INTRODUCTION
Laser-assisted dentistry (LD), often shortened as laser dentistry, is a current opportunity to provide a wide range of oral and dental healthcare using different lasers (Diode, NdYag, Er:Yag, Argon and Erbium) [1–9]. The acronym “laser” stands for “light amplification by stimulated emission of radiation”. The laser produces a very narrow beam of electromagnetic radiation (light) that is monochromatic, collimated, and coherent and has been exploited for some innovative healthcare and dental technologies. Laser-tissue interaction may occur mainly by a process termed photothermolysis because of a direct and irreversible change in the tissues (by primarily protein denaturation) or by a non-ablative process termed photobiomodulation (by predominately stimulatory and biochemically mediated change).
The reported benefits of LD are less bleeding, less invasive, more precise, less painful, better healing, efficient appointments, and wow effect [1]. Other advantages of LD care have been reported: reduced inflammation, better tissue preservation, reduced need for anesthesia, minimized discomfort and postoperative care and complications, no effect on microbial drug resistance, faster healing time by minimizing trauma to surrounding tissues, versatility across a wide range of dental procedures, time-saving potential, and lower air contamination [2,5], compared to conventional dental care [10,11]. Until now, these advantages are sometimes supported by weak clinical efficacy [12–16], cost/benefit analysis [13,17–19], controversial information on molecular, cellular, and tissue mechanisms of action [1,8,20,21], and unclear duration of clinical effects [22].
Our group has been working on infection control and prevention (ICP) measures for years to reduce the possibility that infection can be transmitted from person to person or from hands or inanimate objects within dental environments, also known as cross-infections. Our particular attention is focused on the prevention of the transmission of infectious agents during the use of innovative dental technologies (CAD/CAM, laser, and surgery) or during infectious outbreaks in Italy (respiratory infections, Legionella, Mpox virus, infections caused by mosquitoes, and antibiotic-resistant infections) with possible repercussions for dentistry.
Concerning ICP in dentistry, LD reduces the source of infections. LD decreases the chance of bacterial infection in the gingival crevices, when used as a supplement of conventional periodontal care [1]. In particular, the Nd:YAG laser is mainly used for the disinfection of soft tissues, dental implants, and root canals, Nevertheless, other care with LD reveals some drawbacks during infection control. First, minimal benefit was found for the adjunctive use of laser and antimicrobial photodynamic therapy (aPDT) when compared to LD alone [21]. In addition, few data exists on the comparison between the efficacy of laser-associated care and aPDT and antibacterial agents, systemic antibiotics, probiotics, and a desiccant agent [21] and on the comparison with other activated irrigation in endodontics [22]. Finally, even if skin resurfacing procedures are considered safe, the infection has been reported for ablative and not ablative laser procedures, respectively, in 10% and 3% of adverse events reported from 1999 to 2013 [23], and infections (3.9%) are the main non-cosmetic complications during skin resurfacing procedures [24]. Herpes simplex virus reactivation, particularly after resurfacing of the perioral skin, and some common pathogens (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida) are responsible for post-treatment infections [25].
Despite the reported problems in brief on the safety and efficacy of LD, however, saving time, keeping pain levels low, and better healing time are important to sustain the urgent call for action for better global oral health stated by World Health Organisation (WHO) [26]. All in all, LD will be used increasingly. Today, an estimated 6% of general dentists have a laser for soft tissue applications. The market for dental lasers is expected to grow rapidly during the forecast period with a CAGR of 5.4% (2018–2028) to 9.44% (2022–2029) [27,28]. Then, the rapidly expanding use of LD requires exploring all aspects of laser safety. The occupational hazards associated with dental and medical laser applications remain poorly understood and uncharacterized, although 52% of injuries or deaths occurred in medical facilities [1]. Until now, concerning medical laser use, laser safety issues have mainly been related to the prevention of main tissue damage (eye, non-target oral tissue, and non-target skin) caused by laser beam, fire, and electric shock injuries, stated by the Regulatory framework [1], but minimal issues are on IPC.
This narrative review focuses on the ICP of laser-assisted dental care and includes 158 references. The search was adopted using some essential keywords from documents in databases (PubMed, Scopus) from 2010 to 2023 and the search strategy was reported in detail in the section “MATERIALS AND METHODS”. Concerning IPC, it is important to remember that the outlook for infectious risk is alarming (see section “better IFU and research priorities”), all dental patients are potentially infectious, and as with any other innovative oral-dental health technology, LD cannot claim to be free of healthcare-associated infections [7,8,29–32]. In general, limited data on adverse events and cross infections [33] have been reported for several reasons [34] in dentistry and, this fact is expected for newer technologies, such as lasers.
In terms of our aim, it is important to remember the definition of IPC as a scientific approach and practical solution designed to prevent harm from infection to patients and healthcare workers, following standard and transmission-based precautions [32,33,35]. The standard infection control precautions are the same for all dental technologies, but introducing new technologies, materials, equipment, and updated data requires continuous evaluation of current chemical and ICP and ongoing education of the oral health care team.
Recently, the Centers for Disease Control (CDC) stated that “adherence to infection prevention and control practices is essential to providing safe and high-quality patient care across all settings where healthcare is delivered” [30]. In addition to this, the recent global strategy on ICP indicated “high-quality services and care, and thus clean and safe care is a fundamental component of the right to health” and that “by 2030, everyone accessing or providing health care is safe from associated infections” [29]. Transmission of infectious agents among patients and dental health care personnel (DHCP) in dental settings has been documented rarely from 2003 to 2015 [33], but after 2015 not so rarely in outpatient dental settings (see epidemiological data on blood donors on HBV and HCV and outbreaks caused by Enterococcus, and Mycobacterium non-tuberculosis; antimicrobial resistant infections).
First, our review summarized the main features of different lasers important for ICP and the known advantages of ICP using LD (section “The main features of different lasers important for ICP and Current advantages in ICP using laser-assisted dentistry”). Concerning ICP during LD, the main precautions are the use of proper personal protective equipment (PPE), coaxial water spray, and high-flow suction systems (HVE evacuator) close to the surgical/application site during LD [1,10,32].
Then, the review focused on the updated evidence on ICP, including recent data on aerosol contamination with particulate matter (PM), which worsens indoor air quality, and important issues on reconditioning of laser fibers, tips, and accessories (such as safety goggles) [2,5,8,36].
Data on laser features and LD advantages are reported in sections “The main features of different lasers important for ICP and Current advantages in ICP using laser-assisted dentistry”.
Data on ICP using LD are reported and discussed in section “RESULTS AND DISCUSSION” and concern nine main areas:
- International guidelines and recommendations
- Preprocedural mouthwash before LD
- Indoor air quality during laser dentistry
- Use of PPE
- Hand hygiene
- Reconditioning of laser safety eyewear (LSE)
- Standards for the reconditioning of laser accessories
- Environmental IPC: from the clinical contact surface disinfection to the use of transparent barriers
- Limitations
- Perspectives.
- In addition, there are some subsections:
- Recommendations for indoor air quality in dentistry
- Clinical contact surface disinfection
- The use of transparent barriers
- Selection criteria for disinfectant for contact surfaces in dental clinics
- Better information for users (IFU) and Research priorities
- Infectious risk in dentistry a look to the future
- Airborne transmission of pathogens.
MATERIALS AND METHODS
Focused question
Is an extended recommendation for ICP required for laser-assisted care in the dental setting?
Search strategy
The PICO model (Table 1) (Population, Intervention, Comparison, and Outcome) was used to conduct this review, through a literature search of the PubMed (MEDLINE) and Scopus electronic databases, based on the following three aspects: population, concept, and context.
 | Table 1. This table outlines the PICO model followed. [Click here to view] |
The key indexing terms used, connected with Boolean operators OR, AND, were “patient safety”, “infection control”, “cross-infection”, “laser”, “endodontics”, “sterilization”, “reconditioning”, “critical items”, “semicritical items”, “hand hygiene”, “dental unit water line”, “sharps safety”, “PPE”, “disinfection”, “surgical smoke”, “plumes”, “indoor air”, “guidelines”, “cross-infection”, “needle-stick injuries”, “LSE”, “laser dentistry”, “aerosol”, “information from users”, “recommendation”, “safety”, and dental-care associated infections. Following this, bibliographic material from the papers was used to find other or older appropriate sources. Only some references do not have a DOI or PubMed classification, but the available Internet link and the date accessed have been added. Most of these last references belong to guidelines or IFU from producers.
Inclusion and exclusion criteria
The following inclusion criteria guided our analysis: (I) documents containing recommendations related to ICP in dental and healthcare settings: regulatory framework, guidelines, IFU, expert opinion; (II) study design—observational studies, interventional studies; ex-vivo study; (III) dental setting: clinical dental wards, simulation; (IV) laser-assisted dental care during COVID-19 pandemic. The analysis was limited to studies that satisfied the inclusion criteria. References were excluded for (I) non-method described; (II) duplications; (III) irrelevant data; (IV) content redundancy; (V) irrelevant articles (namely, reviews and articles whose more recent versions are available); (VI) national laws and rules; and (VII) studies with no freely accessible full texts.
Research
This paper is a narrative review regarding the recommendations and guidelines for IPC using LD. The electronic literature search was conducted via the PubMed (MEDLINE) and Google Scholar databases from January 2010 to December 2023. The data extraction process spanned approximately 6 weeks, with the final search conducted on 30/06/2024. Two independent, blind reviewers (L.B. and A.B.) conducted the search, and any disagreements or discrepancies were resolved through consensus or consultation with three additional reviewers (A.S., M.P., and F.S.). All titles and abstracts from the initial search were thoroughly reviewed, and studies that were not relevant were excluded. Relevant articles were listed and carefully examined for any similar studies meeting our inclusion criteria. The full texts of the included studies were thoroughly read, and their findings were documented.
RESULTS AND DISCUSSION
During the document search, the underestimation of ICP for LD became obvious, probably because LD is an unconsolidated and emerging technology, with non-homogeneous clinical procedures. We did not find any Cochrane reviews and protocols on 142 documents on Laser dentistry in the Cochrane Library (from 2017 up to 13/06/2024) (Supplementary Table 1). To make the difficulties of our research clearer, we checked the references of Chapter 5, of a recently published textbook, the most popular among dentists [1] (Supplementary Table 2). It contains 54 references, most (90%) were published within 2015, and only 3.7% between 2016 and 2022. Therefore, the need to include some references older than 1 year in our review was also foreseen.
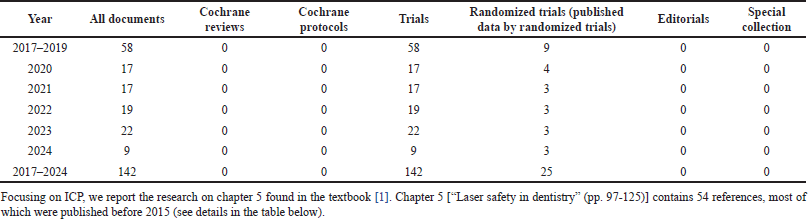 | Supplementary Table 1. Documents on laser dentistry in cochrane library.com/search (accessed on 13/06/2024). [Click here to view] |
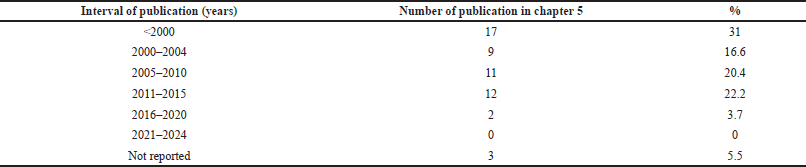 | Supplementary Table 2. Publication date range of the references reported in Chapter 5 entitled “Laser safety in dentistry” by Penny J. Parker and Steven P. A. Parker 2023, 2nd Ed. (pp. 97–125). [Click here to view] |
The main features of different lasers important for ICP
Laser wavelengths commonly available for use in dentistry are in the range visible of visible wavelengths (“Blue” InGaN 445 nm; “Green” GaN” KTP2 532 nm; “Red” GaAs 632/655 nm; InGaAIP 680 nm; GaA/As 810,830,970,980,1064 nm and InGaAsP 940 nm), Near Infrared (Nd:Yag 1064 nm and Nd:YAP 1340 nm); Mild Infrared (Er,Cr:YSGG 2780 nm and Er:YAG 2940 nm); Far Infrared (CO2 9300,9600,10600 nm) [1].
