INTRODUCTION
Lifestyle changes including shifts in instant eating patterns and lack of exercise have triggered increased health risks in many countries, especially in developed countries [1]. In 2016, more than 650 million people were diagnosed with obesity, 13% among them 18 years old or older [2]. The prevalence of obesity in Indonesia for those aged over 18 years from 2007 to 2018 increased from 19.1% to 35.4% [3]. Obesity leads to another complicated health problem and kills more people than being underweight. Apart from obesity, the prevalence of cardiovascular disease (CVD) and immune problems is also increasing. CVD is the leading cause of death globally. Over one-third of the world’s population in 2019 died from CVD, most of which were strokes and heart attacks [4].
Regarding immunity itself, COVID-19, which has hit the world since 2019 and was declared a pandemic, was declared an emergency by the World Health Organization (WHO) on May 5th 2023 [5]. In Indonesia, the President of the Republic of Indonesia, Joko Widodo, declared the end of the COVID-19 emergency status on June 21st, 2023 [6], about a month after WHO. However, the impact of COVID-19 is not over, both health, psychosocial, and economic, positively as an opportunity and vice versa as a challenge. Manfred Eggersdorfer, who is a Professor for Healthy Aging University Medical Center Groningen, said that the pandemic has made the role of supplements more visible because people increasingly understand how to continue to maintain their body’s health [7]. What is more, the health impacts of COVID-19 also leave traces on some sufferers who are called long COVID-19. WHO itself has monitored and continues to carry out enrichment related to the long-term COVID-19 condition so that the quality of human life after the pandemic gradually recovers [8]. At least 65 million people worldwide are estimated to suffer from long COVID-19, with cases continuing to increase every day [9].
In this context, the concept of “Nutraceutical,” which is a combination of the two words “nutrition” and “pharmaceutical,” became one of the wanted supplements for human health. It is proven that there is an increase in the global nutraceutical market trend which will reach more than 423 billion USD in 2022 and is projected to grow at a CAGR of 4.5% from 2023 to 2032 [10]. The prevalence and profitability of the nutraceutical industry make these products potential for development, especially those derived from natural ingredients because of today’s trends. In the exploration of nutraceuticals, active compounds from natural ingredients are attracting attention as potential agents for improving cardiovascular health, overcoming obesity, and modulating immune responses. With the prevalence of this health problem, further understanding of the contribution of these compounds is expected to open up new opportunities in the development of natural source-oriented health therapies.
NUTRACEUTICALS FOR CARDIOVASCULAR, ANTI-OBESITY, AND IMMUNOMODULATOR
Cardiovascular problems, obesity, and immunity are world concerns currently, especially with the increase in cases. Nutraceuticals are one method of prevention to overcome this problem, especially nutraceuticals derived from natural ingredients. The following Table 1 are some nutraceuticals that are used for the prevention and treatment of cardiovascular problems, and act as anti-obesity agents and immunomodulators.
Nutraceuticals for cardiovascular health
The cardiovascular system is a blood circulation organ that consists of the heart, blood components, and blood vessels. Its function is to provide and distribute oxygen and nutrients to all body tissues that are required in the body’s metabolic processes, allowing the body to maintain the quality and quantity of fluids necessary for homeostasis. A healthy cardiovascular system is characterized by a normal circulation process. If circulation is obstructed due to abnormalities in the organs that make up the cardiovascular system, it can cause disease. This is what is called cardiovascular disorders or diseases (CVDs). Dietary factors contribute significantly to CVD risk, either directly or through their effects on other CVD risk factors such as hypertension, dyslipidaemia, and diabetes mellitus. Some researches show that dietary supplements and nutritional food composition could enhance cardiovascular health [64,65].
