INTRODUCTION
The root canal is the primary site for microbial colonization because it causes infection by forming biofilms [1]. Bacterial polysaccharides found in the extracellular matrix play a very important role in biofilm production [2,3]. The ecology of the root canal predominantly consists of both aerobic and facultative anaerobes, the latter of which pose a serious threat and alter the root canal system. Disinfection of the root canal system is one of the most complicated procedures and involves the use of a variety of equipment and techniques, irrigation regimes, and intracanal medications [4,5]. The use of chemo-mechanical instrumentation alone does not guarantee a bacteria-free root canal system. The suppression of biofilms is one of the most important criteria for assessing the effectiveness of antibacterial agents [6].
Enterococcus faecalis is a Gram-positive, facultative anaerobic coccus and is most commonly (incidence) found in failed root canal therapies. It can survive in a microenvironment in the presence of calcium hydroxide, resulting in colonization of the root canal system. Mechanical debridement proves beneficial in biofilm removal by diminishing bacterial presence and eliminating organic substances that might impede the effectiveness of antibacterial agents [2]. While essential for eliminating endodontic biofilms, the incomplete removal of biofilm in certain areas of the root canal during instrumentation indicates the potential persistence of biofilms in these regions. Streptococcus mutans is a facultatively anaerobic, Gram-positive coccus and part of the normal oral flora responsible for tooth decay [6]. This finding emphasizes the importance of utilizing antimicrobial and/or antibiofilm irrigants [7].
Sodium hypochlorite (NaOCl) possesses potent bactericidal and antibiofilm qualities. Nonetheless, its high cytotoxicity and efficacy can be notably influenced by the presence of organic substances. Chlorhexidine (CHX) has unique antimicrobial properties. Positively charged ions generated by CHX can adsorb into dentin, preventing microbial growth on the surface of the dentin [8–10]. Nanoencapsulation might improve drug release while also extending the time of bactericidal irrigants that are delivered to dentinal tissues. Custom-designed nanoparticles enable the sustained and continuous delivery of bactericidal agents, directly reaching the dentinal matrix. Haseeb et al. [11] illustrated the effective self-assembly of PEG-b-PLA bilayer nanoparticles for CHX encapsulation, enhancing drug bioavailability and facilitating precise drug distribution within intricate dentinal matrices.
Cubosomes (CUs) are among the few nanocarriers that are currently being explored for active drug delivery systems. CUs are liquid crystalline nanoparticles with 3D structures. It resembles a honeycomb through bicontinuous domains of water and lipids and is an active drug delivery system [12,13]. The advantages of CU include a highly organized structure, protection of the drug from degradation, controlled drug release, and improved bioavailability with reduced side effects. Considering the contrasting results of previous studies on nanoformulations of chlorohexidine, our study aimed to develop cubosomal CHX for endodontic irrigation [14,15]. The antimicrobial efficacy of the agent was assessed by confocal laser scanning microscopy (CLSM) and colony-forming units (CFUs). The null hypothesis states that novel CU formulations will not influence sustained release or antimicrobial action.
MATERIALS AND METHODS
CHX gluconate (CHX; 20% solution) was obtained from Unilab Chemicals, Maharashtra, India. Glyceryl monooleate was received as a kind gift from Mohini Organics Pvt. Ltd., Maharashtra, India. Polyvinyl alcohol (PVA) and Poloxamer 407 (P407) were obtained from Sigma Aldrich. The cellulose membrane (12 kDa cut-off) for dialysis was purchased from Hi Media.
Synthesis and characterization of CHX gluconate CUs
Preparation of CU
CUs were prepared by emulsification of the lipid phase and surfactant in water containing 0.5% PVA. Glycerol monooleate (GMO) and P407 were completely melted at 60°C. CHX (in its lyophilized powder form) was dissolved in water containing PVA. Then, the melted lipid-surfactant mixture was added to the aqueous phase dropwise while homogenizing it at 8,000 rpm for a duration of 5 minutes using an IKA T25 digital Ultra Turrax high-speed homogenizer [16].
Characterization of CHX CUs
Particle size, zeta potential, polydispersity index (PDI), transmission electron microscopy (TEM), and Fourier transform infrared (FTIR) spectroscopy
To determine the average particle size, zeta potential, and PDI of the prepared CUs, ZetaSizer (Nano ZS, Malvern Instruments; Malvern, UK) was used. To obtain the FTIR spectra, 100 scans were collected in the 500–4,000 cm−1 region (FTIR, Bruker, Alpha II). Structural characterization of the prepared CUs was performed by TEM (FEI Technai-12 G2 120 kV) [17].
Determination of drug entrapment efficiency (EE)
To calculate the amount of drug entrapped inside the CUs, the prepared formulation was transferred into a dialysis bag (which had been soaked previously), and the free drug was removed by dialysis. The dialysis bag was kept for stirring in 250 ml water and at the end of 6 hours, the sample was withdrawn and the absorbance was measured using a UV spectrophotometer at the maximum wavelength of 254 nm. The amount of free drug was calculated from a calibration curve of CHX constructed previously. The amount of drug encapsulated was calculated as the difference between the total amount of drug added and the amount of free drug. Then, the EE was calculated according to the formula:
In vitro drug release study
The amount of drug released from the CUs was measured using the dialysis method. The prepared formulations of CHX CUs (with an appropriate volume corresponding to 20 mg of CHX) and CHX solution (1 ml of 20% CHX) were added to the respective dialysis bags (previously soaked) and dialyzed using Milli-Q water (60 ml) as the receptor medium by stirring for 24 hours at room temperature. Samples were withdrawn at intervals from 0 to 24 hours and replaced with an equal amount of fresh receptor medium. Then, the absorbance of the samples was measured spectrophotometrically at 254 nm. The percentage of drugs released was calculated, and a graph of % drug release versus time was constructed [18].
