INTRODUCTION
The risk of antibiotic-resistant bacteria has rapidly increased due to the widespread use of antibiotics worldwide [1]. Methicillin-resistant Staphylococcus aureus (MRSA) is a dangerous human pathogen that causes severe morbidity and mortality all over the world [2]. According to the World Health Organization (WHO), it has been ranked as a high-priority bacteria for the discovery of new antibiotics [3]. In 2019, over 100,000 deaths were caused by MRSA, based on a recent study in the Lancet [4].
MRSA is a serious concern to public health since it is resistant to many conventional antibiotic treatments and is commonly linked to deadly infections such as pneumonia, endocarditis, and bacteremia [5]. Typically, it is resistant to penicillin-like antibiotics such as penicillin, oxacillin, amoxicillin, and methicillin. [6]. Vancomycin has been a first-line treatment for MRSA since the 1980s. Nevertheless, within the last two decades, Vancomycin-resistant Staphylococcus aureus (VRSA) has emerged and is now a significant worldwide source of morbidity and mortality [7].
One of the primary sources of antibacterial agents is thought to be the secondary metabolites produced by organisms, especially Actinobacteria [8]. Actinobacteria is one of the main microbe factories for the synthesis of new antibiotics since they are the source of around two-thirds of all known antibiotics. Approximately 80% of all antibiotics obtained from Actinobacteria are produced by Streptomyces alone [9]. Streptomyces is a Gram-positive, obligate aerobe bacteria that grows in a variety of environments and has a branching filamentous structure similar to fungi. It is nonmotile, spore-forming, nonfastidious, and has a high DNA content rich in guanine-cytosine [10]. Despite the fact that Streptomyces species have been isolated from a wide range of terrestrial ecosystems across the world, their prevalence and ability to generate bioactive metabolites are dependent on their specific habitat, location, and environment [11].
One of the challenges in the Actinobacteria screening process is finding growing conditions that promote the synthesis of the secondary metabolites [8]. The generation of antimicrobials by Actinobacteria is greatly influenced by key factors such as physical parameters and cultural media [12]. The optimal fermentation conditions (pH, agitation speed, temperature, and so on) and medium components must be determined and optimized in order to develop a production medium [13]. Consequently, the key to improving antibiotic production is to create a suitable medium. Therefore, the aim of this study is to optimize the cultural conditions and nutritional components of Streptomyces tuirus isolated from the rhizosphere of Origanum majorana to produce biologically active compounds against MRSA.
MATERIALS AND METHODS
Strain
Streptomyces tuirus used in this study was isolated from the rhizosphere of Origamum majorana plant grown in Sidon, South Lebanon. Before isolation, the rhizospheric soil was air-dried and pre-treated with calcium carbonate (CaCO3) to stop the growth of other unwanted bacteria. Actinobacteria were isolated by using the soil dilution plate technique on starch casein agar following the Bano et al. [14] method. The agar was prepared by mixing 10 g of soluble starch, 2 g K2HPO4, 2 g KNO3: 2 g, 0.3 g casein, 0.05 g MgSO4.7H2O, 0.02 g CaCO3, 0.01 g FeSO4.7H2O, 15 g agar, in a total volume of 1 l and pH 7.0. The agar was supplemented with nystatin (50 µg/ml) to stop the growth of fungi. The plates were then incubated for 7 days at 28°C. Different bacteria were isolated and their identity was confirmed using 16S rRNA gene sequence analysis.