Briefly, from a strictly practical point of view, the main types within the laser family can be divided into three large groups: solid-state lasers (Nd: YAG, Er: YAG, ErCr: YSGG), gaseous-state lasers (CO2) and semiconductor lasers (diode lasers). Laser applications vary: some of them can be used for soft and hard tissues (Er: YAG, ErCr: YSGG), while others act only on soft tissues (CO2 and diode lasers). The Nd:YAG laser is mainly used for the disinfection of soft tissues, dental implants, and root canals, while its use for cutting hard tissues or treating caries remains controversial, because of the potential damage caused by the strong photo-thermal reaction [1]. Taking into account specifically ICP, briefly, we must consider that the laser features, in terms of power density (irradiance) and exposure time, range from different ablative applications (power density: 1–1012 W/sq cm; time 10-9-1 seconds) to sub-ablative applications (photochemical and photobiomodulation (PBM) effects; Power density: 1 W/sq cm; time 10-9-103 seconds) [1]. They produced different air contamination through different amounts of aerosol and laser plume (LP) [1,2,6]. Then, lasers have been divided into two groups:
- Non-aerosol producing (neodymium-doped yttrium aluminum garnet [Nd:YAG] during soft tissues cares, CO2 –10.3 nm, diodes);
- Aerosol-producing laser (erbium, chromium: yttrium, scandium, gallium garnet [Er,Cr:YSGG]; Erbiumdoped yttrium aluminum garnet [Er:YAG]; CO2 –9.3 nm).
In addition, we should consider that the light energy from a laser can have four different interactions (reflection, transmission, scattering, and absorption) with the target tissue, and these interactions will depend on the optical properties (in terms of type and amount of chromophores (hemoglobin, melanin, water, collagen, enamel, and protein) contained in oral and dental tissues and the photonic wavelength, power density (irradiance) of the beam and exposure time. The thermal effect of laser energy on tissue primarily revolves around the water content of tissue and the temperature rise of the tissue [1]. The observed effects are:
- Hyperthermia and bacterial inactivation (37°C–50°C)
- Coagulation and protein denaturation (>60°C)
- Welding (70°C–80°C)
- Vaporization (100°C–150°C)
- Carbonization (>200°C).
Finally, we cannot rule out the hazard because of microbial and blood contamination by hand touching and air contamination on the laser cart and all the external parts of the laser device, including the laser delivery systems (flexible fiber-optic systems, more rigid fiber glass, semiflexible hollow waveguides, or the articulated arm), the buttons (light button, indicator, and power/select button) on the cordless dental laser pen, the touch screen for the user control panel.
Current advantages in ICP using laser-assisted dentistry
The advantages on ICP using LD are shown in Table 2 [1–7,18,20–22,24,26,33,37–47].
 | Table 2. Main advantages of ICP using LD for dental patients and DHCP [1–7,18,20–22,24,26]. [Click here to view] |
Little is known about the incidence of bacteremia after LD [40]. In a case-control study on 22 patients, 68% had detectable bacteremia after ultrasonic scaling (US) alone, while 36% had detectable bacteremia following laser care plus US [48]. Despite the reduction of the incidence of odontogenic bacteremia, Streptococcus mitis, Streptococcus salivarius, and Streptococcus sanguis were frequently recovered from the bloodstream other than to Haemofhilus spp., Fusobacterium spp., Capnocytophaga spp., Bacterioides spp., and Prevotella melaninogenica. Nowadays, studies by MALDI-TOF mass spectrometry are needed to reveal bacteremia after LD. It is well known that some bacteria, entering the circulation, can produce a heart valve infection in susceptible individuals [49] and bacteremia follows different oral procedures [50]. In addition, we need data to sustain evidence-based recommendations for the use of antibiotic prophylaxis before invasive LD to prevent infective endocarditis [49].
Important areas for improving ICP using LD
In line with the recent WHO, CDC and Dental Safety Association (https://www.myads.org/) focus on ICP and the need to improve safety in dentistry, we have identified nine important areas and discussed the relative weaknesses of ICP using LD [1,2,8,16,30,31,35]. However, we believe that the stress of dental services and DHCP during the COVID-19 pandemic and many commercial interests have limited the studies, even if required by the EU Medical Device Regulation 2017/745, and the recommendations on ICP for LD.
International guidelines and recommendations
Guidelines relating to lasers mainly provide international standards for electrical, electronic, hazard prevention, and biosafety to avoid some tissue damage (eye, non-target oral tissue, and non-target skin). Recommendations are limited or generic when it comes to IPC [1,2,10,51] or are dated [32,51] and are mainly related to reducing aerosol by LD. Two recent documents focused on the ICP benefits of using various laser treatments in daily practice during the COVID-19 pandemic [2,52], and biosafety recommendations related to the type of laser are limited [3]. Knowledge of device functions and the choice of appropriate parameters are important to reduce aerosol and LP formation, as well as the selection of the proper precautions. It is important to choose optimal laser parameters that allow for both concomitant clinical effects and adequate cooling (~8–24 ml/minute), limited aerosol production, and microbial spread by reducing airflow and water spray [1,2,6]. However, the choice is difficult without detailed information for users (IFU) from producers, more interested in clinical efficacy. Concerning health settings, occupational hazards of DHCP caused by surgical LP and indoor air components are an increasing area of interest for international boards involved in ICP [10,51–55] and the CDC’s latest recommendation underscores the need to optimize the use of engineering controls for appropriate indoor air quality [56]. A recent review on the use of lasers in prosthodontics reported that aseptic techniques with strict adherence to the infection control protocols are necessary and it is crucial to prevent any potential cross-contamination or infections [11]. This includes proper sterilization of laser tips or handpieces or any instruments coming in contact during all procedures. Unfortunately, no details have been reported. It is essential that up-to-date guidance on key issues is available, in line with the regulatory framework and improved IFUs from manufacturers and, issued by statutory professional bodies. The laser safety manager and cross-infection control coordinator must work together and have a duty to ensure the safety of patients and the dental team concerning specific dental settings and the classification, type, and use of the laser. The issue of quality (clear guidance), transparency, and review of IFU is emerging [57]. 84% of cross-infection prevention coordinators have had to contact a manufacturer for clarification on the correct cleaning, disinfection, or sterilization of a product and 8% of them have taken the additional step of contacting the Food and Drug Administration for clarifications on the Instructions for Use.
Pre-procedural mouthwash before LD
Data have shown that pre-procedural mouthwashes containing antiseptic agents can help to partially reduce the bacterial or viral burden in the oral cavity or dental aerosols [58]. However, evidence-based efficacy of pre-procedural mouthwash to prevent SARS-CoV-2 and other virus transmission still is lacking [59,60]. The choice of pre-procedural mouthwash for LD deserves some attention. The high temperatures that can occur when using Class IV and certain Class IIIB lasers can themselves cause or contribute to the ignition of flammable dental materials [ethanol (flash point = 14°C), isopropanol (flash point = 11.7°C)] and gases [1]. In the absence of a recommendation, we agree with the indication of precaution described as “No-alcohol based liquids to be used in preparatory or laser-based treatments” to a low-risk assessment [1]. We think that it would be better to use alcohol-free mouthwash [Chlorhexidine digluconate (CHX): 0.05% plus Cetylpyridiniumchloride: 0.05%] or ensure that all oral areas treated with alcoholic mouthwash are completely dry before starting the procedure. This precaution is important because many CHX-based mouth rinses contain 11.6% up to 22.7% alcohol and povidone-iodine-based mouthwash contains 30.5% alcohol (w/v) and ethanol has a flash point of 14°C. In addition, studies are needed on the degradation of CHX by different laser light, increased temperature, and in the presence of other endodontic irrigants to toxic compounds (mainly p-chloroaniline, p-chlorophenylurea, and so on). In particular, P-chloroaniline, is considered to be carcinogenic (Hazardous Substances Data Bank -HSDB-: a database of the National Library of Medicines TOXNET System, 2014).
Indoor air quality during laser dentistry
Indoor air pollution (IAP) is a major cause of diseases (asthma, heart disease, stroke, lung cancer, and possibly dementia) [61] and the occupational consequences of IAP and surgical exposure to LP include irritation of the respiratory tract and eyes, headaches, nausea, and muscle weakness [1,62]. DHCP spend 30%–40% of their time in dental environments and therefore breathe potentially polluted indoor air. Then, researchers and policymakers are increasingly interested in the air quality of dental care facilities [2,4–8,63–65], in the multi-factorial causes and pathological consequences (risk of airborne diseases) of unclean indoor air, and obviously in preventive measures. According to the particle size, PM is classified into:
- coarse particles (particles with aerodynamic equivalent diameter less than 10 µm, PM10)
- fine particles (particles with aerodynamic equivalent diameter less than 2.5 µm, FPs/PM2.5)
- ultrafine particles [particles with aerodynamic equivalent diameter less than 100 nm, ultra?ne particles (UFPs)].
It is known that microbial contamination occurs mainly during aerosol-generating dental procedures (AGP); however, the understanding of the level, spread, and half-life of the contamination and the atomization mechanism is still limited [63,66–69]. Recently, data showed that air contamination (78%) is caused mainly by the cooling water from DUWL contaminated with biofilm [70]. In addition to microbial, endotoxin, and mycotoxin, the main indoor air contaminants are: PM of different dimensions (Ø: nanoparticulate (<1 μm); 1–2.5; 2.5–5; 5–10 μm), volatile organic compounds (VOC), CO2, SiO2, Be, Hg, heavy metals, As, Cd, and Ni [63]. The levels of CO2, PM2.5, and VOCtot present in the indoor air of dental offices are higher than in other healthcare settings [71,72]. A recent review provides an overview of inhalable PM-induced infectious diseases [73]. Interaction between airborne PM and bacteria makes humans more susceptible to otherwise harmless bacteria, particularly in the upper airways. Pathogenesis induced by PM exposure includes damage to airway epithelial cells, alteration of the immune response, dysregulation of the microbiota, and suppression of the host immune response [73]. Then, opportunistic and/or pathogenic bacteria (such as Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, P. aeruginosa, or Staphylococcus aureus) commonly found in dental settings may more easily establish respiratory infections and increase occupational risk.
Particulate, bacterial, and fungal aerosol contamination in five wards (pediatric, periodontics, prosthetics, restorative, and endodontics) of a university dental clinic is very complex [74].
Due to clinical benefits for dental practices, LD was proposed as a winning strategy during the COVID-19 pandemic. The contaminated area during Class I cavity preparation procedure is reduced by 70% using Er,Cr:YSGG laser compared to a high-speed turbine [75]. During caries treatment, the laser use reduced aerosol PM by approximately 50% in the patient area and approximately 10% in the dentist area, compared to using a dental turbine; no difference in indoor air contamination has been observed in the dental nurse area [76]. The number of aerosol particles (PM 0.3–10.0 μm) during caries treatment and debonding of the ceramic crown using three tested Er:YAG lasers is significantly reduced compared to conventional handpieces [5]. When HVE is running, the mean level of aerosol particles measured in the manikin’s mouths (the worst place for measurements) was 64.1, 55.1, 29.4–30.6 × 103, respectively, using a high-speed handpiece, a low-speed handpiece, and three lasers (Morita, Fotona, and LiteTouch). Additionally, using laser plus HVE, aerosol particle reduction is approximately about 45% compared to low-speed handpieces and 55% compared to high-speed handpieces [5]. When using lasers plus a saliva ejector, the reduction is roughly about 45% compared to low-speed handpieces, and 55% compared to high-speed handpieces. Furthermore, in the presence of good office air ventilation and HVE functioning, three Er:YAG lasers did not generate significant changes in aerosol PM levels during the debonding of the orthodontic bracket [5].
