INTRODUCTION
Cancer is a major vital societal, public health, and economic issue over the last decade, affecting individuals, families, healthcare systems, and economies [1,2]. The ongoing rise in cancer incidence in the population is due to a complex interplay of hereditary, environmental, and lifestyle variables. Nowadays, natural compounds derived from plants, marine creatures, fungus, and other sources played anti-cancer effects and some of them show promising therapeutic agents. As of 2022, chemicals derived from the Garcinia genus are gaining popularity as natural sources of cancer treatment [3]. For example, alpha-mangostin is a xanthone found in the pericarp of the mangosteen fruit (Garcinia mangostana). It has been shown to trigger apoptosis in many cancer cells, which is an important mechanism for inhibiting excessive cell proliferation [4]. Gambogic acid, found in the resin of the Garcinia hanburyi tree, has been shown to decrease the activity of nuclear factor-kappa B (NF-κB), a protein linked to inflammation and cell survival [5].
Garcinia schomburgkiana Pierre, also known as Ma-dan in Thailand, is a sour-flavored fruit tree from the Clusiaceae family. It is dominant in Thailand, Cambodia, and Vietnam. This plant has long been used in Thailand to cure coughs, as an expectorant, diabetes, menstrual irregularities, and as a laxative [6]. Various chemicals, such as xanthones [7], anthraquinones [8], flavonoids [9], benzophenones [10], and depsidones [11], have been found in different parts of the plant, and they demonstrate a wide spectrum of biological activity. To investigate other bioactive components from this plant, the methanolic extract of G. schomburgkiana leaves, fruits, and twigs were examined and three known xanthones: norathyriol, macluraxanthone, and 10-O-methylmacluraxanthone were found. In particular, 10-O-methylmacluraxanthone has not been previously reported from this plant and its spectroscopic data was also provided. The biological activity including cytotoxicity and brine shrimp lethality assay (BSLA) were explored and also discussed herein.
MATERIALS AND METHODS
Chemicals and instruments
Solvents for extraction, chromatography, and crystallization were purchased from Tyoto Chemical Component (Thailand) Ltd. and were distilled at their boiling point ranges prior to use. Analytical grade solvents, dimethyl sulfoxide, and methanol as well as potassium dichromate were obtained from Fisher Scientific Korea Ltd. 1H, 13C, and 2D NMR spectra were recorded on a Bruker Ascend™ 400 and Jeol NMR 400 MHz spectrometers. Deuterated chloroform (CDCl3, Merck, Germany), deuterated dimethyl sulfoxide (CD3SOCD3, Merck, Germany), and deuterated acetone (CD3COCD3, Merck, Germany) solvents were used and tetramethylsilane or residual nondeuterated solvent peak was as an internal standard for calibration. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) was recorded on a Bruker micro TOF spectrometer. The chemical compositions were visualized either by ultraviolet light or spraying with 12% H2SO4 in ethanol or anisaldehyde reagents. Column chromatography was performed using silica gel 60 (60–200 µm or 70–230 mesh ASTM, Merck, Germany).
Plant materials
Leaves, fruits, and twigs of Garcinia schomburgkiana Pierre were collected from Bang-Khla, Chachoengsao province, Thailand, in May 2021 (lat. 13°47’26.5”N, long. 101°14’15.3”E). The plant was identified by Asst. Prof. Naowarat Kongkum. A voucher specimen (RRU-SH-0005) was deposited at the Faculty of Science and Technology, Rajabhat Rajanagarindra University, Chachoengsao, Thailand.