 | Table 1. Summary of nutraceuticals for cardiovascular health, anti-obesity, and immunomodulatory. [Click here to view] |
There are bioactive components from everyday food sources that have activities to maintain the health of the cardiovascular system so they have the potential to become nutraceuticals with the action mechanism as shown in Figure 4. The active ingredients include Anthocyanin, Lutein, Policosanol (PC), Coenzyme Q10, and Resveratrol. Following are some explanations regarding these compounds:
Anthocyanin
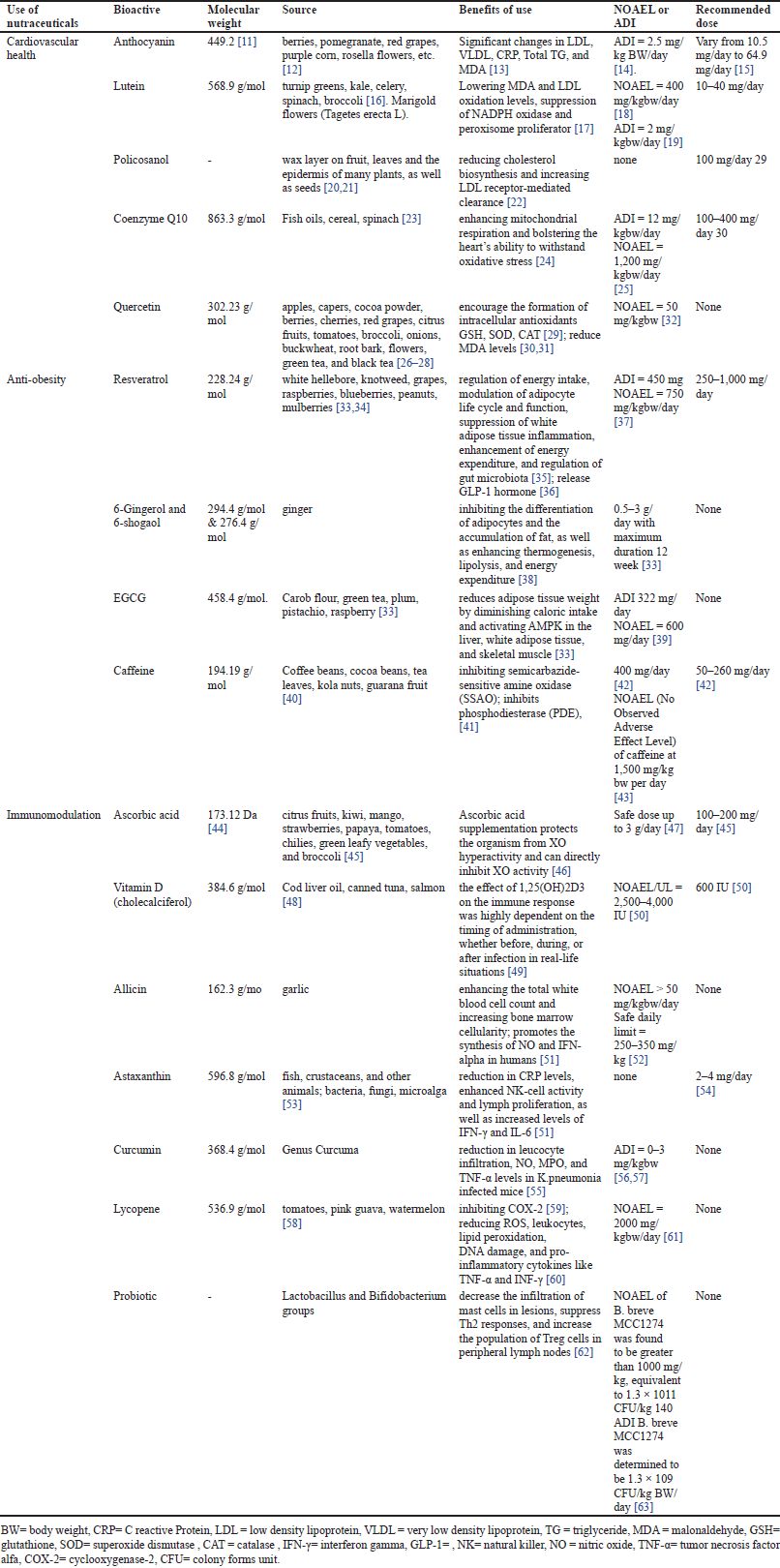
Anthocyanins are flavylium derivative compounds that are reactive and very unstable. This compound has the IUPAC name 2-phenylchromenylium as shown in Figure 1. We can find anthocyanins in the vegetables and fruit we consume every day. Vegetables and fruit that usually contain anthocyanins are those that have striking colored pigments, such as berries, pomegranate, red grapes, purple corn, and rosella flowers [12].
Obtaining this compound can be done using conventional extraction methods such as maceration or nonconventional methods including ultrasound assisted extraction (UAE), microwave assisted extraction (MAE), and enzyme assisted extraction (EAE). Anthocyanin compounds have hydrophilic properties which make them easily soluble in water. This bioactive substance can also dissolve in polar organic solvents such as ethanol, methanol, acetone, and chloroform. A preliminary study of anthocyanin extraction showed that using water as a solvent produced 92% anthocyanin from rosella petals. The addition of methanol extracts 6%, while acidification with HCl (2%) extracts the remaining 2% [66].
