INTRODUCTION
Progressive illnesses known as neurodegenerative diseases (NDs) weaken and eventually kill neurons in the central nervous system (CNS). Disruption of critical neurodevelopmental processes results in neurodevelopmental disorders like autism spectrum disorder, attention-deficit/hyperactivity disorder, and intellectual impairment. The blood–cerebrospinal fluid barrier and the blood–brain barrier (BBB), which block medications from entering the CNS from the systemic circulation, present challenges to the management of illnesses related to neurodevelopment and NDs, which collectively impact 120 million people globally [1]. The nose-to-brain channel can bypass the BBB. It increases the bioavailability of medications taken orally promising to better the management of CNS disorders [2]. Nervous system dysfunction is the end outcome of neurodegenerative illnesses, which are characterized by the progressive and slow death of neural cells [3]. Individual NDs have different aetiologies and manifest in different brain areas. They may act on comparable cellular and molecular pathways. There is still a great need for effective medicines with therapeutic benefits. Although attempts to discover suitable therapeutics for neurodegenerative illnesses are growing, there are still numerous obstacles to overcome [4]. The majority of therapies aim to delay the course of the illness but do not result in a full recovery. Many limiting constraints, such as BBB prevent many active pharmaceutical agents from having the intended therapeutic impact. Therefore, for the successful treatment of NDs, it is imperative to guarantee the delivery of active molecules to the brain securely and effectively [5]. The use of polymers in nose-to-brain microcarrier delivery systems represents an innovative approach to neurotherapeutics. It helps to overcome some of the major limitations of conventional drug delivery methods, offering new possibilities for treating a wide range of neurological conditions. Their ability to enhance drug targeting, stability, and controlled release, combined with their capacity to bypass the BBB, positions them as key players in the advancement of effective and innovative treatments for neurological disorders. Polymers offer immense versatility in the design of microcarriers, allowing them to be customized for specific therapeutic applications. Polymers can be functionalized with various ligands or targeting molecules that enhance their ability to reach and treat the affected brain regions. Polymeric microcarriers are generally biocompatible and can be designed to minimize toxicity. By selecting appropriate polymer materials and optimizing their properties, these microcarriers can safely deliver drugs without causing adverse reactions or triggering immune responses, making them suitable for long-term therapeutic use [6,7]. While various NDs—such as amyotrophic lateral sclerosis, multiple system atrophy, Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease, and others—occur in different brain regions and have distinct aetiologies, accumulating evidence suggests that they share cellular and molecular mechanism [8,9].
BARRIER TO BRAIN
Substances can penetrate the BBB by adsorptive endocytosis, saturable transporters, extracellular pathways, and transmembrane diffusion [10]. The key mechanisms that are particularly significant in drug delivery are transmembrane diffusion and transporters. Transmembrane diffusion is non-saturable and is dependent upon the substance’s physicochemical properties as determined by the first analysis [11]. The blood–cerebrospinal fluid barrier, and the BBB, The cerebrospinal fluid-brain barrier (CBB) are the three barriers that develop between the cerebral vasculature and the brain parenchyma [12,13]. Cerebrospinal fluid (CSF) can exchange molecules with the brain parenchyma’s interstitial fluid and precisely control the entry of blood-borne molecules into the CSF [14]. The BBB plays a major role in regulating biological substances required for the brain’s metabolic activity and neuronal function [15]. The blood vessels that vascularize the CNS have a unique property called the BBB that allows them to precisely regulate the passage of ions, chemicals, and cells between the blood and the brain. Appropriate neuronal activity and protection from toxins and pathogens are made possible by the exact regulation of CNS homeostasis. Changes in these barrier qualities have a substantial impact on pathology and the development of several neurological disorders [16]. The pia mater and astrocytes that make up the CBB show signs of selective permeabilization and aid in the passage of chemicals from the CSF into the brain parenchyma [17,18]. The breakdown of the BBB can be caused by abnormal angiogenesis, vascular regression, hypoperfusion of the brain, rupturing of tight junctions, changes in the way that chemicals are transported from the blood to the brain, and inflammatory reactions [19]. These elements have the potential to initiate or exacerbate a “vicious circle” of medical conditions that eventually cause synapses and neurons to die and malfunction [20].