Extraction and isolation
The leaves, fruits, and twigs of Garcinia schomburgkiana Pierre were dried in a hot air oven at 45oC for 2 days. The samples were ground using a grinder machine. Then, 1 kg of each dried plant was extracted using maceration in methanol (2 l × 5 times). The extracts were concentrated using rotary evaporation under reduced pressure. All extracts were employed for cytotoxicity screening against various cancer cell lines and BSLA assays. Guided by the preliminary screening (see Supplementary data; Table S1), the active methanol extract of fruits was selected for further investigation. A portion of the methanol extract (50 g) was separated by quick column chromatography technique. Elution was conducted initially with hexane (2 l), followed by the various proportions of acetone in hexane (20, 40, 60, 80, and 100%, 2 l each), and methanol in acetone (10%, 20%, 50%, and 100%, respectively, 1 l each), respectively. After the removal of solvents by rotary evaporation under reduced pressure, fractions were collected and combined based on their TLC characteristics to yield 6 subfractions (A1−A6). Subfractions A1−A2 which comprised mainly fats were not investigated. Due to the similarity of their TLC and 1H NMR characteristics, subfractions A3 and A4 were combined (11.56 g). Upon the crystallization from acetone−CH2Cl2 followed by filtration and drying, the dark brown amorphous solid (6.47 g) was obtained. The solid was further purified by silica gel column chromatography eluting with MeOH−CH2Cl2 (0%−100%, 1 l each). The fractions were collected (500 ml each) and combined on the basis of their TLC characteristics to give 5 subfractions (C1−C5). Compounds 2 (537.8 mg) and 3 (20.0 mg) were obtained after recrystallization using MeOH−CH2Cl2 from subfraction C3 and subfraction C2, respectively. Subfraction A5 (6.22 g) was further separated by column chromatography, eluting with various proportions of CH2Cl2:MeOH:H2O (100:3:1) to (10:3:1). Fractions were collected and combined, monitored by TLC. The solvent was removed and dried to give 5 subfractions (B1-B5). Subfraction B2 (2.85 g) was crystalized by the proportion of MeOH−CH2Cl2 giving a yellow solid 1 (1.32 g).
Cytotoxicity assays
Cell lines were cultured in different mediums; human monocytic leukemia cell (THP-1) was cultured in Iscove’s Modified Dulbecco’s Medium, human lung carcinoma cell (A549) cultured in Dulbecco’s Modified Eagle Medium, human hepatocellular carcinoma cell (HepG2), and normal African green monkey kidney cell (Vero) cultured in Minimum Essential Medium (MEM). All cell lines were cultured in a 75 cm2 tissue culture flask. The complete medium contains 1% (v/v) penicillin-streptomycin and 10% (v/v) fetal bovine serum (FBS). Cells were maintained in a 5% CO2 humidity incubator at 37oC. Cells were subcultured when they reached 80% confluence by trypsinization. Cells were seeded at a density of 3 × 104 cells/well for THP-1 while 5 × 104 cells/well for A549, HepG2, and Vero in 100 μl culture medium into 96 well microplates flat bottom. After incubating cell cultures at 37°C and 5 % CO2 for 16 hours, cells were added to the serial dilution of extract and compound with a final concentration of 3.125–200 μM and incubated for 24 hours in CO2 incubator. Then, each sample was added 50 μl of the XTT (sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy6-nitro)benzene sulfonic acid hydrate) and incubate for 2 hours at 37°C and 5 % CO2. The samples were measured the absorbance using a microplate reader at a wavelength of 450 nm. The cytotoxicity assay was expressed as ED50 value [12].
Brine shrimp lethality assay
Brine shrimp (Artemia salina) larvae were used for preliminary cytotoxicity tests [13]. Artificial sea water was prepared by dissolving 33.33 g of sea salt in 1 l of distilled water. The shrimp eggs were spread in a salt solution and cultivated under light exposure and aeration for 48 hours to obtain Artemia nauplii at the instar Stage II. The stock solutions of the extract were prepared by dissolving in artificial sea water and 1% DMSO at different concentrations (1,000, 500, 100, 10, and 5 μg/mL). K2Cr2O7 and artificial sea water with 1% DMSO were used as a positive and negative control, respectively. 10 Brine shrimps larvae were tested in 10 ml of sample solutions in a test tube at room temperature for 24 hours. Each sample and level of concentration was tested in triplicate. The percentage mortality was determined by comparing the mean surviving larvae of the tests and the control as the following equation:
Mortality (%) = [1 - (A1 - A2) / A1] × 100
where A1 is the number of alive brine shrimp in control without the test substance, and A2 is the number of dead brine shrimp with the test substance. The LC50 (Median Lethal Concentration) value is the concentration of sample required to kill 50% of the brine shrimp and was determined by Probit analysis.
