INTRODUCTION
Alzheimer’s disease (AD), a fatal, progressive neurodegenerative disease, is the primary reason for dementia in millions of patients globally. Apart from being the 5th most common reason for mortality globally, AD also adds a heavy financial burden. World Health Organisation estimates that 10 million new patients are added annually and by 2050, about 115 million people are expected to suffer from AD [1–3]. AD results in altered and erratic neuronal functions, disorientation, hallucinations, memory loss, and cognitive dysfunction [4]. Vital features of AD include chronic inflammation, synaptic loss, neuronal deaths, accumulation of insoluble proteins, intracellular neurofibrillary tangles (NFTs) comprising tau proteins, and extracellular plaques made of amyloid beta (Aβ) proteins.
Current treatment focuses on alleviating symptoms and minimizing disease progression [5,6]. Acetylcholinesterase inhibitor therapy for AD includes short-acting galantamine, donepezil, and intermediate-acting rivastigmine [7]. Immunotherapy, with anti Aβ monoclonal antibodies (MAb), targeting Aβ, is emerging because it halts the amyloid cascade and prevents neuronal degeneration [8]. Immunotherapy for neurodegenerative diseases has been widely researched for about two decades, but continues to puzzle researchers because of the difficulty in selectively eliminating the problematic proteins without harming the cell. Many animal models suggest that immunotherapy can significantly diminish the accumulation of Aβ and facilitate its clearance from the brain, thereby improving cognitive function (Table 1) [9].
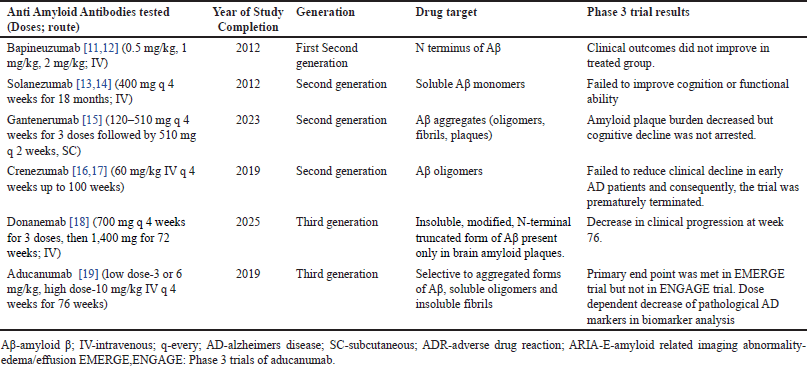 | Table 1. Outcomes of previous and ongoing phase 3 trial results of various anti amyloid antibodies. [Click here to view] |
Understanding the pathology of neurological diseases helps engineer MAb inhibiting toxic pathways, providing target-specific actions, and reducing unwanted outcomes [10]. Many researchers have confirmed the harmful effect of soluble Aβ oligomers on cognitive function, neurotoxicity, and exacerbation of disease. Recently, anti-amyloid agents, in the form of humanized MAb, such as aducanumab and BAN2401 [later named lecanemab] have shown promising results [20]. Aducanumab and lecanemab were approved by the FDA in 2021 and 2023, respectively [21], integrating an immunotherapeutic approach towards neurological disorders. Comparative studies signal a superior risk-benefit ratio and clinical outcomes lecanemab over aducanumab [22]. While lecanemab still awaits approval, aducanumab was rejected by the European Medicines Agency, citing difficulties in monitoring side effects and unsatisfactory phase 3 results [23,24]. This review highlights the multidimensional aspects of lecanemab in terms of pharmacokinetics, pharmacodynamics, clinical applications, and affordability, in addition to safety and efficacy based on clinical trial data.
METHODOLOGY
This review is based on a search of literature done using Google Scholar, Cochrane Library, Scopus, and Pubmed with multiple keywords such as Lecanemab, amyloid β, Alzheimer’s disease, immunotherapy, and MAb.