However, new data on indoor air contamination by LD do raise some doubts about the real advantages of aerosol prevention and biosafety [2–4,6–8,36,77,78]. The chemical and biological hazards of airborne components generated by lasers have been known since the first position paper on laser safety in dentistry [53]. It is known that explosive processes produce tissue ablation and aerosol formation, while thermal actions that create vaporization, produce a smoke plume, containing water (95%) and 5% containing blood, particulate, and microbial matter [2,6,69]. Nevertheless, the different characteristics of dental lasers (section “The main features of different lasers important for ICP”) influence AGP or smoke plume, and the lack of standard irradiation parameters in clinical applications make very difficult the comparisons between studies. Despite the limited available knowledge, we selected some interesting data in the case of different laser care (endodontic therapy, conventional dental procedures, and surgical care):
- Lasers are considered a valuable adjunct treatment in endodontic therapy [1,79,80]. Unfortunately, the evaluation of aerosols during root canal irrigation with all lasers tested showed insignificant differences compared to endodontic needle irrigation alone, and the LP could present the hazard of bacterial spread in simulated endodontic care [81,82]. Because of their strong water absorption, Er:YAG and Er, Cr:YSGG lasers are ideally suited for activating irrigating solution in endodontics by warming (5.5°C–7°C as the limit of acceptable temperature increases on the root surface) and cavitation [1]. Apart from a certain degree of confusion in the terminology used in photodynamic disinfection [1] and the variety of photothermal disinfection protocols, care should be taken to optimize cavitation dynamics, reduce collateral thermal effects on the roots, and avoid carbonization [1]. Importantly, when a liquid is heated and the kinetic energy of molecules increases, there is a reduction in viscosity and surface tension, allowing for better fluid flow and enhanced contact of irrigant solutions with the root canal walls. Unfortunately, this fact could increase the dispersion and airborne particulates, which are removed using HVE supplemented by auxiliary methods.
- More recently, highly increased levels (+40%) of air (UFP, PM0.1) are present during some conventional dental procedures (drilling, grinding, root canal filling), but also at very high levels during some laser periodontal treatments (30.000–250.000 UFP counts/cm3) in multi-chair dental clinics [78]. Despite the high variability, the mean and peak concentration of UFP by laser periodontal treatments is double that of those caused by US and like that by classical endodontic filling [78]. In this case, the efficacy of HVE might not be enough [78]. During some laser periodontal treatments, UFP contamination is like those caused by drilling, grinding, and root canal filling. Recently, Karvely investigated the levels of PM10 and PM2.5 in indoor air during the use of the laser (Er:YAG, 2,940 nm) for the preparation of cavities in human teeth under conditions of insufficient or absent ventilation [36]. During cavity preparation, levels of PM10 and PM2.5 are 10–15 times higher than contamination in the absence of dental activity. VOC, PM10, and PM2.5 levels are higher than what is deemed safe.
- The risk of LP hazards is expected during the use of all class IV lasers (surgical lasers) and in dental caries management [1]. For example, during Er:YAG laser use, any dentist has perceived the odor that spreads into the surrounding environment. The biological tissues vaporize at 100°C–200°C and the smoke is formed in the shape of a plume. 77% of the particles in the surgical smoke produced by lasers have a Ø < 1.1 μm with an average of 0.07 μm, which falls within the inhalable PM range [77]. The diameter of visible PM is at least 20 μm. Surgical LP (Ø 0.1–5 μm) can spread bacteria viruses, VOC (carbon monoxide, hydrogen cyanide, formaldehyde, benzene, and acrolein), and cancer cells [1,2–4,7,8,53–55,82]. A recent literature review identifies the potential hazards of surgical smoke in dentistry [82]. However, electrosurgical smoke seemed to be potentially more hazardous than laser smoke [82]. Although there is no evidence of lasers used in dental operating rooms, E. coli, Staphylococcus aureus, HPV, HIV, and HBV have been detected in surgical LP produced by simulated experiments, dermatology, and otolaryngology [6,53,68,81]. During the treatment of oral HPV-related lesions, DHCP must consider that the surgical LP is infectious due to its possible contamination with HPV-DNA [83]. VOC are also present when using low-power CO2 and Nd:YAG lasers [84]. DHCP should always remember that acrylonitrile hydrogen cyanide and many other VOCs are TOXIC, VOLATILE, and ODORLESS. Even if there are limited data on laser-generated air contaminants, including VOCs, in dental settings, it is expected the exposure and then both acute and chronic health effects in DHCW [1,85]. The detailed health effects of chemicals in surgical smoke and their exposure limits have been reported [82].
Recommendations for indoor air quality in dentistry
Waiting for better data on airborne contamination during LD to sustain evidence-based recommendations, the precautionary principle should be applied. Then, contaminated airborne hazards during LD, must be limited by using different procedures (HVAC, ventilation, evacuation (HVE as close as 1 cm from the target site), extraoral evacuation, and portable air purification system). The evidence is rather cloudy on their different efficacy when it comes to the control of dental indoor air when used alone or concurrently, and, above all, during LD [86–96]. It is well known that the evacuation system is designed to remove fluids, air/gas, and particulates with different efficacy. The following data are then expected. Evacuation systems should remove particles as small as 0.3 μm with at least 80% efficiency. However, during carbon dioxide laser surgery and using the HVE at 2 cm, the evacuation ratio decreases by 50% [85]. Then, it is uncertain whether it is effective to stay far away from 4 cm to remove the LP, based on old evidence [1].
More recent data on HVE efficacy are not reassuring: a) PM1 removal is null at 30 cm, b) PM>0.5, and PM10 removal is approximately 35% using HVE at 8,5 LPM [97,98]. The efficacy in reducing PM (PMtot 0.3–10.0 μm) depends on the type of saliva ejector type (intra vs. extraoral), its shape and diameter, and the distance from the source of contamination [76].
The main strategies (patient placement position; the position of air purification system) to prevent the spread of pathogens are shown in the section “dental facilities” of the latest recommendations [30,56], in addition to the ANSI/ASHRAE/ASHE Standard 170–2021 guidance on ensuring that ventilation systems operate properly, and other options to improve indoor air quality [97]. Concerning indoor air quality, the recommended levels of CO2, PM2.5, and VOC are < 800 ppm, 5 µg/m3, and <150 ppm, respectively [71,72]. For general dentistry, the current recommendation for ventilation, in terms of air changes per hour (ACH), is at least 6 for dental rooms, and 15 during the AGP [98]. The operation of ventilation and portable air purifiers is recommended up to 2 hours after the end of dental work. We also would like to briefly focus on indoor air quality monitoring systems [99], generally called sensors, which we are evaluating in our dental practices to control indoor air quality by CO2, VOC, and PM levels [76,100,101] and to check if the mitigation strategies (HVAC system, HVE use, ventilation, portable HEPA air purification systems, patient orientation) are working properly [102,103]. In addition to engineering control, they are useful for controlling the maintenance costs associated with HEPA filters on air filtration units. Three sensors are shown. They have reasonable costs (350–1000 €), but different parameters, range of use, and the check of specific VOC, partly suitable for LP detection (Table 3) [104–106]. Alternatively, airborne microbiological testing (by culture-based methods and MALDI-TOF MS) can be used [107]. These procedures are time-consuming, tedious, and more expensive for private dental practices, and the relationship between airborne bacteria and PM is unclear [108]. Wells–Riley equation has been used extensively to quantify the risk of indoor airborne infection, and the average indoor CO2 concentration should be kept below 700 ppm to control the risk of indoor airborne infection [109]. To delve deeper into the topic, we recommend the sections “better IFU and research priorities” to readers.
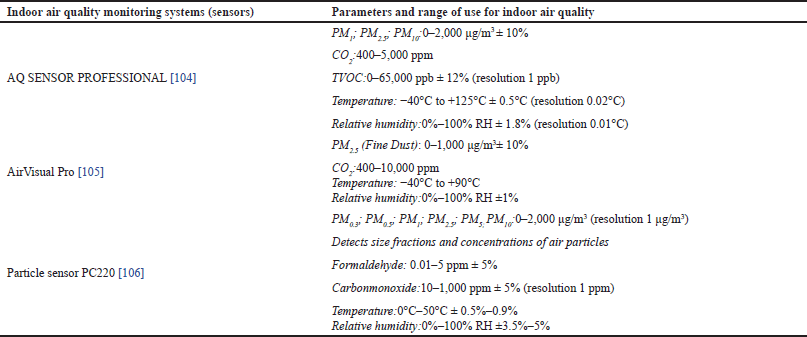 | Table 3. Examples of sensors for indoor air quality and use in private dental settings [76,100,101]. [Click here to view] |
Uses of PPE
PPEs are at the bottom of the hierarchy of defense and the last line of defense against IPC for all DHCP; for this reason, their quality related to filtration or isolation is determinant. In terms of occupational safety, PPE includes disposable clinical gloves, disposable well-fitting (tight face-fit) filtering facepiece (FFP) respirators, face shields/goggles/protective eyewear, and fire-resistant gowns. During LD, the main problem is related to the choice of surgical mask and FFP. During the COVID-19 pandemic, face masks were recommended by most documents (94%), with 91% also specifying the use of goggles or face shields [110]. Face masks (Type II or Type IIR) represent a mitigation strategy to lower airborne disease transmission. The use of N95/FFP2, which removes at least 95% of particles as small as 0.3 μm, is recommended during AGPs [56,111] and surgical procedures, in poorly ventilated areas according to NIOSH recommendation [112].
There is ambiguity regarding aerosol-generating lasers (erbium, chromium: yttrium, scandium, gallium garnet [Er,Cr:YSGG]; erbium-doped yttrium aluminum garnet [Er:YAG]; CO2 -9. 3 nm) and AGPs are “non-risk stratified for transmission” [113]. Although they are not yet included in the dental equipment known to generate aerosols and airborne contamination (ultrasonic scaler, high-speed dental handpiece, air/water syringe, air polishing, and air abrasion), we consider it reasonable that an N95 mask or FFP2 should be used to limit the inhalation of contaminated LP, in line with recent data reported in the section “Indoor air quality during laser dentistry”.
Fluid-resistant surgical facemask for routine care and FFP3 or hood for AGPs is recommended as respiratory protection for healthcare workers during the prevention of 24 suspected or confirmed pathogens showing droplet/airborne routes of transmission [114]. The major international and national health organizations, including the WHO, the European Centre for Disease Prevention and Control, the US CDC and Prevention, and Public Health England, have identified guidance on the reuse or prolonged use of surgical masks or FFP respirators only during the COVID-19 emergency. Surgical masks and FFP/N95 respirators are now considered disposable medical devices.
However, the effectiveness of these PPEs relies heavily on correct usage and a secure fit to the individual’s face. A false form of protection is to cover an N95-FFP respirator with an overlying face mask [115]. Face shields may reduce the inhalation of harmful aerosol particles for a short time, and, to a different extent, concerning the dimension of the particles [65]. When considering the choice and the use of FFP, we must consider that dental lasers have inherent antimicrobial properties and are expected to kill viruses or non-sporulating bacteria with which the beam comes in contact at temperatures in the range of 50°C–60°C [1]. This conclusion does not consider that many bacteria grow in oral biofilm and are much more resistant than planktonic bacteria. Periodontal pathogens are well known to be very resistant, highly adhesive, and biofilm-forming, and little is known about their thermostability [116]. Finally, temperatures (60°C–100°C), normally used for adjunctive nonsurgical periodontal and peri-implant disease laser therapy, do not affect the viability of spores [117,118] and unknown effects on some hyperthermophile infectious agents, multi-drug-resistant (MDR) microorganism, or those having sporulation genes or sulfate-reducing metabolism [119]. Then, it is not surprising that there is low-level evidence that adjunctive use of diode laser for scaling and root planning may provide some additional benefit in terms of reduction of red complex bacterial count and improvement in clinical periodontal parameters [12–16]. So much so that, different nanomaterials have been proposed for the photothermal killing of MDR bacteria to increase laser clinical efficacy [120].
