INTRODUCTION
Chitin, a polysaccharide composed of N-acetyl-?-glucosamine units linked by β (1→4) bonds, stands as the second most abundant natural polymer after cellulose. Transforming chitin through deacetylation produces chitosan, which is a linear polymer consisting of d-glucosamine and N-acetyl-d-glucosamine units [1]. In the pharmaceutical sector, chitosan is prized for its biocompatibility, biodegradability, and non-toxicity. It is used for drug delivery, controlled release formulations, wound healing aids due to its regenerative qualities, and in antimicrobial dressings to prevent infections [2]. Chitin is predominantly present in the exoskeletons of arthropods, fungal cell walls, and the beaks of cephalopods, marking its significant occurrence in nature, where it contributes to their structural support [1]. Within the diverse group of arthropods, crustaceans are a key source of commercial extraction process, largely due to their widespread presence and the substantial amounts of chitin found within their exoskeletons. The chitosan, degree deacetylation (DD), physiochemical properties, and functionality are known to differ with the source species and preparation methods, underscoring the influence of the originating biomass on the polymer’s characteristics [1,3].
Andhra Pradesh, renowned for having India’s second-longest coastline, serves as a fertile ground for the proliferation of various species, notably Penaeus vannamei (White leg Shrimp), Penaeus monodon (Asian Tiger Shrimp), and Scylla serrata (Black Crab). In this study, chitin is extracted from these shrimp and crab species with deproteinization preceding the demineralization step of extraction for foam control and there by optimizing chitin yield as proposed by Divya et al. [4] Subsequent deacetylation of chitin was carried on to produce chitosan. The chitosan obtained was reviewed in terms of the source crustacean genus and the differences in mucoadhesion, and physiochemical characteristics are illustrated, leveraging underutilized marine resources for high-value biomedical uses.
Chitosan’s unique bioadhesive properties make it an indispensable polymer in mucoadhesive drug delivery systems like mucoadhesive buccal tablets (MBT), enhancing the retention of drugs at mucosal sites for improved absorption and therapeutic efficacy [5]. Due to its natural source and its capacity to open tight junctions among epithelial cells, chitosan enables the controlled release of drugs [6], which aligns with the growing demand for environmentally friendly and sustainable pharmaceutical materials. MBT are pivotal in the realm of drug delivery, especially for medications prone to the first-pass effect, as they facilitate the direct transfer of drugs into the bloodstream via the buccal mucosa [7]. Metoprolol tartrate (MT), a beta1-adrenoceptor blocker utilized for hypertension management both alone or in combination, exhibits swift and complete absorption in humans. Nonetheless, oral administration yields plasma levels that are merely half of those achieved via intravenous routes, due to a 50% hepatic first-pass effect predominantly mediated by the cytochrome P450 enzyme system. Furthermore, its metabolites do not significantly contribute to MT’s antihypertensive effect. With an elimination half-life spanning 7.5 hours and 2.8 hours in poor metabolizers and extensive metabolizers respectively, there is a clear indication for exploring an alternative administration pathway for MT. Employing MBT addresses this by bypassing liver metabolism and extending the drug’s release up to 12 hours, offering a sustained therapeutic effect [8]. Taking advantage of the chitosan mucoadhesive nature, this study further aims to prepare the muchoadhesive buccal tablets of MT using the chitosan extracted from different sources to enhance drug delivery, reduce dosing frequency, and mitigate hepatic first-pass metabolism of MT. The approach aims to optimize chitosan’s properties for advanced pharmaceutical applications, focusing on release retardation, and improved mucoadhesion.
MATERIALS AND METHODS
Materials
The shrimps (Penaeus vannamei and Penaeus monodon), and crabs (Scylla serrata) were procured from the local market and their shells were collected separately. Metaprolol tartarate is obtained as a gift sample from AstraZeneca Pharma India, Bangalore. All the chemical reagents used in the present work were procured from Sigma Aldrich.
Ethical approval
The crustacean species utilized in the study included shrimps (Penaeus vannamei, Penaeus monodon) and crabs (Scylla serrata). Their use was approved by the Institutional Animal Ethics Committee (IAEC) under the proposal number 07/IAEC/VPCV/2023-24.
Chitosan extraction from shells of prawn and crab
The shells underwent a cleaning process and were subsequently dried in an oven at 65ºC for a period of 4 days, after which they were ground into a powder. To remove proteins, 30 g of this shell powder was subjected to a 4% NaOH solution at ambient temperature for a duration of 24 hours. Following deproteinization, the solution was removed, and the shells were rinsed thoroughly with water to reach neutrality. For the demineralization step, the shells were then immersed in a 4% Hydrochloric acid solution at 25ºC for 12 hours, resulting in chitin. This acid was also drained away, and the chitin was rinsed and air-dried. Subsequently, the chitin underwent deacetylation through a treatment with 65% NaOH for 24 hours to convert it into chitosan. After removing the alkali, the material was washed and finally, the chitosan was dried at 65ºC and stored. The percentage yield of chitosan from the sources was calculated as percentage yield. The measurements throughout the study were repeated in triplicate, and the data is presented as mean values accompanied by their corresponding standard deviations [4,9]. The chitosan extracted was sterilized in an autoclave for pharmaceutical application.
