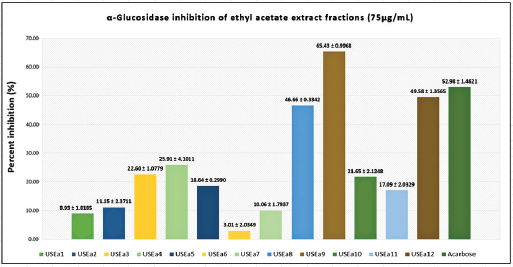 | Figure 3. α-Glucosidase inhibition activity of ethyl acetate extract fractions and acarbose. [Click here to view] |
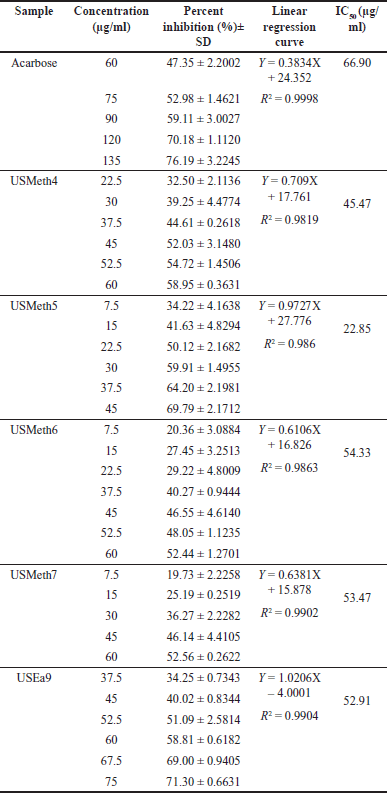 | Table 2. IC50 of α-Glucosidase inhibition for selected fractions and acarbose. [Click here to view] |
Compound profiling of the most active fraction in α-glucosidase inhibition
The most promising fraction as α-Glucosidase inhibitor, USMeth5, underwent further analysis to determine its compound profile using the UPLC-QToF-MS/MS technique. The compound profiling of USMeth5 revealed that this fraction contains alkaloid, flavonoid, and phenol compounds. The alkaloid compounds in USMeth5 are characterized by nitrogen atoms in their structure [33,34], the phenol structure is characterized by the presence of an aromatic ring with one or more hydroxyl group substituents [35], and flavonoid compounds are more specifically characterized by a C6-C3-C6 carbon backbone structure, a benzo-γ-pyrone, and a phenyl ring [36]. The chemical structure of the compounds found in USMeth5 is depicted in Figure 4. Table 3 presents information on compound profiling in USMeth5, including compound formulas, retention times, ion masses, total fragments found, and response.
 | Figure 4. Structure of U. sclerophylla compounds used for docking. [Click here to view] |
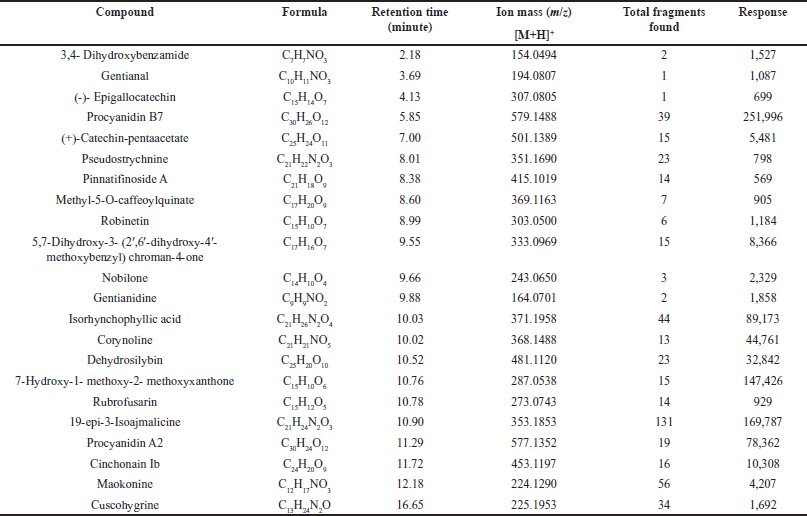 | Table 3. Compounds detected in USMeth5 using LC-MS/MS. [Click here to view] |
Molecular docking
The compounds detected from U. sclerophylla’s most active fraction (Fig. 4) were studied to understand their interaction with the α-Glucosidase enzyme. Using molecular docking techniques, the researchers analyzed the active compounds and their binding with the enzyme. The findings revealed the specific compounds from U. sclerophylla that exhibited the most robust binding to α-Glucosidase with acarbose as drug control. Table 4 and Figure 5 summarize the details of these interactions and their binding potential.