RESULTS AND DISCUSSION
After extraction of air-dried leaves, fruits, and twigs of Garcinia schomburgkiana, along with the filtration, solvent evaporation, and vacuum drying, the crude methanol extract of leaves (175.8 g), fruits (120.2 g), and twigs (77.1 g) were obtained. All extracts were submitted to test cytotoxicity screening against various cancer cell lines and BSLA assay. The preliminary screening guided that the bioactive fruit extract was selected to purify using silica gel column chromatography resulting in three known compounds. The chemicals were identified as norathyriol (1) [14], macluraxanthone (2) [15], and 10-O-methylmacluraxanthone (3) [16] (Fig. 1). Their chemical structures were characterized by means of the spectroscopic data (1D and 2D-NMR, and HR-ESI-MS) and comparison with the literature. Meanwhile, Gunasekera SP and co-workers reported the isolation of 10-O-methylmacluraxanthone from Kayea stylosa Thw. (Guttiferae) in 1975 [16], although the incomplete spectroscopic data were inconclusive. Many researchers employed this data for chemical characterization and identification which proved ineffective. Thus, the 1H and 13C NMR (in both CD3COCD3 and CDCl3 solvents) (Table 1) along with the key heteronuclear multiple bond correlations (HMBCs), homonuclear correlation spectroscopy (1H-1H COSY), and nuclear overhauser effect spectroscopy (NOESY) correlations (Fig. 2) of this compound are presented.
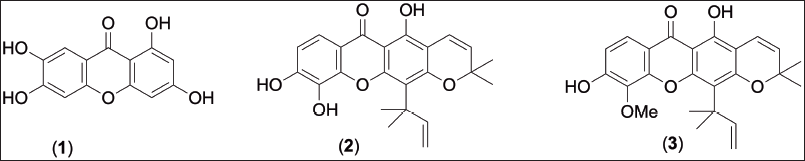 | Figure 1. Structures of isolated compounds 1−3. [Click here to view] |
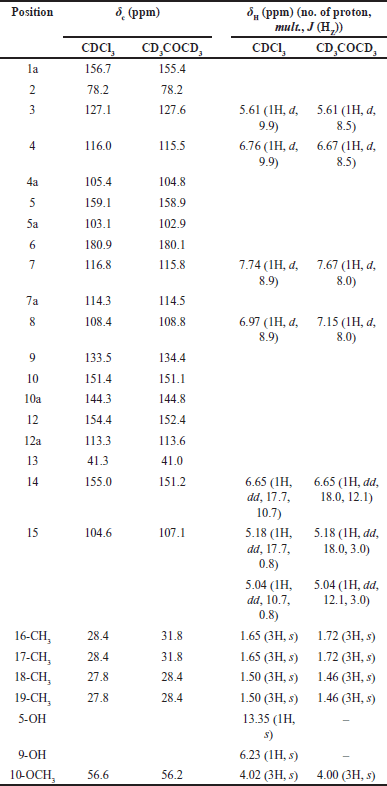 | Table 1. The 1H (400 HMz) and 13C NMR (100 HMz) data of 3. [Click here to view] |
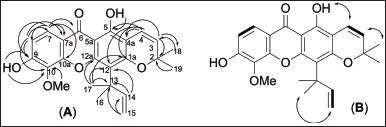 | Figure 2. HMBC (A, single-headed line) and 1H-1H COSY (B, bolded line) and NOESY (B, double-headed line) correlations. [Click here to view] |
Compound 3 was found as a yellow solid. Its molecular formula, C24H24O6, was established by HR-ESI-MS which showed a [M–H]– ion peak at m/z 407.1482 (calcd. for C24H23O6, 407.1489). The EI mass spectrum of this compound showed a base peak at m/z 69 (100% relative intensity), assigned to m/z after the loss of C5H9. This result supported the presence of 1,1-dimethylallyl side chain. The IR spectrum of compound 3 showed absorption at νmax 3447 (chelated O−H stretching), 1651 (chelated C=O stretching), 1290 and 1264 (C−O stretching). The UV spectrum of 3 showed λmax nm (log ε) at 241 (4.23), 283 (4.90), 310 (4.54), and 337 (4.53), supporting the presence of a xanthone chromophore [16]. The 100 MHz 13C-NMR spectrum of compound 3 in CDCl3 showed twenty-two signals for 24 carbons. The combination of 13C-NMR data with DEPT-135 and DEPT-90 spectrum guided the presence of five methine carbons (δ 155.0, 127.1, 116.8, 116.0, and 108.4), one methylene carbon (δ 104.6), two methyl signals for four carbons [δ 28.4 (2 × CH3) and 27.8 (2 × CH3)], thirteen quaternary carbons (δ 180.9, 159.1, 156.7, 154.4, 151.4, 144.3, 133.5, 114.3, 113.3, 105.4, 103.1, 78.2, and 41.3), and one methoxy carbon (δ 56.6) (Table 1). The 1H NMR data