NOSE-TO-BRAIN DELIVERY
One of the many benefits of administering drugs intranasally is that they can enter the brain directly through the olfactory and trigeminal neurons, avoiding the BBB [21]. Due to its huge surface area (150 cm2) and high blood vascularity, the nasal cavity can be used to administer drugs because it allows for improved drug absorption through the nasal epithelium [22,23]. Since the medication enters the systemic circulation by the nose rather than the portal vein, it is especially well-suited for medicines that experience considerable first-pass hepatic inactivation [24] Transport via the trigeminal and olfactory nerve branches that supply the respiratory and olfactory epithelia, respectively, is the mechanism of nose-to-brain delivery. Intranasal (IN) routes are divided into extracellular and intracellular. The olfactory sensory cells initiate the intracellular route by engulfing the medication, which is subsequently transported to their synaptic clefts in the olfactory bulb by axonal transport. Olfactory neurons replicate this transynaptic process, which spreads the drug to other parts of the brain. Under the extracellular pathway, medications enter the cerebral spinal fluid directly after first traversing the nasal epithelium’s paracellular space and then the perineural space, leading to the subarachnoid space of the brain [25].
Factor affecting nose-to-brain delivery
The nasal mucosa has excellent permeability and effective absorption that make, the nasal cavity an ideal site for administering biopharmaceuticals and small-molecule medicines [26]. Therapeutic drugs may be administered noninvasively by IN administration, which circumvents the BBB to give immediate access to the CNS [27]. A fantastic possibility for quick and patient-compliant medication administration is presented by nose-to-brain delivery [28]. As previously shown, there are solid grounds to believe that pharmacological delivery to the CNS would be more likely if the olfactory mucosa were the target [29]. However, there are still certain obstacles to be addressed in terms of the drug’s application, such as the olfactory area, and particularly the olfactory cleft, which is well concealed within the nasal cavity. Also, formulations must have excellent adhesion to stay on the mucosa since the olfactory cleft is located at the top of the nasal cavity [30]. The poor membrane permeability where the epithelial cells are positioned in the nasal mucosa is the main barrier to the absorption of hydrophilic molecules and macromolecules. Tight junctions establish a strong connection between cells and are the main regulators of paracellular transport [31]. Only potent medications may be administered using this route due to the dosage volume limitations for liquids (100–250 µl) and powders (20–50 mg, depending on the powder’s bulk density) [32]. Potent medications that are degraded by the enzymes in the nasal cavity must be shielded from deterioration. Nasal formulations must not irritate the nasal cavity. Furthermore, the administration of medications via the nose-to-brain pathway requires a nasal administration device [33]. Novel IN drug delivery methods have been developed to boost the systemic bioavailability of medications taken via the IN route. Nano- and micro-technologies have become available to improve medication access in brain tissue. Natural or artificial materials make up micro- and nanoparticulate carriers, which interact molecularly with biological structures to change the way NDs are treated [34]. Several studies have looked at the application of microspheres (MSs) to treat NDs via the nose-to-brain route [35].
Microspheres in nose-to-brain delivery
MSs are microscopic solid particles that are spherical and have dimensions between 1 and 1,000 micrometers (μm) [36]. Therapeutic molecules are dissolved, encapsulated, or entrapped into the polymeric matrix of MS. A variety of natural, semi-synthetic, and synthetic materials can be used to produce MSs [37]. Starch, dextran, albumin, and hyaluronic acid are the building blocks to create the MSs [38]. Despite being water-insoluble, every kind of MS that has been employed for nasal delivery absorbs water into its matrix, causing the spheres to inflate and form gel. This gives the formulation more time to stay in the nasal cavity, improves drug-mucosa contact, and increases drug concentration at the deposition site. MSs produce sustained drug release which may help achieve the desired concentration of the drug at the absorption site [39]. Figure 1 depicts the delivery of drug-loaded MSs from the nose to the brain. In several animal models, the bioavailability of various peptides and proteins was enhanced using MSs. Additionally, the delivery of some low-molecular-weight medications in microsphere formulations has been beneficial [40]. MSs have a significantly longer residence duration in the cavity than solutions. They can also improve the absorption of large hydrophilic medicines. Additionally, MSs directly affect the mucosa, causing the epithelial cells’ tight connections to open. Given their recurrent use, starch, and dextran MSs are considered safe dose forms [41]. Animal models are chosen based on anatomical similarities to the human nasal cavity, ease of handling, and established protocols for neurological studies. The most frequently used animals include rats, mice, rabbits, pigs, dogs, and monkeys. These animal models offer unique advantages, helping researchers to optimize nasal drug delivery systems and assess their potential for effective nose-to-brain transport. Sheep are one of the favored animal models for pharmacokinetic (PK) and formulation research in nasal medication administration; therefore, in vivo experiments were conducted on them [42].