Taking all from the IPC point of view, we cannot be sure that laser-assisted treatments destroy all periodontal bacteria and render what is produced without danger of cross-infection. Then, the choice and the correct use of PPE, in particular the adaptation to the face of the surgical face mask, FFP2 or FFP3, is important for DHCP during dental care. Recently, some researchers pointed out criticalities in the use of FFP2 masks related to different professional roles within the overall group of HCWs, stressing the need for an FFP2 human-centered design that accounts not only for physical needs but also for workload and task variability [121]. Finally, DCHP must be vigilant to avoid buying misrepresented respirators, which are all respirators that are falsely marketed and sold as NIOSH-approved respirators or have fake CE marks [122].
Hand hygiene
Hand hygiene is the main standard precautions [29,30,32,55]. Despite the relevant cost-savings associated with hand hygiene, compliance with hand hygiene remains low (educators: 63.7%–78.4%; students: 35.1%–45.8%) in dental school, and violations are significant, even during the COVID-19 pandemic [123,124] and increased attention to limit transmission. Unless hands are visibly soiled, an alcohol-based hand rub (ABHR; 70%–90% alcohol mixture) is preferred to soap and water in most clinical situations due to evidence of better compliance compared to soap and water [125,126]. Because of fire hazards [1], before LD, DHCP must ensure that all alcohol has evaporated and the ABHR bottle is perfectly closed and far from the laser beam. An open bottle of ABHR causes an early alarm for VOC using an AQ sensor [104].
Reconditioning of LSE
During laser care, LSE is needed for both the patient and DHCP [1]. They must have a CE marking, writing down compliance with the PPE regulations and the desired characteristics for laser beam protection [127]. According to PPE Regulation EU 2016/425, manufacturers must specify a rated shelf life and operational life for their products. Indicatively, safety glasses can technically last up to 3 years if they have not been compromised [128]. This creates added costs regarding laser treatments because of the need to replace them following IFU. Polycarbonate lenses are the most popular type used in LSE. They contain a wavelength-absorbent dye and a coating on the surface to limit scratches; scratches can cause the beam to burn through the filter material more quickly, thus causing ocular damage. The LSE would be reconditioned after use avoiding procedures that could cause the filters to crash and damage the elasticized band, required to secure the eyewear around the head. Some indications for LSE reconditioning are present in IFU from different producers, but they are unclear. In general, hand washing is allowed only with a gentle household cleanser. However, immersion or soaking is forbidden. Alternatively, alcohol (about 20% isopropanol) impregnated wipes could be used, while bleach is not recommended as it can damage the lens [129]. In general, it is advisable to be careful when using alcohol-based disinfectants compatible with synthetic materials and to follow the IFU. An IFU does not allow the use of harsh or acidic cleaners isopropyl alcohol or disinfectants [130]. Most LSEs can be adversely affected by disinfectant solutions (solutions of 70% alcohol) indicated for SARS-CoV-2 inactivation [131]. Products for cleaning and/or disinfection with a hydrogen peroxide (pH 2-3) base are never considered in IFU. Never use abrasive cloth or paper-based textiles and never place LSE in a steam autoclave, even when using the “plastic material cycle”. In general, IFU are scarce and focus more on material stability than IPC. See the section “Clinical contact surface disinfection” for the selection criteria for a disinfectant for clinical contact surfaces (as LSE).
Standards for the reconditioning of laser accessories
We agree with those indicated in the “standard 7” of recent guidance [52]. Steam sterilization must become the standard for the reconditioning of laser accessories (fiber, handpieces, and tips) in dentistry. Because improper reconditioning can damage equipment and accessories, producers must give clear indications in the IFU on cleaning, disinfection, and steam sterilization. Recently, some laser handpieces, in particular scalpels and laser handpieces, have some important features (autoclavable design, improved cleanability, reduced porosity of surface, and anodized finishing) leading to easier reconditioning phases. Currently, available handpieces are designed to meet the requirements for sterilization between patients and the ADA recommendations [10]. The main weaknesses of ICP in the three main phases of reconditioning (cleaning, disinfection, and sterilization) [132–134] are:
- A clear indication that plastic delivery tips (called mini tips or disposable tips) are single-use;
- Cleaning procedures of autoclavable tips and laser handpieces (often made of anodized aluminum alloy), with attention to the type of detergent/enzymatic cleaning solution with a neutral pH and to the limited compatibility with alkaline cleaners (normally used in washer-disinfectors);
- Many questions on cleaning procedures of optic fibers. Does it clean immediately after use or within a maximum of 1 hour after the LD? Does it use enzymatic and/or low-foaming detergents? Should aldehyde-based disinfectants be avoided to prevent occupational hazards (asthma) and the reduction of the light transmission of the laser fiber or its damage [133]?
- According to many IFUs, the laser fiber can be inserted in tabletop autoclaves EN 13060 Class B. In general, the cycle for thermolabile items is indicated (normally 121°C for 15 minute). However, the IFUs do not report the maximum number of steam cycles that guarantee the main optical and mechanical properties of the fibers and the connector. It should be noted that so far little data has been reported on the possible effects of sterilization on optical fiber properties [134]. In a steam autoclave, the fibers are exposed to the combination of high temperature and highly concentrated water vapor; this may cause cracking of the polymer coatings and/or deterioration of the glass cladding surface. The strength of the laser fiber also depends on the different coatings of the fiber (i.e., double acrylate, polyimide, silicone/PEEK, and fluoroacrylate/ETFE hard coating). Considering the properties of the laser fiber, the average strength of the fiber was found to be stable when the fibers were exposed to 20 cycles in a gravity autoclave (132°C for 8 minutes) [134]. However, multiple autoclaving cycles caused stress corrosion cracking of the acrylate fiber and slight changes in fiber spectral attenuation for silicone/PEEK and 200/HCS/ETFE fibers alone [134].
Environmental IPC: from the clinical contact surface disinfection to the use of transparent barriers
Clinical contact surface disinfection
DHCPs need to adopt surface disinfection (spray disinfectant/wipe/spray disinfectant decontamination method) [135] in the cases of:
- Large-diameter erbium fiber-optic cable that is not designed for steam sterilization;
- Frequent use of the fiber and handpiece compared to the slowness of the reconditioning process;
- Protective housing around the laser, including the control panel and articulating arm (if applicable), the dental cart, and countertops.
Following IFU, special care must be dedicated to the disinfection of laser devices, buttons, and touch screen, and their accessories before and after their use, and by selecting the appropriate disinfectant [fast active (1–2 minutes contact time), a broad spectrum of activities, compatible (containing 20% alcohol or less) for plastic, metals, and laser fibers] [135].
The use of transparent barriers
Further precautions must be taken, including the use of disposable transparent barriers (food use barriers, medical grade barriers, adhesive disposable protective film) for two main reasons. First, the aim is to protect the laser tips and avoid direct contact with oral tissues during low-level laser PBM; alternatively, the tip should be sterilized. Second, to shield the dental laser cart and countertop instrument, in particular, the switch on/off from environmental contamination and the contaminated glove touch of the clinicians at the end of LD. The reduction of laser output power (at red and infrared wavelengths) by using two latex-free protective materials [polyethylene and polyvinyl chloride (PVC)] has been reported by Nogueira Rodrigues et al. [136]. PVC is the most suitable for the protection of the tip of low-power lasers (both red and infrared wavelengths) because its main absorbance is in the range of 200–300 nm, while polyethylene absorbance has peaks at 720 nm, about 732 nm, about 1190 nm, and 1,250 nm [136].
Selection criteria for disinfectant for contact surfaces in dental clinics
IFU of lasers and its accessories contains limited indication about the importance of disinfection efficacy. The 2003 CDC guideline for dentistry specifies that after each patient the clinical contact surfaces (as LSE) must be cleaned and disinfected with a certified low-level (against HIV and HBV) or medium-level (against TBC) disinfectant when the surface is visibly contaminated with blood or other potentially infectious materials [32]. The list of the EPA can help DHCP with the choice of disinfectants [137]. The EPA (Environmental Protection Agency, USA) regulates the claims on?disinfectant product labels and periodically updates different Lists (indicated with S, C, D, E, F, N, Q, and K) for disinfectants. Each is a searchable and sortable list of products for use against specific infective agents or groups of them. For example, list N contains disinfectants for use against SARS-CoV2 and its variants, and the recent list S those against HIV, HBV, and HCV.
There are not so many appropriate commercial disinfectants (fast active (1-2 minute contact time), with a broad spectrum of activities, and compatible with plastic materials (containing 20% alcohol or less) [135]. Ensure that all areas treated with commonly used disinfectants, and potentially flammable liquid preparations are completely dry before starting LD. Be careful to use the commonly used disinfectants for clinical contact surfaces, because of ethanol and isopropanol concentrations are often in the range of 35%–62% and 1%–35%, respectively [135].
Limitations
The limitations of the present narrative review are the limited number of current studies and recommendations and the heterogeneity of the data specifically regarding ICP in LD, which makes a quantitative assessment of the data by meta-analysis impractical.
There is still important work to be done on ICP during LD in the future. In the dental setting, recommendations, guidelines, and legislation concerning standard precautions for ICP must be evidence-based or based on international best practices. Recommendations, guidelines, and regulations should then be developed in consultation with the dental team and extended to the laser safety manager and the IPC coordinator who are experts in clinical LD and updated on current laser technologies and ICP. We need to encourage dental staff to speak up and report adverse events, near misses, and technical difficulties during the decontamination and sterilization of laser parts or accessories. The Dental Patient Safety Foundation is a way to report patient safety events (adverse events, near misses, or unsafe conditions) safely, voluntarily, and confidentially [138]. This is a strategy to know errors and develop evidence-based recommendations. Regarding ICP during LD, research on aerosol generation is minimal [6]. Future research should include routine real-time monitoring of LP with appropriate sensors, which will provide valuable information to clarify the influence of different lasers and the relationship between LP, its composition (i.e., in terms of VOC, PM, and CO2), and potential toxicity and development of respiratory disease in DHCP. At the same time, the chemical toxicity and microbiological hazard of LP should be assessed using in vitro cellular studies and a lung-on-a-chip model, and finally at the organ level in animal models. Data are needed on amalgam melting by LD and the possible release of mercury vapor and laser fiber resistance [1].
Perspectives
In the reported sections, we reported the standard infection control precautions, the current limitation for application during LD, and if known, hazard severity. The summary of key and specific recommendations for ICP in LD, the rationale, and open questions are shown in Table 4. This table is expected to strengthen the operative applicability of those reported.