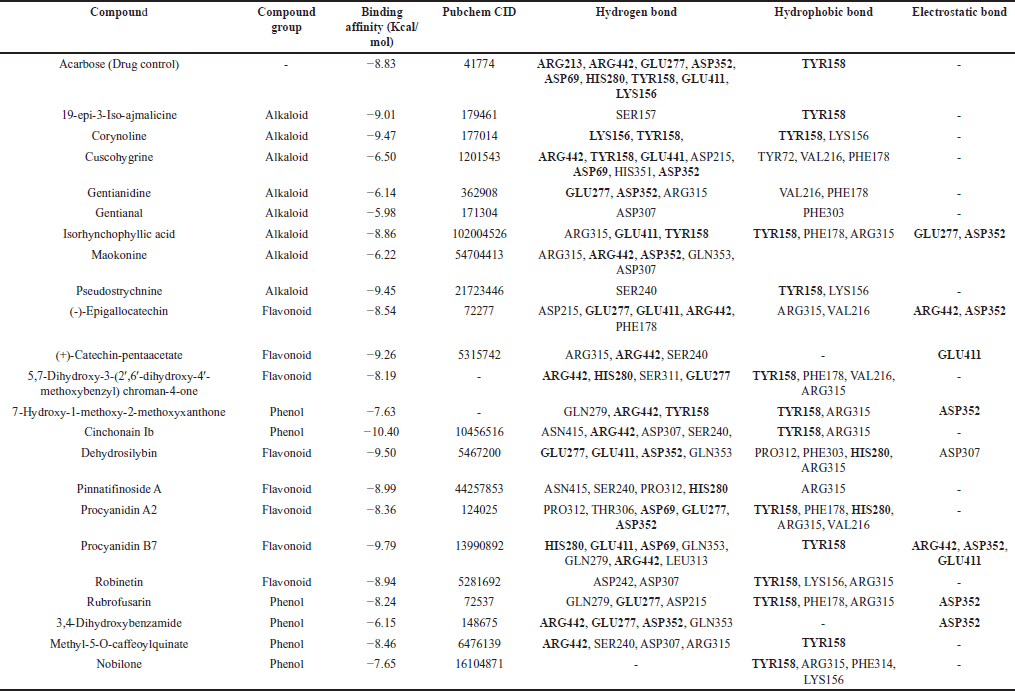 | Table 4. Molecular docking of compounds contained in USMeth5. [Click here to view] |
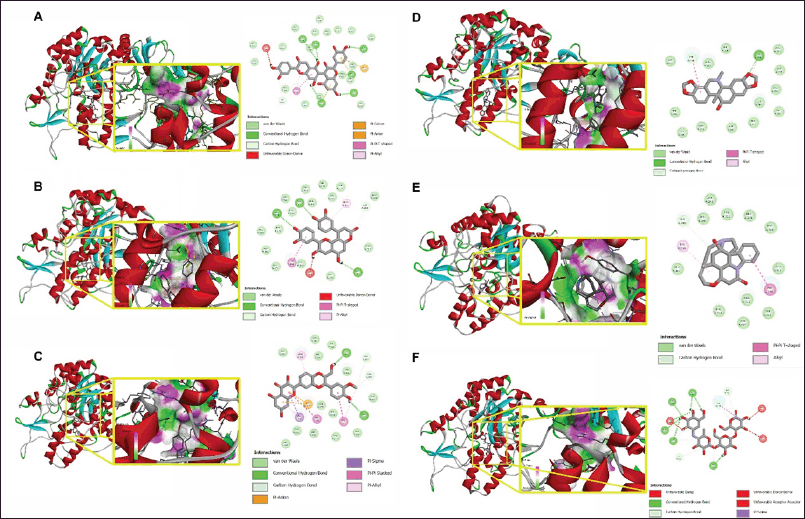 | Figure 5. Molecular docking interaction of the five compounds with the best binding affinity (A) Cinchonain Ib, (B) Procyanidin B7, (C) Dehydrosilybin, (D) Corynoline, (E) Pseudostrychnine, along with Acarbose (F) as standard. [Click here to view] |
ADMET analysis
The evaluation of ADME and toxicity properties for the studied compounds adhered to standard pharmacokinetic criteria, with Lipinski’s rule of five as a foundational guideline. This rule stipulates that compounds with molecular weights (MWs) ≤500, hydrogen bond donors (HBDs) ≤5, hydrogen bond acceptors (HBAs) ≤10, and log P ≤5 are more likely to exhibit favorable oral bioavailability [32]. The compounds evaluated in this study largely conformed to these parameters, highlighting their potential as viable drug candidates.
Human intestinal absorption (HIA) values for nearly all compounds exceeded 30%, indicating good absorption capabilities. The volume of distribution at steady state (VDss) was greater than 0.45 log l/kg, further supporting their potential for effective systemic distribution. Moreover, the absence of significant metabolism by cytochrome P450 enzymes, specifically CYP2D6 and CYP3A4, suggests minimal interaction risks with these metabolic pathways, which can reduce adverse drug reactions. Importantly, none of the compounds tested positive for AMES toxicity, underscoring their safety profile in terms of mutagenicity [31]. These results provide a promising pharmacokinetic and safety outlook for the compounds, laying a strong foundation for further drug development investigations. The detailed ADMET parameters, as summarized in Table 5, underscore their suitability for progressing into advanced stages of drug development.