 | Figure 1. Chemical structure of (a) anthocyanin, (b) lutein, (c) policosanol, (d) coenzyme Q10, and (e) quercetin. [Click here to view] |
Intervention studies in humans and experimental animal models using anthocyanin-containing food or pure anthocyanin-rich extracts have shown significant changes in the oxidation of low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), C-reactive protein (CRP), total triglycerides, malondialdehyde (MDA), and also, reducing comorbid diseases. Apart from that, improving the clinical condition of CVD patients, in vivo and clinical trials have succeeded in showing the benefits of anthocyanins in preventing and improving the quality of life of CVD patients [13]. Anthocyanins increase Nitric Oxide (NO) bioactivity and upregulate Nrf2, which induces endogenous antioxidant production and limits oxidative stress. Anthocyanins can reduce inflammation by downregulating NF-κB and reducing the expression and production of cytokines such as TNF-α, IL-1β, IL-6, ICAM-1, and VCAM-1. NF-κB inhibits TGF-β expression, resulting in reduced ECM buildup and minimal fibrosis [67].
The daily intake of anthocyanins is determined by the population’s eating patterns, which are impacted by socioeconomic, demographic, and lifestyle characteristics. In the United States, the average reported consumption is 12.6 mg/day for women and 10.5 mg/day for men. In Europe, it ranges from 64.9 (Italy) to 19.8 mg/day (Netherlands) for men and 44.1 (Italy) to 18.4 mg/day (Spain) for women [15]. Meanwhile, the ADI (acceptable daily intake) for anthocyanins has been established as 2.5 mg/kg BW/day (EFSA, 2013) [14]. According to the Bars-Cortina in 2022 [68], taking into account the European Union Food Safety Authority (EFSA) report as a whole although it does not have enough data regarding the long-term toxicity or carcinogenicity of anthocyanins, taking into account the JECFA ADI and estimates of anthocyanin exposure in European populations, anthocyanins can be considered safe as food additives. Thus, a daily anthocyanin intake of between 49 and 133 mg for an average adult body weight of 70 kg can be tolerated quite well.
Lutein
Lutein is a yellow xanthophyll carotenoid that has the IUPAC name (3R,3′R,6′R) β, ε-carotene-3-3′-diol) as shown in Figurer 1. Lutein can be found in yellow maize, egg yolks, orange, melon, and other fruits, but it is most commonly found in dark green vegetables including turnip greens, kale, celery, spinach, and broccoli [16]. Lutein is the main compound found in marigold flowers (Tagetes erecta L). Lutein is a pigment that is sensitive to heat, so when food containing lutein is subjected to extreme conditions such as cooking, frying, and baking, it can reduce the lutein content and activity in it [69].
Lutein has nonpolar properties that bind to one or two fatty acids (known as lutein fatty acid esters or lutein esters), so vegetable oils are one candidate to replace the use of organic solvents during extraction. The initial stage in extracting lutein involves suspending marigold powder in canola oil (0.01 g/ml), vortexed then sonicated and centrifuged at 10,000 rpm for 2 minutes in repetition until the residue became pale. Then, each supernatant was diluted one hundredfold in hexane and spectrophotometrically analyzed at 350–600 nm UV-Vis Spectrophotometer. The recovery value was determined by comparing the amount of lutein ester recovered in oil to that extracted in organic solvents (acetone and n-hexane) as a reference [70].
The antioxidant and anti-inflammatory activity of the lutein compound has been proven to have a positive influence on improving heart health and reducing the risk of Coronary Artery Disease [16]. In vivo studies demonstrate that lutein can protect atherosclerosis by lowering malondialdehyde and LDL oxidation levels, as well as inflammatory cytokines like interleukin (IL-)10. Furthermore, in ApoE-deficient animals administered lutein for 24 weeks, NADPH oxidase was suppressed and peroxisome proliferator (activated receptor shedding) was elevated, which protected against high fat-induced atherosclerosis [17].
In general, the recommended dose for lutein is 10–40 mg/day. Research conducted by Ravikrishnan in 2011 [18] showed no observed-adverse-effect level (NOAEL) for 400 mg/kgbw/day, the highest dose tested. Based on the Joint FAO/WHO Expert Committee on Food Additives (JECFA), the ADI when consuming lutein is 2 mg/kgbw/day [19].
Policosanol
PC is a mixture of high molecular weight (20–36 carbon atoms) primary aliphatic long-chain alcohols, mainly including docosanol, tetracosanol, hexacosanol, octacosanol, and triacontanol [20]. PC as shown in Figure 1 has synonyms, including n-octacosanol; Octacosyl alcohol; Octanosol; Montanyl alcohol; Cluytyl alcohol. As for sources containing this compound, apart from beeswax, octacosanol is also found in the superficial wax layer on fruit, leaves, and the epidermis of many plants, as well as seeds. The main commercial source is a by-product of the sugar cane industry, but it is also found in alfalfa leaves, wheat, and also from various other sources [20,21].