Optimization of culture conditions affecting antibacterial compound production
Optimization of physical parameters
In order to optimize the antibacterial activity of the extracted metabolites produced by S. tuirus, a series of experiments were conducted where one variable was changed at a time while the other parameters were kept constant at a specific set of conditions [15]. The different parameters studied for optimization were medium, temperature, incubation period, and agitation speed. To determine the most effective medium for metabolite production, the isolate was grown in three different culture media: starch-casein broth (SCB), nutrient broth (NB) (g/l: 10 g peptone, 3 g beef extract, and 5 g NaCl) and tryptone yeast extract ISP1 (g/l: 5 g tryptone, 3 g yeast extract) at 28°C, 120 rpm for 7 days. Based on the optimal medium, further optimal fermentation-process experiments were carried out by single-factor tests. For the incubation period, the optimum medium was inoculated and incubated separately for 7 days, 14 days, and 21 days at 28°C. For culture temperature tests, the culture temperatures were carried on at 25°C, 30°C, and 35°C for 7 days. Similarly, the optimum agitation rate was carried out at 3 different speeds: 100 rpm, 120 rpm, and 150 rpm at 28°C for 7 days.
Optimization of biochemical parameters
The biochemical parameters used for optimization were various sources of carbon and nitrogen. Carbon sources such as glucose, lactose, and sucrose at a concentration of 1% were supplemented separately into the SCB. As for the diverse nitrogen sources used, sodium nitrate, tryptone, and peptone were individually added into the fermentation medium at a final concentration of 1% as well. The flasks were placed in a shaking incubator set at 120 rpm for 7 days at 28°C.
Extraction of released metabolites
The extraction of the released metabolites was done according to Ibnouf et al. [16]. In brief, after incubation under the appropriate conditions, the cultures were centrifuged for 20 minutes at 4,000 rpm. To the collected supernatants, an equivalent volume of ethyl acetate was added, and mixed, and then the upper organic layer was separated and evaporated in a water bath at 40°C. The weight of the crude extract was measured, and the extract was dissolved in 5% dimethylsulfoxide (DMSO) to a final concentration of 2 mg/ml.
Determination of antibacterial effect
The antibacterial effect of the isolated metabolites was determined using the Agar well diffusion method. MRSA was diluted using sterile normal saline, and the concentration of the bacterial culture was adjusted to 0.5 McFarland standard. 100 µl of standardized cell suspensions were spread on a Mueller–Hinton agar. 100 µl of the crude extracts were added to the wells created using a 6 mm sterile cork-borer and then incubated at 37°C. DMSO was used as a negative control while vancomycin was used as a positive control. The zones of inhibition (ZOI) were measured and examined following a 24-hour incubation period.
Statistical analysis
All experiments were conducted in triplicate. The obtained values are presented as mean ± standard error of the mean (SEM). One-way ANOVA was used to calculate the statistically significant differences between groups, followed by the Tukey test. A p-value of < 0.05 was considered significant. GraphPad Prism version 10.1.1 (270) was used for analysis and drawing graphs.
RESULTS AND DISCUSSION
Globally, bacterial infections are becoming a serious issue due to the substantial mortality and morbidity caused by antibiotic-resistant strains [17]. Notably, MRSA stands out as a significant organism among these drug-resistant strains [18]. The WHO and CDC have identified MRSA as one of the pathogenic bacteria that poses serious and crucial risks [4]. To address this challenge, researchers are investigating alternative reservoirs of antimicrobial agents, and Actinobacteria is considered a viable option [17].
In this study, the emphasis was on optimizing culture conditions and nutritional components to improve the production of anti-MRSA agents by S. tuirus. We utilized a single-factor analysis to enhance the antimicrobial effectiveness against MRSA. To maximize the antibacterial activity of the isolate, we carried out small-scale fermentation in three different media with different incubation times, temperatures, agitation speeds, and carbon and nitrogen sources.