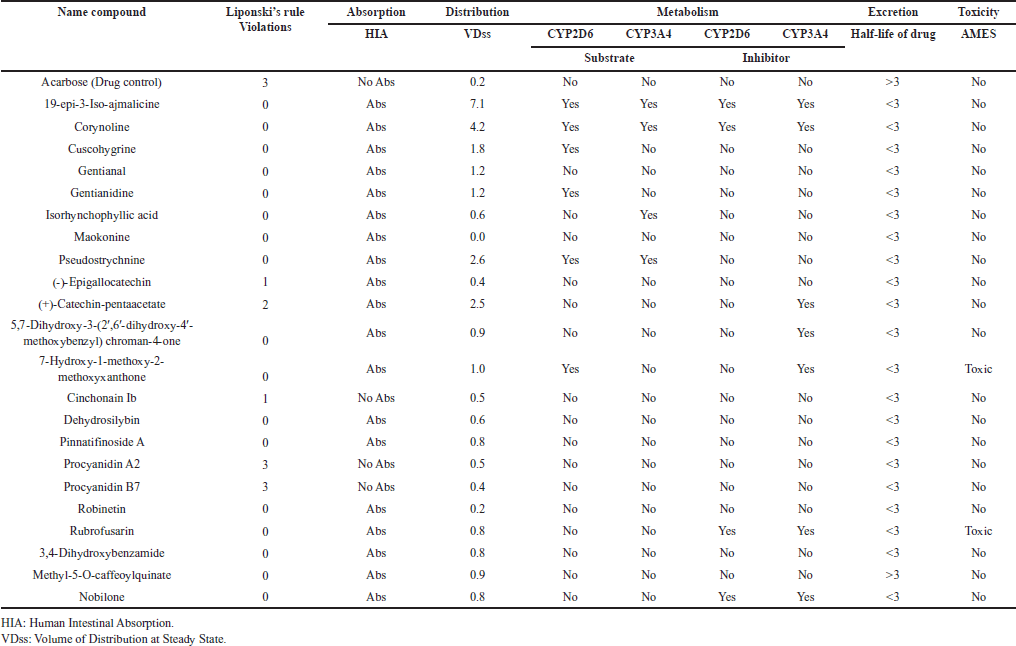 | Table 5. ADMET analysis of compounds contained in USMeth5. [Click here to view] |
DISCUSSION
The U. sclerophylla plant has long been renowned as a medicinal plant traditionally used by Kalimantan, Indonesia, for many years. Our previous study reported that the stem and twig parts of the U. sclerophylla plant have inhibitory activity against α-Glucosidase [27]. This current research explores the potential of U. sclerophylla leaves as an α-Glucosidase inhibitor, both in vitro and in silico. The leaves were extracted using four organic solvents simultaneously, employing a four-graded maceration technique to obtain compounds with various polarities contained in the leaves. The results showed that the methanol and ethyl acetate extracts exhibited the best activity in inhibiting α-Glucosidase, with an inhibition percentage of 81.39% ± 1.6333% and 55.88% ± 2.0631% at a sample concentration of 75 µg/ml.
Activity exploration continued to obtain the most effective fraction in α-Glucosidase inhibition using column chromatography fractionation on ethyl acetate and methanol extracts. Several fractions exhibited inhibitory activity above 60%, and the analysis continued to determine the IC50 value to confirm the extent of its activity. The fractions analyzed for IC50 (USMeth4-7 and USEa9) showed better activity than acarbose, a positive comparator, with an IC50 range of 54.33–22.85 µg/ml. This demonstrates the superior potential of U. sclerophylla leaves as an α-Glucosidase inhibitor in treating diabetes.
USMeth5 is the most active fraction with IC50 22.85 µg/ml, indicating the potential of this fraction to be further investigated for the compounds contained therein, including prediction of the potential of these compounds in silico, including ADMET analysis. USMeth5 consists of alkaloid, phenol, and flavonoid compounds known for their potential as antidiabetics [35–40] and as inhibitors of α-Glucosidase [41–43]. In addition to being detected from USMeth5 as the most active fraction of U. sclerophylla leaves, the compound 19-epi-3-iso-ajmalicine was also detected from U. hirsuta [44] and U. attenuata [45]. The compound cuscohygrine found in certain plants shows potential in treating diabetes [46–48], as does the compound pseudostrychnine [49]. Phenol and flavonoid compounds contained in USMeth5 have been reported to have antidiabetic activity, namely the compound chinchonain 1b, which is reported to be able to induce insulin secretion in vivo and in vitro [50] and is also able to inhibit proteins that are important in diabetes therapy, namely α-Glucosidase and dipeptidyl peptidase-4 and has activity in glucose uptake [51], as well as rubrofusarin with its activity in inhibiting the PTP1B protein [52]. (-)-Epigallocatechin also has the potential for antidiabetic activity; this is supported by a study report where compounds with a catechin backbone have the potential for antidiabetic activity [53–55 ], including in the inhibition of α-Glucosidase [56,57]. Dehydrosilybin has been reported for its in vivo activity in overcoming diabetes complications and cardiomyopathy [58] and also has the potential as a glucokinase and PPARγ dual-target agonist [59]. Robinetin with antidiabetic potential [60] was also detected in USMEth5; this compound can alleviate liver metabolic failure and has hypoglycemic effects [61,62]. The compound 7-hydroxy-1-methoxy-2-methoxyxanthone was reported to be contained in fractions that can lower blood sugar levels [63] and in plants with the potential as α-Glucosidase inhibitors [64]. Procyanidins were reported to be contained in natural materials that can prevent postprandial hyperglycemia and have the potential to inhibit dipeptidyl peptidase-4 [65–67]. Some of the compounds detected in USMeth5 have never been reported for their antidiabetic activity, including inhibition of α-Glucosidase; this opens opportunities for exploring these compounds’ activity in treating diabetes.