GENESIS AND MECHANISM OF ACTION
Understanding the mechanism of action of lecanemab (humanized IgG1 MAb) requires a deep insight into the pathophysiology of AD. The two hallmark lesions of AD in the brain are extracellular Aβ plaques and intracellular tau protein NFTs [25,26]. Apolipoprotein E (APOE) gene encodes APOE, which regulates lipid traffic in the central nervous system. APOE gene has multiple variants such as APOE2, APOE3, and APOE4, among which APOE4, is a predictor of late-onset AD and plays a role in neuroinflammation, Aβ, and tau pathology [27]. APOE4, by impeding Aβ degradation and clearance, is implicated in greater Aβ burden. Additionally, APOE4 also heightens tau hyperphosphorylation and NFT formation [28]. Aβ is a product of the cleavage of amyloid precursor protein (APP) [29]. Tau protein stabilizes microtubules, a part of the neuronal cytoskeleton [30]. In a healthy brain, APP processing involves alpha-secretase enzyme, generating sAPPα, for neuronal function. In AD, beta secretase enzyme cleaves APP, producing sAPPβ, initiating apoptotic pathways and neuronal death. Gamma secretase enzyme further cleaves APP to form Aβ monomers that assemble further into oligomers, protofibrils, and amyloid fibrils (Fig. 1). Large, insoluble Aβ fibrils assemble into Aβ plaques that impair synaptic transmission. Plaque formation triggers microglia and astrocytes to release reactive oxygen species, which hyperphosphorylate tau proteins, damaging microtubules and generating NFTs, ultimately destabilizing the neuronal cytoskeleton [31,32]. Disease progression in AD may be slowed down by clearing or inhibiting the synthesis of Aβ plaques. MAbs clear up plaques by activating an immune response against Aβ [33]. The murine version of lecanemab, mAb158, was designed with a conformation-dependent epitope to attack soluble aggregated forms of Aβ protofibrils, with a weak affinity to Aβ monomers [34]. Intravenous (IV) lecanemab does not bind to Aβ monomers in blood, thereby inhibiting its peripheral sequestration. Only a small amount of lecanemab penetrates the blood–brain barrier and binds and removes soluble oligomers and protofibrils as well as insoluble amyloid plaques. Protofibrils are primarily composed of Aβ42 peptides (peptides that are 42 amino acids long) that are toxic and exist in a soluble aggregated form [35]. Lecanemab could selectively bind to and remove toxic soluble Aβ protofibrils. The action of lecanemab was confirmed by cerebrospinal fluid (CSF) analysis that showed reduced levels of protofibrils [36]. Removal of protofibrils neutralized by lecanemab occurs by microglial activation and phagocytosis [37].
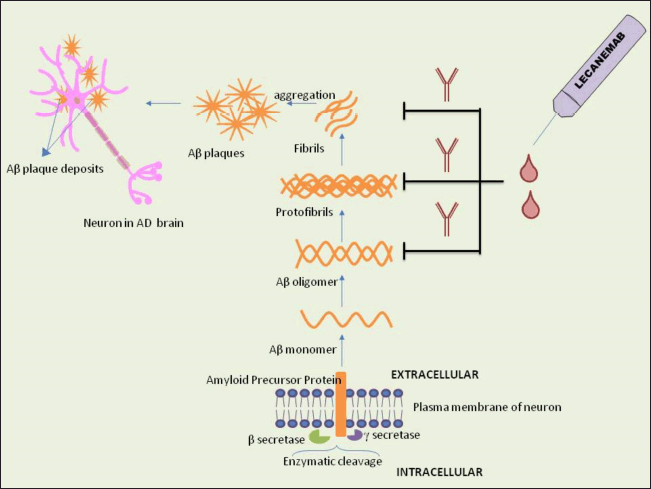 | Figure 1. Mechanism of action of lecanemab. APP in the brain is cleaved enzymatically to produce Aβ plaques, resulting in neuronal damage. Lecanemab inhibits the formation and aggregation of Aβ plaques by binding to Aβ oligomers, protofibrils, and fibrils. (Aβ-Amyloid β, AD-Alzheimer’s disease). [Click here to view] |
SAFETY AND EFFICACY STUDIES OF LECANEMAB
Preclinical phase
The mAb158, murine version of lecanemab, prevented Aβ accumulation in transgenic mice with high Aβ burden. mAb158 greatly diminished soluble Aβ protofibrils and insoluble Aβ plaques in early AD. The binding of mAb158 to Aβ protofibrils in cadaver AD brain formed a humanised version of BAN2401 [38,39] (Table 2).
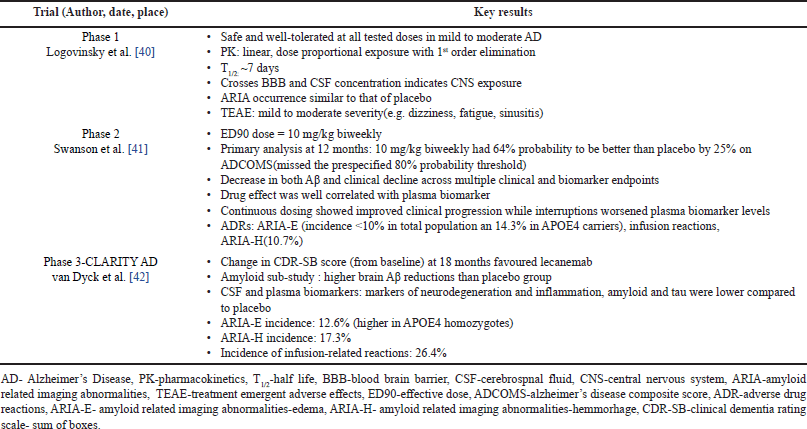 | Table 2. Clinical trials of Lecanemab. [Click here to view] |
Phase 1
The phase 1 trial of lecanemab (n = 80) assessed safety, pharmacokinetics, and effect on CSF and plasma biomarkers. Subjects aged 50 years and above, with mild to moderate AD—based on National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria and Mini Mental State Examination scores of 16–28—were randomly allocated to single ascending doses of 0.1, 0.3, 1, 10, and 15 mg/kg and multiple ascending doses of 0.3, 1, and 3 mg/kg. Safety was evaluated by conducting magnetic resonance imaging (MRI) to monitor Amyloid Related Imaging Abnormalities that is ARIA with edema and effusion (ARIA-E) and ARIA with microhemorrhages and superficial siderosis (ARIA-H). Pharmacokinetics and biomarker effects were monitored with CSF and plasma sampling [40].