Tooth preparation
A total of 90 single-rooted teeth with straight canals were selected for the study and divided into 8 groups (Table 1 and 2), with a sample size of 10 teeth each (n = 10). The two teeth were kept for scanning electron microscopic observation and the rest 8 were discarded were discarded. The study protocol was approved by the Institutional Ethics Committee, Kasturba Hospital, Manipal (Approval No: IEC933/2019). Radiographs were taken to confirm single canals, the teeth were decoronated with a carborundum disc, and the root length was standardized. The working length for all the teeth was determined by a radiographic method, and biomechanical preparation was performed by K-files. Simultaneous irrigation was performed with 5.25% NaOCl and 17% EDTA at 5 ml/minute each for a duration of 1 minute between each file size and irrigated with normal saline at 5 ml/minute as the final rinse. After the working length was measured, the canals were enlarged to 35 k files (Dentsply, USA), and the apices were sealed with sticky wax. All the teeth were subjected to autoclaving. Forty teeth were subjected to CFU determination. In the remaining 40 teeth, deep dentinal grooves were placed in the buccal and lingual halves of the tooth using a Diamond disc for splitting for observation via CLSM.
Determination of antimicrobial efficacy
CFU determination
Preparation of Bacterial Inocula: The American Type Culture Collection strains of E. faecalis (ATCC 29212) and S. mutans (ATCC 25175) were used in this study. The culture was inoculated in brain heart infusion broth and incubated for 48 hours at 37°C. Forty sterilized teeth were inoculated with this fresh bacterial culture (20 samples for E. faecalis and 20 samples for S. mutans) and incubated in a bacteriological incubator for approximately 72 hours. After 72 hours of incubation, the teeth were irrigated with their respective groups with a 27-gauge needle in a 5 ml syringe for 1 minute twice. Then, the incubated suspension was serially diluted from 101 to 109 by following standard procedures, and each dilution was plated (0.1 ml) on 5% sheep blood agar and incubated at 37°C for 24 hours. After 24 hours of incubation, each dilution was checked, and at the optimum dilution, the number of colonies could be counted easily, while that of the dilution was taken for further CFU calculation. CFU was calculated by following the formula.
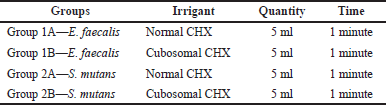 | Table 1. Irrigation regimen used for CFUs. [Click here to view] |
TDF: 105
Volume of culture plated in ml: 0.1 ml
Scanning electron microscopy (SEM)
Two samples innoculated with E.faecalis and s.mutans were subjected to SEM for visualization of bacterial growth.
Confocal laser scanning microscopy
Two halves of the roots were removed from the remaining forty samples, which underwent an irrigation schedule, and were then used for CLSM. A coverslip (20 mm in diameter and 0.17 mm thick) was used to place one randomly chosen side of the tooth on the glass SYTO9 and propidium iodide dyes were added to the canal for 5 minutes at a 1:1 ratio (total volume of ¼ 40 ml) to assess the bacterial viability. Following the instrumentation of the canals, the mesial and distal portions of the root were separated, and each sample was taken out of the device. A randomly chosen tooth side was set on a glass coverslip that measured 20 mm in diameter and 0.17 mm in thickness. Bacterial viability was determined using a BacLightTM kit L13152 from Molecular Probes, Inc., located in Eugene. Whereas the propidium iodide probe only penetrates bacteria with damaged plasma membranes, the SYTO9 probe labels every bacterium in a population [19]. For SYTO9 and propidium iodide, the microscope was set to 473 and 559 nm, respectively. For SYTO9 and propidium iodide, the excitation/emission maxima are 480/500 and 490/635 nm, respectively. For 5 minutes, dyes were added to the canal in a 1:1 ratio (for a total volume of ¼ 50 ml). CLSM (Olympus Europa Holding GmbH, Hamburg, Germany) was used to view the fluorescence from the labeled cells. The Olympus FluoView Version 1.7 software (Olympus Europa Holding GmbH, Hamburg, Germany) was utilized to gather CLSM images. The program was set to 512 pixels2 resolution and a magnification factor of 1.0, resulting in a final pixel resolution of 0.41 mm/pixel. Red and green fluorescence were displayed simultaneously using dual-channel imaging. The last millimeter of the apical third of the root was not included in the evaluation since it was not covered by the chemomechanical preparation. From every specimen, ten micrometer-deep scans with a 0.5 mm step size and 20 slices per scan were acquired. Using 60X magnification oil immersion objectives with a confocal pinhole set to 30 mm in diameter and a numerical aperture of 1.4 mm, the mounted specimens were viewed. CLSM (Olympus Europa Holding GmbH, Hamburg, Germany) was used to view the fluorescence from the labeled cells.
 | Table 2. Irrigation regimen used in the CLSM analysis among the study groups. [Click here to view] |
Data were analyzed using the statistical package SPSS Software (version 16.0) was used to analyze the data. Initially, data were screened in a Chi-Square test then followed by one-way ANOVA for calculating the significance difference between the data groups. A p-value <0.05 was considered statistically significant.