Ethyl acetate was employed for the extraction of bioactive compounds since it can effectively dissolve a greater variety of secondary metabolites, thereby contributing to antibacterial activity [19]. The antibacterial activity of the crude extract obtained from S. tuirus at various conditions was evaluated using the agar well diffusion method (Fig. 1)). Three different media namely SCB, NB, and ISP1 were used to grow the S. tuirus to determine the one that produces the best antibacterial metabolites against MRSA. All three media yielded antibacterial metabolites as seen in Figure 2. The largest ZOI (18.87 ± 0.09 mm) was obtained from SCB metabolites, followed by NB and ISP1 with ZOI of 14.85 ± 0.16 mm and 10.67 ± 0.23 mm, respectively. Considering the nutritional factors of each media, it is possible to predict that the components of SCB may contribute to higher antimicrobial activity. For example, starch is an important carbon source needed by bacteria and casein is a main protein source that is necessary for growth [20]. This result is in complete accordance with other studies showing that SCB was the best production medium for Streptomyces [21]. Similar findings were observed with Amycolatopsis sp. ST-28 [22].
In fact, the incubation duration of Actinobacteria also had an important effect on the production of the antibacterial metabolites [23]. The anti-MRSA activity obtained from the isolate was evaluated on days 7, 14, and 21 of the fermentation process. The effect of fermentation duration on the antibacterial activity against MRSA is shown in Figure 3. The 7 days of fermentation yielded the best activity with ZOI of 18.92 ± 0.04 mm. This activity decreased slightly at day 14 (ZOI = 17.76 ± 0.21mm), until reaching the lowest zone of inhibition at day 21 (ZOI = 14.86 ± 0.22 mm). A previous study also demonstrated that the 7-day fermentation period produced the highest antimicrobial activity [24]. Similarly, a study done by ELaasser [15] reported that 7 days of incubation was the ideal duration for Streptomyces griseoplanus NRRL-ISP 5009 against the tested bacteria [15].
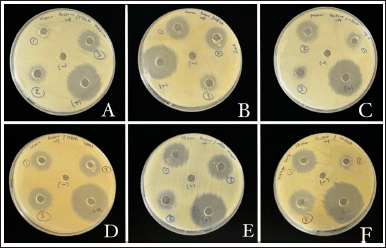 | Figure 1. Antibacterial activity of crude extract obtained from S. tuirus against MRSA at various conditions. (A): Culture medium (1: ISP1, 2: NB, 3: SCB), (B): Incubation days (1: 7 days, 2: 14 days, 3: 21 days), (C): Incubation temperature: (1: 25°C, 2: 30°C, 3: 35°C), (D): Agitation rate (1: 100 rpm, 2: 120 rpm, 3: 150 rpm), (E): Carbon source: (1: Glucose, 2: Lactose, 3: Sucrose), and (F): Nitrogen source (1: Peptone, 2: Tryptone, 3: Sodium nitrate). [Click here to view] |
Temperature is one of the important factors that affect antibiotic production [18]. It is one of the key elements that influence the growth of Actinobacteria and the synthesis of bioactive compounds [25]. To determine the optimal temperature for the isolate to produce secondary metabolites against MRSA, S. tuirus was incubated at various temperatures. As shown in Figure 4, the best antibacterial activity (ZOI = 19.35 ± 0.07 mm) was recorded at 30°C. On the other hand, the lowest antibacterial activity (ZOI = 10.54 ± 0.22 mm) was obtained at 35°C. Thus, 30?°C proved to be a favorable choice for S. tuirus in the production of secondary metabolites. These results were compatible with the results of Balachandar et al. [12] who likewise revealed that the highest antibacterial activity of Amycolatopsis sp.-AS9 against the tested bacteria was achieved at 30°C [12]. On the other hand, Abd-Elnaby et al. [26] found that the highest antibacterial activity of Streptomyces parvus against Aeromonas hydrophila was at 35°C.
Agitation plays an essential role in the synthesis of antimicrobial compounds. It facilitates the transfer of oxygen from the gaseous phase into the aqueous phase[15]. Different antibacterial activities were obtained from the extract isolated from the S. tuirus at different agitation speeds tested (100,120, and 150 rpm). A gradual increase in the antibacterial activity was noted in the present investigation until the maximum activity was achieved at an agitation speed of 150 rpm (ZOI = 21.04 ± 0.19 mm). In contrast, the lowest antibacterial activity (ZOI = 17.16 ± 0.23 mm) occurred at an agitation speed of 100 rpm as illustrated in Figure 5. This result is in agreement with the previous report indicating that 150 rpm was the optimum agitation speed for maximum metabolite activity [15]. Moreover, Al Ghazali and Omran [27] stated that the intensity of agitation during the fermentation process may affect cell damage, growth rate, antibiotic production, and metabolism through the transport of nutrients and enzymes.