Phase 2
Phase 2b (proof-of-concept, dose-finding study) used a novel Bayesian adaptive design, aimed to establish the effective dose of 90% (ED90), enrolled 856 patients with confirmed Aβ pathology and the placebo arm consisted of 56 subjects with more males in the lecanemab group than in the placebo group. The 5 lecanemab arms (n = 28 in each cohort) received three ascending doses, namely, 2.5 mg/kg, 5 mg/kg, 10 mg/kg biweekly, and 5 mg/kg, 10 mg/kg monthly. 50 subjects were randomized to each cohort based on Alzheimer’s Disease Composite Score (ADCOMS). APOE4 carriers were excluded from high-dose lecanemab due to the risk of ARIA as per a protocol amendment. The primary outcome was the Bayesian analysis of 12-month change on ADCOMS for the ED90 dose. Secondary outcomes included (a) positron emission tomography (PET) analysis of reduction in brain amyloid, (b)measurement of clinical decline across scales such as ADCOMS, Clinical Dementia Rating Sum of Boxes (CDR-SB), AD Assesment Scale—Cognitive Subscale14 (ADAS-cog14), and (c) alterations in CSF biomarkers and total hippocampal volume using volumetric MRI [41].
Phase 3
“Clarity AD”, an 18 month, double blind trial (n = 1795), enrolled 50–90 year old patients with early AD (mild cognitive impairment). Subjects were assigned randomly in two groups namely Placebo group (n = 897) and Lecanemab group (n = 898). Lecanemab group received IV lecanemab 10 mg/kg biweekly. The primary outcome was measured by the change in CDR-SB over 18 months from the start of therapy. Secondary outcomes included (a) amyloid PET imaging, (b) ADAS-Cog14, (c) ADCOMS, and (d) Alzhiemer’s Disease Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment and safety assessments [43].
Additional ongoing trials include AHEAD 3-45 trial (initiated in 2020) and the DIAN-TU (Dominantly Inherited Alzheimer Network Trials Unit) Next Generation trial initiated in 2012 (results awaited) [44]. AHEAD 3-45, the first secondary prevention trial using plasma biomarkers for eligibility screening, evaluated lecanemab’s capacity to retard tau deposition in early preclinical and preclinical stages of AD, exploring the possibilities of reducing cognitive decline upon early intervention [45].
Söderberg et al. [46] compared the binding characteristics of three IgG1 MAbs aducanumab, gantenerumab, and lecanemab using various in vitro assays and reported that lecanemab demonstrated the highest binding affinity to soluble Aβ protofibrils. Lecanemab’s tenfold stronger binding to protofibrils than fibrils may be the reason for its superior efficacy and minimal frequency of ARIA-E when compared to aducanumab and gantenerumab [46].
The appropriate IV dose was reported as 10 mg of lecanemab per kilogram of body weight, biweekly from the phase 2b dose finding trial [47]. Meta-analysis by Abdelazim et al. [48] confirms the efficacy of lecanemab—10 mg/kg biweekly demonstrating uniform reduction across ADCOMS, CDR-SB, and ADAS-cog14 scores, without significant heterogeneity between clinical trial results. However, there was significant heterogeneity between trial results on the safety profile, reflected by Treatment Emergent Adverse Events (TEAEs) [48].
DOSING, ADMINISTRATION, AND APPROPRIATE USE RECOMMENDATIONS FOR LECANEMAB
The recommended dose of lecanemab is 10 mg/kg twice a week as per US-FDA. Lecanemab is available as an injectable IV solution in single-dose vials of strengths 200 mg/2 ml and 500 mg/5 ml. Lecanemab must be diluted in 250 ml of 0.9% sodium chloride before IV administration and infused over a period of 1 hour. IV line must be fitted with a terminal low protein binding 0.2 micron inline filter. Dose interruptions may be necessary according to ARIA events and regular MRI scans [49].