Characterization of chitosan extracted
Composition analysis
The chitosan extracted was visually inspected for color determination. Moisture content and residue on ignition were analyzed according to the methods of the Association of Official Analytical Chemists [4]. The product is tested for the presence of microorganisms using the turbidimetric method.
Viscosity and pH
The chitosan powder extracted (0.5 g) was added to 2% acetic acid and stirred for 3 hours to form a homogenous mixture. The pH was determined using pH meter and a Brookfield digital viscometer was used to measure the viscosity [10].
Degree of deacetylation
In each titration, 30 ml of 1% w/v solution (in 0.1 N hydrochloric acid) was used for assessment with methyl orange as an indicator. It is titrated with 0.1 N sodium hydroxide solution until a distinct color transition from pink to yellow–orange observed [11].
Solubility of extracted chitosan
A known excess amount of chitosan was added to 1% acetic acid and vigorously mixed for 1 hour using a mechanical shaker at ambient temperature. Subsequently, the solution underwent filtration through the Whatman No. 1 filter, and the residual sample was weighed to ascertain the amount of solubilized chitosan [4].
Fourier transform infrared spectroscopy (FTIR)
The structural composition of the synthesized chitin and chitosan was analyzed using Bruker Optik GmbH, Ettlingen, Germany. Spectra in the mid-infrared range were captured with a resolution of 2 cm-1 [12].
Preparation of mucoadhesive buccal tablets
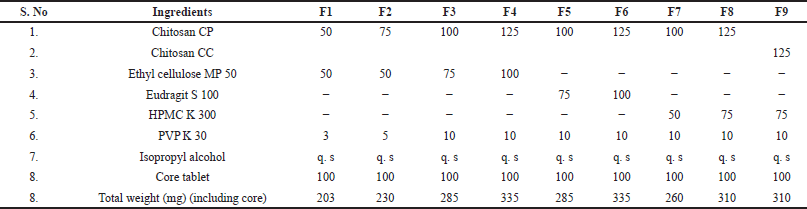
The drug MT was accurately weighed and combined with Avicel pH101 and HPMC K100M according to the specified quantities outlined in the table. Following this, lubricant and glidant were added and the blend was unified for 10 minutes. Subsequently, 100 mg of the blend was compressed using a 16-station automatic tablet punching machine (Cadmach Pvt Ltd., Ahmedabad, India) equipped with 6 mm flat-faced punches to form the core tablet. For the chitosan granules, a precise amount of Chitosan & PVP K 30 was moistened with IPA to create a damp mass suitable for granule preparation in a mortar. The dough was then pressed over a sieve #16 to generate chitosan granules, which were left to air-dry. The core tablet was positioned at the center of an 8 mm punch and coated with chitosan granules poured onto one side before recompression. Following this, the backing layer was applied to the other side after lifting the upper punch, resulting in the formation of multilayered MBT with a thickness ranging between 1.7 and 1.8 mm. Core composition is maintained uniformly with 50 mg drug and 50 mg diluent with a total weight of 100 mg. Preliminary trials were conducted to select the Bioadhesive polymer among the two chitosan types extracted as depicted in Table 1.
Evaluation of MBT
MBT underwent various tests to assess their physical and chemical properties. Tablet hardness, friability, weight uniformity, and drug content were assessed using the TH 1050M hardness tester (LABINDIA), FT 1020 friability tester (LABINDIA), Analytical Balance Cy 224c (Aczet Pvt Ltd.), UV 3000+ UV/VIS Spectrophotometer (LABINDIA), respectively. Hardness was measured on a sample of six tablets, while friability was assessed for tablets totaling 6.5 g at a speed of 25 rpm for 4 minutes [13]. To ensure weight uniformity, twenty tablets were precisely weighed, allowing the calculation of average weight and percentage deviation to be determined [14]. The MT content in twenty randomly selected tablets was quantified in a pH 6.8 phosphate buffer solution, with the MT concentration determined at 275 nm, in line with Indian Pharmacopoeia [15].
Mucoadhesive time or in vitro residence time
The assessment of mucoadhesion time (conducted in triplicate) involved the application of buccal tablets to freshly obtained sheep buccal mucosa. The mucosa was secured onto a glass slide, and the chitosan side of the tablet was moistened with a single droplet of phosphate buffer at pH 6.8 before being attached. Subsequently, the slide was placed in a glass beaker with 200 ml of the same buffer, at 37°C ± 1°C, with a stirring speed of 50 rpm. The duration until the tablet disengagement is documented [16].
Ex vivo mucoadhesive strength (N)
An adapted version of the balance technique was employed. Buccal mucosa from sheep was initially rinsed with distilled water and phosphate buffer solution at standard temperature. To achieve equilibrium, both pans were balanced by adjusting weights. A section of the mucosa was affixed to the beaker’s surface beneath the right pan, moistened with buffer. To the underside of the right side pan the tablet was attached with glue. Water was incrementally added to a previously weighed beaker on the left side pan till the tablet was detached from the buccal mucosa. The force necessary for the detachment is determined as the mucoadhesive strength. This procedure was conducted in triplicate, and the average value was considered [17]. The N is equivalent to the force applied for detachment and was determined using the given formula.