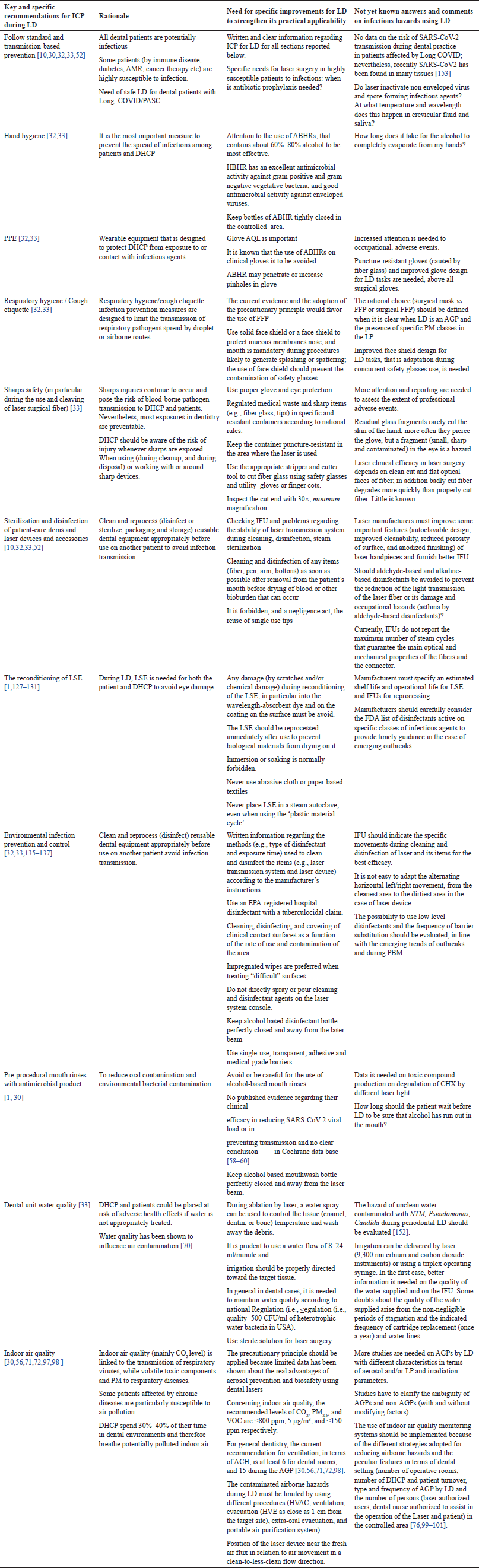 | Table 4. Summary of key and specific recommendations for ICP that are expected to strengthen its practical applicability, rationale and open questions concerning ICP in LD. [Click here to view] |
Better IFU and research priorities
As part of sound recommendations or best practices against IPC in LD, better, unambiguous IFU and deeper knowledge are needed. Recently, the WHO reported global research priorities for antimicrobial resistance in human health. In panel 5, infection prevention and control shows a high research priority score (0.90) [139].
We think that better and more clear IFUs from producers, in line with the requirements of the Regulations EU 2017/745, are needed for:
- The reconditioning (cleaning-disinfection-sterilization) of dental laser components, parts, and accessories;
- Laser fiber and connector stability after steam autoclave cycles;
- The reconditioning of LSE and its shelf life and working life according to PPE Regulation [93].
For the evidence-based recommendation for ICP, research priorities are :
- To clarify the ambiguity of AGP and non-AGP (with and without modifying factors) [113] during the use of lasers in dentistry;
- To quantify the air and aerosol contamination using different types of lasers and different clinical protocols;
- To select evidence-based standard activities for better air quality on the components of LP, particularly those caused by laser-assisted endodontics, periodontics, and surgery, focusing on microorganisms, VOC, and PM contamination;
- To develop air sensors suitable and sensitive enough to identify the main VOCs produced by LD;
- To verify the efficacy of FFP2 and surgical mask during LD.
Looking to the future, molecular biology tests, sensors for indoor air quality in dental settings, and artificial intelligence (AI) will be very useful approaches to investigation. AI is profoundly transforming dentistry. For our specific objective, it is expected to improve the diagnostic accuracy of microbially caused oral diseases, while molecular biology plus AI the extent of bacteremia after LD. Mainly, AI will be useful for monitoring the application of standard precautions (e.g., procedure and duration of hand washing, disinfection, and so on) or the quality of indoor air interfaced with automatic ventilation modulation systems.
Infectious risk and cross-infection hazard in dentistry: a look to the future
Predicting infectious hazards is very complicated. Nevertheless, the outlook for infectious risks is alarming [140–142] and SARS-CoV2 different variants continue to go on spreading. Climate and land-use changes are predicted to increase the frequency of zoonotic spillover events, which have been the cause of most modern epidemics. Meadows’s group estimated that some pathogens (SARS Coronavirus 1, Filoviruses, Machupo virus, and Nipah virus) would cause four times the number of spillover events and 12 times the number of deaths in 2050, compared with 2020 [140]. So the real question is, not if there will be a pandemic, but when there will be. Other causes of disease emergence are: changes in human demographics and behaviors (i.e., HIV, tubercolosis, and Mpox clade 2), international travel and commerce (SARS, mPox clade 1), health care technology (Enterococcus, HCV, HBV, and so on), and microbial changes (infections with antibiotic-resistant strains). In addition, we live in a very dynamic word for infective agents [143]. The 2024 WHO BPPL covers 24 pathogens, spanning 15 families of antibiotic-resistant bacterial pathogens. Remarkable among these are Gram-negative bacteria resistant to last-resort antibiotics, at least three of them are of dental interest (drug-resistant strains of Mycobacterium tuberculosis, P. aeruginosa, and Staphylococcus aureus). But on the podium of the winners of the 2024 BPPL update, there are also Klebsiella pneumoniae and Acinetobacter baumannii, which are “new entries” for ICP in dentistry [143–145]. Fungal infections are continually increasing and oral Candida infection is certainly the most widespread mycosis in denture wearers, immunosuppressed patients, and patients with saliva secretory dysfunctions. Candida albicans and Candida auris are included in the WHO fungal pathogens priority list [146] and also C. auris seems to be present in denture wearers and adheres to dental implant materials [147]. The risk of new pandemics will recur, and the economic consequences are of concern to all economic and healthcare activities, including dentistry. Dentistry is also concerned with emerging pathogens (i.e., Enterovirus, Candida auris, measles virus, and so on) [142,148,149] and many of them could be a hazard for elderly and susceptible persons. Some NHS and epidemiologists reported that measles outbreaks is expected in the near future [149]. The new mutations of SARS-CoV-2 virus indicate that it remains a bit of a wild card, where it is always hard to predict what he will do next. Dental care-associated infections do not seem so rare in the last years [33,49,148–152]. The prospect caused by new SARS-CoV2 variants and the long-term presence of SARS-CoV2 in many tissues during Long COVID is not reassuring [153]. Antimicrobial resistance is a global public health hazard with serious economic consequences [49,50]. Photodynamic and photothermal therapy seems a promising strategy to combat antimicrobial-resistant bacteria [21,22,154].
Airborne transmission of pathogens
First, it is important to note that R0, referred to as the reproduction number, is a mathematical term that indicates how contagious an infectious disease is; R0 is in the range 0–18. For measles, poliomyelitis/rhinovirus/small pox, SARS-Co-V2, and influenza H1N1, the estimated R0 values are 18, 6, 3, and 1.5, respectively.
Then, numerous respiratory and other viruses replicate in the oral cavity and are transmitted via aerosol or “droplet nuclei” (5–100 μm) and mainly at a distance of >1 to 2 m away from the infected individual and via droplets (>100 μm) [155]. Droplets can travel less than 1 meter, and fall to the ground in under 5 seconds. SARSCoV, influenza-, parainfluenza-, metapneumo-, rhino-, adeno-, and respiratory syncytial viruses are transmitted by aerosols and droplets, as well as direct and indirect contact (fomites). Other diseases spread by respiratory and oral fluids are: tubercolosis, diphtheria, pneumonia, meningitis, sinusitis, conjunctivitis, bacterial bronchitis, CMV disease, mononucleosis, measles, rubella, and mumps. In particular, mycobacterium and rubeola (measles) virus are considered aerosol transmitted. Aerosols produced by an infected individual may contain infectious viruses, and studies have shown that viruses are enriched in small aerosols. The transport of virus-laden aerosols is affected by the physicochemical properties of aerosols themselves and environmental factors (temperature, relative humidity, ultraviolet radiation, airflow, and ventilation). Aerosols up to 100 mm can be inhaled. Depending on their size, they deposit in different regions of the respiratory tract, based on one of several key mechanisms (inertial impaction, gravitational sedimentation, Brownian diffusion, electrostatic precipitation, and interception). Aerosols >5 mm deposit primarily (87% to 95%) in the nasopharyngeal region. Only aerosols that are sufficiently small (0.001–2 μm, mainly in the range 0.001–0.3 μM) can reach and deposit in the alveolar region. Recent data shows that ambient CO2 concentration correlates with SARS-CoV-2 aerostability and infection risk [156]. Higher aerostability results also from a moderate increase in the atmospheric CO2 concentration (e.g., 800 ppm).
Recently, Zemouri’s group showed data on the modeling of the transmission of Coronaviruses, Measles Virus, Influenza Virus, Mycobacterium tuberculosis, and Legionella pneumophila in Dental Clinics (ref) in a high scenario [(The DHCP does not wear a medical face mask, nor covers the nose with the medical face mask and works in poor indoor air quality (>1,500 PPM CO2)] and low-risk scenario (The DCHP wears an FFP-2 mask and works in good indoor air quality (400–800 PPM CO2) [157]. The high-risk scenario leads to the highest transmission probabilities of measles virus (100%), coronaviruses (99.4%), influenza virus (89.4%), and M. tuberculosis (84.0%). The low-risk scenario leads to transmission probabilities of 4.5% for the measles virus and 0% for the other pathogens. From the sensitivity analysis, the transmission probability is strongly driven by indoor air quality, followed by patient infectiousness, and the least by respiratory protection from medical face mask use. Nevertheless, in-depth studies are necessary to evaluate the influence of saliva composition on the decay of viral viability and viral transmission.
Up to now, great progress has been made in understanding the adverse effects of ambient fine and ultrafine PM on human health [73,158]. However, more studies are needed on the effects of them on various organs and their specific action mechanisms.
Finally, it is known that DHCPs work and are exposed in an area within 1.5–2 m of the source of contamination (patient’s head). We think that the data reported in the section “Indoor air quality during laser dentistry”, relating to aerosol and PM production during LD, do not allow us to exclude the hazard of airborne transmissible infections.
CONCLUSION
Epidemiological data estimated for the future indicate that dental teams must learn to live with an infectious risk in dentistry and LD. Unfortunately, it will be variable, fluctuating and intermittent, sometimes known and re-emerging, and at other times unknown and emerging. ICP’s goal will always remain the same: prevent infections for vulnerable patients in a vulnerable context.
In general, the debate on the safety of LD is silenced, perhaps caused by the many commercial interests. But today more than ever, 360° safety is essential, not optional, for whatever dental technology, including LD and IPC. IPC is one of the main strategies for patient and occupational safety. LD partly has some advantages (low AGP, drug-free, and reduction of drug prescriptions), but cannot be considered completely free of dental-care-associated infections. The review aims to throw a stone into the dormant water of LD for IPC. Our review shows that current recommendations for LD have been limited or generic to IPC or are outdated.
Unfortunately in our review, some burning questions remain unanswered due to the limitations of available data. Some experts exclude adverse events during LD in DHCP, but are they right with recent data on PM contamination during LD? How long could we passively wait for more reliable data on aerosol contamination to support evidence-based recommendations? Available data shows reduced bacteremia after periodontal LD, but remains of concern the infective endocarditis is secondary to its level. There are no data on bacteremia during surgical LD, but can we exclude it? Rationally, we cannot. More in-depth research is needed that takes advantage of the potential of molecular biology, sensors, and AI.
We have reported the available information, the weak points, and useful information for making informed choices for the prevention of cross-infection, often supported by the precautionary principle. We hope our specific indications are useful for the activities of the infection control coordinator, laser safety manager, and dental teams.
We hope that our review is a useful “crutch” while waiting for an evidence-based recommendation or at least an updated Ad Interim best practice on IPC in LD issued by accredited international organizations (CDC, OSAP, UK NHS, and so on), in line with recent WHO indications for IPC and the legal requirements for healthcare safety. More clear and detailed IFU for ICP should be available for any type of laser medical device and laser care in line with the requirements of the Regulations EU 2017/745 and someone should check the documents before the laser commercialization.
ACKNOWLEDGMENTS
The authors would like to thank Maté Jarai (Creative Writing Ma Creative Writing Bachelor’s in Philosophy, UK), for English language revision. The last version has been revised by Write Full and Grammarly.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE)requirements/guidelines.
FINANCIAL SUPPORT
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF AI-ASSISTED TECHNOLOGY
The authors declare that they have not used AI-tools for writing and editing the manuscript, and no images were manipulated using AI.