AUR instructs clinicians to select patients similar to participants of lecanemab trials that demonstrated safety and efficacy. Infusion reactions are likely, and may be managed with
prophylactic anti-inflammatory therapies (Table 3) [50]. A study assessed the pharmacokinetics, bioavailability, safety, and immunogenicity of a single fixed dose of 700mg lecanemab administered subcutaneously (SC). SC lecanemab was well tolerated with 77.5% bioavailability and a half-life of 21 days. The occurrence of anti-lecanemab antibodies was low and did not impact safety or pharmacokinetics [54].
 | Table 3. Recommendations for appropriate use of lecanemab [51]. [Click here to view] |
PHARMACOKINETICS
Pharmacokinetic/pharmacodynamic simulations showed that a twice-weekly dose of 10 mg/kg rapidly decreased Aβ plaques, as estimated by PET. After treatment discontinuation, Aβ re-accumulation was slower than the recovery of plasma Aβ42/40 ratio and p-tau181 (soluble biomarkers of amyloid pathology). Terminal half life was estimated to be ~5–9.5 days. The clearance (CL) and volume of distribution (Vd) in the central compartment increased with body weight. CL and Vd were slightly decreased for women when compared to men. Also, the CL of lecanemab decreased with rising albumin levels. However, AD risk factors such as age and APOE4 carrier status had no significant effects on the pharmacokinetics of lecanemab. Trials indicate that lecanemab clearance is potentially influenced by anti-drug antibody-positive status, sex, body weight, and albumin (Table 4) [52,55].
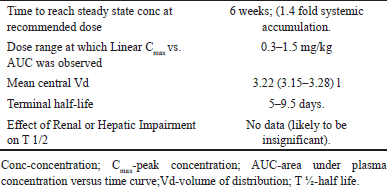 | Table 4. Pharmacokinetics [52]. [Click here to view] |
ADVERSE EFFECTS FROM TRIALS
Phase 1 trial data suggests that lecanemab was well tolerated without any severe TEAEs. Asymptomatic ARIA-H occurred in 5 subjects (1 from placebo). In the phase 2 study, TEAEs were comparable between placebo and treatment groups. Infusion reactions were managed with prophylactic medications. The severity of ARIA-E was mild to moderate in general and in APOE4 carriers. In the phase 3 study, about 50% of the population had no infusion reactions beyond the first dose and did not need any prophylactic management [53]. Analysis of anti-drug antibody profile of lecanemab showed no significant effect on pharmacokinetics, pharmacodynamics, safety, and efficacy thus making lecanemab a low-risk molecule for immunogenicity [56]. Johannesson et al. [35] showed the minimal binding of lecanemab to cerebral amyloid angiopathy fibrils, a property that reduces the likelihood of ARIA associated with lecanemab when compared to other AD antibodies (Table 5).
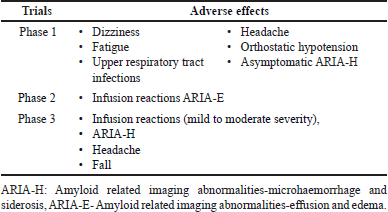 | Table 5. Adverse effects from trials [53]. [Click here to view] |
ECONOMIC CONSTRAINTS
The treatment of AD is largely supportive, including individualized care plans, caregiver education and support, and pharmacologic therapy for symptom management. Given the increasing population with AD and the human and economic impact of the disease, there is a tremendous need for disease-modifying drugs that slow down or halt the course of AD [57]. After providing billions of hours of unpaid care, caregivers encounter numerous detrimental physical, psychological, and emotional effects. Little is known regarding the lecanemab cost-effectiveness. This FDA-approved drug has a list price of $26,500 per year [58]. Direct costs of health care in the United States (US) related to AD have been estimated to be about $321 billion in 2022 and are anticipated to reach slightly under $1 trillion in 2050 [59]. A majority of key stakeholders (such as manufacturers, clinicians, health insurance delegates, and patient advocates) in the US found that current evidence is insufficient to show lecanemab’s net health benefit when compared to standard of care (SoC) alone [58]. Nguyen et.al. [60] from the US compared targeted lecanemab treatment and treatment unrestricted by APOE4 genotype with SoC for mild dementia due to AD and showed that the former is not cost effective than SoC and suggested that it would be cost-effective in some settings if priced below $5,100 per year[60]. Another study estimated the potential value-based price (VBP) of lecanemab + SoC compared to SoC alone and showed that lecanemab +SoC improved overall non-treatment expenses by $8707 compared to SoC alone for individuals with early AD, and it increased QALYs (Quality Adjusted Life Years) by 0.61. Additionally, lecanemab’s potential yearly VBP was predicted to be between $92,49 and $35,605 (with a $50,000 to $200,000 WTP threshold for each QALY achieved) [61]. Monfared et al. [61], from the US showed that lecanemab was linked to 0.73 incremental life years and 0.75 incremental QALYs, and the caregiver QALYs lost was decreased by 0.03 years. The Institute for Clinical and Economic Review (ICER) analyses suggest lecanemab would meet conventional thresholds for cost-effectiveness if priced between $8,900–$21,500 annually [58]. Concerns regarding lecanemab pricing have been voiced by the European Union. If the drug’s price were the same as in the US, the annual cost of treatment would surpass 133 billion EUR, or more than half of all pharmaceutical spending in the EU. In certain European nations, patients may not be able to afford the treatment due to pricing comparable to what has been revealed for the US market [62]. A few crucial strategies pertaining to lecanemab affordability and access were suggested by ICER. The intention is to alert stakeholders and decision-makers to the possibility that the health system may not be able to absorb the full amount of additional healthcare costs associated with a new service in the near future without displacing other necessary services, imposing stringent access restrictions on payers, or increasing the growth of health insurance premiums in a way that might jeopardise all patients’ sustained access to high-value care [58,63]. For instances to improve cost-effectiveness, reimbursement could be restricted to only subgroups of patients (e.g., male sex, APOE4 non-carrier status, and elderly) within the overall target indication who will have better therapeutic benefits [64]. Restricting reimbursement to only subgroups would have the combined effect of lowering the cost per QALY ratio as well as the financial impact. The uncertainty around the long-term therapeutic benefits needs to be considered when assessing the value of the therapy. Lecanemab’s cost-effectiveness needs to be evaluated by independent investigators (without influence or direct support from the manufacturers, payers, or health technology assessment agencies), with careful consideration of the uncertainty in the underlying therapeutic and economic statistics, and the methodologies and results published clearly and evidently.