RESULTS
Preparation of CUs
GMO and phytantriol are the two most commonly used lipids for the preparation of CUs. Out of these, the literature suggests that GMO is safer as compared to phytantriol as the latter showed higher cytotoxicity to tested cell lines [20,21]. Therefore, to develop a safe formulation to be used as a root canal irrigant, we used GMO for the preparation of CUs. The emulsification of GMO and P407 in water formed CUs that were loaded with CHX. Due to its well-established property as a stabilizer, P407 is widely used in the preparation of CUs [22]. P407 is a triblock copolymer consisting of hydrophilic (polyethylene glycol) and hydrophobic (polypropylene glycol) chains. PVA was used in the formulation of CU because it adsorbs onto the surface of the emulsion droplets and stabilizes them [23]. It also helps to form easily dispersible particles in aqueous media [24].
Characterization of CHX CUs: particle size, zeta potential, PDI, TEM, and FTIR spectroscopy
The properties of the CHX-loaded CUs were characterized. We obtained particles with an average size of approximately 157 nm, which shows that they are in the nanoscale range and have a PDI of approximately 0.4, which is within the acceptable range and shows that the formulation was homogeneous in distribution. The particles had a positive zeta potential of approximately 19 mV, indicating that the CUs were stable. The average size, zeta potential, and PDI of the prepared CHX CUs are displayed in Table 3.
The morphology of the prepared CUs was visualized via TEM, and the results are shown in Figure 1. The nanoparticles had a polyangular shape, and a cubic structure was visible [13]. However, most of the nanoparticles tended to form spherical shapes, as reported previously, with some amount of aggregation [12].
The FTIR spectra of pure CHX (Fig. 2) showed characteristic peaks at 2,926 cm−1 (N-H stretching, a secondary amine salt), 1,520 cm−1, 1,405 cm−1 (aromatic C=C stretching modes), 1,241 cm−1 (C-N stretching, a secondary aromatic amine), and 1,018 cm−1 (C-N stretching), in accordance with previously reported literature [25,26]. The peaks in the FTIR spectrum of pure CHX were retained in the FTIR spectrum of the CHX CUs (Fig. 3), which showed that there were no significant interactions between the drug and excipients.
 | Table 3. Particle size, zeta potential, and PDI of CHX CUs. [Click here to view] |
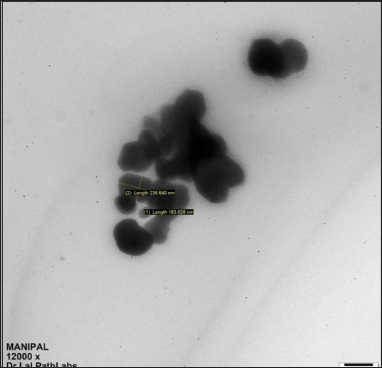 | Figure 1. TEM image of CHX CUs. [Click here to view] |
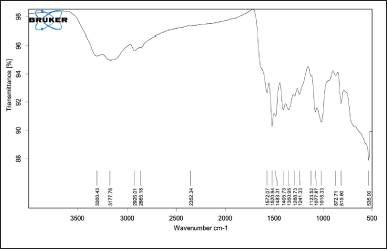 | Figure 2. FTIR spectra of pure CHX. [Click here to view] |
Determination of drug EE
A UV/visible spectrophotometric method was used to quantify CHX. The λmax for CHX was 254 nm. A calibration curve was constructed using different concentrations of the drug against the obtained absorbance values. A calibration curve was used to calculate the drug EE. The dialysis method was used to determine the EE. The EE of the CHX CUs was 47%.
Drug release studies
To quantify CHX in the drug release studies, a UV/Vis spectroscopy method was used. A curve of % of drug released versus time was constructed, as shown in Figure 4. Upon comparison between the curves obtained for CHX CUs and plain CHX solution, it is clear that the plain drug solution showed 100% release in 6 hours, whereas in the case of the prepared CUs formulation, approximately 77% of the drug was released at the end of 6 hours. CHX was completely released from CU at the end of 24 hours. Therefore, the CHX CUs showed slower and more controlled release than the plain CHX solution, which demonstrated rapid release.
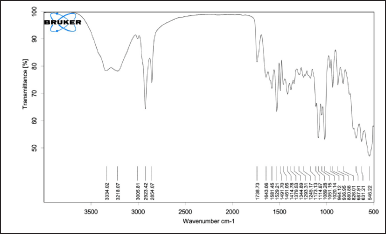 | Figure 3. FTIR spectra of CHX CUs. [Click here to view] |
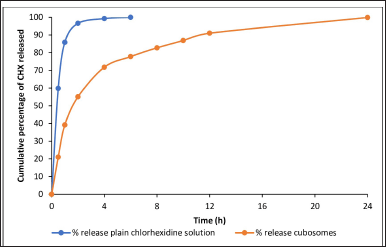 | Figure 4. Drug release profiles of plain CHX solution and CHX CUs. [Click here to view] |
CFU calculation
The CFU calculations of E. faecalis and S. mutans against CHX and the cubosomal formulations are shown in Figures 5 and 6.