The Uncaria genus has the potential to be developed as an antidiabetic agent. Various studies have been conducted to ensure its activity, including in vitro studies on the leaves and stems of U. gambir, U. acida, U. cordata, U. longiflora, U. lucida, and U. callophylla [26,68]. In vivo studies on U. tomentosa showed hypoglycemic activity and the ability to delay diabetes progression [69]. Various α-Glucosidase inhibitor compounds have also been successfully isolated from the genus Uncaria; this strengthens the potential of this genus as an antidiabetic agent [70].
The molecular docking profiling of compounds from U. sclerophylla was conducted by comparing them to acarbose as a drug control. Ten compounds exhibited binding affinity values ranging from −10.4 to −8.86, namely cinchonain Ib, procyanidin B7, dehydrosilybin, corynoline, pseudostrychnine, (+)-catechin-pentaacetate, 19-epi-3-iso-ajmalicine, pinnatifinoside A, robinetin, and isorhynchophyllic acid, which showed better binding processes compared to acarbose with a value of −8.83 (Table 4). Meanwhile, the other 12 compounds had binding affinity values ranging from −8.54 to −5.98. Lower binding affinity values indicate stronger binding and more effective inhibition potential against α-Glucosidase, suggesting that some compounds from U. sclerophylla may be more effective than the drug control in inhibiting the activity of this enzyme.
The docking results show that acarbose interacts through hydrogen bonds with the amino acids ARG213, ARG442, GLU277, ASP352, ASP69, HIS280, TYR158, GLU411, and LYS156, as well as through hydrophobic interactions with TYR158. Compounds from U. sclerophylla exhibit similar interaction patterns. For example, cinchonain Ib interacts with the residues ASN415, ARG442, ASP307, and SER240 through hydrogen bonds and with TYR158 and ARG315 through hydrophobic interactions. Procyanidin B7 forms more hydrogen bonds with residues such as HIS280, GLU411, ASP69, and GLN353, which are also involved in interactions with acarbose, and adds strong electrostatic interactions with ARG442, ASP352, and GLU411. Other compounds, such as dehydrosilybin, also show interactions with some of the same residues as acarbose, such as GLU411 and ASP352, which may contribute to their inhibitory potential. Although corynoline and pseudostrychnine exhibit simpler interaction patterns than acarbose, they still bind to the residue TYR158, which is key in acarbose’s inhibition mechanism. This is shown in Table 4 and Figure 5.
Cinchonain Ib exhibits the lowest interaction energy, with hydrogen bonding at the ARG442 residue and hydrophobic interaction at the TYR158 residue, which is also key in the inhibitory mechanism of acarbose against the α-Glucosidase enzyme. This suggests that cinchonain Ib has the potential to be a more effective inhibitor than acarbose, with stable interactions at the enzyme’s active site. In addition to cinchonain Ib, several other compounds from U. sclerophylla also show similar interaction patterns, indicating the presence of competitive inhibitor potential within these compounds.
The physicochemical properties of compounds from U. sclerophylla and acarbose (drug control) were analyzed based on Lipinski’s rule, which includes criteria such as MW ≤500, HBD ≤5, HBA ≤10, and log P ≤5 [32]. The analysis revealed that almost all compounds comply with these rules, except for (-)-epigallocatechin, (+)-catechin-pentaacetate, cinchonain Ib, procyanidin A2, procyanidin B7, and acarbose which exhibit some deviations. Acarbose has a local effect in the small intestine, with less than 2% absorption into the systemic circulation [71]. This differs from other α-Glucosidase inhibitors, such as miglitol, with a high absorption profile in the systemic circulation [72]. A compound’s ADMET properties are crucial parameters in drug discovery [73]. A compound is considered to have a favorable ADMET profile if it meets established criteria. One critical ADMET parameter is HIA, which measures how much a compound can be absorbed from an orally administered solution. The results of this study indicate that nearly all compounds have an HIA value above 30%, demonstrating good absorption capability through the human small intestine [31]. Another critical parameter is the VDss, which represents the theoretical volume required for the total drug dose to be uniformly distributed and produce the same concentration as in blood plasma [74]. A higher VDss value suggests that more of the drug is distributed into tissues rather than plasma. The predicted VDss value for a given compound is expressed in log l/kg. In this study, a compound is considered to have a favorable volume of distribution if its VDss value exceeds 0.45. The results show that nearly all compounds exhibit high VDss values, with none showing values below −0.15. The detailed results can be found in Table 5.