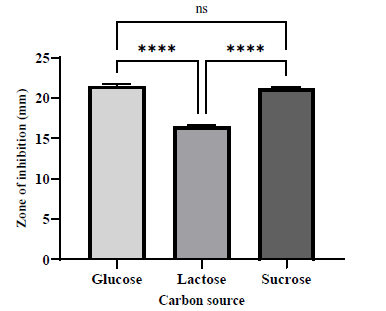
It is well known that antibiotic production by Actinobacteria is significantly influenced by the nutritional sources of carbon and nitrogen [25]. Carbon is the most significant component of the medium, serving as both an energy source for microorganisms and a crucial factor in their growth and the production of primary and secondary metabolites [13]. The carbon sources glucose, sucrose, and lactose were supplemented with SCB. Glucose or sucrose supplementation enhanced the antibacterial activity with glucose exhibiting the strongest antibacterial activity (ZOI = 21.49 ± 0.14 mm) followed by sucrose (ZOI = 21.19 ± 0.09 mm) and then lactose (ZOI = 16.45 ± 0.13 mm) as shown in Figure 6. Among the different carbon sources evaluated, glucose was one of the most effective carbon sources for antibacterial activity. It is widely recognized that the majority of microbes that produce antibiotics use glucose as a readily assimilated carbon source [28]. This finding is consistent with several studies that have shown that the antibacterial effect of Streptomyces species was increased by glucose[18,27]. In contrast, a previous study revealed that the supplementation of glucose to culture media prevented Streptomyces sp. KB1 from producing bioactive compounds [10].
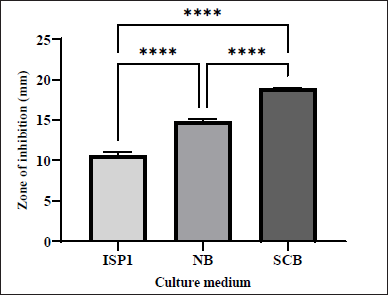 | Figure 2. Effect of various culture media on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. ****, p ≤ 0.0001. [Click here to view] |
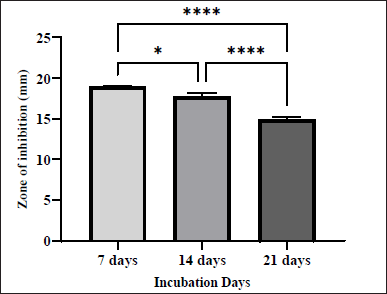 | Figure 3. Effect of different incubation days on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. *, p ≤ 0.05; ****, p ≤ 0.0001. [Click here to view] |
Similar to carbon, the choice of nitrogen source in the media also significantly influences metabolite production [13]. Among the nitrogen sources tested, sodium nitrate and peptone were found to have a potent effect on the antibacterial metabolite production. Sodium nitrate in SCB showed the highest effect of the secondary metabolite produced with ZOI of 22.07 ± 0.29 mm, followed by peptone (ZOI = 20.51 ± 0.22 mm), while tryptone addition yielded the smallest ZOI of 12.51 ± 0.23 mm as shown in Figure 7. Our results confirmed that sodium nitrate had a stimulatory effect on the production of bioactive compounds from our isolate. It was previously reported that the antibacterial effect of Streptomyces sp. AS11 was increased by sodium nitrate [12]. On the other hand, our results revealed that tryptone significantly reduced the antimicrobial activity against MRSA. This may be due to the fact that nitrogen molecules have an inhibiting influence on the production of metabolites in certain circumstances [13]. Hence, using inappropriate amino acids can reduce the production of secondary metabolites, while using the right amino acids can enhance the activity [29].