REFERENCES
1. Coluzzi DJ, Parker SPA. Lasers in dentistry—current concepts. 2nd ed. Cham, Switzerland: Springer Nature; 2023. CrossRef
2. Arnabat-Dominguez J, Del Vecchio A, Todea C, Grzech-Le?niak K, Vescovi P, Romeo U, et al. Laser dentistry in daily practice during the COVID-19 pandemic: benefits, risks, and recommendations for safe treatments. Adv Clin Exp Med. 2021;30:119–25. CrossRef
3. Neves Lago AD, Cordon R, Machado Gonçalves L, Sousa Menezes CF, Silva Furtado G, Nogueira Rodrigues FC, et al. How to use laser safely in times of COVID-19: systematic review. Spec Care Dentist. 2021;41:463–73. CrossRef
4. Malekzadeh M, Zare H. Laser in dentistry during COVID-19 pandemic: a brief review of literature. Avicenna J Dent Res. 2022;14:96–101. CrossRef
5. Grzech-Lesniak K, Matys J. The effect of Er:YAG lasers on the reduction of aerosol formation for dental workers. Materials. 2021;14:2857. CrossRef
6. Kumar NK, Thomas PM, Sowmya KR, Yavagal C, Hariprasad L, Preetham HS. Laser: a boon during the COVID pandemic in aerosol mitigation—a systematic review. J Indian Assoc Public Health Dent. 2023;21:4–10. CrossRef
7. Hamedani S, Farshidfar N, Ziaei A. Application of high-power lasers in dentistry during COVID-19 outbreak: an equivocal issue. Int J Med Rev. 2022;9:283–7.
8. Mortazavi H, Baharvand M, Mokhber-Dezfuli M, Rostami N, Doost-Hoseini M, Alavi O, et al. Lasers in dentistry: is it really safe? Dent Hypotheses. 2016;7:123–7. CrossRef
9. Barenghi A, Barenghi L, Pulicari F, Pellegrini M, Di Blasio A, Spadari F. Problems and perspectives on accelerated orthodontic tooth movement by low-level laser photobiomodulation. Dent Res Oral Health. 2023;6:21–4. CrossRef
10. Australian Dental Association (ADA). Guidelines for the use of lasers in dentistry. Sydney, Australia: ADA [cited 2024 Jun 15]. Available from: https://www.ada.org.au/ADA-guidelines-lasers-in-dentistry-Doc.aspx
11. Binrayes A. An update on the use of lasers in prosthodontics. Cureus. 2024;16:e57282. CrossRef
12. Pardo A, Butera A, Giordano A, Gallo S, Pascadopoli M, Scribante A, et al. Photodynamic therapy in non-surgical treatment of periodontitis: a systematic review and meta-analysis. Appl Sci. 2023;13:1086. CrossRef
13. Mills MP, Rosen PS, Chambrone L, Greenwell H, Kao RT, Klokkevold PR, et al. American Academy of Periodontology best evidence consensus statement on the efficacy of laser therapy used alone or as an adjunct to non-surgical and surgical treatment of periodontitis and peri-implant diseases. J Periodontol. 2018;89(7):737–42. CrossRef
14. Patel S, Awan KH, Freitas CMT, Bhandi S, Licari FW, Patil S. Diode laser targeting red-complex bacteria in periodontitis: a systematic review. Eur Rev Med Pharmacol Sci. 2023;27:11806–16.
15. Mulder-van Staden S, Holmes H, Hille J. In vivo investigation of diode laser application on red complex bacteria in non-surgical periodontal therapy: a split-mouth randomised control trial. Sci Rep. 2020;10:21311. CrossRef
16. Theodoro LH, Chiérici Marcantonio RA, Wainwright M, Garcia VG. LASER in periodontal treatment: is it an effective treatment or science fiction? Braz Oral Res. 2021;35:e099. CrossRef
17. Pearson S. The effectiveness of laser application in stage 3/4 periodontal disease. Br Dent J Team. 2023;10:10–12. CrossRef
18. Clem DS, Heard R. Treatment of periodontal diseases with laser: assessing the evidence. Compend Contin Educ Dent. 2021;42(1):44–6.
19. Clem D, Heard R, McGuire M, Scheyer ET, Richardson C, Toback G, et al. A comparison of Er,Cr laser to minimally invasive surgical technique in the treatment of intrabony defects: twelve-month results of a multicenter, randomized, controlled study. J Periodontol. 2024 Jul;95(7):621–31. CrossRef
20. Santonocito S, Polizzi A, Cavalcanti R, Ronsivalle V, Chaurasia A, Spagnuolo G, et al. Impact of laser therapy on periodontal and peri-implant diseases. Photobiomodul Photomed Laser Surg. 2022;40(7):454–62.
21. Barbato L, Cavalcanti R, Rupe C, Scartabelli D, Serni L, Chambrone L, et al. Clinical efficacy of adjunctive methods for the non?surgical treatment of peri?implantitis: a systematic review and meta?analysis. BMC Oral Health. 2023;23:375. CrossRef
22. Cheung AWT, Lee AHC, Cheung GSP. Clinical efficacy of activated irrigation in endodontics: a focused review. Restor Dent Endod. 2021;46(1):e10. CrossRef
23. Tremaine AM, Avram MM. FDA MAUDE data on complications with lasers, light sources, and energy-based devices. Lasers Surg Med. 2015;47:133–40. CrossRef
24. Halepas S, Lee KC, Higham ZL, Ferneini EM. A 20-year analysis of adverse events and litigation with light-based skin resurfacing procedures. J Oral Maxillofac Surg. 2020;78(4):619–28. CrossRef
25. Prohaska J, Hohman MH. Laser complications. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Aug 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532248/
26. WHO. Global oral health status report: towards universal health coverage for oral health by 2030. Geneva, Switzerland: WHO; 2024 [cited 2024 Jun 16]. Available from:: https://www.who.int/publications/i/item/9789240061484
27. Data Bridge Market Research. Global dental lasers market size, share, and trends analysis report—industry overview and forecast to 2031. Pune, India: Data Bridge Market Research; 2024 [cited 2024 Jun 16]. Available from: https://www.databridgemarketresearch.com/reports/global-dental-lasers-market
28. Mordor Intelligence. Dental lasers market size & share analysis—growth trends & forecasts (2024-2029). Hyderabad, India: Mordor Intelligence; 2024 [cited 2024 Jun 16]. Available from: https://www.mordorintelligence.com/industry-reports/dental-lasers-market
29. WHO. Global strategy on infection prevention and control. Geneva, Switzerland: WHO; 2023 [cited 2024 Jun 04]. Available from: https://www.who.int/publications/m/item/global-strategy-on-infection-prevention-and-control
30. CDC. CDC’s core infection prevention and control practices for safe healthcare delivery in all settings. Atlanta, GA: CDC; 2024 [cited 2024 Jun 20]. Available from: https://www.cdc.gov/infectioncontrol/guidelines/core-practices/index.html
31. ECDC. Consideration for infection prevention and control in relation to respiratory viral infections in health care settings. Solna, Sweden: ECDC; 2024 [cited 2024 Jun 06]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Considerations%20for%20IPC%20respiratory%20viral%20infections%20in%20HC%20settings.pdf
32. CDC, USA. Guidelines for infection control in dental health-care settings—2003. Atlanta, GA: CDC; 2024 [cited 2024 Jun 04]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.html
33. CDC, USA, Dept of Health and Human Services. Summary of infection prevention practices in dental settings: basic expectations for safe care. Atlanta, GA: Centers for Disease Control and Prevention; 2016 [cited 2024 Jun 04]. Available from: https://www.cdc.gov/oralhealth/infectioncontrol/summary-infection-prevention-practices/index.html
34. Padmanabhan V, Islam MS, Rahman MM, Chaitanya NC, Sivan PP. Understanding patient safety in dentistry: evaluating the present and envisioning the future—a narrative review. BMJ Open Qual. 2024;13:e002502. CrossRef
35. World dental Federation (FDI) Policy Statement. Revision: to be adopted by the general assembly 27-29 September 2021, Sydney, Australia. Adopted by the general assembly: September 2019, San Francisco, United States of America. Original version adopted by the General Assembly: September 2009, Singapore, Singapore. Infection prevention and control in dental practice Adopted by the General Assembly. Int Dent J. 2020;70:17–8. Available from: https://www.fdiworlddental.org/sites/default/files/2021-10/EN%20-%20WDPS6_Infection%20Prevention%20and%20Control%20in%20Dental%20Practice.pdf
36. Karveli A, Tzoutzas IG, Raptis PI, Tzanakakis EGC, Farmakis ETR, Helmis CG. Air quality in a dental clinic during Er:YAG laser usage for cavity preparation on human teeth—an ex-vivo study. Int J Environ Res Public Health. 2021;18:10920. CrossRef
37. Pereira MC, Weber Mello F, Ribeiro DM, Porporatti AL, da Costa Junior S, Flores-Mir C, et al. Prevalence of reported percutaneous injuries on dentists: a meta-analysis. J Dent. 2018;76:9–18. CrossRef
38. Huang J, Li N, Xu H, Liu Y, An N, Cai Z. Global prevalence, risk factors, and reporting practice of needlestick and sharps injuries among dental students: a systematic review and meta-analysis. J Hosp Inf. 2022;129:89–101. CrossRef
39. Iwamatsu-Kobayashi Y, Watanabe J, Kusama T, Endo H, Ikeda S, Tokuda K, et al. A 19-year study of dental needlestick and sharps injuries in Japan. Int Dent J. 2023;73:114–20. CrossRef
40. Kaminer R, Liebow C, Margarone JE 3rd, Zambon JJ. Bacteremia following laser and conventional surgery in hamsters. J Oral Maxillofac Surg. 1990;48:45–8. CrossRef
41. Henneberry K, Hilland S, Haslam S. Are dental hygienists at risk for noise-induced hearing loss? A literature review. Can J Dent Hyg. 2021;55:110–9.
42. Kawtharani A, Chemeisani A, Salman F, Younes AH, Msheik A. Neck and musculoskeletal pain among dentists: a review of the literature. Cureus. 2023;15:e33609. CrossRef
43. Soo SY, Ang WS, Chong CH, Tew IM, Yahya NA. Occupational ergonomics and related musculoskeletal disorders among dentists: a systematic review. Work. 2023;74:469–76. CrossRef
44. Lin K, Wink C, Dolan B, Osann K, Habib AA, Gehring J, et al. A novel ergonomic curette design reduces dental prophylaxis-induced muscle work and fatigue. Dent J. 2023;11(12):272. CrossRef
45. Kaur M, Thakur V, Bhalla M. Dental laser: a boon in dentistry & its significance in COVID-19. J Curr Med Res Opin. 2020;3:682–91. CrossRef
46. Smith WAJ, Al-Bayaty HF, Matthews RW. Percutaneous injuries of dental personnel at the University of the West Indies, school of dentistry. Int Dent J. 2006;56:209–14. CrossRef
47. Dukka H, Byrd P, Qian C, Baughman G, Butt S, Rai SN. Occupational percutaneous injuries and exposures in a dental teaching environment: a 10-year report. J Dent Educ. 2021;85:1729–38. CrossRef
48. Assaf M, Yilmaz S, Kuru B, Dirikan S, Noyun U, Kadir T. Effect of the diode laser on bacteremia associated with dental ultrasonic scaling: a clinical and microbiological study. Photomed Laser Surg. 2007;25(4):250–6. CrossRef
49. Thornhill M, Prendergast B, Dayer M, Frisby A, Peter Lockhart P, Baddour LM. New evidence calls into question NICE’s endocarditis prevention guidance. Br Dent J. 2024;236(9):702–8. CrossRef
50. Martins CC, Lockhart PB, Firmino RT, Kilmartin C, Cahill TJ, Dayer M, et al. Bacteremia following different oral procedures: systematic review and meta-analysis. Oral Dis. 2024;30(3):846–54. CrossRef
51. Medicines and Healthcare products Regulatory Agency, UK. Lasers, intense light source systems and LEDs—guidance for safe use in medical, surgical, dental and aesthetic practices. London, UK: Medicines and Healthcare products Regulatory Agency; 2024 [cited 2024 Jun 04]. Available from: https://www.gov.uk/government/publications/guidance-on-the-safe-use-of-lasers-intense-light-source-systems-and-leds
52. Dubai Health Authority. Standards for the use of laser in dentistry. Standard 7. Sterilization and infection control. Dubai, United Arab Emirates (UAE): Dubai Health Authority [cited 2024 Jun 27]. Available from: https://www.dha.gov.ae/uploads/042022/Standards%20for%20Laser%20in%20Dentistry2022459602.pdf
53. Laser Safety Committee, Academy of Laser Dentistry. Laser safety in dentistry: a position paper. J Laser Dent. 2012;20:2.