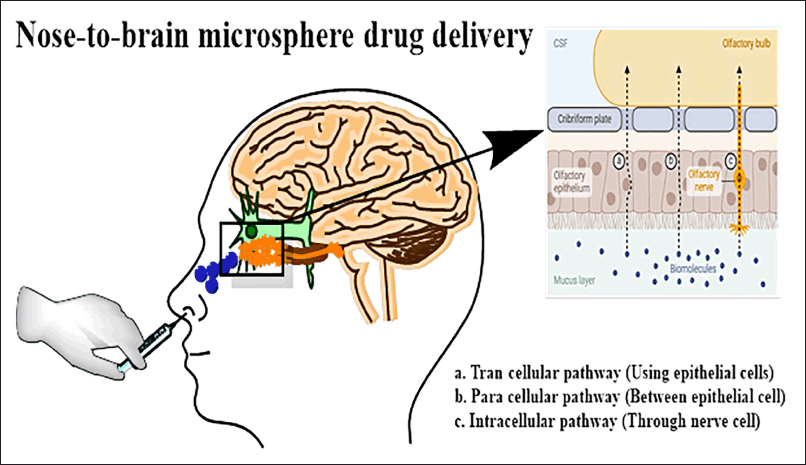 | Figure 1. Drug-loaded MSs delivered from the nose to the brain. [Click here to view] |
MSs in the nose-to-brain delivery for migraine
Migraine, an episodic headache condition, is characterized by recurring episodes of intense, usually unilateral, undulating pain that are typically accompanied by nausea, vomiting, photophobia, and phonophobia [43]. Dysfunction of the brain’s sensory processing that is likely cyclical and impacted by both heredity and environment gives rise to migraine attacks [44]. Migraine is the second most typical reason for impairment in young and middle-aged people [45,46]. One of the main indicators of several psychiatric and mental illnesses, such as anxiety and sadness, is migraine [47]. Neurogenic inflammation of the trigeminal nerve in the cranial dura mater is the cause of migraine headaches. Trigeminal neurons may be activated and sensitized by these central stimulations [48]. For the treatment of acute migraines, the IN route is known to provide a high brain drug concentration and a quick beginning of action as given in Table 1 [49].
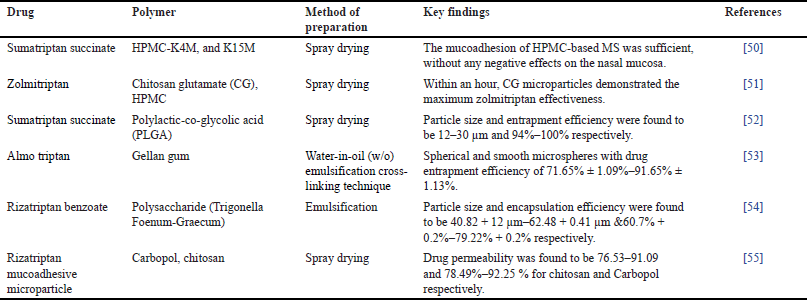 | Table 1. MSs in nose to brain delivery for Migraine. [Click here to view] |
MSs in nose to brain delivery for Alzheimer
AD is a major global cause of dementia or memory loss that primarily affects older folks. The hallmark of AD is the progressive deterioration and loss of brain cells, especially neurons, which results in a reduction in cognitive ability [56]. AD symptoms progressively get worse over time, making it harder for a person to do everyday tasks and ultimately resulting in a serious deterioration in cognitive and functional abilities. However, with today’s lifestyle, it appears to affect people at a younger age, a condition called as younger-stage AD [57]. Most of the FDA-approved pharmaceuticals for treating AD symptomatology are sold as traditional oral medications [58,59]. AD is linked to the buildup of aberrant protein deposits in the brain, such as tau tangles and beta-amyloid plaques, which obstruct neuronal transmission and promote cell death [60]. Gene therapy, stem cell treatment, and innovative drug delivery methods like nose-to-brain distribution using MSs are examples of emerging techniques meant to increase therapeutic efficacy and more precisely target certain brain areas [61]. Table 2 highlights some research based on nose-to-brain delivery of MS for the treatment of Alzheimer’s.