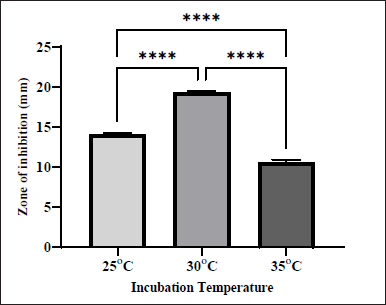 | Figure 4. Effect of different incubation temperatures on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. ****, p ≤ 0.0001. [Click here to view] |
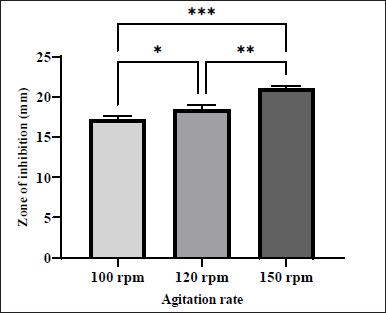 | Figure 5. Effect of various agitation rates on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. *, p ≤ 0.05, **, p ≤ 0.01, ***, p ≤ 0.001. [Click here to view] |
 | Figure 6. Effect of different nitrogen sources supplemented to SCB on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. ns, not significant; p > 0.05; ****, p ≤ 0.0001. [Click here to view] |
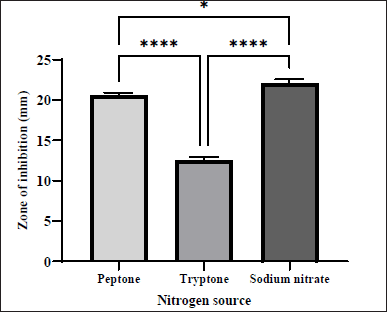 | Figure 7. Effect of different nitrogen sources supplemented on SCB on the production of antibacterial agents by S. tuirus against MRSA. Data are expressed as the mean ± SEM of the triplicate experiment. *, p ≤ 0.05; ****, p ≤ 0.0001. [Click here to view] |
In this investigation, the mass of crude extract ranged between 26.03 ± 0.75 mg/l and 121.56± 1.03 mg/l under different conditions as shown in Table 1. This variation in the mass of crude extract, or in the antibacterial activity as we discussed, could be due to the changes in the composition of the crude extract or in the quantity of bioactive compounds produced under each fermentation condition. A previous study focused on the production of erythromycin by Saccharopolyspora erythraea MTCC 1103 showed that erythromycin production was maximum in a medium containing corn steep liquor (416 mg/l). In contrast, a medium supplemented with peptone resulted in a much lower concentration of only 120 mg/l, making it the least effective [30]. Moreover, Pudi et al. [25] demonstrated the impact of various conditions on alkaloid production produced by marine Actinomycetes strain (KU375127). The study revealed that raising the temperature to 30°C and using dextrose as a carbon source enhanced alkaloid production. Conversely, temperatures above 30°C and the use of maltose or galactose as a carbon source resulted in a significant decline in alkaloid production [25]. In general, the mass of crude extract produced by Actinobacteria varied in many studies. In 2024, Prastya et al. [31] reported that the mass of crude extract obtained by three different actinobacterial strains under the same conditions ranged between 104.5 mg/l and 474.5 mg/l. Another study showed that 653 mg of crude extract was obtained from the 10 l scale-up fermentation of the Aeromicrobium ponti strain [32]. Moreover, a study done by Singh and Dubey [33] showed that the fermentation process of actinobacterial strain produced approximately 120 mg/l of crude extract. Furthermore, in our study, we have found that in some conditions the highest antibacterial activity was not accompanied by the highest accumulation of crude extract. A previous study done by Siti Junaida et al. [24] confirmed this issue by illustrating that the highest antimicrobial activity was achieved using Thronton media, but it was associated with the lowest mass of crude extract (0.5 mg/400 ml). Also, the same study reported that the maximum activity was produced during the 7-day fermenting phase, while the highest accumulation of crude extract was obtained on day 21 [24]. Larasati et al. [34] stated in a previous study that the quantity of crude extract, whether high or low, did not impact the size of the inhibition zone. Thus, increasing the mass of crude extract did not necessarily result in greater inhibition activity [34]. Hence, the qualitative and quantitative aspects of antibiotic production by microorganisms vary based on the strains, species, nutritional factors, and cultural conditions employed [22].