As visualized under SEM, there was strong adherence to the roots, and clusters of bacteria were also observed. The SEM images (Figs. 7 and 8) suggested that the strong biofilms of both isolates were concordant.
Confirmation of biofilm by CLSM studies
Two-dimensional analysis of CLSM pictures was carried out, and bio-Image software (The MathWorks, Inc., Natick, MA) [25] was used for quantitative analysis to assess viability. The color segmentation techniques used by MATLAB (The Mathworks) underpin the bioImage L software, which produces data on both the whole biofilm population and separate subpopulations that are symbolized by red and green fluorescent colors. The percentage (%) survival of biofilm cells as determined by the LIVE/DEAD approach was obtained by dividing the biovolume of the green subpopulation by the biovolume of the biofilm as a whole. By contrasting the biovolume of biofilms before treatment with the biovolume of biofilms following antimicrobial exposure, the elimination of biofilm cells was estimated. Each solution was considered an experimental group.
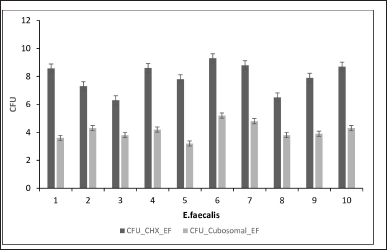 | Figure 5. CFU of E. faecalis against CHX and cubosomal formulation. [Click here to view] |
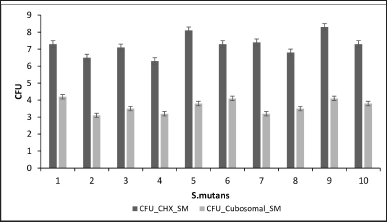 | Figure 6. CFU of S. mutans against CHX and cubosomal formulation. [Click here to view] |
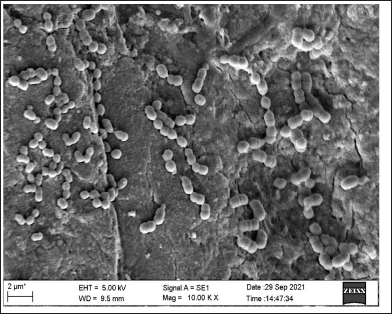 | Figure 7. SEM image showing biofilm of S. mutans. [Click here to view] |
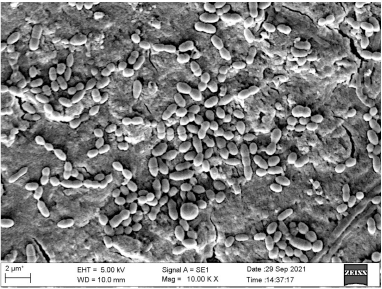 | Figure 8. SEM image showing biofilm of E. faecalis. [Click here to view] |
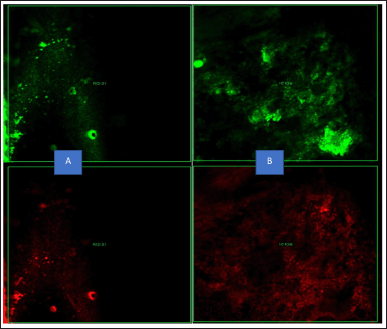 | Figure 9. CLSM showing the intensity of the biofilm production, red fluorescence indicating the dead bacteria and green fluorescence indicating the live bacteria by normal CHX at 48 hours of incubation (A: Biofilm intensity of E. faecalis; B: Biofilm intensity of S. mutans). [Click here to view] |
Confocal images revealed greater E. faecalis biofilm removal in the apical third when irrigated with CHX and CU than in the other thirds. Biofilm eradication among the groups showed a significant difference. The biofilm density was greater in the middle third in both groups. The latter decreased the biofilm to 4 ± 3, 14 ± 3, and 15 ± 2 from the apical root portion to the coronal third, respectively. In contrast, the CHX decreased to 51.4 ± 4.86 ± 9.58 ± 8 from the apical third.