Mucoadhesive strength (N) = Weight of water (Kg) × 9.8 m/sec2
Tablet surface pH
The investigation of the tablets’ surface pH was conducted, given that a pH too acidic or too alkaline might irritate the buccal lining. Tablets were placed in distilled water (1 ml) with a pH close to 6.5 ± 0.05 for 2 hours at standard temperature and allowed to swell. Subsequently, the pH measurement involved contacting the tablet’s surface with the electrode and allowing a settling time of 1 minute for pH equilibrium before the reading was taken [18].
Swelling test
Each buccal tablet was weighed initially (W1) and then placed on a 2% agar gel surface within individual Petri dishes, ensuring the core was in contact with the gel, and incubated at 37°C ± 1°C. Over a 6-hour period, at 1-hour intervals, tablets were carefully retrieved, and any residual surface moisture was gently blotted away with filter paper. After drying, the tablets’ swelling was measured by reweighing (W2). The swelling index (SI) was calculated as the variance between the final weight of the swollen tablet (W2) and its initial weight (W1), divided by the initial weight and is expressed as a percentage [16].
 | Table 1. Composition of metoprolol mucoadhesive multilayer tablets. [Click here to view] |
In vitro dissolution test
The study was carried out following the guidelines outlined in the Indian Pharmacopoeia 2018, employing the USP II dissolution apparatus to evaluate the cumulative percentage of drug released. A set of six tablets was subjected to dissolution testing in 900 ml of a pH 6.8 phosphate buffer solution at 37°C ± 0.5°C. At 1-hour intervals, 5 ml of the solution was sampled and subsequently replaced with an equal quantity of fresh buffer. The drug content was quantified via UV spectrophotometry at a wavelength of 275 nm. Drug release patterns were determined by fitting the in vitro data to drug release mathematical models, with the best fit identified by plot linearity [19,20].
Ex vivo permeation study
The study utilized a Keshary-Chien–type glass diffusion apparatus, maintaining a temperature of 37°C ± 0.2°C. The sheep buccal mucosa was secured between the donor and receptor chambers. The tablet with its chitosan layer oriented towards the mucosa, was then inserted, and the chambers were tightly sealed. The donor side received 1 ml of a pH 6.8 phosphate buffer, while the receptor side, capable of holding up to 25 ml, had pH 6.8 phosphate buffer. To ensure consistent movement within the receptor chamber, a magnetic bead provided continuous, gentle stirring. Samples of 1 ml were collected at set intervals to measure the drug concentration, using UV spectrophotometry against a placebo sample for accuracy [16].
Stability analysis of optimized formulation
Optimized formulations were distributed into two groups, each sealed in airtight bottles, and placed in humidity-controlled environments at 30°C ± 2°C with 70% ± 5% RH, and 40°C ± 2°C with 75% ± 5% RH, adhering to ICH and WHO guidelines for zone IV and accelerated stability testing, respectively [21]. After periods of 3 and 6 months, these buccal tablets were evaluated for the tablet properties and in vitro dissolution efficiency. The stability of the dissolution rates pre- and post-storage under both long-term and accelerated conditions was assessed using the similarity factor (f2) [22].
RESULTS AND DISCUSSION
Chitin was successfully extracted from both prawn and crab sources and further deacetylated to obtain chitosan, and pulverized, passed through mesh 60# to regulate particle size. The chitosan collected from prawn and crab sources will be denoted as CP and CC, respectively, throughout the discussion. The overall yield is higher accounted to the change in the sequence of the extraction process, deproteinization followed by the demineralization step; however, the percentage yield was higher from black crab shells compared to the shrimp shells. The percentage yield is subjective to the influence of the percentage of weight lost during the deacetylation process.
Notably, CC exhibited a brown color, consistent with previous reports on chitosan extracted from various crab species (Fig. 1). However, no chemical treatment for decoloration was applied in this study to avoid compromising the water-binding capacity of chitosan, which was observed in past literature [23]. CP and CC showed no characteristic odor or taste. The turbidimetric analysis confirmed the absence of viable content in both CP and CC, it can also be an indication of their potential antimicrobial activity. The pH of CP and CC was observed to be near neutral, with values of 6.2 and 6.5, respectively. Additionally, CC showed a higher residual content on ignition compared to CP, suggesting a higher mineral content attributed to the marine environment where crabs inhabit (Table 2). However, both CC and CP met the criteria for high-grade chitosan with residual content <1%. Furthermore, the source of chitin significantly (p < 0.05) influenced the viscosity of the resultant chitosan, with CC exhibiting lower viscosity (370.92 cP) compared to CP (407.44 cP). This difference in viscosity may stem from variations in chitin composition, DD, and potential mineral content between crab and shrimp shells. Comparing the compressibility index and Hausner’s ratio of CP and CC, CC has a slightly higher compressibility index and Hausner’s ratio compared to CP. This suggests that CC may exhibit slightly poorer flow and compaction properties compared to CP. However, both chitosan samples demonstrate acceptable tableting properties, with compressibility indices and Hausner’s ratios within acceptable ranges for pharmaceutical tablet formulation. Overall, these differences in tableting properties between CP and CC may influence their suitability for specific tablet formulations and manufacturing processes.