Results of the Quality of Life (QoL) study of participants and care-givers of the Clarity AD trial, combined with earlier reports of benefits, highlight lecanemab’s potential. Cohen et al. [65] reported that lecanemab therapy was linked to good health-related QoL and a lower increase in caregiver burden. Results were obtained based on scales such as European QoL-5 dimensions, QoL in AD, and Zarit Burden Interview [65]. A study on long-term health outcomes associated with lecanemab in early AD, found that lecanemab plus SoC improved mean survival, QALYs and prolonged the onset of AD dementia or need for institutional care compared to SoC alone [66].
IS LECANEMAB PROMISING ENOUGH?
Lecanemab, which received FDA approval soon after aducanumab, is not a cure for AD because it only slows down cognitive decline in early stage AD patients. FDA approval of aducanumab has paved the way for controversies about the potential of anti amyloid therapies. However, lecanemab has shown greater therapeutic efficacy and lower risk of brain edema and bleeding than aducanumab. Lecanemab phase 3 trials failed to improve AD in women and APOE4 carriers; despite constituting the majority of enrolled subjects (women have a 2-fold risk of AD than men). Lecanemab induced cognitive decline and ARIA in APOE4 homozygotes [45,67]. Despite the high annual cost ($26,500), and the need for frequent MRI scans (towards ARIA diagnosis), iv infusions, and so on, opinion is divided on the duration of lecanemab treatment in AD [68–70].
A few studies reported deaths from lecanemab therapy because of severe bleeding, cerebral edema, and seizures or due to a probable drug interaction between lecanemab and apixaban [71,72]. The high risk of antithrombotic-induced cerebral bleeding is a hurdle for patients who need concurrent antithrombotics with lecanemab therapy. This is a major challenge, especially for elderly patients prone to developing cerebrovascular stroke, pulmonary embolism, or myocardial infarction that need management with drugs such as tissue plasminogen activator, IV heparin, dual antiplatelet therapy or low-molecular-weight heparin [73]. The efficacy of SC lecanemab is being evaluated, possibly lowering the costs of therapy. Diagnostic tests and screening that facilitate early detection of AD will increase lecanemab use because it is more effective in treating early AD [64].
CONCLUSION
Lecanemab signifies a major milestone in AD treatment. This humanized Mab eliminates Aβ plaques, a hallmark feature of AD. Clinical trials have yielded optimistic outcomes, with lecanemab being able to slow cognitive decline in early-stage AD patients. Nonetheless, significant obstacles persist. Lecanemab is not a cure, and its efficacy seems to be reduced in women and APOE4 carriers, who constitute a major portion of the AD population. Additionally, the high cost of therapy, potential adverse events, and ambiguity around long-term benefits raise concerns about cost-effectiveness and clinical utility. Cost cutting measures such as optimizing patient selection criteria, and investigating alternative routes of administration (e.g., SC injection) offer hope. Despite limitations, lecanemab marks a new beginning in immunotherapy for AD.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval was not needed as this is a review article.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Aisen PS, Jimenez-Maggiora GA, Rafii MS, Walter S, Raman R. Early-stage Alzheimer disease: getting trial-ready. Nat Rev Neurol. 2022 Jul;18(7):389–99.
2. Alzheimer’s disease - PubMed [Internet]. [cited 2024 Jan 10]. Available from: https://pubmed.ncbi.nlm.nih.gov/33667416/
3. Ma C, Hong F, Yang S. Amyloidosis in Alzheimer’s gisease: pathogeny, etiology, and related therapeutic directions. Molecules. 2022 Feb 11;27(4):1210.
4. Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019 Jul 19;14:5541–54.
5. Shi M, Chu F, Zhu F, Zhu J. Impact of Anti-amyloid-β Monoclonal Antibodies on the pathology and clinical profile of Alzheimer’s disease: a focus on aducanumab and lecanemab. Front Aging Neurosci. 2022;14:870517.
6. Chopade P, Chopade N, Zhao Z, Mitragotri S, Liao R, Chandran Suja V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng Transl Med. 2023;8(1):e10367.
7. Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021 Jun 1;190:108352.
8. Liu J, Yang B, Ke J, Li W, Suen WC. Antibody-based drugs and approaches against amyloid-β species for Alzheimer’s disease immunotherapy. Drugs Aging. 2016 Oct;33(10):685–97.
9. Bittar A, Sengupta U, Kayed R. Prospects for strain-specific immunotherapy in Alzheimer’s disease and tauopathies. NPJ Vaccines. 2018;3:9.
10. Gklinos P, Papadopoulou M, Stanulovic V, Mitsikostas DD, Papadopoulos D. Monoclonal antibodies as neurological therapeutics. Pharmaceuticals (Basel). 2021 Jan 26;14(2):92.
11. Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014 Jan 23;370(4):322–33.
12. Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, et al. Effect of Immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol. 2012 Aug 1;69(8):1002–10.
13. Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimer’s Dement. 2012 Jul 1;8(4):261–71.
14. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease | NEJM [Internet]. [cited 2024 Jan 21]. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1312889
15. Bateman RJ, Smith J, Donohue MC, Delmar P, Abbas R, Salloway S. Two Phase 3 Trials of Gantenerumab in Early Alzheimer’s Disease. N Engl J Med [Internet]. 2023 Nov [cited 2024 Jan 24];389(20):1862–76. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2304430
16. Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, et al. Abby. Neurology. 2018 May 22;90(21):e1889–97.
17. Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS, et al. Evaluating the safety and efficacy of crenezumab versus placebo in adults with early Alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol. 2022 Nov 1;79(11):1113–21.
18. Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023 Aug 8;330(6):512–27.
19. Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022 Apr 1;9(2):197–210.
20. Tolar M, Abushakra S, Hey JA, Porsteinsson A, Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res Ther. 2020 Aug 12;12(1):95.
21. Commissioner of the FDA. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Silver Spring, MD: FDA; 2023 [cited 2024 Jan 11]. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment
22. Leqembi (Lecanemab) versus Aduhelm (Aducanumab) For Alzheimer’s: what’s the difference? [Internet] Augusta, ME: Local Infusion. [cited 2024 Jan 28]. Available from: https://mylocalinfusion.com/blog/lecanemab-vs-aducanumab
23. Reuters. EU regulator delays decision on Eisai-Biogen Alzheimer’s drug [Internet]. London, UK: Reuters; 2024 Mar 22 [cited 2024 Apr 6]. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/eu-drug-regulator-does-not-refer-eisai-biogen-alzheimers-drug-meeting-notes-2024-03-22/
24. Biogen announces withdrawal of marketing authorisation application for aducanumab for the treatment of Alzheimer’s disease. 2024 Mar 22 [cited 2024 Apr 6]; Available from: https://www.alzheimer-europe.org/news/biogen-announces-withdrawal-marketing-authorisation-application-aducanumab-treatment?language_content_entity=en
25. Vickers JC, Mitew S, Woodhouse A, Fernandez-Martos CM, Kirkcaldie MT, Canty AJ, et al. Defining the earliest pathological changes of Alzheimer’s disease. Curr Alzheimer Res. 2016 Mar;13(3):281–7.
26. National Institute on Aging [Internet]. [cited 2024 Mar 30]. What Happens to the Brain in Alzheimer’s Disease? Bethesda, MD: National Institute on Aging. Available from: https://www.nia.nih.gov/health/alzheimers-causes-and-risk-factors/what-happens-brain-alzheimers-disease
27. Fernández-Calle R, Konings SC, Frontiñán-Rubio J, García-Revilla J, Camprubí-Ferrer L, Svensson M, et al. APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol Neurodegen. 2022 Sep 24;17(1):62.
28. Troutwine BR, Hamid L, Lysaker CR, Strope TA, Wilkins HM. Apolipoprotein E and Alzheimer’s disease. Acta Pharmaceutica Sinica B. 2022 Feb 1;12(2):496–510.
29. Sehar U, Rawat P, Reddy AP, Kopel J, Reddy PH. Amyloid Beta in aging and Alzheimer’s disease. Int J Mol Sci. 2022 Jan;23(21):12924.
30. Medina M, Hernández F, Avila J. New features about Tau function and dysfunction. Biomolecules. 2016 Apr 19;6(2):21.
31. Chen G fang, Xu T hai, Yan Y, Zhou Y ren, Jiang Y, Melcher K, et al. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017 Sep;38(9):1205–35.
32. Siddappaji KK, Gopal S. Molecular mechanisms in Alzheimer’s disease and the impact of physical exercise with advancements in therapeutic approaches. AIMS Neurosci. 2021 Mar 19;8(3):357–89.
33. Olloquequi J, Ettcheto M, Cano A, Sanchez-López E, Carrasco M, Espinosa T, et al. Impact of new drugs for therapeutic intervention in Alzheimer’s disease. Front Biosci (Landmark Ed). 2022 May 6;27(5):146.