PC’s extraction could be obtained by hydrolysis or saponification reaction with NaOH or KOH base with various parameters included. The use of methanolysis in supercritical carbon dioxide, using high ultrasound as a catalyst can also use pressurized solvent extraction. The PC purification stage must use an organic solvent for purification and continue with crystallization and using a silica column for purification [20].
This active ingredient is claimed to be as effective as currently available lipid-lowering drugs, such as statins, in lowering plasma cholesterol. PC was first developed in Cuba and is used as the main heart medication in the Caribbean region. Recent studies also show that PC lowers cholesterol levels by reducing cholesterol biosynthesis and increasing LDL receptor-mediated clearance [22]. PC acts as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase by decreasing cholesterol synthesis from the acetate pathway. HMG-CoA reductase is a key enzyme in the first step of cholesterol biosynthesis which converts acetyl CoA into mevalonate. It increases LDL metabolism by LDL binding, uptake, and degradation in a dose-dependent manner in cultured fibroblasts [71].
The daily dose of PC to provide a therapeutic effect that can reduce cholesterol is routinely between 5 and 20 mg/day. Consuming 20–40 mg/day could reduce the risk of diabetes and lower lipid serum. Therefore, the recommended dose for PC as a supplement is 100 mg/day. PC exists in other forms including nutraceuticals which are considered clinically used supplements in 10 mg dose [72].
Coenzyme Q10
Coenzyme Q10 (CoQ10) also often called ubiquinone is a natural lipophilic compound that has fat-soluble antioxidant activity and is synthesized endogenously from tyrosine in the human body. From the name (Q10), Q refers to the quinone group and 10 is related to the number of isoprenoid subunits in the tail [23]. CoQ10 consists of 3 forms, namely the completely oxidized form of ubiquinone (CoQ10), the semiquinone radical intermediate ubisemiquinone CoQ10H, and the reduced form of ubiquinol CoQ10H2 [73]. Food sources containing CoQ10 are very diverse, including fish oil, cereals (in the range of 1–10 mg/kg), fruit and vegetables, especially spinach. CoQ10 is most abundant in meat, fish, nuts, and several types of oil, containing around 10–50 mg/kg [23].
The extraction of CoQ10 from various samples is commonly carried out using the liquid–liquid extraction method or ultrasound extraction with a mixture of hexane and 2-propanol [74]. In the case of fresh tobacco leaves and longan skin, CoQ10 is extracted using ultrasonic extraction with ethanol and hexane [75]. Both extraction techniques result in the production of harmful chemicals, posing significant risks to the environment and human health. Therefore, it is advisable to seek alternative extraction methods that do not involve the use of toxic solvents. Accelerated solvent extraction (ASE) with ethanol and heat-acid treatment with petroleum ether extractant have been found to be effective in extracting CoQ10 from pollen collected by bees and Agrobacterium tumefaciens [74].
Clinical studies have provided evidence that the addition of CoQ10 to one’s diet is easily tolerated so levels of CoQ10 in the bloodstream increased. This increasing level reduces oxidative stress and the risk of CVD-related mortality [24]. The findings of the study indicate that heart failure patients may experience various benefits from CoQ10 through three specific mechanisms. First, it can enhance the generation of adenosine triphosphate (ATP) and cellular energy by facilitating electron transfer in the electron transport chain. Second, it can reduce oxidative stress, a well-established marker of mortality in heart failure, and prevent the oxidation of membranes and peroxidation of lipids. Finally, it can stabilize calcium-dependent ion channels in the myocardium, thereby promoting increased ATP synthesis.
Although no nutritional reference values have been established for CoQ10, the average daily intake of this compound is approximately 5.4 mg and 3.8 mg for men and women [23]. Meanwhile, the recommended daily dose given is 100–400 mg/day in clinical trials for heart disease. According to Rabanal-Ruiz et al. [24], clinical data indicates that the administration of CoQ10 at dosages of 200 mg/day or more over extended periods is considered safe. The ADI is determined to be 12 mg/kg/day, which is derived from a no adverse effect level (NOAEL) of 1,200 mg/kg/day obtained from a 52-week chronic toxicity study conducted on mice. For individuals weighing 60 kg, this translates to a recommended daily intake of 720 mg/day [25].
Quercetin
Quercetin (3,3′,4′,5,7- pentahydroxyflavone) in Figure 1 is a compound in the flavonoid class of the flavonol subclass which is abundant in nature, especially in food sources such as vegetables, fruit, and tea. This particular flavonol can be discovered in a wide range of foods including apples, capers, cocoa powder, berries, cherries, red grapes, citrus fruits, tomatoes, broccoli, onions, buckwheat, root bark, flowers, green tea, and black tea [26–28].