Degree deacetylation
The deacetylation process is pivotal in modifying the chemical structure and characteristics of chitosan by removing acetyl groups and forming reactive amino groups [24]. This alteration significantly influences key properties such as solubility, chemical reactivity, and biodegradability [25]. DD of chitosan derived from shrimp shell (90.15% ± 2.33%) was notably higher compared to that from crab shell (83.6% ± 1.95%). This difference in DD can be credited to factors, including the inherent composition of chitin in the source material and the duration of treatment with NaOH for deacetylation. It’s noteworthy that longer deacetylation periods, as employed in this study (24 hours), typically result in higher DD values for both shrimp and crab-derived chitosan. Previous literature has also reported lower DD values when deacetylation is carried out for shorter periods, highlighting the significant influence of treatment duration on the degree of deacetylation [23].
 | Figure 1. Chitosan varieties extracted A) CC B) CP. [Click here to view] |
Solubility of extracted chitosan
The solubility assessment of chitosan is crucial to ensure its purity and assess potential side effects stemming from residual minerals and proteins, which could adversely affect its solubility and biological properties [23]. In this study, CP and CC exhibited high solubility, with values of 95.62% and 91.37%, respectively. The higher solubility of CP compared to CC may be attributed to the differences in their respective deacetylation degrees. A higher DD generally results in a more hydrophilic chitosan structure, enhancing its solubility in aqueous solutions. Additionally, chitosan’s solubility in acidic aqueous solutions is facilitated by the protonation of its primary amine groups, a process directly influenced by its degree of deacetylation. This reveals the significance of considering extraction source and deacetylation degree when assessing chitosan solubility and its potential biological applications.
FTIR analysis
The FTIR spectra of CP and CC are provided in Figure 2, and the principal characteristic peaks of chitosan are identified in both the sample and discussed. The Stretching vibration of -OH and -NH groups of the amines are recorded at 3,434 and 3,442 cm-1 CP and CC, respectively, higher DD in CP made the peak more prominent in CP with higher intensity, corresponding to an increase in amino groups. The peaks of C=O stretching of the amide I band, the N–H (N-acetylated residues, amide II band) bending vibrations and the C-H bending, OH bending of CP are identified at 1,654.26 cm-1, 1,570.13 cm-1, 1,421.07 cm-1, and 1,375.16 cm-1, respectively. In the case of CC, the peaks are observed at 1,657.98 cm-1, 1,572.24 cm-1, 1,423.20 cm-1, and 1,377.95 cm-1, respectively. The amide group peaks recorded at in the range of 1,550–1,650 cm-1 showed a shift to a lower wavenumber in CP compared to CC. The decrease in acetyl groups might cause this shift. The stretching vibrations of C-H group are recorded at 2,923.15 cm-1 and 2,921.32 cm-1 in CP and CC, respectively. The CP and CC showed the same functional groups at diverse peak wavelengths because both have differences in sources of extraction and DD of the sample [26]. The FTIR spectra of MT combined with the chitosan (Fig. 3) ruled out the possibility of interaction by retaining the peaks characteristic of the chitosan with a small shift, which was observed at 1,574 cm–¹ and 1,378 cm–¹. The MT shows prominent broad peaks at 3,397.63 cm–¹ and 3,148.66 cm–¹, which were attributed to the O-H and N-H bonds stretching, respectively. Notably, the N-H stretch merges with the aromatic hydrogen stretch, contributing to the observed peak at 3,148.66 cm–¹. Other significant peaks include the stretching vibrations of C-O-C, C-N, and aromatic C=C at 1,242.23 cm–¹, at 1,563.76 cm–¹, at 1,114.24 cm–¹, respectively. While the C-H stretching is observed at 2,923.97 cm–¹, and C-O at 1,385.12 cm–¹. However, the intensity of the peaks was enhanced which was observed at 2,940 cm–¹, 1,574 cm–¹, and 1,242 cm–¹ due to the presence of the drug [27].
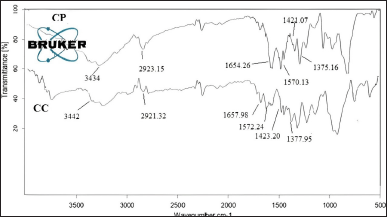 | Figure 2. FTIR spectra of chitosan extracted from different sources. [Click here to view] |
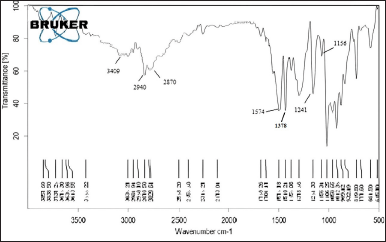 | Figure 3. FTIR spectrum of MT in combination with chitosan. [Click here to view] |
Preparation of MBT
Based on the initial analysis comparing the purity and DD of CC and CP, the optimization of polymer quantities for MBT is conducted using CP first, followed by a comparison with CC. Among the three polymers considered for backing layers—Ethyl cellulose MP 50, Eudragit S 100, and HPMC K 300, Ethyl cellulose MP 50 stands out for its exceptional film-forming properties and impermeability, making it well-suited as a backing layer. Eudragit S 100, being pH-sensitive, ensures controlled drug release by demonstrating limited dissolution in acidic pH conditions (pH <7), thereby enabling tailored drug delivery profiles. HPMC K100M is selected for its ability to form a protective barrier, minimizing drug absorption through avenues other than the buccal mucosa and thereby meeting the requirement for controlled drug release. For the first time in buccal delivery, HPMC and Eudragit have been scrutinized as potential backing layers in this study. In preparation for the mucoadhesive layer, chitosan is granulated to achieve uniform coating over the core for optimal mucoadhesion. Additionally, granulated chitosan exhibits improved flow ability and compressibility, as evidenced by the angle of repose, Carr’s index, and Hausner’s ratio values of 27, 15, and 1.10, respectively. All formulations prepared demonstrate uniform weight, good mechanical integrity, and drug content within standard requirements, as indicated in Table 3.