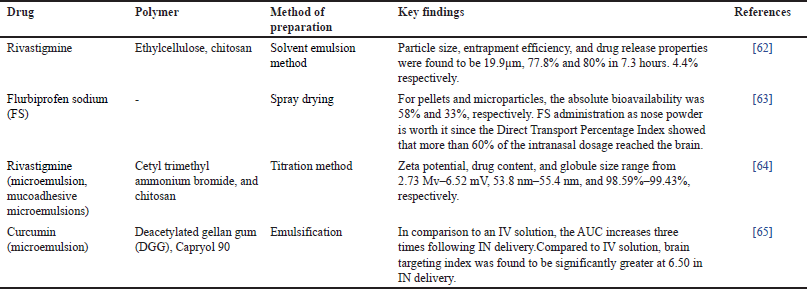 | Table 2. MSs in the nose to brain delivery for Alzheimer. [Click here to view] |
MSs in nose-to-brain delivery for Parkinsonism
PD is a prevalent and intricate neurological condition [66]. Changes in the neuronal cytoskeleton that occur in a small number of vulnerable kinds of nerve cells cause this illness. Lewy bodies and Lewy neurites are eventually produced by damaged neurons in their perikaryal and neuronal processes have an impact on mobility [67]. It happens gradually, with symptoms that frequently begin mildly and get worse with time. Among the main signs and symptoms of PD are involuntary shaking, which often begins in one hand, slowness of motion, which makes basic things challenging, Stiffness in the trunk or limbs, which can hurt and restrict range of motion, decreased coordination and balance, which raises the possibility of falls. Cognitive impairment, emotional issues, and alterations in speech, writing, and facial expressions are possible additional symptoms [68]. The substantia nigra, a part of the brain, is where dopamine-producing neurons are lost in PD [69]. Levodopa and other medications raise dopamine levels in the brain, which helps with movement issues [70]. With the goal of better management and, perhaps, a cure, research on novel medicines and the underlying processes of PD is still ongoing [71,72]. The role of MS in the treatment of PD has been discussed in Table 3.
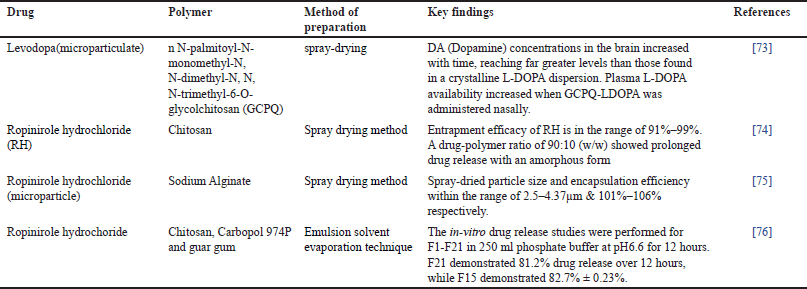 | Table 3. MSs in the nose to brain delivery for Parkinsonism. [Click here to view] |
Miscellaneous application of MSs for nose-to-brain
Solid microparticles based on chitosan or methyl-β-cyclodextrin were used to increase the nose-to-brain transport of deferoxamine mesylate (DFO), a neuroprotector that is unable to pass through the BBB and has detrimental peripheral effects. Beta-cyclodextrin is a cyclic oligosaccharide with a hydrophobic interior and a hydrophilic exterior. This amphiphilic structure allows it to form inclusion complexes with various drugs, improving their solubility and stability. In nose-to-brain delivery, the most crucial feature of beta-cyclodextrin is its ability to enhance drug bioavailability. By encapsulating lipophilic drugs within its hydrophobic core, beta-cyclodextrin can protect the drugs from enzymatic degradation in the nasal cavity, thereby increasing the amount of drug that reaches the brain. Additionally, its hydrophilic exterior ensures good compatibility with the nasal mucosa, facilitating efficient drug transport across the nasal epithelium. Chitosan is a natural polysaccharide known for its biocompatibility and mucoadhesive properties. The primary chemical feature that makes chitosan crucial for nose-to-brain delivery is its positive charge, which arises from its amino groups. This positive charge allows chitosan to interact with the negatively charged cell membranes in the nasal mucosa, enhancing adhesion and prolonging the residence time of the drug at the absorption site. Furthermore, chitosan can transiently open tight junctions between epithelial cells, promoting paracellular transport and improving drug permeation across the nasal barrier. This property is particularly valuable for delivering larger molecules, such as peptides and proteins, directly to the brain [77,78]. Solid microparticle formulations as nasal drug delivery vehicles could augment the transfer of DFO from the nose to the brain. Spray drying was used to create spherical chitosan chloride microparticles loaded with DFO DCH and methyl-βcyclodextrin microparticles loaded with DFO MCD. The aerodynamic diameters of microparticles were approximately 1.1 μm and the volume-surface diameters varied from 1.77 ± 0.06 μm DCH to 3.47 ± 0.05 μm MCD. As demonstrated by ex vivo permeation investigations across pig nasal mucosa, MCD improved DFO permeability across lipophilic membranes in comparison