of 3 in CDCl3 (Table 1) displayed a low field signal at δ 13.35 (1H, s) which was assigned to be a chelated-phenolic protons (OH-5) as deduced from HMBC correlation of OH-5/C-5 (Fig. 2A). A pair of doublets at δ 7.74 (1H) and 6.97 (1H) with coupling constant (J ) of 8.9 Hz were assigned to the ortho-aromatic protons at positions C-7 and C-8, respectively. Based on heteronuclear single quantum coherence (HSQC) correlations, the protons H-7 (δ 7.74) and H-8 (δ 6.97) connected to C-7 (δ 116.8) and C-8 (δ 108.4), respectively. The COSY correlation of H-7 and H-8 supported that they were adjacent. In HMBC correlations, proton H-7 showed the cross-peaks with C-6 (δ 180.9), C-7a (δ 114.3), C-8 (δ 108.4), C-9 (δ 133.5), and C-10a (δ 144.3). In addition, proton H-8 gave the HMBC correlations with C-7a (δ 114.3), C-7 (δ 116.8), C-9 (δ 133.5), and C-10 (δ 151.4). The presence of 2,2-dimethylchromene ring displayed a shape single signal at δ 1.50 (6H, 2x(-CH3)), a pair of doublets at δ 5.61 (1H, J = 9.9 Hz, H-3) and 6.76 (1H, J = 9.9 Hz, H-4). The HSQC correlations showed the cross-peaks of H-18 (δ 1.50)/C-18 (δ 27.8), H-19 (δ 1.50)/C-19 (δ 27.8), H-3 (δ 5.61)/ C-3 (δ 127.1), and H-4 (δ 6.76)/ C-4 (δ 116.0). This group was plated at C-1a and C-4a of xanthone ring elucidated from HMBC data of H-3/C-4a, H-4/C-1a, H-4/C-5, H-4/12, H-4/C-4a, H-17/C-1a (Fig. 2A) along with the NOESY correlation of OH-5/H-4 (Fig. 2B). In addition, 1,1-dimethylallyl group gave a shape signals at δ 1.65 (6H, 2x(-CH3)), an AMX proton system at δ 6.65 (1H, dd, J = 17.7, 10.7 Hz, H-14), 5.18 (1H, dd, J = 17.7, 0.8 Hz, Ha-15), and 5.04 (1H, dd, J = 10.7, 0.8 Hz, Hb-15). The HSQC data displayed the correlation H-16 (δ 1.65)/C-16 (δ 28.4), H-17 (δ 1.65)/C-17 (δ 28.4), H-14 (δ 6.65)/C-14 (δ 155.0), Ha-15 (δ 5.18)/C-15 (δ 104.6), Hb-15 (δ 5.04)/C-15 (δ 104.6). The present group located at C-12 based on HMBC correlations of 17-CH3/C-1a, 17-CH3/C-12, 17-CH3/C-12a, and H-14/C-12. A sharp singlet signal at δ 4.02 (1H) showed HSQC correlation with δ 56.6 (-OCH3) assignable to be a methoxy group. This group was placed at C-10, confirming HMBC correlation of 10-OCH3/C-10. In addition, a singlet signal at δ 6.23 (OH) was assumed as a hydroxy proton. This group orientated at C-9 due to the HMBC correlations of those signals with δ 133.5 (C-9). Moreover, the complete assignment of each carbon was performed by HMBC correlations as shown in Figure 2.
To determine their biological activity, all isolated compounds were evaluated for cytotoxicity and BSLA assays, and the findings are shown in Table 2. The criteria used to classify the active compound with cytotoxicity on various cancer cell lines with EC50 cutoff at ≤10 μM – good activity; 10 μM < EC50 ≤30 μM – moderate activity, 30 μM < EC50 ≤100 μM – weak activity, EC50 > 100 μM – inactive [17]. In this study, the BSLA activity is interpreted based on the range of LC50 as follows: LC50 < 100 µM –toxic; 100 μM < LC50 <500 μM – moderate toxic; 500 μM < LC50 < 1000 μM – weakly toxic; LC50 > 1,000 μM – nontoxic according to the literatures [18,19].