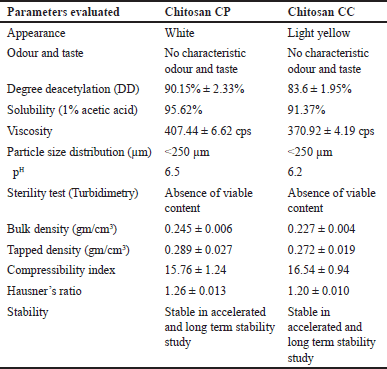 | Table 2. Physiochemical properties of extracted chitosan varieties. [Click here to view] |
Mucoadhesive time
Based on the ex vivo mucoadhesion results (Table 4), it is evident that varying the chitosan concentration in the formulations significantly (p < 0.05) influences the mucoadhesive strength of the buccal tablets. Formulations with chitosan concentrations near or above 100 mg, such as F3, F4, F5, F6, F8, and F9, exhibited longer mucoadhesion times compared to formulations with lower concentrations, such as F1 and F2. This observation suggests that increasing chitosan concentration enhances the mucoadhesive properties of the tablets. On the contrary, formulations with lower concentrations of chitosan demonstrated shorter mucoadhesion times, indicating weaker adhesion to the buccal mucosa. These results suggest that insufficient chitosan concentration may compromise the mucoadhesive strength of the tablets, leading to premature detachment from the mucosa. It is essential to note that the objective to develop buccal tablets capable of retarding drug release for more than 12 hours needs longer mucoadhesion. Hence, formulations with chitosan concentration above 100 mg, aligned with the formulation objective.
Ex vivo mucoadhesive strength
As observed in the results in Table 4, formulations with higher concentrations of chitosan generally exhibited increased mucoadhesive strength. For instance, formulations F4, F6, and F8, demonstrated stronger mucoadhesive properties compared to formulations with lower chitosan concentrations. This can be attributed to the uniform, thick coating layer and also the greater availability of chitosan molecules for interaction with mucosal surfaces, leading to enhanced adhesion. Moreover, the type of chitosan used also played a crucial role in determining mucoadhesive strength. Comparing formulations F8 and F9, which utilized different types of chitosan (CP and CC, respectively), it is evident that formulation F8 with CP exhibited higher mucoadhesive strength compared to formulation F9 with CC.
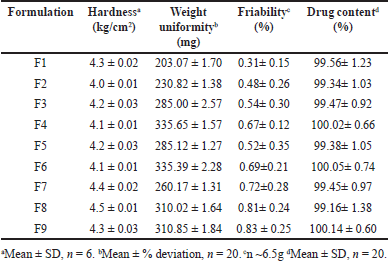 | Table 3. Tablet characteristics of Metoprolol MBT. [Click here to view] |
While the mucoadhesive strength and residence time are influenced by the concentration of chitosan in the formulation, it is also essential to consider the DD of chitosan. Higher DD typically results in increased availability of amino groups, which can enhance the interaction between chitosan and mucosal surfaces, thereby improving mucoadhesion. Therefore, formulations with chitosan (CP) of higher DD may exhibit stronger mucoadhesive properties and longer residence times compared to those with lower DD (CC).
Tablet surface pH
The surface pH of buccal tablets is a critical parameter that can influence their compatibility with the buccal mucosa. In this study, formulations F1 to F8, containing chitosan extracted from prawn (CP), exhibited similar surface pH values, ranging from 6.48 to 6.73. Additionally, the addition of PVP K 30, showed limited influence on the tablet pH across all formulations, likely due to the low concentration used. However, formulation F9, which incorporated chitosan extracted from crab (CC), displayed a notably lower surface pH of 6.22, this emphasizes the importance of considering the source of chitosan in buccal tablet formulations. Maintaining surface pH within a range similar to that of saliva (typically around 6.2–7.6) can offer several advantages in buccal formulations. First, it promotes mucosal compatibility, reducing the likelihood of irritation or discomfort upon administration. Additionally, this can enhance patient acceptance and compliance by minimizing any unpleasant sensations associated with the formulation.
Swelling test
The SI of buccal formulations is a crucial parameter that influences drug release and mucoadhesion. The swelling behavior of formulations (F1–F9) was evaluated over time as shown in Table 4, revealing notable trends and insights. Initially, during the first hour, the SI across formulations were relatively comparable, indicating similar initial hydration and swelling kinetics. However, as time progressed, variations in swelling became evident, suggesting differences in water permeation and hydration capacity among the formulations. In particular, formulations featuring lower concentrations of chitosan demonstrated increased swelling over the 6-hour period, suggesting thinner layers with enhanced channels for water permeation. F9 containing CC exhibited reduced swelling compared to CP at an equivalent concentration, a distinction attributed to the higher deacetylation of CP in contrast to CC.