34. Rizoska B, Zachrisson O, Appelkvist P, Boström E, Björklund M, Rachalski A, et al. Disease modifying effects of the amyloid-beta protofibril-selective antibody mAb158 in aged Tg2576 transgenic mice. Mol Cell Neurosci. 2024 Sep 1;130:103950.
35. Johannesson M, Söderberg L, Zachrisson O, Fritz N, Kylefjord H, Gkanatsiou E, et al. Lecanemab demonstrates highly selective binding to Aβ protofibrils isolated from Alzheimer’s disease brains. Mol Cell Neurosci. 2024 Sep 1;130:103949.
36. Tucker S, Möller C, Tegerstedt K, Lord A, Laudon H, Sjödahl J, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43(2):575–88.
37. Loeffler DA. Antibody-mediated clearance of brain Amyloid-β: mechanisms of action, effects of natural and monoclonal anti-Aβ antibodies, and downstream effects. J Alzheimers Dis Rep. 2023;7(1):873–99.
38. Lord A, Gumucio A, Englund H, Sehlin D, Sundquist VS, Söderberg L, et al. An amyloid-β protofibril-selective antibody prevents amyloid formation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009 Dec 1;36(3):425–34.
39. Cummings J, Osse AML, Cammann D, Powell J, Chen J. Anti-Amyloid monoclonal antibodies for the treatment of Alzheimer’s disease. BioDrugs. 2024 Jan 1;38(1):5–22.
40. Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. Safety and tolerability of BAN2401—a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers Res Ther. 2016 Apr 6;8:14.
41. Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody - PMC Alzheimers Res Ther [Internet]. 2021 [cited 2024 Jan 12];13:80. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8053280/
42. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023 Jan 5;388(1):9–21.
43. Eisai Inc. A placebo-controlled, double-blind, parallel-group, 18-month study with an open-label extension phase to confirm safety and efficacy of BAN2401 in subjects with early Alzheimer’s disease [Internet]. Nutley, NJ: Eisai Inc.; 2023 Jun [cited 2024 Jan 1]. Report No.: NCT03887455. Available from: https://clinicaltrials.gov/study/NCT03887455
44. Washington University School of Medicine. A Phase II/III multicenter randomized, double-blind, placebo-controlled platform trial of potential disease modifying therapies utilizing biomarker, cognitive, and clinical endpoints in dominantly inherited Alzheimer’s disease [Internet]. St. Louis, MO: Washington University School of Medicine; 2023 Oct [cited 2024 Jan 1]. Report No.: NCT01760005. Available from: https://clinicaltrials.gov/study/NCT01760005
45. Rafii MS, Sperling RA, Donohue MC, Zhou J, Roberts C, Irizarry MC, et al. The AHEAD 3–45 Study: Design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 2023 Apr;19(4):1227–33.
46. Söderberg L, Johannesson M, Nygren P, Laudon H, Eriksson F, Osswald G, et al. Lecanemab, aducanumab, and gantenerumab—binding profiles to different forms of amyloid-Beta might explain efficacy and side effects in clinical trials for Alzheimer’s disease. Neurotherapeutics. 2023 Jan;20(1):195–206.
47. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. NEJM [Internet]. 2023[cited 2024 Jan 13];388:9–21. Available from: https://www.nejm.org/doi/10.1056/NEJMoa2212948?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
48. Abdelazim K, Allam AA, Afifi B, Abdulazeem H, Elbehiry AI. The efficacy and safety of lecanemab 10 mg/kg biweekly compared to a placebo in patients with Alzheimer’s disease: a systematic review and meta-analysis of randomized controlled trials. Neurol Sci. 2024 Aug 1;45(8):3583–97.
49. Leqembi (lecanemab) dosing, indications, interactions, adverse effects, and more [Internet] New York, NY: WebMD LLC. [cited 2024 Jan 13]. Available from: https://reference.medscape.com/drug/leqembi-lecanemab-4000310
50. Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362–77.
51. Rafii MS. Appropriate use recommendations for lecanemab. J Prev Alzheimers Dis. 2023 Sep 1;10(3):356.
52. FDA. Eisai Inc. Highlights of prescribing information for LEQEMBI [Internet]. Silver Spring, MD: FDA; 2023. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269s000lbl.pdf
53. Chowdhury S, Chowdhury NS. Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer’s disease: a systematic review. Int J Immunopathol Pharmacol. 2023 Oct 30;37:03946320231209839.
54. Rawal S, Duong A, Landry I, Aluri J, Boyd P, Yagi T, et al. Absolute bioavailability of a single, fixed subcutaneous dose of lecanemab in healthy subjects. Alzheimer’s Dement. 2022;18(S10):e069438.
55. Hayato S, Takenaka O, Sreerama Reddy SH, Landry I, Reyderman L, Koyama A, et al. Population pharmacokinetic-pharmacodynamic analyses of amyloid positron emission tomography and plasma biomarkers for lecanemab in subjects with early Alzheimer’s disease. CPT Pharmacometrics Syst Pharmacol. 2022 Dec;11(12):1578–91.