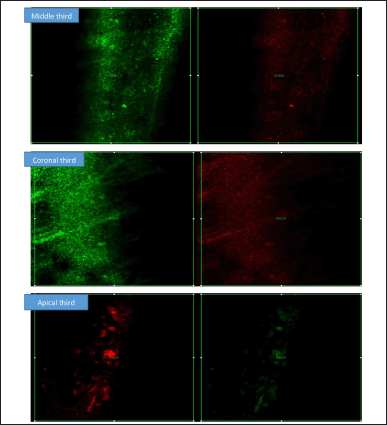 | Figure 10. CLSM showing the intensity of the biofilm production at Apical, Medial, and Coronal areas. Red fluorescence indicating the dead bacteria and green fluorescence indicating the live bacteria by CHX. [Click here to view] |
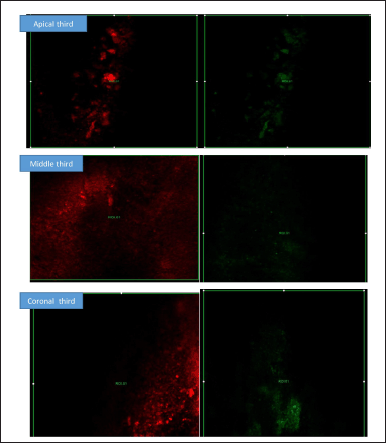 | Figure 11. CLSM showing the intensity of the biofilm production at Apical, Medial, and Coronal areas. Red fluorescence indicating the dead bacteria and green fluorescence indicating the live bacteria by Cubosomal CHX at 0 and 48 hours of incubation. [Click here to view] |
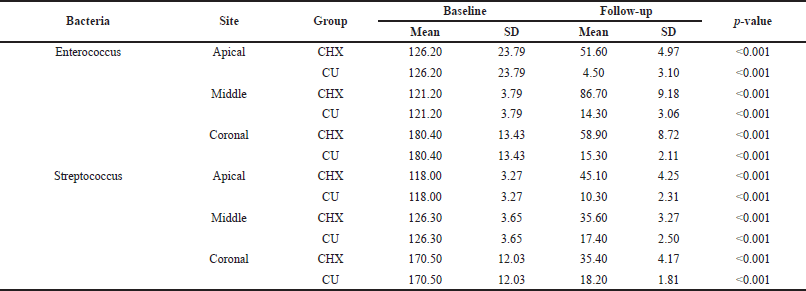 | Table 4. Intra-group comparison. [Click here to view] |
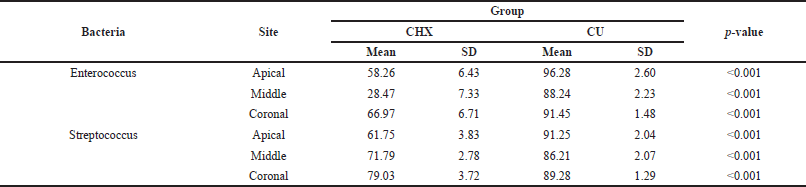 | Table 5. Inter-group comparison. [Click here to view] |
In the streptococcal group, both irrigants were effective at eradicating biofilms. CU showed 18% ± 1.81%, 17% ± 2.5%, and 10% ± 2.3% reductions in biofilms from the coronal to apical thirds. Similarly, CHX decreased the bacterial density to 35 ± 4, 35 ± 3, and 45 ± 3 from the coronal portion. CHX was not successful in eliminating CHX from the apical portion, where biofilms constituted the majority. There was a significant difference in eradication between the CU group and the CHX group.
The intergroup comparisons revealed significant differences among groups. The intra- and intergroup comparisons are shown in Tables 4 and 5, respectively. CLSM images showing the intensity of biofilm production are shown in Figures 9–11.
DISCUSSION
To the best of our knowledge, this study is one of the few CHX nanoformulations that were tested on extracted teeth. This study demonstrated the number of bacterial biofilms adhering to roots, which was further confirmed by microscopic evidence. Bacteria within the root canal system can penetrate deep inside the tubules, leading to biofilm formation [27]. In failed cases of root canal-treated teeth, most irrigants have proven to be ineffective. By developing nanocubosomal CHX formulations, better penetration ability, and sustained release can be achieved [28].
At dosages lower than those utilized for topical treatments, CHX inhibits bacterial membrane-induced cell activation [29]. The integrity of the bacterial cytoplasmic membrane is disrupted by these cationic irrigants, resulting in the release of intracellular contents. They bind to not only lipopolysaccharide but also lipoteichoic acid from Gram-positive bacteria. Several authors have developed nanoformulations of CHX that have proven to be beneficial [30].
CUs possess distinctive cubic structures that enable the incorporation of highly lipophilic, hydrophilic, and amphiphilic drugs. Moreover, the lipid components utilized in CUs preparation, such as monoolein and phytantriol, are both biodegradable and biocompatible, indicating the safety of these cubic nanoparticles for drug delivery purposes. CUs maintain a stable cubic shape, promoting slower dissociation rates, enhanced drug retention, and targeted drug delivery. The architecture of cubic particles offers more advantageous drug delivery systems compared to other lipid-based nanocarriers like solid lipid nanoparticles, primarily due to their capacity to efficiently deliver drugs to the nanoparticle surface [31]. This study was successful in the development of Cubosomal CHX, for which the average particle size was approximately 150 nm. The TEM image showed a nearly cuboidal or hexagonal shape, which is in agreement with other studies.
The dialysis method was used to determine the drug EE. The EE of the CHX CUs was 47%. Yan et al. [7] reported similar EE values for CHX in mesoporous silica nanoparticles for antibiofilm activity. Nevertheless, some groups have obtained lower entrapment, for example, in the case of nanoporous silica nanoparticles and other kinds of nanoparticles developed for the mitigation of bacterial infections and dental applications [23]. The unique structure of CUs allows for the encapsulation of both hydrophilic and hydrophobic drugs. The bicontinuous water channels present in the structure are the hydrophilic regions where the CHX was entrapped. As the space occupied by the lipids in the cubosomal structure is much greater, the entrapment observed in the case of hydrophilic drugs is not very high [32].