Quercetin compounds, like other flavonoid compounds, can be extracted using both conventional and nonconventional methods. A recent study successfully extracted quercetin from the leaves of the Trigonella foenum-graecum plant. The study highlighted the significance of solvents in the extraction process, with ethanol, a highly polar solvent, demonstrating a higher flavonoid content compared to hexane and ethyl acetate. The identification of quercetin was achieved by directly comparing its retardation factor. The isolated quercetin constituents were further characterized using FT-IR, NMR, and mass spectroscopy. Interestingly, the isolated quercetin compound exhibited an enhanced antioxidant activity as the processed concentrations increased. This extraction method proves to be a simple, rapid, and highly efficient approach for obtaining bioactive components from plants [76].
The quercetin compound has a strong antioxidant effect so it can prevent the accumulation of ROS formation in endothelial cells. This compound can encourage the formation of intracellular antioxidants GSH, SOD, and CAT, thereby maintaining normal body function and preventing cell damage, especially in the cardiovascular system [29]. The quercetin compound can also reduce levels of MDA, a marker of oxidative stress, which is the final product of the lipid peroxidation chain reaction [30,31]. Based on in vitro and in vivo research, shows that quercetin can work as an angiotensin converter enzyme (ACEi) inhibitor, the mechanism of which is by preventing the action of the ACE enzyme which converts angiotensin I into angiotensin II which is a potent vasoconstrictor via the AT1 and AT2 receptors, so that blood pressure is lower. Height becomes normal and hypertension can be prevented [77].
Even though this compound is a common constituent in daily food sources, quercetin has not received a definite ADI dose recommendation from the World Health Organization (WHO) [78]. However, it should be noted that a study in animals showed that administration of high doses of quercetin, namely 100 mg/kgBW, showed pro-oxidant activity rather than antioxidant effects, resulting in harmful effects in healthy mice. Throughout the investigation, no adverse reactions were noted following the administration of quercetin at a dosage of 50 mg/kgBW to the mice [32].
Nutraceuticals for anti-obesity
According to WHO, overweight and obesity are described as an abnormal or excessive accumulation of fat that poses a threat to health. A body mass index (BMI) above 25 is considered overweight, while a BMI above 30 is classified as obese. Obesity is characterized by the abnormal buildup of fat or adipose tissue in the body, which is associated with an increased risk of developing conditions such as diabetes mellitus, CVD e, hypertension, and hyperlipidemia [79].
Obesity is a challenging health problem, and its prevalence along with its comorbidities is increasing worldwide. Based on epidemiological reports from the World Health Organization and the Organization for Economic Co-operation and Development, it has been identified that overweight and obesity rank as the fifth leading cause of death worldwide. The prevalence of obesity poses a significant challenge, greatly impacting the overall well-being of individuals [80].
Managing obesity requires a comprehensive treatment approach and may involve lifelong care. Even a modest weight loss of 5%–10% can lead to significant improvements in health, quality of life, and the economic burden on individuals and society [81]. Traditional approaches to combat obesity typically rely on synthetic compounds and surgical interventions, which carry numerous risks and the potential for recurring complications so nutraceuticals could be one approach that is safer and more attractive [80].
Various plants and some phytochemical constituents are responsible for the anti-obesity activity. When it comes to obesity, nutraceuticals from nature have many modes of action as shown in Figure 5; as a regulator of nutrient absorption (e.g., EGCG), appetite regulator (e.g., ginger), energy expenditure modulator (e.g., curcumin and caffeine), and fat metabolism (e.g., resveratrol).
Resveratrol
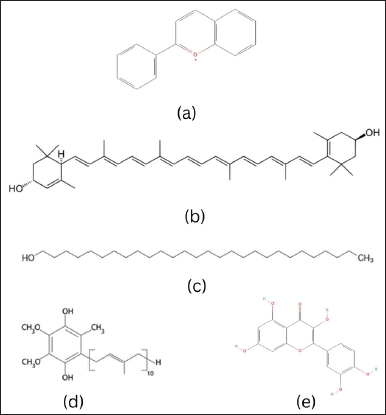
Resveratrol (3,5,4’-trihydroxystilbene) has a molecular weight of 228.24 g/mol with a melting point of 254°C. This compound is in the form of a yellowish–white powder solid. Its solubility in water is 3 mg/100 ml; easily soluble in organic solvents such as ethanol, DMSO, and dimethylformamide at concentrations of around 65 mg/ml; solubility in PBS (phosphate salt solution) at pH 7.2 is approximately 100 μg/ml [82].