to DCH. Additionally, MCD may increase DFO permeability via PC 12 cell monolayers (which are like neurons). However, unlike DCH, it was unable to alter the DFO permeation pattern through Caco-2 monolayers (which are similar to epithelium). When 200 μg of DFO encapsulated in microparticles was administered nasally to rats, the microparticles’ absorption into the CSF was observed. Thirty minutes after insufflation, peak values ranged from 3.83 ± 0.68 μg/ml DCH to 14.37 ± 1.69 μg/ml MCD. DCH and MCD nasal delivery produced DFO systemic absolute bio availabilities of 6% and 15%, respectively [79].Quercetin (Que), a potent antioxidant, has limited absorption upon oral treatment and poor solubility restricts its beneficial effects. It has been discovered that the physicochemical characteristics of Que were improved by complexation with two distinct cyclodextrin (CD) derivatives (hydroxypropyl-β-CD and methyl-β-CD) using the neutralization/lyophilization process. Additionally, following in vitro and ex vivo testing, mixes of the lyophilized powders with mannitol/lecithin microparticles (MLMPs) have been suggested as candidates for IN administration. Wistar rats were used in a comparative PK investigation comparing the IN versus. oral administration of Que lyophilized powders and their mixes with MLMPs (75:25 w/w). The results demonstrated the efficacy of IN administration in either brain targeting or bloodstream penetration. At both locations, significant amounts of the chemical were obtained, in contrast to negligible levels following oral delivery. These findings support the possible systemic and nose-to-brain distribution of the produced Que nasal powders for the prevention and/or therapy of neuroinflammatory degenerative diseases including Parkinson’s and AD [80]. A sprayable powder delivery system for of dexamethasone sodium phosphate (DSP) was developed to target the brain. DSP-loaded MSs were combined with soluble inert carriers (lactose monohydrate or mannitol) after being optimized using the Quality-by-Design technique. Compared to lactose, mannitol offered superior powder mix flow characteristics. Mannitol-blended MSs improved DSP permeability across epithelial model barriers and maintained or expanded their mucoadhesive characteristics. The proposed powder platform can provide specific olfactory stimuli, as evidenced by the 17.0% DSP dosage fraction deposited in the olfactory area. The influence of nasal cavity asymmetry was shown to be significant, indicating the need for an individual strategy when targeting the olfactory area [81]. The use of egg whites, starch, and DEAE dextran (diethyl aminoethyl-dextran) MSs was advised to help the nasal cavity gradually absorb water and produce a gel-like coating. Half of the delivered egg whites and starch MSs and 60% of the dextran MSs had been present at the testimonial site for 3 hours after the organization. As indicated, the degradable starch MS program increased the virtual IN bioavailability of human growth hormone in sheep from 0.1 % for explanation to 2.7 % [82].
CONCLUSION
Nose-to-brain delivery avoids the BBB and gives a direct path to the brain, the unique methodologies based on lipid-based microsize spherical carriers to target the brain through the nasal cavity were summarised in this review paper, along with its applicability in treating NDs including PD, AD, and migraines, avoiding the BBB and giving a direct path to the brain.
ACKNOWLEDGEMENT
We would like to express our sincere gratitude to the president of Uttaranchal University Shri Jitendra Joshi for his valuable support and guidance.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Clementino AR, Marchi C, Pozzoli M, Bernini F, Zimetti F, Sonvico F. Anti-inflammatory properties of statin-loaded biodegradable lecithin/chitosan nanoparticles: a step toward nose-to-brain treatment of neurodegenerative diseases. Front Pharmacol. 2021;12:716380.
2. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dementia. 2021;17:1966–75.
3. Brown RC, Lockwood AH, Sonawane^ BR. Research I Mini-monoaraph neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect [Internet]. 2005;1250–6. Available from: http://dx.doi.org/iOnline
4. Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, et al. An overview of oxidative stress, neuroinflammation and neurodegenerative diseases. Int J Mol Sci. 2022;23(11):5938.
5. Zecca L, Youdim MBH, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–73.
6. Haider MS, Mahato AK, Kotliarova A, Forster S, Böttcher B, Stahlhut P, et al. Biological activity in vitro, absorption, BBB penetration, and tolerability of nanoformulation of BT44:RET agonist with disease-modifying potential for the treatment of neurodegeneration. Biomacromolecules. 2023;24:4348–65.
7. Xu J, Yang X, Ji J, Gao Y, Qiu N, Xi Y, et al. RVG-functionalized reduction sensitive micelles for the effective accumulation of doxorubicin in brain. J Nanobiotechnology. 2021;19:251.
8. Volkman R, Offen D. Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells. 2017;35(8):1867–80.
9. Bonferoni MC, Rassu G, Gavini E, Sorrenti M, Catenacci L, Giunchedi P. Nose-to-brain delivery of antioxidants as a potential tool for the therapy of neurological diseases. Pharmaceutics. 2020;12:1–21.
10. Delche NA, Kheiri R, Nejad BG, Sheikhi M, Razavi MS, Rahimzadegan M, et al. Recent progress in the intranasal PLGA-based drug delivery for neurodegenerative diseases treatment. Iran J Basic Med Sci. 2023;26:1107–19.
11. Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9:1–5.
12. Ale Y, Nainwal N. Progress and challenges in the diagnosis and treatment of brain cancer using nanotechnology. Mol Pharm. 2023;20(10):4893–921.
13. Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4): 497–511.
14. Akhtar A, Andleeb A, Waris TS, Bazzar M, Moradi AR, Awan NR, et al. Neurodegenerative diseases and effective drug delivery: a review of challenges and novel therapeutics. J Control Release. 2021;330: 1152–67.
15. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1): 13–25.
16. Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–50.
17. Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–75.
18. Zhang S, Gan L, Cao F, Wang H, Gong P, Ma C, et al. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 2022;190:69–83.
19. Nainwal N. Recent advances in transcranial focused ultrasound (FUS) triggered brain delivery. Curr Drug Targets. 2017;18:1225–32.
20. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13.
21. Ghadiri M, Young PM, Traini D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics. 2019;11(13):113.
22. Arora P Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today. 2002;7(18):967–75.
23. Illum L. Nasal drug delivery—Possibilities, problems and solutions. J Controld Release. 2003;87: 187–98.
24. Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2:215–22.
25. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52.
26. Passoni A, Favagrossa M, Colombo L, Bagnati R, Gobbi M, Diomede L, et al. Efficacy of cholesterol nose-to-brain delivery for brain targeting in Huntington’s Disease. ACS Chem Neurosci. 2020;11:367–72.
27. Zlokovic BV. The blood-brain barrier in Health and chronic neurodegenerative disorders. Neuron. 2008;57: 178–201.
28. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–28.
29. Aranaz I, Paños I, Peniche C, Heras Á, Acosta N. Chitosan spray-dried microparticles for controlled delivery of venlafaxine hydrochloride. Molecules. 2017;22:1980.
30. Vaka SRK, Murthy SN. Enhancement of nose-brain delivery of therapeutic agents for treating neurodegenerative diseases using peppermint oil. Pharmazie. 2010;65:690–2.
31. Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018 Aug 3;10(3):116.
32. Claus S, Weiler C, Schiewe J, Friess W. How can we bring high drug doses to the lung? Eur J Pharm Biopharm. 2014;86:1–6.
33. Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008 Dec 10;9 Suppl 3(Suppl 3):S5.
34. Marcello E, Chiono V. Biomaterials-enhanced intranasal delivery of drugs as a direct route for brain targeting. Int J Mol Sci. 2023 Feb 8;24(4):3390.