To quantify CHX in the drug release studies, a UV/Vis spectroscopy method was used. The curve of the percentage of drugs released versus time was constructed as shown in Figure 4. By comparing the drug release curves obtained for CHX CUs and plain CHX solution, it was clear that the plain drug solution showed 100% release after 6 hours, whereas in the case of the prepared novel CU formulation, approximately 77% of the drug was released at the end of 6 hours. Therefore, the CHX CUs showed slow and controlled release compared to plain CHX solution, which demonstrated rapid release [31,33,34]. The plain CHX solution releases CHX rapidly because the drug is free and not encapsulated. This exhausts the CHX completely from the solution within a period of 6 hours. The slower release of CHX from the CUs was because of the encapsulation of CHX inside the CUs, leading to its release from the CUs over a long period of time [35]. Because of this sustained-release property, the CUs acted as effective root canal irrigants by promoting the residence of CHX inside the root canal for an extended time. Thus, the CUs act as a reservoir for CHX, allowing its localization at the target site (root canal) and releasing it slowly while killing the bacteria. Hence, the null hypothesis was rejected, which indicated that the novel CU formulation does not influence the sustained release of CHX. In the studies by Cetin et al. [15] and Beyth et al. [14], the results were similar to this study’s results. Sahoo et al. [24] have also shown the controlled release of drugs from CU without any burst release.
In a study conducted by Priyadarshini et al. [36], they developed a nanocapsule of poly(ε-caprolactone) (PCL)-coated CHX, in which the CHX particles were degraded, and sustained release was observed for up to 25 days. Moreover, the notable antibacterial effectiveness of nano-PCL/CHX against S. mutans and E. faecalis was observed to be significant [37]. In a study by Seneviratne et al. [8], they synthesized mesoporous silica nanoparticles in which CHX was successfully loaded. This formulation had antibiofilm activity and showed sustained release [8]. Our results, which are consistent with previous reports of nanoformulations, suggest that the controlled release of CHX from CU could be a good option for dental applications.
In this study, compared with normal CHX, Cubosomal CHX significantly reduced the bacterial count. Our study is in agreement with previous nanoformulations that were synthesized. However, the microorganisms used by most of the previous authors were E. faecalis and S. mutans. E. faecalis and S. mutans were selected because of their ability to penetrate deep inside dentinal tubules and form biofilms. Additionally, these two pathogens are the predominant pathogens involved in endodontic infections. In addition, the bacterium could coaggregate with some strains of S. mutans. E. faecalis is commonly found in cases in which root canal treatment fails. Streptococcus mutans is one of the most common organisms isolated from primary endodontic infections. The foreseeable reason for the increased effectiveness of Cubosomal CHX may be the sustained release of CHX from CUs up to 24 hours [8]. CHX is a widely used antibacterial agent. Gomeset et al. [38] investigated the chemical composition of CHX and its antibacterial efficacy because it is a hydrophobic, positively charged chemical. The fundamental mechanism of CHX is to interact with the bacterial cell membrane, and CHX is also effective against the cell wall of bacteria [10]. Khademi et al. [33] reported that treatment with CHX for 5 minutes may be effective at removing biofilms for up to 4 weeks.
In this study, CU was effective at decreasing the bacterial count from the baseline value. This results from CUs penetrating deeply into the dentinal tubules and the extended release of CHX. Bacteria in the apical third were less resistant to CU than were those in the coronal and middle thirds. Our study is in disagreement with other studies in which more biofilms were detected in the root portion. There may be various factors that help in elimination, such as the depth of needle penetration, configuration factors, and biofilm development. Additionally, another reason in the case of this study may be attributed to the lower number of tubules in the apical third and the depth of irrigation. The middle third had more biofilms than the coronal third. This can be explained by support from previous studies, as there is a complex morphology in the middle third compared to the coronal third. Additionally, irrigants are easily penetrable in the coronal third. Viscosity and surface tension are key determinants affecting the flow and depth of penetration of irrigants. It was observed that 2% CHX irrigant exhibited superior penetration into dentinal tubules compared to conventional syringe irrigation, which is consistent with the results of previous studies.
Kishen et al. [34] showed that CLSM is a method for detecting bacterial adherence and biofilm intensity both in vivo and under in vitro conditions. CLSM can be used to analyze and differentiate between live and dead bacteria on any surface [34]. Zapata et al. [39] in their study, showed that CLSM has more advantages over other conventional methods of studying bacterial biofilm, especially in the dentinal tubules. The SYTOX and propidium iodide stains are used predominantly to study biofilms and show 100% accuracy. In this study, better contrast was demonstrated among the live and dead bacteria in the roots, which were analyzed at 0 hours and after 48 hours in the case of both CHX and CU formulations. Both S. mutans and E. faecalis were inoculated in conventional CHX and CU formulations (Tables 2 and 3) onto the roots of the teeth and it was observed that both bacteria were inhibited (to a lesser extent) by the CU formulation compared to those in the normal CHX solution. The effects of the CU formulation were significantly greater than those of the 2% CHX solution (p < 0.05). In our study, CLSM showed very good results compared to the conventional colony count method. CLSM provides a real-time picture of the remaining cells of microorganisms and easily estimates the antibacterial properties of the irrigants used for the study [40,41]. In a previous study the intensityof the biofilm for both dead and live bacteria was not significantly different [39]. In our study, a significant difference (p = < 0.05) was obtained between normal CHX and cubosomal CHX at 0 and 48 hours of incubation. The mean differences subjected to statistical analysis were also significant [42].