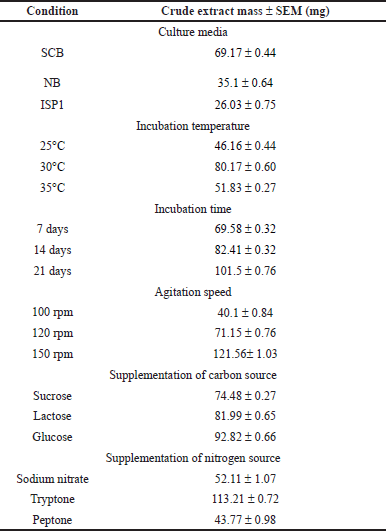 | Table 1. Crude extract mass obtained in different cultural and nutritional conditions (n = 3). [Click here to view] |
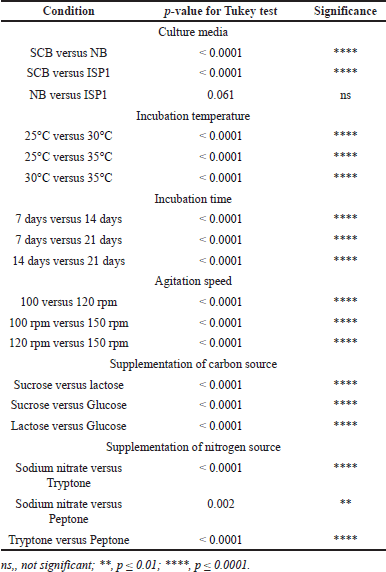 | Table 2. P-values of the crude extract mass obtained at different cultural and nutritional conditions. [Click here to view] |
CONCLUSION
Our study reveals that the crude extract of S. tuirus has potent antimicrobial activity against MRSA. It produced the maximum activity when inoculated in SCB for 7 days at 30°C and an agitation speed of 150 rpm. Also, the supplementation of glucose as a carbon source, and sodium nitrate as a nitrogen source produced the most powerful activity against MRSA. Further characterization and purification of the antibacterial compounds present in this crude extract are very important to discover novel compounds with beneficial biological properties.
ACKNOWLEDGMENT
The authors would like to notify the journal that they acknowledge Beirut Arab University as the institution where the study was performed.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Zhang W, Wei L, Xu R, Lin G, Xin H, Lv Z, et al. Evaluation of the antibacterial material production in the fermentation of Bacillus amyloliquefaciens-9 from Whitespotted Bamboo Shark (Chiloscyllium plagiosum). Mar Drugs. 2020;18(2):119. CrossRef
2. Pusparajah P, Letchumanan V, Law JWF, Ab Mutalib NS, OngYS, Goh BH, et al. Streptomyces sp.—a treasure trove of weapons to combat methicillin-resistant Staphylococcus aureus biofilm associated with biomedical devices. Int J Mol Sci. 2021;22(17):9360. CrossRef
3. WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva, Switzerland: WHO; 2017.
4. Ali Alghamdi B, Al-Johani I, Al-Shamrani JM, Musamed Alshamrani H, Al-Otaibi, BG, Almazmomi K, et al. Antimicrobial resistance in methicillin-resistant staphylococcus aureus. Saudi J Biol Sci. 2023;30(4):103604. CrossRef
5. Tamang MD, Bae J, Park M, Jeon B. Potentiation of β-Lactams against methicillin-resistant Staphylococcus aureus (MRSA) using octyl gallate, a food-grade antioxidant. Antibiotics. 2022;11(2):266. CrossRef
6. Liu WT, Chen EZ, Yang L, Peng C, Wang Q, Xu Z, et al. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: a comprehensive review. Microb Pathog. 2021;156:104915. CrossRef
7. Belete MA, Gedefie A, Alemayehu E, Debash H, Mohammed O, Gebretsadik D, et al. The prevalence of vancomycin-resistant Staphylococcus aureus in Ethiopia: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2023;12(1):86. CrossRef
8. Djinni I, Djoudi W, Boumezoued C, Barchiche H, Souagui S, Kecha M., et al. Statistical medium optimization for the production of anti-methicillin-resistant Staphylococcus aureus metabolites from a coal-mining-soil-derived Streptomyces rochei CMB47. Fermentation. 2023;9(4):381. CrossRef
9. Maiti PK, Das S, Sahoo P, Mandal S. Streptomyces sp SM01 isolated from Indian soil produces a novel antibiotic picolinamycin effective against multi drug resistant bacterial strains. Sci Rep. 2020;10(1):10092. CrossRef
10. Lertcanawanichakul M, Sahabuddeen T. Characterization of Streptomyces sp. KB1 and its cultural optimization for bioactive compounds production. Peer J. 2023;11:e14909. CrossRef
11. Al-Joubori B, Saadoun I, Hotchin N, Cunningham D, Alderwick L.The isolation of novel terrestrial Streptomyces strains with antimicrobial and cytotoxic properties. Arab J Basic Appl Sci?. 2023;30(1):285–98. CrossRef
12. Balachandar R, Karmegam N, Subbaiya R, Boomi P, Karthik D, Saravanan M. Optimization of culture medium for improved production of antimicrobial compounds by Amycolatopsis sp. -AS9 isolated from vermicasts. Biocatal Agric Biotechnol. 2019;20:101186. CrossRef
13. Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi, CKM. Strategies for fermentation medium optimization: an in-depth review. Front Microbiol. 2017;7:2087. CrossRef
14. Bano N, Siddiqui S, Amir M, Zia Q, Banawas S, Iqbal D, et al. Bioprospecting of the novel isolate Microbacterium proteolyticum LA2(R) from the rhizosphere of Rauwolfia serpentina. Saudi J Biol Sci. 2022;29(3):1858–68. CrossRef
15. Elaasser M. A new trial for bioformation of antimicrobial agent controlling multi drug resistant microorganism. Al-Azhar J Pharm Sci. 2020;62(2):72–95. CrossRef
16. Ibnouf EO, Aldawsari MF, Ali Waggiallah H. Isolation and extraction of some compounds that act as antimicrobials from Actinomycetes. Saudi J Biol Sci. 2022;29(8):103352. CrossRef
17. Shikuku B, Kiruki S, Kuria E, Mayo D, Ogolla, F. Effect of pH, carbon and nitrogen sources on antibiotic production by Actinomycetes isolates from River Tana and Lake Elementaita, Kenya. Asian J Res Biochem. 2023;13:42–51. CrossRef
18. Al-Ansari M, Kalaiyarasi M, Almalki MA, Vijayaraghavan P. Optimization of medium components for the production of antimicrobial and anticancer secondary metabolites from Streptomyces sp. AS11 isolated from the marine environment. J King Saud Univ Sci. 2020;32(3):1993–8. CrossRef
19. Rante H, Alam G, Pakki E, Usmar U, Ali A. Identification and antibacterial activity of Actinomycetes isolated from medicinal plant Andrographis paniculata Rhizosphere Soil. Crescent J Med Biol Sci. 2020;7(4):467–73.