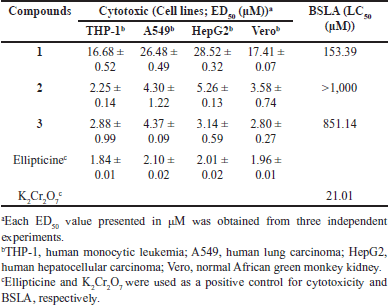 | Table 2. Cytotoxicity and BSLA of isolated compounds 1−3. [Click here to view] |
For the cytotoxicity study, compound 1 (norathyriol) exhibited moderate cytotoxicity against all tested cell lines with ranging from 16.68 ± 0.52 to 28.52 ± 0.32 μM. Its cytotoxicity (high ED50 value) was lower compared to compounds 2 and 3, which had low ED50 value. Based on our comprehensive search for biological activities, including cytotoxicity similar to a previous study, our results are consistent with the findings reported in the literature. For example, norathyriol isolated from Garcinia mckeaniana leaves showed cytotoxicity ED50 ranging from 15.36 ± 1.5 to 29.23 ± 2.0 μM on KB, HepG2, Lu-1, and MCF-7 cell lines [20]. In the anti-cancer mechanistic study, norathyriol (1), an active metabolite derived from mangiferin, induces apoptosis by inhibiting the nuclear translocation of NF-κB through the suppression of NF-κB-inducing kinase activation in multiple myeloma cell lines [21]. This compound also inhibited cell proliferation by inducing G2–M arrest and attenuating AP-1 activity in SKH-1 hairless mice exposed to solar UV [22].
Compound 2 exhibited good cytotoxicity against THP-1, A549, HepG2, and Vero cells with ED50 values of 2.25 ± 0.14, 4.30 ± 1.22, 5.26 ± 0.13, and 3.58 ± 0.74 μM, respectively. In addition, compound 3 also showed good cytotoxicity against all tested cell lines with ED50 2.88 ± 0.99, 4.37 ± 0.09, 3.14 ± 0.59, and 2.80 ± 0.27 μM, respectively.
Compound 2, macluraxanthone, isolated from Mesua beccariana, Mesua ferrea and Mesua congestiflora displayed high cytotoxicity against several cancer cell lines, with ED50 ranging from 1.40 to 5.28 μM [23]. In addition, the cytotoxicity study of macluraxanthone derived from the bark of Garcinia schomburgkiana showed IC50 values ranging from 1.45 to 1.93 μM on KB, HeLa S-3, HT-29, MCF-7, and HepG2 [8]. Moreover, macluraxanthone demonstrated antileukemic therapy in a xenograft murine model showing the induction of apoptotic mitochondrial pathway in chronic lymphocytic leukemia cells [24].
The structure of compound 3 or 10-O-methylmacluraxanthone was closely related to macluraxanthone (2). The main difference is that the hydroxy group at C-10 in 2 was placed by methoxy group found in 3. The present finding suggests that the cyctoxicity of both compounds is comparable. However, macluraxanthone isolated from roots of Cratoxylum cochinchinense showed good cytotoxicity, with IC50 value ranging from 1.18 ± 0.04 to 9.57 ± 0.74 μM on KB, Hela S-3, HT-29, MCF-7, and HepG2 cell lines while 10-O-methylmacluraxanthone showed moderate cyctotoxicty with IC50 value of 53.01 ± 4.88 and 35.12 ± 2.63 μM against only KB and Hela S-3 cancerous cells, respectively [17]. From our search in literature [17], the complete chemical identification was unsatisfatory. Thus, it might be duduced that the 10-O-methylmacluraxanthone found in that research was related compounds.