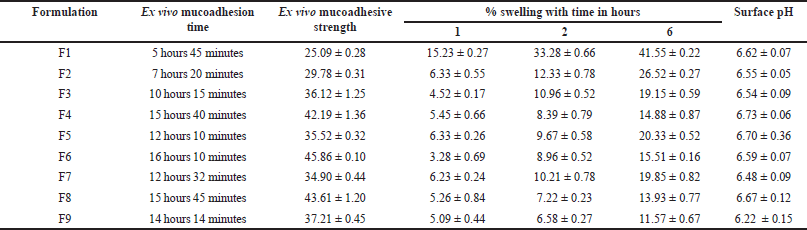 | Table 4. The ex vivo mucoadhesion time, ex vivo mucoadhesion strength, % swelling and surface pH of duration of metoprolol MBT. [Click here to view] |
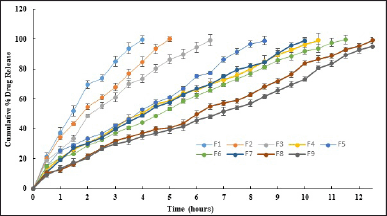 | Figure 4. Cumulative percentage drug profile of Metoprolol MTBT. [Click here to view] |
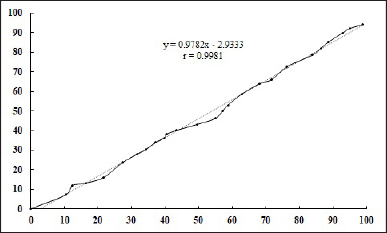 | Figure 5. Correlation between in vitro drug released and ex vivo drug permeation study of optimized formulation (F8). [Click here to view] |
In vitro dissolution test
The drug release profiles of the buccal tablets (Fig. 4) revealed intriguing insights into the interplay between chitosan concentration, type of backing layer, and dissolution characteristics. Formulations F1 and F2 with lower concentrations of chitosan, generally exhibited faster dissolution rates compared to those with higher concentrations, they were unable to hold the drug for more than 5 hours due to the low coat-to-core ratio (1:1), 100 mg of coat (chitosan plus backing layer) was insufficient to extent the release for more than 12 hours. Further increase in coat-to-core ratio to 2.5:1 (F3-F9) significantly (p < 0.05) improved the release retardation. The buccal cavity being frequently prone to movement and agitation/turbulence, thin and improper coating of core by the backing layer, may lead to two-way release of the drug from the core causing complete drug release in a shorter period of time. This signifies the importance of optimizing both chitosan and backing layer concentration. Upon comparing the release profiles of F1 and F2, it becomes apparent that an increase in chitosan concentration (while maintaining a constant backing layer concentration) correlates with a reduction in drug release. This observation aligns with the principle that higher concentrations of chitosan create denser matrices, thereby retarding drug release by hindering water penetration.
Additionally, the choice of backing layer polymer significantly impacted the dissolution behavior of MBT at identical chitosan concentrations. HPMC K 300 (F7, F8), and Eudragit S 100 (F5, F6) backing layers demonstrated slower drug release profiles compared to Ethyl cellulose (F3, F4) attributable to their swelling nature and pH-dependent dissolution characteristics, respectively. The controlled release achieved with HPMC K 300 and Eudragit S 100 establishes their suitability as a backing layer for buccal tablets for the first time, particularly in formulations where sustained drug release is desired. Though F3-F9 formulations maintained a release of less than 25% in 1st our, only F8 formulation with HPMC K 300 at 75 mg, successfully met the standard drug release requirements of USP (<25%, 20%–40%, 40%–60%, >80% in 1 hour, 4 hours, 8 hours, and 10 hours respectively) showing complete release (99.08% ± 1.85%) in 12.5 hours. This observation aligns with the well-known properties of HPMC, including its ability to form gel layers upon hydration, which can act as a barrier to drug diffusion. As a result of achieving a thick gel at 75 mg of HPMC K 300, a higher concentration of 100 mg was not investigated in this study. The utilization of ethyl cellulose as a backing layer effectively shielded the drug release from the core for approximately 10 hours, with the release duration extending beyond 10.5 hours when combined with 125 mg of chitosan. While it is possible that increasing the concentration of ethyl cellulose could potentially further prolong the release, the objectives were already achieved with lower concentrations of other polymers. Therefore, additional trials with higher concentrations of ethyl cellulose were not included in this study.