Our study demonstrated that the apical region of the root is of key importance in preventing reinfection, and the CU formulation showed very good effectiveness in preventing biofilm formation even after 48 hours of incubation in the apical third followed by the medial and coronal thirds. A normal 2% CHX solution was least effective at eradicating the bacterial biofilm in the apical third of the root. Both bacterial biofilms showed noticeably less intensity in the apical third, followed by the medial and coronal thirds of the root [43]. It was also found that the bacterial load was greater in the middle third than in the other two-thirds of the root section, which can be attributed to the variation in root canal morphology, inadequate penetration of the irrigant into the dentinal tubules, and the presence of a heavy bacterial load.
It is evident from this study that when compared with normal CHX, cubosomal CHX showed better and more promising results, possibly because CUs act as a reservoir owing to their structure and their sustained release property, which enhances bacterial eradication.
CONCLUSION
In this study, a novel and unique cubosomal nanocarrier for CHX delivery in endodontic irrigation was developed and proven to be successful. The sustained release of CHX improved the antimicrobial efficacy in contrast to the regular 2% solution. Both colony-forming assays and CLSM showed that the antimicrobial efficacy of cubosomal CHX improved. These findings could have important implications for developing CHX nanocarriers specifically for in situ root canal treatment.
ACKNOWLEDGMENT
The authors are grateful to Manipal College of Dental College Sciences, Manipal Academy of Higher Education (MAHE), Manipal and Manipal College of Pharmaceutical Sciences, Manipal academy of Higher Education, MAHE, Manipal for providing the facilities. The authors sincerely thank the Council of Scientific and Industrial Research (CSIR), Govt of India for providing a Senior Research Fellowship to Ruchira Raychaudhuri.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Ethics Committee of Kasturba Medical College, MAHE, Manipal, India (Approval No: IEC933/2019).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Antunes HS, Rôças IN, Alves FR, Siqueira Jr JF. Total and specific bacterial levels in the apical root canal system of teeth with post-treatment apical periodontitis. J Endod. 2015;41(7):1037–42. CrossRef
2. Johnson EM, Flannagan SE, Sedgley CM. Coaggregation interactions between oral and endodontic Enterococcus faecalis and bacterial species isolated from persistent apical periodontitis. J Endod. 2006 Oct 1;32(10):946–50. CrossRef
3. Byström A, Sundqvist G. Bacteriologic evaluation of the efficacy of mechanical root canal instrumentation in endodontic therapy. Eur J Oral Sci. 1981;89(4):321–8. CrossRef
4. Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42(4):288–302. CrossRef
5. Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod. 2004 Feb 1;30(2):110–2. CrossRef
6. Loesche WJ. Chemotherapy of dental plaque infections. Oral Sci Rev. 1976 Jan 1;9:65–107.
7. Yan H, Yang H, Li K, Yu J, Huang C. Effects of chlorhexidine-encapsulated mesoporous silica nanoparticles on the anti-biofilm and mechanical properties of glass ionomer cement. Molecules. 2017;22(7):1225. CrossRef
8. Seneviratne CJ, Leung KCF, Wong CH, Lee SF, Li X, Leung PC, et al. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS One. 2014;9(8):e103234. CrossRef
9. Tronstad L, Barnett F, Cervone F. Periapical bacterial plaque in teeth refractory to endodontic treatment. Dent Traumatol. 1990;6(2):73–7. CrossRef
10. Gomes B, Souza SFC, Ferraz CCR, Teixeira FB, Zaia AA, Valdrighi L, et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36(4):267–75. CrossRef
11. Haseeb R, Lau M, Sheah M, Montagner F, Quiram G, Palmer K, et al. Synthesis and characterization of new chlorhexidine-containing nanoparticles for root canal disinfection. Materials. 2016;9(6):452. CrossRef
12. Wei Y, Zhang J, Zheng Y, Gong Y, Fu M, Liu C, et al. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019;9(11):6287–98. CrossRef
13. Mohsen AM, Younis MM, Salama A, Darwish AB. Cubosomes as a potential oral drug delivery system for enhancing the hepatoprotective effect of coenzyme Q10. J Pharm Sci. 2021;110(7):2677–86. CrossRef
14. Beyth N, Redlich M, Harari D, Friedman M, Steinberg D. Effect of sustained-release chlorhexidine varnish on Streptococcus mutans and Actinomyces viscosus in orthodontic patients. Am J Orthod Dentofacial Orthop. 2003;123(3):345–8. CrossRef
15. Çetin EÖ, Buduneli N, Atl?han E, K?r?lmaz L. In vitro studies on controlled-release cellulose acetate films for local delivery of chlorhexidine, indomethacin, and meloxicam. J Clin Periodontol. 2004;31(12):1117–21. CrossRef
16. Elgindy NA, Mehanna MM, Mohyeldin SM. Self-assembled nano-architecture liquid crystalline particles as a promising carrier for progesterone transdermal delivery. Int J Pharm. 2016;501(1–2):167–79. CrossRef