54. CDC-NIOSH, USA. Health and safety practices survey of healthcare workers. Surg Smoke. 2015. [cited 2024 Jun 04]. Available from: https://www.cdc.gov/niosh/topics/healthcarehsps/smoke.html#print
55. CDC, USA. Surgical smoke inhalation: dangerous consequences for the surgical team. Atlanta, GA: CDC; 2024 [cited 2024 Jun 04]. Available from: https://blogs.cdc.gov/niosh-science-blog/2020/06/18/surgical-smoke/
56. CDC, USA. Interim infection prevention and control recommendations for healthcare personnel during the Coronavirus Disease 2019 (COVID-19) pandemic. Atlanta, GA: CDC; 2024 [cited 2024 Jun 04]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
57. APIC. Modernizing medical device instructions for use (IFUs). Arlington, VA: APIC; 2024. Available from: https://apic.org/modernizing-medical-device-instructions-for-use-ifus/
58. Weber J, Bonn EL, Auer DL, Kirschneck C, Buchalla W, Scholz KJ, et al. Preprocedural mouthwashes for infection control in dentistry—an update. Clin Oral Investig. 2023;27(Suppl 1):33–44. CrossRef
59. Hasan F, Chiu HY, Salamanca E, Ridwan ES, Wiratama BS, Budi HS. Effects of chlorhexidine and povidone-iodine on the SARS-CoV-2 load: a systematic review and meta-analysis. Eur J Dent. 2023;17:587–601. CrossRef
60. Rahman GS, Alshetan AAN, Alotaibi SSO, Alaskar BMI, Baseer MA. Is chlorhexidine mouthwash effective in lowering COVID-19 viral load? A systematic review. Eur Rev Med Pharmacol Sci. 2023;27:366–77.
61. Van Tran V, Park D, Lee YC. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int J Environ Res Public Health. 2020;17:2927. CrossRef
62. Polednik B. Exposure of staff to aerosols and bioaerosols in a dental office. Build Environ J. 2021;187:107388. CrossRef
63. Chatoutsidou SE, Saridaki A, Raisi L, Katsivela E, Tsiamis G, Zografakis M, et al. Airborne particles and microorganisms in a dental clinic: variability of indoor concentrations, impact of dental procedures, and personal exposure during everyday practice. Indoor Air. 2021;31:1164–77. CrossRef
64. Cocârta DM, Prodana M, Demetrescu I, Lungu PEM, Didilescu AC. Indoor air pollution with fine particles and implications for Workers’ Health in Dental Offices: a brief review. Sustainability. 2021;13:599. CrossRef
65. Tzoutzas I, Karoussis I, Maltezou HC. Air quality in dental care facilities: update to current management and control strategies implementing new technologies: a comprehensive review. Vaccines. 2022;10:847. CrossRef
66. Dabiri D, Conti SR, Pour NS, Chong A, Dadjoo S, Dabiri D, et al. A multi-disciplinary review on the aerobiology of COVID-19 in dental settings. Front Dent Med. 2021;2:726395. CrossRef
67. Nóbrega TMC, da Rosa MoreiraBastos RT, Mecenas P, de Toledo IP, Richardson-Lozano R, Altabtbaei K, et al. Aerosol generated by dental procedures: a scopingreview. J Evid Based Med. 2021;14:303–12. CrossRef
68. Kayahan E, Wu M, Van Gerven T, Braeken L, Stijven L, Politis C, et al. Droplet size distribution, atomization mechanism and dynamics of dental aerosols. J Aerosol Sci. 2022;166:106049. CrossRef
69. Kumar P, Subramanian K. Demystifying the mist: sources of microbial bioload in dental aerosols. J Periodont. 2020;91:1113–22. CrossRef
70. Meethil AP, Saraswat S, Chaudhary PP, Dabdoub SM, Kumar PS. Sources of SARS-CoV-2 and other microorganisms in dental aerosols. J Dent Res. 2021;100:817–23. CrossRef
71. Baudet A, Baures E, Blanchard O, Le Cann P, Gangneux JP, Florentin A. Indoor carbon dioxide, fine particulate matter and total volatile organic compounds in private healthcare and elderly care facilities. Toxics. 2022;10:136. CrossRef
72. Baudet A, Baurès E, Guegan H, Blanchard O, Guillaso M, Le Cann P, et al. Indoor air quality in healthcare and care facilities: chemical pollutants and microbiological contaminants. Atmosphere. 2021;12:1337. CrossRef
73. Zomuansangi R, Lalbiaktluangi C, Gupta VK, Medders AA, Vidal JE, Singh BP, et al. Interaction of bacteria and inhalable particulate matter in respiratory infectious diseases caused by bacteria. Atmos Pollut Res. 2024;15:102012. CrossRef
74. Mirhoseini SH, Koolivand A, Bayani M, Sarlak H, Moradzadeh R, Ghamari F, et al. Quantitative and qualitative assessment of microbial aerosols in different indoor environments of a dental school clinic. Aerobiologia. 2021;37:217–24. CrossRef
75. Abdelkarim-Elafifi H, Arnabat-Artés C, Parada-Avendaño I, Polonsky M, Arnabat-Domínguez J. Aerosols generation using Er,Cr:YSGG laser compared to rotary instruments in conservative dentistry: a preliminary study. J Clin Exp Dent. 2021;13:e30–6. CrossRef
76. Matys J, Grzech-Lesniak K. Dental aerosol as a hazard risk for dental workers. Materials (Basel). 2020;13:5109. CrossRef
77. Dixon K, Dasgupta P, Vasdev N. A systematic review of the harmful effects of surgical smoke inhalation on operating room personnel. Health Sci Rev. 2023;6:100077. CrossRef
78. Tang F, Wen X, Zhang X, Qi S, Tang X, Huang J, et al. Ultra?ne particles exposure is associated with speci?c operative procedures in a multi-chair dental clinic. Heliyon. 2022;8:e11127. CrossRef
79. Anagnostaki E, Mylona V, Parker S, Lynch E, Grootveld M. Systematic review on the role of lasers in endodontic therapy: valuable adjunct treatment?. Dent J (Basel). 2020;8:63. CrossRef
80. Huang Q, Li Z, Lyu P, Xhou X, Fan Y. Current applications and future directions of lasers in endodontics: a narrative review. Bioengineering. 2023;10:296. CrossRef
81. McKinley Jr IB, Ludlow MO. Hazards of laser smoke during endodontic therapy. J Endod. 1994;20:558–9. CrossRef
82. Padmanabhan AK, Prabhuji MLV, Mampuzha S, Subramanya AP. Surgical smoke in dental practice: a potential biohazard. Dentist Case Rep. 2020;4:01–4.
83. Fox-Lewis A, Allum C, Vokes D, Roberts S. Human papillomavirus and surgical smoke: a systematic review. Occup Environ Med. 2020;77:809–17. CrossRef
84. Kokosa JM, Eugene J. Chemical composition of laser-tissue interaction smoke plume. J Laser. 1989;3:59–63. CrossRef
85. OSHA Hazard Information Bulletins Hazard of Laser Surgery Smoke [cited 2024 Jun 04]. Available from: https://www.osha.gov/publications/hib19880411
86. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–37. CrossRef
87. Jacks ME. A laboratory comparison of evacuation devices on aerosol reduction. J Dent Hyg. 2022;76:202–6.
88. Blackley BH, Anderson KR, Panagakos F, Chipps T, Virji MA. Efficacy of dental evacuation systems for aerosol exposure mitigation in dental clinic settings. J Occup Environ Hyg. 2022;19:281–94. CrossRef
89. Fennelly M, Callagher C, Harding M, Hellebust S, Wenger J, O’Sullivan N, et al. Real-time monitoring of aerosol generating dental procedures. J Dent. 2022;120:104092. CrossRef
90. Nulty A, Lefkaditis C, Zachrisson P, Van Tonder Q, Yar R. A clinical study measuring dental aerosols with and without a high-volume extraction device. Br Dent J. 2020;20:1–8. CrossRef
91. Maurais MT, Kriese J, Fournier M, Langevin L, MacLeod B, Blier S, et al. Effectiveness of selected air cleaning devices during dental procedures. Mil Med. 2023;188:e80–5. CrossRef
92. Watanabe J, Iwamatsu-Kobayashi Y, Kikuchi K, Kajita T, Morishima H, Yamauchi K, et al. Visualization of droplets and aerosols in simulated dental treatments to clarify the effectiveness of oral suction devices. J Prosthodont Res. 2024;68:85–91. CrossRef
93. Liu MH, Chi-Tsung C, Li-Chuan C, Wen-Ming L, Gwo-Hwa W. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS One. 2019;14:e0225644. CrossRef
94. Comisi JC, Ravenel TD, Kelly A, Teich ST, Renne W. Aerosol and splatter mitigation in dentistry: analysis of the effectiveness of 13 setups. J Est Restor Dent. 2021;1:1–14. CrossRef
95. Böke ES, Kele? A, Keskin C, Çayci YT, Turk T. Are aerosol control devices effective in preventing the spread of dental aerosol? Peer J.2022;10:e13714. CrossRef
96. Hallier C, Williams DW, Potts AJ, Lewis MAO. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br Dent J. 2010;209:E14. CrossRef
97. ANSI/ASHRAE/ASHE Standard 170-2021—Ventilation of Health Care Facilities [cited 2024 Jun 04]. Available from: https://blog.ansi.org/ansi-ashrae-ashe-170-2021-health-care-ventilation/
98. OSAP. Strategies: for air quality management using engineering controls. Infect Control Pract [cited 2024 Jun 04]. Available from: https://www.osap.org/assets/docs/education-training/icip/icip-2023-1-february.pdf
99. CDC, USA. C. Air [cited 2024 Jun 15]. Available from: https://www.cdc.gov/infection-control/hcp/environmental-control/air.html. see section d: Air Sampling
100. Saini S, Dutta M, Marques G. A comprehensive review on indoor air quality monitoring systems for enhanced public health. Sustainable Env Res. 2020;30:6. CrossRef
101. Zhang H, Srinivasan R. A systematic review of air quality sensors, guidelines, and measurement studies for indoor air quality management. Sustainability. 2020;12:9045. CrossRef
102. Alvarengaa MOP, Diasb JMM, Limac BJLA, Gomesd ASL, Monteiro GQM. The implementation of portable air-cleaning technologies in healthcare settings—a scoping review. J Hosp Inf. 2023;132:93–103. CrossRef
103. Cao R, Qiu P, Xu B, Lin J, Chu D, Fan Z. Effectiveness of interventions to reduce aerosol generation in dental environments: a systematic review. Prev Med Rep. 2023;35:102383. CrossRef
104. Odontes—AQ Sensor Professional—Sensore di qualità dell’aria interna [cited 2024 Jun 04]. Available from: https://odontes.it/prodotto/aq-sensor-professional-sensore-di-qualita-dellaria-interna/
105. IQ Air—Air Quality Monitors [cited 2024 Jun 04]. Available from: https://www.iqair.com/air-quality-monitors
106. Trotec—Particle Counter PC200 [cited 2024 Jun 04]. Available from: https://uk.trotec.com/products-services/measuring-devices/air-quality/particle-counter/pc200-particle-counter/
107. Chawla H, Anand P, Garg K, Bhagat N, Varmani SG, Bansal T, et al. A comprehensive review of microbial contamination in the indoor environment: sources, sampling, health risks, and mitigation strategies. Front Public Health. 2023;1:1285393. CrossRef
108. Akbari M, Atoof F, Nazari-Alam A, Nasab ZS, Bagher M, Mirzaei MN. Assessment of bacterial bioaerosols and particulate matters characteristics in the indoor air of dentistry clinics. Int Arch of Health Sci. 2023;10(3):130–6.