For BSLA data, compound 1 exhibited moderate toxicity with brine shrimps’ larvae at LC50 153.39 μM. Compounds 2 and 3 were nontoxic and low toxic with LC50 >1,000 and 851.14 μM, respectively. By comparison the biological investigation, many studies have found a correlation between cytotoxicity evaluation on cancer cells and the BSLA. Some studies have shown that compounds with high cytotoxicity against cancer cells may also exhibit high lethality in the BSLA, suggesting a degree of positive correlation. However, this correlation is not consistent across all compounds due to the differences in biological targets and mechanisms of action. For example, phrymarolin II and ursolic acid isolated from Phryma leptostachya L. revealed the BSLA with LD50 value as 0.0013 ± 0.0001 µg/ml and 27 ± 3.9 µg/ml, respectively. These compounds were evaluated the cyctotoxicty against cancer cell lines; murine leukemia (L1210), lung adenocarcinoma (A549), ovarian (SK-OV-3), skin melanoma (SK-MEL-2), CNS (XF498), and colon (HCT15). The results showed that the cytotoxicity of phrymarolin II were IC50 value > 20 µg/ml (considered as inactive) while ursolic acid were IC50 value ranging from 3.70 ± 0.20 to 11.12 ± 3.11 µg/ml [25]. In addition, Luo and co-workers exhibited biological activities including brine shrimp assay and in vitro antiproliferative assay against mammary cancer (F10) and lung cancer (HvEvc) cell lines of natural compounds isolated from the stem bark of Micromelum falcatum. The results showed that 7-methoxy-8-(2-hydroxmethyl-1-O-isovaleryl-4-butenyl)-coumarin gave the toxicity against brine shrimp larvae at LD50 at 6.8 µg/ml and the cytotoxicity against lung cancer HvEvc cell line at IC50 35.7 µg/ml while no cytotoxicity against mammary cancer (F10) was found. Arscotin, 6-hydroxy-7,8-dimethoxycoumarin, showed toxicity against brine shrimp assay with LD50 118.5 µg/ml whereas it was inactive with two cancerous cell lines tested [26]. The study of Solis and co-workers revealed that the correlation of brine shrimp toxicity and KB cell-based assays may be unpredictable for tested compounds. Emetine was more toxic to KB cells than villastonine but the converse was found to be against brine shrimp. Similarly, although podophyllotoxin and actinomycin D were equitoxic to KB cells, the brine shrimp toxicity of podophyllotoxin was >50,000 times more sensitive than actinomycin D [19]. Hence, the correlation between cytotoxic evaluation on cancer cells and brine shrimp is not always straightforward. Occasional cross-monitoring in the more specific assay systems would be necessary to ensure that the activities continue to correlate. An in-depth mechanistic investigation of macluraxanthone and its derivative, 10-O-methylmacluraxanthone, is required for further exploration.
Xanthones belong to an important class and are mainly isolated from many plant species, such as Garcinia [27], Cratoxylum [28], Mesua [29]. Traditionally plants containing xanthones have long been used as folk medicine in Southeast Asia, especially, in Thailand [30]. Among xanthone derivatives, linear pyranoxanthone with 1,1-dimethylchromen system fused at C-1a and C-4a (numbering outline in Fig. 2) possesses various important biological activities including anticancer against a broad array of mammalian cancerous cell lines [31]. This skeleton was crucial for synthesis to study the structure-activity relationship (SAR) [32−34]. Interestingly, this finding agreed well with the literature to support the cytotoxicity of 2,2-dimethylchromene linear pyranoxanthone structure, macluraxanthone and 10-O-methylmacluraxanthone [8,17,23,35,36]. In particular, 10-O-methylmacluraxanthone is more attention for further chemical synthetic derivatizations and SAR investigations.
CONCLUSION
The anti-cancer and brine shrimp lethality activities of extracts and isolated components from Garcinia schomburgkiana Pierre were investigated. Using bioassay-guided fractionation, the bioactive fruit extract was purified by various chromatographic techniques yielding three distinct xanthones: norathyriol (1), macluraxanthone (2), and 10-O-methylmacluraxanthone (3). Chemical characterization of the isolated compounds was carried out using spectroscopic methods. In particular, 10-O-methylmacluraxanthone (3) was identified for the first time in this plant. Furthermore, thorough spectroscopic data for 3 were presented. Macluraxanthone (2) and 10-O-methylmacluraxanthone (3) demonstrated notable cytotoxicity across all tested cancerous cell lines, with calculated ED50 values ranking from 2.25 ± 0.14 to 5.26 ± 0.13 μM whereas norathyriol showed moderate toxicity in BSLA with LD50 153.39 μM. These findings underscore the prospective cytotoxicity of the isolated xanthones originating from Garcinia schomburgkiana Pierre.
ACKNOWLEDGMENTS
The authors thank the Faculty of Science and Technology, Rajabhat Rajanagarindra University for partial financial support. We also thank Ms. Jitra Limthongkul, Department of Microbiology, Faculty of Science, Mahidol University for the comments on the cytotoxicity test. We acknowledged the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Mahidol University for spectroscopic instruments. The authors thank Asst. Prof. Naowarat Kongkum from Surindra Rajabhat University, Thailand for plant identification.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
This research article encompasses all the data that has been generated and analyzed, which is readily available within this document (Supplementary data, SI.).