35. Ruby JJ, Pandey VP. Formulation and evaluation of olanzapine loaded chitosan nanoparticles for nose to brain targeting an in vitro and ex vivo toxicity study. J Appl Pharm Sci [Internet]. 2016 [cited 2024 Jul 23];6:034–40. Available from: https://japsonline.com/abstract.php?article_id=1976&sts=2
36. Sahil K, Akanksha M, Premjeet S, Bilandi A, Kapoor B. Microsphere: a review. IJRPC [Internet]. 2011;4(4):1184–98. Available from: www.ijrpc.com
37. Wang Z, Xiong G, Tsang WC, Schätzlein AG, Uchegbu IF. Nose-to-brain delivery. J Pharmacol Exp Therap. 2019;370:593–601.
38. Jeong SH, Jang JH, Lee YB. Drug delivery to the brain via the nasal route of administration: exploration of key targets and major consideration factors. J Pharm Investig. 2023;53:119–52.
39. Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88:8–27.
40. Patel MM, Patel BM. Crossing the blood–brain barrier: recent advances in drug delivery to the brain. CNS Drugs. 2017;31:109–33.
41. Pereswetoff-Morath L, Morath M. Microspheres as nasal drug delivery systems 1. Adv Drug Deliv Rev. 1998;29:185–94.
42. Chen BK, Staff NP, Knight AM, Nesbitt JJ, Butler GW, Padley DJ, et al. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion (Paris). 2015;55:1013–20.
43. Nagini KSS. Formulation and evaluation of anti migraine nasal microparticles [Master’s thesis]. Bangalore, India: Rajiv Gandhi University of Health Sciences; 2013.
44. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev [Internet]. 2017;97:553–622. Available from: www.prv.org
45. Hansen JM, Charles A. Differences in treatment response between migraine with aura and migraine without aura: lessons from clinical practice and RCTs. J Headache Pain. 2019;20:96.
46. Assadpour S, Shiran MR, Asadi P, Akhtari J, Sahebkar A. Harnessing intranasal delivery systems of sumatriptan for the treatment of migraine. Biomed Res Int. 2022;2022:3692065.
47. Goadsby PJ. Migraine pathophysiology. J Head Face Pain. 2005;45:S14–25.
48. Abo El-Enin HA, Mostafa RE, Ahmed MF, Naguib IA, Abdelgawad MA, Ghoneim MM, et al. Assessment of nasal-brain-targeting efficiency of new developed mucoadhesive emulsomes encapsulating an anti-migraine drug for effective treatment of one of the major psychiatric disorders symptoms. Pharmaceutics. 2022;14:410.
49. Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–23.
50. Jain SA, Chauk DS, Mahajan HS, Tekade AR, Gattani SG. Formulation and evaluation of nasal mucoadhesive microspheres of Sumatriptan succinate. J Microencapsul. 2009;26:711–21.
51. Gavini E, Rassu G, Ferraro L, Beggiato S, Alhalaweh A, Velaga S, et al. Influence of polymeric microcarriers on the in vivo intranasal uptake of an anti-migraine drug for brain targeting. Eur J Pharm Biopharm. 2013;83:174–83.
52. Mistry R, Mandale D, Chauhan NN. Development, characterization and in-vitro/Ex-vivo evaluation of mucoadhesive microsphere of sumatriptan succinate for nasal delivery. Int J Pharm Res. 2021;13:430–5.
53. Abbas Z, Marihal S. Gellan gum-based mucoadhesive microspheres of almotriptan for nasal administration: formulation optimization using factorial design, characterization, and in vitro evaluation. J Pharm Bioallied Sci. 2014;6:267–77.
54. Sharma M, Sharma N, Sharma A. Rizatriptan benzoate loaded natural polysaccharide based microspheres for nasal drug delivery system. Int J Appl Pharm. 2018;10:261–9.
55. Jadhav S, Mishra S. The spray-dried mucoadhesive microparticles of rizatriptan with chitosan and carbopol in migraine. Egypt Pharm J. 2022;21:293–301.
56. Liu WH, Song JL, Liu K, Chu DF, Li YX. Preparation and in vitro and in vivo release studies of huperzine a loaded microspheres for the treatment of Alzheimer’s disease. J Control Release. 2005;107:417–27.
57. Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dementia. 2019;15:17–24.
58. Wen MM, El-Salamouni NS, El-Refaie WM, Hazzah HA, Ali MM, Tosi G, et al. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: technical, industrial, and clinical challenges. Journal of Controlled Release. 2017;245:95–107.