109. Park S, Doosam Song D. CO2 concentration as an indicator of indoor ventilation performance to control airborne transmission of SARS-CoV-2. J Inf Pub Health. 2023;16(7):1037–44. CrossRef
110. Robertson C, Clarkson JE, Aceves-Martins M, Ramsay CR, Richards D, Thibault Colloc T, et al. A review of aerosol generation mitigation in International Dental Guidance. Int Dent J. 2022;72:203–10. CrossRef
111. Li R, Zhang M, Wu Y, Tang P, Sun G, Wang L, et al. What we are learning from COVID-19 for respiratory protection: contemporary and emerging issues. Polymers. 2021;13:4165. CrossRef
112. CDC, USA Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. See section 3. Setting-specific considerations: Dental Facilities [cited 2024 Mar 18]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
113. Virdi MK, Durman K, Deacon S. The debate: what are aerosol generating procedures in dentistry? a rapid review. JDR Clin Transl Res. 2021;6(2):115–27. CrossRef
114. NHS England. Appendix 11a: Aide memoire for optimal patient placement and respiratory protective equipment (RPE) for infectious agents in hospital inpatients (based on evidence from WHO, CDC and UKHSA). Redditch, UK: NHS England; 2023 [cited 2024 Jun 15]. Available from: https://www.england.nhs.uk/publication/national-infection-prevention-and-control-manual-for-england-appendices/
115. Mueller JT, Holly D, Geyer HD, Karimi S, Poterack KA, Seville MTA, et al. Two masks can be worse than one: N95 respirator failure caused by an overlying face mask. Infect Control Hosp Epidemiol. 2023;44:1529–31. CrossRef
116. Russell AD. Lethal effects of heat on bacterial physiology and structure. Sci Prog. 2003;86:115–37. CrossRef
117. Wolf A, Moissl-Eichinger C, Perras A, Koskinen K, Tomazic PV, Thurnher D. The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: a pilot study. Sci Rep. 2017;7(1):5867. CrossRef
118. Du GH, Wang YF, Chen JJ, Deng YW, Han XZ, Tang GY. Potential association between Fusobacterium nucleatum enrichment on oral mucosal surface and oral lichen planus. Oral Dis. 2020;26:122–30. CrossRef
119. Morrison AG, Sarkar S, Umar S, Lee STM, Thomas SM. The contribution of the human oral microbiome to oral disease: a review. Microorganisms. 2023;11:318. CrossRef
120. Yougbaré S, Chinmaya Mutalik C, Krisnawati DI, Kristanto H, Jazidie A, Nuh M, et al. Nanomaterials for the photothermal killing of bacteria. Nanomaterials. 2020;10:1123. CrossRef
121. Cremasco M, Vigoroso L, Solinas C, Caffaro F. Discomfort in use and physical disturbance of FFP2 masks in a group of Italian doctors, nurses and nursing aides during the COVID-19 pandemic. Safety. 2023;9:40. CrossRef
122. C.D.C USA. Counterfeit respirators/misrepresentation of NIOSH approval. Atlanta, GA: CDC; 2024 [cited 2024 May 22]. Available from: https://www.cdc.gov/niosh/npptl/usernotices/counterfeitResp.html
123. Resende KKM, Neves LF, de Rezende Costa Nagib L, Oliveira Martins LJ, Rezende Costa CR. Educator and student hand hygiene adherence in dental schools: a systematic review and meta-analysis. J Dent Educ. 2019;83:575–84. CrossRef
124. Caggiano M, Acerra A, Martina S, Galdi M, D’Ambrosio F. Infection control in dental practice during the COVID-19 pandemic: what is changed? Int J Environ Res Public Health. 2023;20:3903. CrossRef
125. CDC. Hand hygiene guidance. Atlanta, GA: CDC; 2024 [cited 2024 Jun 04]. Available from: https://www.cdc.gov/handhygiene/providers/guideline.html
126. Glowicz JB, Landon E, Sickbert-Bennet EE, Aiello AE, deKay K, Hoffmann KK, et al. SHEA/IDSA/APIC practice recommendation: strategies to prevent healthcare-associated infection through hand hygiene: 2022 update. Inf Control Hosp Epidemiol. 2023;44:355–37. CrossRef
127. Blackman G. PPE directive revision impacts user safety eyewear suppliers. Laser Systems Europe. Spring; 2018 [cited 2024 Jun 27]. Available from: https://www.lasersystemseurope.com/feature/ppe-directive-revision-impacts-laser-safety-eyewear-suppliers
128. Xamax Workplace Solutions. How long do Goggles and safety glasses last? [cited 2024 Jun 27]. Available from: https://www.xamax.co.uk/blog/how-long-does-ppe-last.html
129. DiOptika. Cleaning laser safety glasses [cited 2024 Jun 27]. Available from: https://www.lasersafetyglasses.com.au/blog/cleaning-laser-safety-glasses/
130. Laser Safety Industries. How to clean laser safety glasses [cited 2024 Jun 27]. Available from: https://lasersafetyindustries.com/blogs/laser-safety-articles/how-to-clean-and-care-for-your-laser-safety-glasses
131. MIT. Guidelines for the use and disinfection of shared PPE [cited 2024 Jun 27]. Available from: https://ehs.mit.edu/wp-content/uploads/2020/06/MITEHS_shared_ppe_guidance.pdf
132. iLase User Manual P/N 5400230 Rev.J. Cleaning and sterilization. Section 6. Avaialble from: https://www.biolase.com/media/5400230-RevJ_ilase-UM.pdf
133. Kakaboura A, Tzoutzas J, Pitsinigos D, Vougiouklakis G. The effect of sterilization methods on the light transmission characteristics and structure of light-curing tips. J Oral Rehabilitation. 2004;31:918–23. CrossRef
134. Stolov AA, Slyman BE, David T, Burgess DT, Hokansson AS, Li J, et al. Effects of sterilization methods on key properties of specialty optical fibers used in medical devices. Conference Paper in Proceedings of SPIE—The International Society for Optical Engineering; Bellingham, WA: SPIE—The International Society for Optical Engineering; March 2013. Avaialble from: www.researchgate.net/profile/Andrei-Stolov/publication/271541382_Effects_of_sterilization_methods_on_key_properties_of_specialty_optical_fibers_used_in_medical_devices/links/5678d4f308ae0ad265c83fb0/Effects-of-sterilization-methods-on-key-properties-of-specialty-optical-fibers-used-in-medical-devices.pdf
135. Barenghi L. Clean, disinfect and cover: top activities for clinical contact surfaces in dentistry. Orange, CA: Kerr Dental; 2018 [cited 2024 Jun 04]. Available from: https://www.ultimatedental.com/uploads/KerrDrLiviaBarenghi.pdf
136. Nogueira Rodrigues FC, de Araújo JGL, dos Santos Araújo EM, Lago ADN, Mantilla TF, de Freitas PM. Influence of biosafety materials of the laser output power. Lasers Med Sci. 2021;36:311–5. CrossRef
137. EPA, USA. Selected EPA-registered disinfectants. Washington, DC: EPA. Available from: https://www.epa.gov/pesticide-registration/selected-epa-registered-disinfectants
138. Dental Patient Safety Foundation [cited 2024 Jun 19]. Available from: https://www.dentalpatientsafety.org/
139. Bertagnolio S, Dobreva Z, Centner CM, Olaru ID, Donà D, Burzo S, et al. WHO global research priorities for antimicrobial resistance in human health. Lancet Microbe. 2024 [cited 2024 Aug 12]. doi: https://doi.org/10.1016/S2666-5247(24)00134-4 CrossRef
140. Meadows AJ, Stephenson N, Madhav NK, Oppenheim B. Historical trends demonstrate a pattern of increasingly frequent and severe spillover events of high-consequence zoonotic viruses. BMJ Glob Health. 2023;8:e012026. CrossRef
141. WHO. 2023. Available from: https://www.weforum.org/publications/quantifying-the-impact-of-climate-change-on-human-health/
142. Cannon RD. Oral fungal infections: past, present, and future. Front Oral Health. 2020;3:838639. CrossRef
143. WHO Bacterial Priority Pathogens List, 2024. Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva, Switzerland: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO.
144. Liu J, Spencer N, Utter DR, Grossman A, Santos NCD, Shi W, et al. Persistent enrichment of multidrug-resistant Klebsiella in oral and nasal communities during long term starvation. Microbiome. 2024;12:132. CrossRef
145. Smiline Girija AS. Acinetobacter baumanii as an oro dental pathogen: a red alert. J Appl Oral Sci. 2024;32:e20230382. CrossRef
146. WHO. WHO fungal priority pathogens list to guide research, development and public health action. Geneva, Switzerland: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
147. Le PH, Linklater DP, Aburto-Medina A, Nie S, Williamson NA, Crawford RJ, et al. Apoptosis of multi-drug resistant Candida species on microstructured titanium surfaces. Adv Mater Interfaces. 2023;10:2300314 CrossRef
148. Drago F, Ciccarese G, Merlo G, Trave I, Javor S, Rebora A, et al. Oral and cutaneous manifestations of viral and bacterial infections: not only COVID-19 disease. Clin Dermatol. 2021;39:384–404. CrossRef
149. ADA Huddles. Global measles outbreak sees ‘alarming’ increase. ADA Huddles. February 2024. Available from: https://adanews.ada.org/huddles/global-measles-outbreak-sees-alarming-increase/
150. Bah M, Wilseck ZM, Lin LY, Peterson AJ, Chaudhary M, Gemmete JJ. The interplay among a dental procedure, infective endocarditis, and an acute ischemic stroke. JADA. 2024;155:P244–50. CrossRef
151. Caminada S, Mele A, Ferrigno L, Alfonsi V, Crateri S, Iantosca G, et al. Risk of parenterally transmitted hepatitis following exposure to invasive procedures in Italy: SEIEVA surveillance 2000-2021. J Hepatol. 2023;79:61–8. CrossRef
152. Singh J, O’Donnell K, Nieves DJ, Adler-Shohet FC, Arrieta C, Negar Ashouri N, et al. Invasive Mycobacterium abscessus outbreak at a pediatric dental clinic. Open Forum Infect Dis. 2021 Jun;8(6):ofab165. CrossRef
153. Zuo W, He D, Liang C, Du S, Hua Z, Nie Q, et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China. Lancet Inf Dis. 2024;24:845–55. CrossRef
154. Willis JA, Cheburkanov V, Chen S, Soares JM, Kassab G, Blanco KC, et al. Breaking down antibiotic resistance in methicillin-resistant Staphylococcus aureus: combining antimicrobial photodynamic and antibiotic treatments. PNAS. 2022;119(36):e2208378119. CrossRef
155. Wang CC, Prather KA, Sznitman J, Jimenez JL, Lakdawala SS, Tufekci Z, et al. Airborne transmission of respiratory viruses. Science. 2021;373:9eabd9149. CrossRef
156. Haddrell A, Oswin H, Otero-Fernandez M, Robinson JF, Cogan T, Alexander R, et al. Ambient carbon dioxide concentration correlates with SARS-CoV-2 aerostability and infection risk. Nat Commun. 2024;15:3487. CrossRef
157. Zemouri C, Awad SF, Volgenant CMC, Crielaard W, Laheij AMGA, de Soet JJ. Modeling of the transmission of Coronaviruses, Measles virus, influenza virus, Mycobacterium tuberculosis, and Legionella pneumophila in dental clinics. J Dent Res. 2020;99(10):1192–8. CrossRef
158. Zhang J, Chen Z, Shan D, Wu Y, Zhao Y, Li C, et al. Adverse effects of exposure to fine particles and ultrafine particles in the environment on different organs of organisms. J Env Sci. 2024;135:449–73. CrossRef