SUPPLEMENTARY MATERIAL:
The supplementary material can be accessed at the journal’s website: Link here [https://japsonline.com/admin/php/uploadss/4475_pdf.pdf].
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Alzehr A, Hulme C, Spencer A, Morgan-Trimmer S. The economic impact of cancer diagnosis to individuals and their families: a systematic review. Support Care Cancer. 2022;30(8):6385–404. CrossRef
2. Muralidharan S, Gore M, Katkuri S. Cancer care and economic burden–a narrative review. J Family Med Prim Care. 2023;12(12):3042–7. CrossRef
3. Divyalakshmi MV, Thoppil JE. Cytotoxicity and antioxidant activity evaluation of endemic variety of the Western Ghats Garcinia gummi-gutta var. papilla and Garcinia xanthochymus. Proc Natl Acad Sci India Sect B Biol Sci. 2024;13:1–13. CrossRef
4. Lee CH, Ying TH, Chiou HL, Hsieh SC, Wen SH, Chou RH, et al. Alpha-mangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget. 2017;8(29):47425. CrossRef
5. Liu Y, Chen Y, Lin L, Li H. Gambogic acid as a cndidate for cancer therapy: a review. Int J Nanomedicine. 2020;22:10385–99. CrossRef
6. Mungmee C, Sitthigool S, Suttisri R, Buakeaw A. Xanthones and biphenyls from Garcinia schomburgkiana wood and their cytotoxicity. Thai J Pharm Sci. 2012;36:6–9. CrossRef
7. Lien Do TM, Duong TH, Nguyen VK, Phuwapraisirisan P, Doungwichitrkul T, Niamnont N, et al. Schomburgkixanthone, a novel bixanthone from the twigs of Garcinia schomburgkiana. Nat Prod Res. 2021;35(21):3613–8. CrossRef
8. Kaennakam S, Mudsing K, Rassamee K, Siripong P, Tip-Pyang S. Two new xanthones and cytotoxicity from the bark of Garcinia schomburgkiana. J Nat Med. 2019;73:257–61. CrossRef
9. Meechai I, Phupong W, Chunglok W, Meepowpan P. Antioxidant properties and phytochemical contents of Garcinia schomburgkiana Pierre. J Appl Pharm Sci. 2016;6(6):102–7. CrossRef
10. Sukandar ER, Kaennakam S, Wongsuwan S, Chatwichien J, Krobthong S, Yingchutrakul Y, et al. Schomburginones A?J, geranylated benzophenones from the leaves of Garcinia schomburgkiana and their cytotoxic and anti-inflammatory activities. Phytochemistry. 2023;211:113701. CrossRef
11. Sukandar ER, Siripong P, Khumkratok S, Tip-Pyang S. New depsidones and xanthone from the roots of Garcinia schomburgkiana. Fitoterapia. 2016;111:73?7. CrossRef
12. Tanagornmeatar K, Chaotham C, Sritularak B, Likhitwitayawuid K, Chanvorachote P. Cytotoxic and anti-metastatic activities of phenolic compounds from Dendrobium ellipsophyllum. Anticancer Res. 2014;34(11):6573?9.
13. Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982;45(05):31?4.
14. Meechai IM, Phupong WO, Chunglok W, Meepowpan P. Anti-radical activities of xanthones and flavonoids from Garcinia schomburgkiana. Int J Pharm Sci. 2016;8(9):235?8.
15. Silva-Castro LF, Derbré S, Le Ray AM, Richomme P, García-Sosa K, Peña-Rodriguez LM. Using 13C-NMR dereplication to aid in the identification of xanthones present in the stem bark extract of Calophyllum brasiliense. Phytochem Anal. 2021;32(6):1102?9. CrossRef
16. Gunasekera SP, Selliah S, Sultanbawa MU. Chemical investigation of ceylonese plants. Part XV. Extractives of Kayea stylosa Thw.(Guttiferae). J Chem Soc Perkin Trans. 1975(16):1539?44. CrossRef
17. Natrsanga P, Jongaramruong J, Rassamee K, Siripong P, Tip-Pyang S. Two new xanthones from the roots of Cratoxylum cochinchinense and their cytotoxicity. J Nat Med. 2020;74:467?73.
18. Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK, et al. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanzan J Health Res. 2010;12(1):63–7. CrossRef
19. Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993;59(03):250–2.
20. Thi Thu HN, Minh QP, Van CP, Van TN, Van KP, Thanh TN, et al. The SN. Cytotoxic and α-glucosidase inhibitory xanthones from Garcinia mckeaniana leaves and molecular docking study. Chem Biodivesity. 2021;18(11):e2100396.
21. Takeda T, Tsubaki M, Kino T, Yamagishi M, Iida M, Itoh T, et al. Mangiferin induces apoptosis in multiple myeloma cell lines by suppressing the activation of nuclear factor kappa B-inducing kinase. Chem Biol Interact. 2016;251:26–33. CrossRef
22. Li J, Malakhova M, Mottamal M, Reddy K, Kurinov I, Carper A, et al. Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases. Cancer Res. 2012;72(1):260–70.
23. Teh SS, Ee GC, Mah SH, Lim YM, Ahmad Z. Cytotoxicity and structure-activity relationships of xanthone derivatives from Mesua beccariana, Mesua ferrea and Mesua congestiflora towards nine human cancer cell lines. Molecules. 2013;18(2):1985–94. CrossRef
24. Loisel S, Le Ster K, Meyer M, Berthou C, Youinou P, Kolb JP, et al. Therapeutic activity of two xanthones in a xenograft murine model of human chronic lymphocytic leukemia. J Hematol Oncol. 2010;3:1–3. CrossRef
25. Lee S, Min B, Kho Y. Brine shrimp lethality of the compounds from Phryma leptostachya L. Arch Pharm Res. 2002;25:652–4. CrossRef
26. Luo X, He W, Yin H, Li Q, Liu Q, Huang Y, et al. Two new coumarins from Micromelum falcatum with cytotoxicity and brine shrimp larvae toxicity. Molecules. 2012 Jun 6;17(6):6944–52. CrossRef
27. Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol. 2008;46(10):3227?39. CrossRef
28. Boonnak N, Chantrapromma S, Tewtrakul S, Sudsai T. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW264.7 macrophages by isolated xanthones from the roots of Cratoxylum formosum ssp. pruniflorum. Arch Pharm Res. 2014;37:1329?35. CrossRef
29. Chukaew A, Saithong S, Chusri S, Limsuwan S, Watanapokasin R, Voravuthikunchai SP, et al. Cytotoxic xanthones from the roots of Mesua ferrea L. Phytochemistry. 2019; 157:64?70. CrossRef
30. Reutrakul V, Anantachoke N, Pohmakotr M, Jaipetch T, Sophasan S, Yoosook C, et al. Cytotoxic and anti-HIV-1 caged xanthones from the resin and fruits of Garcinia hanburyi. Planta Med. 2007;73(01):33?40. CrossRef
31. Castanheiro RA, Silva A, Campos NA, Nascimento MS, Pinto MM. Antitumor activity of some prenylated xanthones. Pharmaceuticals. 2009; 2(2):33?43. CrossRef
32. Perchellet EM, Ward MM, Skaltsounis AL, Kostakis IK, Pouli N, Marakos P, et al. Antiproliferative and proapoptotic activities of pyranoxanthenones, pyranothioxanthenones and their pyrazole-fused derivatives in HL-60 cells. Anticancer Res. 2006;26(4B):2791?804.
33. Kolokythas G, Daniilides K, Pouli N, Marakos P, Pratsinis H, Kletsas D. Design, synthesis, and cytotoxic activity evaluation of new linear pyranoxanthone aminoderivatives. J Heterocycl Chem. 2011;48(4):927?35.
34. Lewis JR, Reary JB. The synthesis of pyranoxanthenones containing a dimethylchromen system. J Chem Soc C. 1970;12:1662?4. CrossRef
35. Lakornwong W, Kanokmedhakul K, Masranoi J, Tontapha S, Yahuafai J, Laphookhieo S, et al. Cytotoxic and antibacterial xanthones from the roots of Maclura cochinchinensis. Nat Prod Res. 2022;36(23):6021?30. CrossRef
36. Pailee P, Kuhakarn C, Sangsuwan C, Hongthong S, Piyachaturawat P, Suksen K, et al. Anti-HIV and cytotoxic biphenyls, benzophenones and xanthones from stems, leaves and twigs of Garcinia speciosa. Phytochemistry. 2018;147:68?79. CrossRef