20. Sapkota A. Starch Casein Agar (SCA)—composition, principle, preparation, results. Microbe Notes. 2022 Jan. Available from:https://microbenotes.com/starch-casein-agar-sca-composition-principle-preparation-and-results/
21. Kavitha A, Savithri HS. Biological significance of marine Actinobacteria of East Coast of Andhra Pradesh, India. Front Microbiol. 2017 Jul;8. Available from:https://www.frontiersin.org/articles/10.3389/fmicb.2017 .01201
22. Alam M, Jha, DK. Optimization of culture conditions for antimetabolite production by a rare tea garden actinobacterial isolate, Amycolatopsis Sp ST-28. Afr J Clin Exp Microbiol. 2019 Jul;20 (3):209–20. CrossRef
23. Amin DH, Abdallah NA, Abolmaaty A, Tolba, S, Wellington EMH. Microbiological and molecular insights on rare Actinobacteria harboring bioactive prospective. Bull Natl Res Cent?. 2020;44(1):5. CrossRef
24. Siti Junaidah A, Suhaini S, Mohd Sidek H, Basri DF, Zin NM. Anti-methicillin resistant Staphylococcus aureus activity and optimal culture condition of Streptomyces sp. SUK 25. Jundishapur J Microbiol. 2015;8(5):e16784. CrossRef
25. Pudi N, Varikuti GD, Badana AK, Gavara MM, Kumari S, Malla R. Studies on optimization of growth parameters for enhanced production of antibiotic alkaloids by isolated marine Actinomycetes. J Appl Pharm Sci. 2016;6:181–8. CrossRef
26. Abd-Elnaby H, Abo-Elala G, Abdel-Raouf U, Abd-elwahab A, Hamed M. Antibacterial and anticancer activity of marine Streptomyces parvus: optimization and application. Biotechnol Biotechnol Equip. 2016;30(1):180–91. CrossRef
27. Al-Ghazali LH, Omran R. Optimization production conditions of antibacterial metabolite from streptomyces sp. Asian J Pharm Clin Res?. 2017;10(9):386–91. CrossRef
28. Rammali S, Rahim A, El Aalaoui M, Bencharki B, Dari K, Habach A, et al. Antimicrobial potential of Streptomyces coeruleofuscus SCJ isolated from microbiologically unexplored garden soil in Northwest Morocco. Sci Rep. 2024;14(1):3359. CrossRef
29. Ullah H, Ullah A, Khan MW. Fermentation optimization for actinomycetes as a single cell protein: a comprehensive review [Preprint]. 2020:2020040498. CrossRef
30. Subathra Devi C, Saini A, Rastogi S, Jemimah Naine S, Mohanasrinivasan V. Strain improvement and optimization studies for enhanced production of erythromycin in bagasse based medium using Saccharopolyspora erythraea MTCC 1103. 3 Biotech. 2015;5:23–31. CrossRef
31. Prastya M, Simbolon S, Jepri Agung P, Hasidu LOAF, Permatasari V, Primahana G, et al. Antibacterial and antibiofilm activities from soil Streptomyces spp. isolated from Muna Island, Indonesia against multidrug-resistant clinical isolates. Res Sq [Preprint]. 2024;8. CrossRef
32. Gos FMWR, Savi DC, Shaaban KA, Thorson JS, Aluizio R, Possiede YM, et al. Antibacterial activity of endophytic actinomycetes isolated from the medicinal plant Vochysia divergens (Pantanal, Brazil). Front Microbiol. 2017;8:1642. doi:10.3389/fmicb.2017.01642
33. Singh R, Dubey AK. Isolation and characterization of a new endophytic actinobacterium Streptomyces californicus strain ADR1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms. 2020;8(6) :929. CrossRef
34. Larasati A, Ryandini D, Oedjijono O, Kusharyati D. Optimization of medium and incubation time in the production of antibacterial compounds by Streptomyces sp. SA404. Adv Biol Sci Res. 2021;15:37–43. CrossRef