In the metabolism phase, Cytochrome P450 is a crucial class of detoxification enzymes predominantly found in the liver [75]. CYP450 enzymes play a key role in the catabolism of xenobiotics, facilitating their excretion through urine. Subgroups of these enzymes, such as CYP2D6 and CYP3A4, are particularly significant in drug metabolism [76,77]. This study identified that 19-epi-3-iso-ajmalicine, corynoline, and pseudostrychnine act as substrates and inhibitors for CYP2D6 and CYP3A4 enzymes. Additionally, to assess the mutagenic potential of these compounds, AMES toxicity tests were conducted using bacterial models [78]. The results indicated that 7-hydroxy-1-methoxy-2-methoxyxanthone and rubrofusarin are predicted to have toxicity based on the AMES test. Detailed results can be found in Table 5.
CONCLUSION
The leaves of U. sclerophylla have demonstrated potential as an antidiabetic agent by inhibiting α-Glucosidase. Methanol and ethyl acetate extracts from the leaves effectively inhibited α-Glucosidase, with IC50 values of 42.70 and 75.75 µg/ml, respectively. Further analysis of these extracts using column chromatography led to the discovery of USMeth5 as a more potent α-Glucosidase inhibitor with an IC50 of 22.85 µg/ml, outperforming acarbose. USMeth5 was found to contain various alkaloids, phenols, and flavonoids, and molecular docking revealed five compounds with better binding affinity than acarbose, including cinchonain Ib, procyanidin B7, dehydrosilybin, corynoline, and pseudostrychnine. These compounds were determined to be non-toxic and exhibited diverse pharmacokinetic profiles based on ADMET analysis. Further research is needed to explore additional antidiabetic mechanisms and to solidify the plant’s potential in developing antidiabetic treatments.
ACKNOWLEDGMENT
The authors would like to express their gratitude to the Indonesian Education Scholarship (BPI), the Indonesia Endowment Fund for Education (LPDP), and the Center for Higher Education Funding (BPPT) for their financial support in conducting this research. Additionally, the authors appreciate the assistance provided by the Phytochemistry and Drug Development Laboratory at the Faculty of Pharmacy, Universitas Indonesia, and the Phytochemistry Research Laboratory at the Faculty of Pharmacy, Universitas Muhammadiyah Banjarmasin, for their support with research facilities and instrumentation.
AUTHOR CONTRIBUTIONS
The authors have made significant contributions to the research, including the conception, design, data collection, analysis, and interpretation. The authors have been involved in drafting the article or critically revising it for intellectual content. The authors have agreed to the publication in the current journal, have given final approval for the version to be published, and have decided to be responsible for all aspects of the work. According to the guidelines of the International Committee of Medical Journal Editors (ICMJE), the authors are eligible to be considered as authors.
CONFLICTS OF INTEREST
The authors declare that no conflict of interest could have influenced the result reported in this paper.
ETHICAL APPROVALS
This research does not involve human subjects or animals in the experiments.
DATA AVAILABILITY
The research data and analysis have all been presented in this article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hossain MJ, Al-Mamun M, Islam MR. Diabetes mellitus, the fastest growing global public health concern: early detection should be focused. Heal Sci reports. 2024 Mar;7(3):e2004.
2. Antar SA, Ashour NA, Sharaky M, Khattab M, Ashour NA, Zaid RT, et al. Diabetes mellitus: classification, mediators, and complications; a gate to identify potential targets for the development of new effective treatments. Biomed Pharmacother [Internet]. 2023;168:115734. Available from: https://www.sciencedirect.com/science/article/pii/S0753332223015329
3. Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes. 2021;14:3567–602.
4. Chen M, Pu L, Gan Y, Wang X, Kong L, Guo M, et al. The association between variability of risk factors and complications in type 2 diabetes mellitus: a retrospective study. Sci Rep [Internet]. 2024;14(1):6357. CrossRef
5. Mansour A, Mousa M, Abdelmannan D, Tay G, Hassoun A, Alsafar H. Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Front Endocrinol (Lausanne) [Internet]. 2023;14. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2023.1143067
6. Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–39.
7. Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet [Internet]. 2023 Jul 15;402(10397):203–34. CrossRef
8. Shi Q, Nong K, Vandvik PO, Guyatt GH, Schnell O, Rydén L, et al. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ [Internet]. 2023 Apr 6;381:e074068. Available from: http://www.bmj.com/content/381/bmj-2022-074068.abstract
9. Chong K, Chang JK-J, Chuang L-M. Recent advances in the treatment of type 2 diabetes mellitus using new drug therapies. Kaohsiung J Med Sci [Internet]. 2024 Mar 1;40(3):212–20. CrossRef
10. Singh A, Singh K, Sharma A, Kaur K, Kaur K, Chadha R, et al. Recent developments in synthetic α-glucosidase inhibitors: acomprehensive review with structural and molecular insight. J Mol Struct [Internet]. 2023;1281:135115. Available from: https://www.sciencedirect.com/science/article/pii/S0022286023002156
11. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the COMPREHENSIVE type 2 diabetes management algorithm—2020 executive summary. Endocr Pract [Internet]. 2020;26(1):107–39. CrossRef