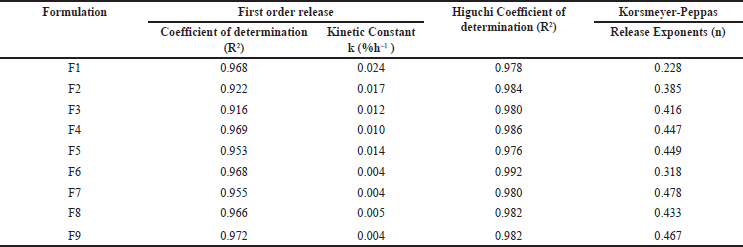 | Table 5. In vitro dissolution kinetics of Metoprolol MBT. [Click here to view] |
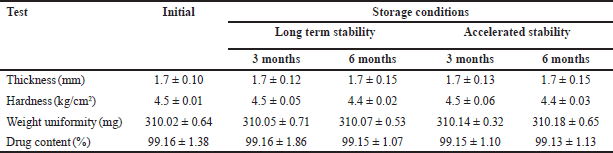 | Table 6. Stability assessment of optimized metoprolol MBT. [Click here to view] |
The dissolution data underscores the effectiveness of the multilayer mucoadhesive tablet design in extending drug release. As the drug is not physically mixed with the coating layer but rather must diffuse through it, the multilayer configuration provides a controlled pathway for drug release. This is particularly evident in F1 and F2, where even relatively low concentrations of chitosan polymer effectively sustain drug release over the 5-hour dissolution period. Notably, the gradual increase in drug release percentages observed at each time point suggests a regulated diffusion process facilitated by the chitosan layers. Such a multilayer approach holds promise for achieving prolonged and controlled drug release, offering potential benefits for patient treatment regimens and therapeutic efficacy, especially in buccal drug delivery where the high weight of the tablet effects patient compliance.
Notably, F8 and F9, incorporating CC, displayed distinct dissolution profiles despite having similar chitosan concentrations. F9 showed a release of 94.98% ± 1.31% in 12.5 hours. This discrepancy underscores the importance of considering the type of chitosan, as variations in deacetylation levels can significantly impact dissolution behavior. Collectively, this emphasizes the need for meticulous optimization of formulation parameters to accomplish desired drug release profiles for therapeutic efficacy.
The gradual increase in swelling observed with chitosan layers in the buccal formulations correlates with enhanced drug release over time, indicating a direct relationship between swelling behavior and release kinetics. Moreover, formulations with higher concentrations of chitosan displayed gradual swelling, leading to improved retardation of drug release and prolonged therapeutic action. For all batches, the drug release conformed to a first-order kinetic model, while the Korsmeyer-Peppas model n values stretched between 0.228 and 0.478 (Table 5), suggesting the Fickian release mechanism dominated by diffusion rather than erosion. This indicates efficient drug release without compromising mucoadhesion throughout the duration of release. Formulation F8 was selected as the optimized formulation depending on its in vitro drug release (95.23% ± 2.79% at 12 hours), SI (13.93 ± 0.77 at 6 hours), and ex vivo mucoadhesive strength (43.61 ± 1.20 g), demonstrating favorable drug release coupled with adequate mucoadhesion.
Ex vivo permeation study
An ex vivo buccal mucosal permeation study was performed with the formulation F8. The results revealed a drug permeation of 94.11% ± 2.14% over a 12.5-hour period. From Figure 5, a positive correlation exists between the in vitro drug release rate and ex vivo drug permeation across the sheep buccal mucosa, with a correlation coefficient (r) of 0.9981. Chitosan’s positively charged amino groups interact with negatively charged mucin molecules in the mucus layer, leading to improved adhesion and prolonged residence time on mucosal surfaces. This interaction alters the mucus layer’s viscosity and hydration status, promoting the paracellular transport of drugs across the mucosal membrane [6]. This possible mechanism explains the better permeation of the drug and also the difference in mucoadhesion with the difference in the amino groups (DD).
Stability assessment of optimized formulation
The stability assessment outlined in Table 6 revealed no observable alterations in the MBT under both long-term and accelerated conditions. Analysis of cumulative percentage drug release from F8 indicated consistency between pre- and post-storage samples, as corroborated by the high similarity factor (f2) values of 96.52 and 92.10 for long-term and accelerated conditions, respectively. Moreover, the integrity of the coating thickness remained intact, effectively shielding the drug from external factors. Both the percentage drug content and drug release remained unchanged, underscoring the product’s stability in accordance with ICH guidelines.
CONCLUSION
In conclusion, the study underscores the pivotal role of chitosan in formulating multilayer mucoadhesive tablets for controlled drug delivery. Through meticulous examination of chitosan properties such as DD, mucoadhesive strength, and solubility, we discerned that chitosan derived from shrimp shells (CP) exhibited enhanced solubility and dissolution characteristics compared to that from crab shells (CC). This suggests that chitosan with higher DD facilitates more efficient drug release. Furthermore, the CC and CP extracted retarded the drug release with considerable mucoadhesive strength. Optimization of HPMC concentration as a backing layer and formulation parameters may offer opportunities to fine-tune the release kinetics and tailor drug delivery profiles according to specific therapeutic requirements. Additionally, the study emphasizes the efficacy of the multilayer approach in sustaining drug release, even at lower chitosan concentrations. By adopting a multilayer design, prolonged drug release was achieved, while ensuring consistent mucoadhesion. The findings offer valuable insights into optimizing drug delivery systems, with implications for enhancing therapeutic efficacy and patient adherence.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The crustacean species utilized in the study included shrimps (Penaeus vannamei, Penaeus monodon) and crabs (Scylla serrata). The study protocol was approved by the Institutional Animal Ethics Committee, Department of Pharmaceutics, Vignan Pharmacy College, Guntur, India with approval number 07/IAEC/VPCV/2023-24.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Singh KS, Majik MS, Tilvi S. Vibrational spectroscopy for structural characterization of bioactive compounds. In: Comprehensive analytical chemistry. Amsterdam, The Netherlands: Elsevier; 2014. Vol. 65, pp. 115–48.