17. Shreya AB, Managuli RS, Menon J, Kondapalli L, Hegde AR, Avadhani K, et al. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: in vitro and in vivo performance evaluations. J Liposome Res. 2016;26(3):221–32. CrossRef
18. Rarokar NR, Saoji SD, Raut NA, Taksande JB, Khedekar PB, Dave VS. Nanostructured cubosomes in a thermoresponsive Depot system: an alternative approach for the controlled delivery of docetaxel. AAPS PharmSciTech. 2016 Apr 1;17(2):436–45. CrossRef
19. Vitkov L, Hannig M, Krautgartner WD, Herrmann M, Fuchs K, Klappacher M, et al. Ex vivo gingival-biofilm consortia. Lett Appl Microbiol. 2005;41(5):404–11. CrossRef
20. Jagielski J, Przysiecka ?, Flak D, Diak M, Pietralik-Moli?ska Z, Kozak M, et al. Comprehensive and comparative studies on nanocytotoxicity of glyceryl monooleate- and phytantriol-based lipid liquid crystalline nanoparticles. J Nanobiotechnol. 2021 Jun 3;19(1):168. CrossRef
21. Hinton TM, Grusche F, Acharya D, Shukla R, Bansal V, Waddington LJ, et al. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol Res. 2014 Jan 1;3(1):11–22. CrossRef
22. Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discov Today. 2016;21(5):789–801. CrossRef
23. Hans ML, Lowman AM. Biodegradable nanoparticles for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6(4):319–27. CrossRef
24. Sahoo SK, Panyam J, Prabha S, Labhasetwar V. Residual polyvinyl alcohol associated with poly (D, L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J Control Release. 2002;82(1):105–14. CrossRef
25. Onnainty R, Onida B, Páez P, Longhi M, Barresi A, Granero G. Targeted chitosan-based bionanocomposites for controlled oral mucosal delivery of chlorhexidine. Int J Pharm. 2016;509(1–2):408–18. CrossRef
26. Garala K, Joshi P, Shah M, Ramkishan A, Patel J. Formulation and evaluation of periodontal in situ gel. Int J Pharm Investig. 2013;3(1):29. CrossRef
27. Tartari T, Wichnieski C, Bachmann L, Jafelicci Jr M, Silva RM, Letra A, et al. Effect of the combination of several irrigants on dentine surface properties, adsorption of chlorhexidine and adhesion of microorganisms to dentine. Int Endod J. 2018;51(12):1420–33. CrossRef
28. Mahendra A, Koul M, Upadhyay V, Dwivedi R. Comparative evaluation of antimicrobial substantivity of different concentrations of chlorhexidine as a root canal irrigant: an in vitro study. J Oral Biol Craniofacial Res. 2014;4(3):181–5. CrossRef
29. Akram Z, Aati S, Ngo H, Fawzy A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J Nanobiotechnol. 2021;19(1):1–16. CrossRef
30. Tirali RE, Turan Y, Akal N, Karahan ZC. In vitro antimicrobial activity of several concentrations of NaOCl and Octenisept in elimination of endodontic pathogens. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2009;108(5):e117–20. CrossRef
31. Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36(2):100–5. CrossRef
32. Oliveira C, Ferreira CJO, Sousa M, Paris JL, Gaspar R, Silva BFB, et al. A versatile nanocarrier—cubosomes, characterization, and applications. Nanomaterials. 2022 Jan;12(13):2224. CrossRef
33. Khademi AA, Mohammadi Z, Havaee A. Evaluation of the antibacterial substantivity of several intra-canal agents. Aust Endod J. 2006 Dec 1;32(3):112–5. CrossRef
34. Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008;34(7):850–4. CrossRef
35. Kapoor K, Pandit V, Nagaich U. Development and characterization of sustained release methotrexate loaded cubosomes for topical delivery in rheumatoid arthritis. Int J Appl Pharm. 2020;12(3):33–9. CrossRef
36. Priyadarshini BM, Selvan ST, Lu TB, Xie H, Neo J, Fawzy AS. Chlorhexidine nanocapsule drug delivery approach to the resin-dentin interface. J Dent Res. 2016;95(9):1065–72. CrossRef
37. Jain K, Agarwal P, Jain S, Seal M, Adlakha T. Alexidine versus chlorhexidine for endodontic irrigation with sodium hypochlorite. Eur J Dent. 2018;12(03):398–402. CrossRef
38. Gomes B, Ferraz CCR, Vianna ME, Berber VB, Teixeira FB, Souza?Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34(6):424–8. CrossRef
39. Zapata RO, Bramante CM, de Moraes IG, Bernardineli N, Gasparoto TH, Graeff MS, et al. Confocal laser scanning microscopy is appropriate to detect viability of Enterococcus faecalis in infected dentin. J Endod. 2008;34(10):1198–201. CrossRef
40. Koburger T, Hübner NO, Braun M, Siebert J, Kramer A. Standardized comparison of antiseptic efficacy of triclosan, PVP–iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother. 2010;65(8):1712–9. CrossRef
41. Cherian B, Gehlot PM, Manjunath MK. Comparison of the antimicrobial efficacy of octenidine dihydrochloride and chlorhexidine with and without passive ultrasonic irrigation—an in vitro study. J Clin Diagn Res JCDR. 2016 Jun;10(6):ZC71–77. CrossRef
42. Salah S, Mahmoud AA, Kamel AO. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017;24(1):846–56. CrossRef
43. Shahadeh M, Jabban MO, Altaki Z. Evaluation of antimicrobial effectiveness of two endodontic irrigation solutions on the microbial reduction of Enterococcus faecalis in infected root canals (in vitro study). Int Dent Med J Adv Res. 2015;1(1):1–6. CrossRef