Resveratrol, a compound found in various plants like grapes (Vitis vinifera L.), raspberries (Rubus idaeus L.), blueberries (Vaccinium corymbosum L.), peanuts (Arachis hypogaea L.), and mulberries (Morus alba Hort. ex Loudon L.) [34], is classified as a phytochemical along with flavonoids and lignans. It is synthesized by plants as a defense mechanism against pathogens like bacteria and fungi [34]. Resveratrol was initially discovered in 1940 from the roots of white hellebore (Veratrum album L.), and later in 1963 from knotweed (Polygonum cuspidatum Sieb. et Zucc.), which is known to be one of the richest natural sources of resveratrol [33]. The concentration of resveratrol in knotweed ranges from 296–377 µg.g [83], and it holds significant importance in ancient Chinese and Japanese traditional medicine [33].
The resveratrol extraction procedure through reflux extraction using solvents such as ethanol or methanol, and hydrolysis of glycosides (polydatin) is carried out by adding an acid solution, increasing the yield of resveratrol approximately 4-fold, and the mixture is then extracted liquid–liquid to remove unwanted compounds. Extraction results at various stages showed very high levels of resveratrol content, reaching more than 73.8% in the final product. Quantitative analysis and testing are carried out using spectrophotometric or chromatographic methods, and the final results are evaluated for the presence of impurities [84].
The anti-obesity effects of resveratrol are believed to be mediated through various molecular mechanisms. These mechanisms encompass the regulation of energy intake, modulation of adipocyte life cycle and function, suppression of white adipose tissue inflammation, enhancement of energy expenditure, and regulation of gut microbiota [35]. In addition, resveratrol has shown its ability to stimulate the release of the hormone GLP-1, which plays a role in regulating blood sugar and providing a sensation of fullness. Resveratrol also shows an activating effect on SIRT-1, a type of sirtuin protein. Activation of SIRT-1 by resveratrol has been linked to inhibition of lipogenesis, namely the formation and accumulation of fat in cells. Through this mechanism, resveratrol can contribute to controlling fat gain by reducing the lipogenesis process. Thus, resveratrol not only affects blood sugar regulation via GLP-1 but also inhibits fat accumulation via SIRT-1, making it a potential candidate in anti-obesity strategies [36].
The suggested dosage of resveratrol ranges from 250 to 1,000 mg taken orally daily for a maximum of 3 months. Generally, resveratrol is well received by individuals in good health; nevertheless, adverse effects like nephrotoxicity and gastrointestinal issues such as nausea, diarrhea, and abdominal discomfort have been documented in human participants. It has been observed that at dosages exceeding 1,000 mg per day, resveratrol alters the function of cytochrome P450 isoenzymes, leading to potential drug interactions [85].
Studies with resVida® (trans-purified resveratrol) show that trans-resveratrol is a substance with low oral toxicity. An ADI of 450 mg of trans-resveratrol in food has been deemed acceptable, surpassing the natural intake of this compound in the diet. The determination of this ADI is grounded on NOAELs of 750 mg/kg bw/day from a 13-week developmental toxicity study conducted through the dietary route, with a standard safety margin of 100 [37].
Gingerol and shogaol
6-Gingerol ((5S)-5-hydroxy-1-(3-hydroxy-4-methoxyphenyl)decan-3-one) has a molecular weight of 294.4 g/mol with a melting point of 32°C (Fig. 2). This compound is a sharp crystalline solid, white to pale yellow in color, slightly soluble in water, but soluble in nonpolar organic solvents such as ethanol, chloroform, or ether. Meanwhile, 6-Shogaol (1-(4-hydroxy-3-methoxyphenyl)dec-4-en-3-one) has a molecular weight of 276.4 g/mol with a melting point of 32°C (Fig. 2). This compound is in the form of a powdered solid, yellow or yellowish brown, less soluble in water but more soluble in nonpolar organic solvents such as ethanol, chloroform, or ether [86,87].
Throughout history, ginger has been utilized in Ayurvedic, Chinese, and Greek medicine to alleviate symptoms such as nausea, rheumatoid arthritis, muscle pain, indigestion, sore throat, constipation, and fever. It is also recognized for its stimulant and carminative properties for the digestive system. Ginger’s health benefits have been extensively researched, leading various regulatory bodies to classify it as a safe nutraceutical [88].The procedure for isolating gingerol and shogaol compounds from ginger rhizomes involves various extraction and purification methods. Conventional extraction methods such as solvent extraction or maceration, soxhlet extraction, and steam distillation are commonly used but have disadvantages such as large use of organic solvents, long extraction duration, thermal degradation of target compounds, and negative environmental impacts. Therefore, current research focuses on more environmentally friendly and economical extraction methods, such as liquid–liquid extraction (MAE), supercritical fluid extraction (SFE), UAE, pressurized liquid extraction, and EAE. At the purification stage, the high-speed counter-current chromatography method is considered the best method for obtaining these two compounds [89].