12. Kalra S. Alpha glucosidase inhibitors. J Pak Med Assoc. 2014;64(4):474–6.
13. Hahr AJ , Molitch ME. Management of diabetes mellitus in patients With CKD: core curriculum 2022. Am J Kidney Dis [Internet]. 2022;79(5):728–36. CrossRef
14. Sohrabi M, Binaeizadeh MR, Iraji A, Larijani B, Saeedi M, Mahdavi M. A review on α-glucosidase inhibitory activity of first row transition metal complexes: a futuristic strategy for treatment of type 2 diabetes. RSC Adv [Internet]. 2022;12(19):12011–52. CrossRef
15. Lu H, Xie T, Wu Q, Hu Z, Luo Y, Luo F. Alpha-glucosidase inhibitory peptides: sources, preparations, identifications, and action mechanisms. Nutrients. 2023;15.
16. Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed Pharmacother. 2019;111(December 2018):947–57.
17. Salleh NH, Zulkipli IN, Mohd Yasin H, Ja’afar F, Ahmad N, Ahmad W, et al. Systematic review of medicinal plants used for treatment of diabetes in human clinical trials: An ASEAN perspective. Evid Based Complement Alternat Med. 2021;2021:5570939.
18. Alam S, Sarker MMR, Sultana TN, Chowdhury MNR, Rashid MA, Chaity NI, et al. Antidiabetic phytochemicals from medicinal plants: prospective candidates for new drug discovery and development. Front Endocrinol (Lausanne). 2022;13:800714.
19. Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, Latinwo L, et al. The management of diabetes mellitus using medicinal plants and vitamins. Int J Mol Sci. 2023 May;24(10).
20. Hoyos MN, Sánchez-Patán F, Masis RM, Martín-Álvarez PJ, Ramirez WZ, Monagas MJ, et al. Phenolic assesment of Uncaria tomentosa L. (cat’s claw): leaves, stem, bark and wood extracts. Molecules. 2015;20(12):22703–17.
21. Qin N, Lu X, Liu Y, Qiao Y, Qu W, Feng F, et al. Recent research progress of Uncaria spp. based on alkaloids: phytochemistry, pharmacology and structural chemistry. Eur J Med Chem [Internet]. 2021;210:112960. CrossRef
22. Zebua E, Silalahi J, Julianti E. Hypoglicemic activity of gambier (Uncaria gambir robx .) drinks in alloxan-induced mice. in: international conference on agriculture, environment, and food security. Bristol, UK: IOP Publishing; 2018. pp. 1–8.
23. Aprely KJ, Misfadhila S, Asra R. A review: the phytochemistry, pharmacology and traditional use of gambir (Uncaria gambir (Hunter) Roxb). EAS J Pharm Pharmacol [Internet]. 2021;0990(1):21–5. Available from: https://www.easpublisher.com
24. Abdullah NH, Salim F, Ahmad R. Chemical constituents of malaysian U. cordata var. ferruginea and their in vitro α-glucosidase inhibitory activities. Molecules. 2016;21(5):1–11.
25. Wang ZW, Wang JS, Luo J, Kong LY. α-Glucosidase inhibitory triterpenoids from the stem barks of Uncaria laevigata. Fitoterapia. 2013;90(October):30–7.
26. Ahmad R, Hashim H, Noor Z, Ismail N, Salim F, Lajis N, et al. Antioxidant and antidiabetic potential of Malaysian Uncaria. Res J Med Plant. 2011;5(5):587–95.
27. Triadisti N, Elya B, Hanafi M, Hashim NM. Phytochemicals, antioxidant and inhibitory activity against α-glucosidase in Uncaria Sclerophylla twigs and stems. Int J Agric Biol. 2024;31(3):199–206.
28. Yamamoto K, Miyake H, Kusunoki M, Osaki S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010 Oct;277(20):4205–14.
29. Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol. 2015;1263:243–50.
30. Eberhardt J, Santos-Martins D, Tillack AF, Forli S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J Chem Inf Model. 2021 Aug;61(8):3891–8.
31. Myung Y, de Sá AGC, Ascher DB. Deep-PK: deep learning for small molecule pharmacokinetic and toxicity prediction. Nucleic Acids Res. 2024 Jul;52(W1):W469–75.
32. Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004 Dec;1(4):337–41.
33. Dey P, Kundu A, Kumar A, Gupta M, Lee BM, et al. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv Nat Prod Anal. 2020:505–67.
34. Rampogu S, Balasubramaniyam T, Lee JH. Phytotherapeutic applications of alkaloids in treating breast cancer. Biomed Pharmacother [Internet]. 2022;155:113760. Available from: https://www.sciencedirect.com/science/article/pii/S0753332222011490
35. Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016 Oct;21(10):1374.
36. Yi X, Dong M, Guo N, Tian J, Lei P, Wang S, et al. Flavonoids improve type 2 diabetes mellitus and its complications: a review. Front Nutr. 2023;10:1192131.