56. Landry I, Kanekiyo M, Aluri J, Li D, Hussein Z, Reyderman L, et al. Lecanemab (ban2401) infusion reactions and immunogenicity: results from randomized phase 2 study and an open-label extension. Alzheimer’s Dement J Alzheimer’s Assoc. 2022;18(S10):e066289. Available from: https://alz-journals.onlinelibrary.wiley.com/doi/abs/10.1002/alz.066289
57. Wright AC, Lin GA, Whittington MD, Agboola F, Herron-Smith S, Rind D, et al. The effectiveness and value of lecanemab for early Alzheimer disease: a summary from the Institute for clinical and economic review’s California Technology Assessment Forum. J Manag Care Spec Pharm. 2023 Sep;29(9):1078.
58. ICER. ICER. [cited 2024 May 16]. ICER Publishes Final Evidence Report on Lecanemab for Alzheimer’s Disease. Boston, MA: ICER; 2024. Available from: https://icer.org/news-insights/press-releases/icer-publishes-final-evidence-report-on-lecanemab-for-alzheimers-disease/
59. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022 Apr;18(4):700–89.
60. Nguyen HV, Mital S, Knopman DS, Alexander GC. Cost-effectiveness of lecanemab for individuals with early-stage Alzheimer disease. Neurology. 2024 Apr 9;102(7):e209218.
61. Tahami Monfared AA, Tafazzoli A, Chavan A, Ye W, Zhang Q. The potential economic value of Lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2022 Sep 1;11(3):1285–307.
62. Jönsson L, Wimo A, Handels R, Johansson G, Boada M, Engelborghs S, et al. The affordability of lecanemab, an amyloid-targeting therapy for Alzheimer’s disease: an EADC-EC viewpoint. Lancet Regional Health—Europe [Internet]. 2023 Jun 1 [cited 2024 May 16];29:100657. Available from: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00076-5/fulltext
63. Lin GA, Whittington MD, Wright A, Agboola F, Herron-Smith S, Pearson SD, et al. Beta-amyloid antibodies for early Alzheimer’s disease: effectiveness and value; evidence report. Institute for Clinical and Economic Review; 2023. Available from: https://icer.org/assessment/alzheimers-disease-2022/#timeline
64. Frederiksen KS, Arus XM, Zetterberg H, Gauthier S, Boada M, Pytel V, et al. Focusing on earlier diagnosis of Alzheimer’s disease. Fut Neurol. 2024 Mar 26;19(1):2337452.
65. Cohen S, van Dyck CH, Gee M, Doherty T, Kanekiyo M, Dhadda S, et al. Lecanemab clarity AD: quality-of-life results from a randomized, double-blind phase 3 trial in early Alzheimer’s disease. J Prev Alzheimers Dis. 2023 Nov 1;10(4):771–7.
66. Tahami Monfared AA, Ye W, Sardesai A, Folse H, Chavan A, Aruffo E, et al. A path to improved Alzheimer’s care: simulating long-term health outcomes of Lecanemab in early Alzheimer’s disease from the CLARITY AD Trial. Neurol Ther. 2023 Jun 1;12(3):863–81.
67. Kurkinen M. Lecanemab (Leqembi) is not the right drug for patients with Alzheimer’s disease. Adv Clin Exp Med. 2023 Sep;32(9):943–7.
68. Qiao Y, Chi Y, Zhang Q, Ma Y. Safety and efficacy of lecanemab for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. Front Aging Neurosci [Internet]. 2023 May 5 [cited 2024 Mar 24];15:1169499. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1169499
69. What the FDA Approval of Lecanemab Means for Patients and Families: A Q&A with MBWC Clinicians - Memory and Brain Wellness Center [Internet] Seattle, WA: Memory and Brain Wellness Centre, UW Medicine, University of Washington. [cited 2024 Mar 21]. Available from: https://depts.washington.edu/mbwc/news/article/lecanemab
70. Yoon C, Groff C, Criss O. Lecanemab: a second in class therapy for the management of early Alzheimer’s disease. Innov Pharm [Internet]. 2024 Mar 18 [cited 2024 Mar 24];15(1). Available from: https://pubs.lib.umn.edu/index.php/innovations/article/view/5787
71. American Association for the Advancement of Science. Second death linked to potential antibody treatment for Alzheimer’s disease [Internet]. Washington, DC: American Association for the Advancement of Science; 2024 [cited 2024 Apr 10]. Available from: https://www.science.org/content/article/second-death-linked-potential-antibody-treatment-alzheimer-s-disease
72. Solopova E, Romero-Fernandez W, Harmsen H, Ventura-Antunes L, Wang E, Shostak A, et al. Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer’s disease. Nat Commun. 2023 Dec 12;14:8220.
73. Ko D, Pascual-Leone A, Shah SJ. Use of lecanemab for patients with cardiovascular disease: the challenge of uncertainty. JAMA [Internet]. 2024 Mar 15 [cited 2024 Mar 24];331:1089–90. Available from: https://doi.org/10.1001/jama.2024.2991