The presence of gingerol and shogaol compounds in ginger rhizomes [90] brings forth numerous advantages. Among these benefits, one stands out as ginger’s potential as an anti-obesity agent, as highlighted by Ishfaq et al. [91]. Ginger acts as an anti-obesity agent through various processes that promote weight loss. The active compounds in ginger stimulate TRPV1, triggering the sympathetic nervous system and NE release. This leads to increased UCP-1 expression, thermogenesis, and cyclic adenosine monophosphate (cAMP) production, enhancing HSL activity and lipolysis. In addition, ginger’s components activate PPARδ(β), reducing lipogenesis enzyme expression in adipose tissue [92].
Besides that, ginger’s mechanism is the reduction of adipogenesis, which is achieved by decreasing the expression of peroxisome proliferator-activated receptor gamma. In addition, ginger can inhibit fat absorption in the intestine by reducing the activity of the lipase enzyme. Moreover, ginger has a modulatory effect on 5-hydroxy tryptamine (5-HT or serotonin) and its receptors. This modulation of serotonin receptors may lead to a decrease in appetite, potentially through binding to 5-HT2 receptors in the central nervous system. However, it is important to note that ginger may also act as an appetite stimulant through 5-HT3 receptors in the intestine.
Overall, the comprehensive mechanism of action of ginger in combating obesity involves the regulation of the nervous system, thermogenesis, lipolysis, lipogenesis, adipogenesis, and fat absorption. This holistic approach makes ginger an effective option for weight loss [92].
Ginger is commonly ingested in oral doses ranging from 0.5 to 3 grams per day, with a recommended maximum duration of 12 weeks. While it is generally regarded as safe for the majority of individuals when consumed in moderation, excessive or prolonged use may lead to potential adverse effects. Ingesting doses exceeding 5 g per day heightens the likelihood of experiencing mild side effects, including heartburn, diarrhea, belching, and general gastric discomfort. In addition, there have been reported cases of arrhythmia and low blood pressure. It is important to note that ginger can exacerbate gallstone formation by increasing the secretion of bile acids [33]. In a study regarding the acute toxicity test of ginger rhizome extract, no deaths or clinical signs of toxicity were observed at the maximum recommended dose.
Epigallocatechin gallate
Epigallocatechin Gallate ([(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate) as shown in Figure 2 has a molecular weight of 458.4 g/mol with a melting point of 140°C–142°C. This compound is a crystalline solid, white or light yellow, soluble in hot water, and also has good solubility in organic solvents such as methanol, ethanol, and acetone [93].
EGCG, the most abundant catechin in green tea (Camellia sinensis L.) [94], can also be found in smaller quantities in various other foods such as carob flour (Ceratonia siliqua L.), blackberries (Rubus plicatus L. Weihe & Nees), apple (Malus domestica (Suckow) Borkh.), raspberry (Rubus idaeus L.), plum (Prunus domestica L.), pistachio (Pistacia vera L.), peach (Prunus persica (L.) Batsch), and avocado (Persea americana Mill.) [33]. In the production of green tea, the tea leaves undergo a process of heating to deactivate enzymes, rolling, and drying to prevent oxidation and stabilize the tea compounds. Catechins, including EGCG, make up around 30%–42% of the dry weight in brewed green tea, with a 250 ml cup of tea containing approximately 260 mg of catechins, of which about 96 mg is EGCG [95].
The EGCG isolation process begins with the extraction of the soluble compound from tea leaves into a tea infusion, which is then concentrated and dried to obtain a product in powder form. There are several methods for producing crude tea extract, including boiling water–ethanol extraction methods, membrane ultrafiltration, and gradual extraction using water. However, crude tea extract still contains other compounds such as caffeine and chlorophyll. Simply put, the method for isolating EGCG from tea leaves includes extraction, making a mixture of catechins, decaffeination, and individual isolation by optimizing various techniques such as chromatography and supercritical extraction [96].
 | Figure 2. Chemical structure of (a) resveratrol, (b) gingerol, (c) shogaol, (d) EGCG, and (e) caffeine. [Click here to view] |
EGCG, classified as a polyphenol, exhibits remarkable anti-inflammatory, antioxidant, and anti-obesity properties. As an anti-obesity agent, EGCG effectively curbs weight gain and reduces adipose tissue weight by diminishing caloric intake and activating AMPK in the liver, white adipose tissue, and skeletal muscle. Moreover, it impedes the absorption of lipids, cholesterol, triacylglycerol, and leptin, while simultaneously stimulating energy expenditure, fat oxidation, and elevating high-density lipoprotein levels. In addition, EGCG aids in the excretion of lipids through feces [33].