59. Lista S, Dubois B, Hampel H. Paths to Alzheimer’s disease prevention: from modifiable risk factors to biomarker enrichment strategies. J Nutr Health Aging. 2015;19:154–63.
60. Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - Insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol. 2017;13:612–23.
61. Perl DP. Neuropathology of Alzheimer’s disease. Mount Sinai J Med. 2010;77:32–42.
62. Gao Y, Almalki WH, Afzal O, Panda SK, Kazmi I, Alrobaian M, et al. Systematic development of lectin conjugated microspheres for nose-to-brain delivery of rivastigmine for the treatment of Alzheimer’s disease. Biomed Pharmacother. 2021;141:111829.
63. Tiozzo Fasiolo L, Manniello MD, Banella S, Napoli L, Bortolotti F, Quarta E, et al. Flurbiprofen sodium microparticles and soft pellets for nose-to-brain delivery: serum and brain levels in rats after nasal insufflation. Int J Pharm. 2021;605:120827.
64. Shah BM, Misra M, Shishoo CJ, Padh H. Nose to brain microemulsion-based drug delivery system of rivastigmine: formulation and ex-vivo characterization. Drug Deliv. 2015;22:918–30.
65. Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281: 139–77.
66. Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
67. Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247:3–10.
68. Rinaldi F, Seguella L, Gigli S, Hanieh PN, Del Favero E, Cantù L, et al. inPentasomes: an innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J Control Release. 2019;294:17–26.
69. Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, MY Lee V, et al. Short communication aggregation of a-synuclein in lewy bodies of sporadic Parkinson’s disease and dementia with lewy bodies. Am JPathol. 1998;152:879–84.
70. Dawson TM, Dawson VL. Rare genetic mutations shed light on the pathogenesis of Parkinson disease. J Clin Investig. 2003;111:145–51.
71. Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7(4):306–18.
72. Md S, Bhattmisra SK, Zeeshan F, Shahzad N, Mujtaba MA, Srikanth Meka V, et al. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J Drug Deliv Sci Technol. 2018;43:295–310.
73. Dimiou S, Lopes RM, Kubajewska I, Mellor RD, Schlosser CS, Shet MS, et al. Particulate levodopa nose-to-brain delivery targets dopamine to the brain with no plasma exposure. Int J Pharm. 2022;618:121658.
74. Hussein NR. Bioadhesive microparticles and liposomes of anti parkinsons drugs for nasal delivery [Doctoral dissertation]. Preston, UK: University of Central Lancashire; 2014.
75. Hussein N, Omer H, Ismael A, Albed Alhnan M, Elhissi A, Ahmed W. Spray-dried alginate microparticles for potential intranasal delivery of ropinirole hydrochloride: development, characterization and histopathological evaluation. Pharm Dev Technol. 2020;25:290–9.
76. Mantry S, Balaji A. Formulation design and characterization of ropinirole hydrochoride microsphere for intranasal delivery. Asian J Pharm Clin Res. 2017;10:195–203.
77. Jain AK, Mishra K, Thareja S. In Silico docking of anti cancerous drugs with β-cyclodextrin polymer as a prominent approach to improve the bioavailability. Anticancer Agents Med Chem [Internet]. 2020 [cited 2024 Aug 30];21:1275–83. Available from: https://www.eurekaselect.com/article/110632
78. Yang B, Dong Y, Wang F, Zhang Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules. 2020;25(20):4613.
79. Rassu G, Soddu E, Cossu M, Brundu A, Cerri G, Marchetti N, et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: a first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J Control Release. 2015;201:68–77.
80. Manta K, Papakyriakopoulou P, Nikolidaki A, Balafas E, Kostomitsopoulos N, Banella S, et al. Comparative serum and brain pharmacokinetics of quercetin after oral and nasal administration to rats as lyophilized complexes with β-cyclodextrin derivatives and their blends with Mannitol/Lecithin microparticles. Pharmaceutics. 2023;15:2036.
81. Nodilo LN, Ugrina I, Špoljari? D, Klari? DA, Brala CJ, Perkuši? M, et al. A dry powder platform for nose-to-brain delivery of dexamethasone: formulation development and nasal deposition studies. Pharmaceutics. 2021;13:795.
82. Potdar MB, Jain NK, Rathod SS, Patil KD. A Study on the nose to brain drug delivery system. Int J Pharm Sci Res [Internet]. 2022;13:569.doi: http://dx.doi.org/10.13040/IJPSR.0975-8232.13