37. Behl T, Gupta A, Albratty M, Najmi A, Meraya AM, Alhazmi HA, et al. Alkaloidal phytoconstituents for diabetes management: exploring the unrevealed potential. Molecules. 2022 Sep;27(18).
38. Xiao J. Recent advances in dietary flavonoids for management of type 2 diabetes. Curr Opin Food Sci [Internet]. 2022;44:100806. Available from: https://www.sciencedirect.com/science/article/pii/S2214799322000029
39. Menezes R, Matafome P, Freitas M, García-Conesa M-T. Updated Information of the Effects of (Poly)phenols against type-2 diabetes mellitus in humans: reinforcing the recommendations for future research. Nutrients. 2022 Aug;14(17).
40. Deka H, Choudhury A, Dey BK. An overview on plant derived phenolic compounds and their role in treatment and management of diabetes. J Pharmacopuncture. 2022 Sep;25(3):199–208.
41. Swargiary A, Roy MK, Mahmud S. Phenolic compounds as α-glucosidase inhibitors: a docking and molecular dynamics simulation study. J Biomol Struct Dyn [Internet]. 2023 Jun 13;41(9):3862–71. CrossRef
42. Lam T-P, Tran N-VN, Pham L-HD, Lai NV-T, Dang B-TN, Truong N-LN, et al. Flavonoids as dual-target inhibitors against α-glucosidase and α-amylase: a systematic review of in vitro studies. Nat Products Bioprospect. 2024 Jan;14(1):4.
43. Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness [Internet]. 2014;3(3):136–74. Available from: https://www.sciencedirect.com/science/article/pii/S2213453014000329
44. Xin W, Gu P, Chou G, Wang Z. Studies on alkalodial constituents in leaves of Uncaria hirsuta. Zhongguo Zhong yao za zhi. 2008 Sep;33(17):2124–8.
45. Martins D, Nunez CV. Secondary metabolites from Rubiaceae species. Molecules. 2015;20(7):13422–95.
46. Behl T, Kotwani A. Chinese herbal drugs for the treatment of diabetic retinopathy. J Pharm Pharmacol. 2017;69:223–35.
47. Pramanick DD. Herbal medicine-a natural cure to diabetes ( an overview). Int J Adv Res Biol Sci. 2021;8(10):96–140.
48. Monira KM, Munan SM. Review on datura metel: a potential medicinal plant. Glob J Res Med Plants Indig Med. 2018;1(4):123–32.
49. Bhati R, Singh A, Saharan VA, Ram V, Bhandari A. Strychnos nux-vomica seeds?: Pharmacognostical standardization , extraction , and antidiabetic. J Ayurveda Integr Med. 2012;3(2):2–6.
50. Qa’dan F, Verspohl EJ, Nahrstedt A, Petereit F, Matalka KZ. Cinchonain Ib isolated from eriobotrya japonica induces insulin secretion in vitro and in vivo. J Ethnopharmacol. 2009 Jul;124(2):224–7.
51. Teerapongpisan P, Praparatana R, Noppradit B, Laphookhieo S, Puttarak P. Anti-diabetic compounds from Uvaria dulcis Dunal. Heliyon [Internet]. 2024;10(5):e26962. Available from: https://www.sciencedirect.com/science/article/pii/S2405844024029931
52. Paudel P, Seong SH, Jung HA, Choi JS. Rubrofusarin as a dual protein tyrosine phosphate 1b and human monoamine oxidase-a inhibitor: an in vitro and in silico study. ACS Omega. 2019 Jul;4(7):11621–30.
53. Li W, Zhu C, Liu T, Zhang W, Liu X, Li P, et al. Epigallocatechin-3-gallate ameliorates glucolipid metabolism and oxidative stress in type 2 diabetic rats. Diabetes Vasc Dis Res. 2020;17(6):1479164120966998.
54. James A, Wang K, Wang Y. Therapeutic activity of green tea epigallocatechin-3-gallate on metabolic diseases and non-alcoholic fatty liver diseases: the current updates. Nutrients. 2023;15:3022.
55. Zhu T, Li M, Zhu M, Liu X, Huang K, Li W, et al. Epigallocatechin-3-gallate alleviates type 2 diabetes mellitus via β-cell function improvement and insulin resistance reduction. Iran J Basic Med Sci. 2022 Apr;25(4):483–8.
56. Wen L, Wu D, Tan X, Zhong M, Xing J, Li W, et al. The role of catechins in regulating diabetes?: an update review. Nutrients. 2022;14(4681):1–19.
57. Patanè GT, Putaggio S, Tellone E, Barreca D, Ficarra S, Maffei C, et al. Catechins and proanthocyanidins involvement in metabolic syndrome. Int J Mol Sci. 2023;24:9228.
58. Stolf AM, Cardoso CC, Acco A. Effects of silymarin on diabetes mellitus complications: a review. Phyther Res. 2017;31(3):366–74.