2. Verma AK. Collagen-Based Biomaterial as Drug Delivery Module. In: In: Mazumder N, Chakrabarty S, editors. Collagen Biomaterials. London, UK: IntechOpen; 2022.
3. Minke RA, Blackwell J. The structure of α-chitin. J Mol Biol. 1978 Apr 5; 120(2):167–81.
4. Divya K, Rebello S, Jisha MS. A simple and effective method for extraction of high purity chitosan from shrimp shell waste. In Proceedings of the international conference on advances in applied science and environmental engineering-ASEE; 141–5; Kuala Lumpur, Malaysia: 2014.
5. M. Ways TM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers. 2018 Mar 5;10(3):267.
6. Mohammed MA, Syeda JT, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017 Nov 20;9(4):53.
7. Gilhotra RM, Ikram M, Srivastava S, Gilhotra N. A clinical perspective on mucoadhesive buccal drug delivery systems. J Biomed Res. 2014 Mar;28(2):81.
8. Validus Pharmaceuticals. Lopressor, Metaprolol Tartarate Tablets. Jersey, NJ: Food and Drug Administration; 2013. pp 3276452..
9. Hisham F, Akmal MM, Ahmad FB, Ahmad K. Facile extraction of chitin and chitosan from shrimp shell. Mat Today Proc. 2021 Jan 1;42:2369–73.
10. Faisal M, Elhussieny A, Ali KA, Samy I, Everitt NM. Extraction of degradable bio polymer materials from shrimp shell wastes by two different methods. In IOP Conf Ser Mat Sci Eng. 2018 Nov 1;464(1):012004.
11. Dutta J. A facile approach for the determination of degree of deacetylation of chitosan using acid-base titration. Heliyon. 2022 Jul 1;8(7):e09924.
12. Vaingankar PN, Juvekar AR. Fermentative production of mycelial chitosan from zygomycetes: media optimization and physico-chemical characterization. Adv Biosci Biotechnol. 2014 Oct 31;5(12):940.
13. Indian Pharmacopoeia, Govt of India. Ghaziabad, India: Ministry of Health and Family Welfare, IPC; 2018. Vol. 2.5.5, pp 309.
14. Indian Pharmacopoeia, Govt of India. Ghaziabad, India: Ministry of Health and Family Welfare, IPC; 2018. Vol. 2.5.3, p 308.
15. Indian Pharmacopoeia, Govt of India. Ghaziabad, India: Ministry of Health and Family Welfare, IPC; 2018. Vol. 2, p 1118.
16. Patel VM, Prajapati BG, Patel MM. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS Pharmscitech. 2007 Mar;8:E147–54.
17. Koirala S, Nepal P, Ghimire G, Basnet R, Rawat I, Dahal A, et al. Formulation and evaluation of mucoadhesive buccal tablets of aceclofenac. Heliyon. 2021 Mar 1;7(3):e06439.
18. Krishna PS. Advances and challenges in mucoadhesive drug delivery systems: a comprehensive review. Asian J Pharm. 2024 Jun 15;18(02);401–10.
19. Shirsand SB, Suresh S, Keshavshetti GG, Swamy PV, Reddy PV. Formulation and optimization of mucoadhesive bilayer buccal tablets of atenolol using simplex design method. Int J Pharm Investig. 2012 Jan;2(1):34.
20. The United States Pharmacopeial Convention. USP-NF Metoprolol succinate extended release tablets monograph. Rev Bull. 2018;3:1–4.
21. Tangri P, Bisht B. Who role and guidelines in stability study of pharmaceuticals: a regulatory perspective. Int J Res Pharm Biomed Sci. 2012;3:1379–86.
22. Zeeshan F, Lin PY, Sheshala R. Application of similarity factor (f 2) and time required to drug release (t%) indicators for dissolution profiles comparison of paracetamol tablets. Indian J Pharm Edu Res. 2020;54(3):647–53.
23. Sarbon NM, Sandanamsamy S, Kamaruzaman SF, Ahmad F. Chitosan extracted from mud crab (Scylla olivicea) shells: physicochemical and antioxidant properties. J Food Sci Technol. 2015 Jul;52:4266–75.
24. Yadav M, Kaushik B, Rao GK, Srivastava CM, Vaya D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: a review. Carbohydr Polym Technol Appl. 2023 Jun 1;5:100323.
25. Issahaku I, Tetteh IK, Tetteh AY. Chitosan and chitosan derivatives: recent advancements in production and applications in environmental remediation. Environ Adv. 2023 Apr 1;11:100351.
26. Islam S, Arnold L, Padhye R. Comparison and characterisation of regenerated chitosan from 1-Butyl-3-methylimidazolium chloride and chitosan from crab shells. BioMed Res Int. 2015;2015(1):874316.
27. Cesme M, Tarinc D, Golcu A. Spectrophotometric determination of metoprolol tartrate in pharmaceutical dosage forms on complex formation with Cu (II). Pharmaceuticals. 2011 Jun 28;4(7):964–75.