59. Zhang Z, Meng Y, Wang Z, Mei Y, Gao S, Wu Y, et al. Discovery of potent glucokinase and PPAR γ dual-target agonists through an innovative scheme for regioselective modification of silybin. ACS Omega. 2022;7(4):3812–22.
60. Ajiboye BO, Iwaloye O, Owolabi OV, Ejeje JN, Okerewa A, Johnson OO, et al. Screening of potential antidiabetic phytochemicals from Gongronema latifolium leaf against therapeutic targets of type 2 diabetes mellitus: multi-targets drug design. SN Appl Sci [Internet]. 2022;4(1). CrossRef
61. Hong Z, Xie J, Hu H, Bai Y, Hu X, Li T, et al. Hypoglycemic effect of Moringa oleifera leaf extract and its mechanism prediction based on network pharmacology. J Futur Foods [Internet]. 2023;3(4):383–91. Available from: https://www.sciencedirect.com/science/article/pii/S2772566923000277
62. Song JH, Kim HJ, Lee J, Hong SP, Chung MY, Lee YG, et al. Robinetin alleviates metabolic failure in liver through suppression of p300-CD38 axis. Biomol Ther (Seoul). 2024 Mar;32(2):214–23.
63. Lazuardi M, Anjani QK, Budiatin AS, Restiadi TI. Efficacy of quercetin-like compounds from the mistletoe plant of Dendrophthoe pentandra L. Miq, as oral random blood sugar lowering treatment in diabetic rats. Vet Q. 2024 Dec;44(1):1–14.
64. Rosmalena R, Senlia AO, Hanafi M, Artanti N, Eldafira E, Handayani SI, et al. Phytochemical, antioxidant and antidiabetic properties of senna alexandrina leaf extract. Res J Pharm Technol. 2022;15(12):5835–40.
65. Yamashita Y, Okabe M, Natsume M, Ashida H. Cacao liquor procyanidins prevent postprandial hyperglycaemia by increasing glucagon-like peptide-1 activity and AMP-activated protein kinase in mice. J Nutr Sci. 2019;8:e2.
66. González-Abuín N, Martínez-Micaelo N, Blay M, Pujadas G, Garcia-Vallvé S, Pinent M, et al. Grape seed-derived procyanidins decrease dipeptidyl-peptidase 4 activity and expression. J Agric Food Chem [Internet]. 2012 Sep 12;60(36):9055–61. CrossRef
67. de Paulo Farias D, de Araújo FF, Neri-Numa IA, Pastore GM. Antidiabetic potential of dietary polyphenols: a mechanistic review. Food Res Int [Internet]. 2021;145:110383. Available from: https://www.sciencedirect.com/science/article/pii/S0963996921002829
68. Arundita S, Kurniawan F, Ismed F, Rita RS, Putra DP. In vitro alpha glucosidase activity of Uncaria gambir roxb. and syzygium polyanthum (wight) walp. from West Sumatra, Indonesia. Maced J Med Sci. 2020;8(A):810–7.
69. .Domingues A, Sartori A, Assis M, Maria L, Valente M, Camargo L, et al. Prevention of experimental diabetes by Uncaria tomentosa extract: Th2 polarization, regulatory T cell preservation or both?? J Ethnopharmacol [Internet]. 2011;137(1):635–42. CrossRef
70. Kim TH. A novel α-glucosidase inhibitory constituent from Uncaria gambir. J Nat Med. 2016;70(4):811–5.
71. Zheng X, Zhang Z, Xie J. The effect of acarbose on the gut microbiota: an evidence-based review. Front Med Sci Res. 2021;3(3):4–11.
72. Ahr HJ, Boberg M, Brendel E, Krause HP, Steinke W. Pharmacokinetics of miglitol. Absorption, distribution, metabolism, and excretion following administration to rats, dogs, and man. Arzneimittelforschung. 1997 Jun;47(6):734–45.
73. Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA. 2020 Mar;323(9):844.
74. Yahata M, Ishii Y, Nakagawa T, Watanabe T, Miyawaki I. Applicability of the Øie-Tozer model to predict three types of distribution volume (Vd) in humans: Vd in central compartment, Vd at steady state, and Vd at beta phase. Biopharm Drug Dispos. 2020 Apr;41(4–5):151–65.
75. Guengerich FP. Roles of cytochrome P450 enzymes in pharmacology and toxicology: past, present, and future. Adv Pharmacol. 2022;95:1–47.
76. Molden E, Juki? MM. CYP2D6 reduced function variants and genotype/phenotype translations of cyp2d6 intermediate metabolizers: implications for personalized drug dosing in psychiatry. Front Pharmacol. 2021;12:650750.
77. Murugan S, Nehru J, Arputharaj DS, Catherine Paul A, Gunasekaran P, Dege N, et al. Synthesis of 3-Methoxy-6- [(2, 4, 6-trimethyl-phenylamino)-methyl]-phenol Schiff base characterized by spectral, in-silco and in-vitro studies. Heliyon. 2022 Aug;8(8):e10070.
78. Guy RC. Ames test. In: Encyclopedia of toxicology. Amsterdam, The Netherlands: Elsevier; 2024. pp. 377–9.