Positive impacts on body weight were noted in adults who ingested up to 460 mg per day, but doses exceeding 800 mg/kg led to adverse effects. While EGCG is deemed safe in moderate quantities from food, there are worries about its toxicity in high doses or concentrated forms. Research on humans and animals indicates that the liver is particularly vulnerable to toxicity [97].
The reported ADI for an adult weighing 70 kg is 322 mg of EGCG per day. The NOAEL is reported to be 600 mg per day. According to the EFSA, consuming equivalent to or above 800 mg of EGCG per day can lead to liver damage in humans, as indicated by increased transaminases. However, a review found no evidence of liver toxicity when consuming EGCG below 600 mg per day through green tea infusion or GTE tea-based beverages. In a recent study involving 39 women (aged 18–40 years), a dose of 720 mg of EGCG (taken for at least a month), either alone or in combination with uterine myoma management drugs, was found to be well-tolerated without any associated liver toxicity [39].
Caffeine
Caffeine (1,3,7-trimethylpurine-2,6-dione) has a molecular weight of 194.19 g/mol with a melting point of 236.2°C. This compound is in the form of white crystals, soluble in ethanol, methanol, and hot water [98]. This particular compound belongs to a category of alkaloids that can be naturally found in over 60 different plant species. Some of these plants include coffee beans (Coffea arabica L.), cocoa beans (Theobroma cacao L.), tea leaves (Camellia sinensis (L.) Kuntze), kola nuts (Cola acuminata (P.Beauv.) Schott & Endl., Cola nitida (Vent.) Schott & Endl.), guarana fruit (Paullinia cupana Kunth), yerba mate (Ilex paraguariensis A.St.-Hil.), and guayusa (Ilex guayusa Loes.) [40].
In general, caffeine can be isolated from coffee beans by extracting them in an aqueous solution or using two-phase extraction with an organic solvent such as chloroform or methylene chloride. Other methods include the use of adsorbents for the isolation of caffeine from other soluble substances. Isopropanol is also used as a safer solvent in some methods. After extraction, the organic solvent is evaporated to produce a crude caffeine preparation which is then further purified [99].
The isolation process involves additional steps such as tannin precipitation, the use of Ca(OH)2, and further purification through recrystallization or sublimation. It is important to note that ethyl acetate is considered a more suitable solvent for caffeine isolation, and methods for isolating and purifying caffeine from green tea still have limited literature compared with coffee and black tea [99].
Numerous epidemiological studies have established a connection between caffeine intake and various health benefits for moderate coffee drinkers. These benefits include lower mortality rates, reduced risk of cancer, decreased chances of developing type 2 diabetes, management of Parkinson’s disease, slower progression of dementia, and delayed advancement of various liver diseases. In the realm of sports, caffeine is widely utilized for its performance-enhancing effects, with caffeine supplements proving to enhance muscle strength, endurance, jumping ability, running speed, and overall movement speed. Moreover, consuming caffeine before morning workouts seems to aid in weight loss, as indicated by research findings [33].
According to the effects of caffeine on weight loss, several studies implicated that caffeine consumption can reduce body weight, BMI, and body fat [100]. Caffeine acts as an anti-obesity agent by two main mechanisms. First, through inhibiting semicarbazide-sensitive amine oxidase (SSAO), caffeine reduces SSAO activity involved in weight gain. Second, caffeine inhibits phosphodiesterase (PDE), increasing the concentration of cAMP, which in turn suppresses processes that contribute to fat accumulation and increased body weight. By combining these two effects, caffeine exerts an anti-obesity impact by inhibiting the causes of weight gain and regulating metabolic processes that support fat reduction [41].
The recommended oral dose of caffeine ranges from 50 to 260 mg per day. For most healthy adults, caffeine is considered safe when consumed in doses up to 400 mg per day, which is approximately equivalent to four cups of coffee. However, it is important to note that prolonged use or exceeding the daily limit of 400 mg can lead to various side effects such as insomnia, nervousness, restlessness, nausea, increased heart rate, and more. In addition, higher doses of caffeine may result in headaches, anxiety, and chest pain. It is crucial to avoid caffeine overdose as it can have serious consequences including hypokalemia, hyponatremia, impaired iron and zinc absorption, rhabdomyolysis, and circulatory collapse [42].
A single dose of caffeine, not exceeding 200 mg or approximately 3 mg/kg BW for a 70 kg adult, does not result in any significant changes in blood pressure, myocardial blood flow, hydration status, or body temperature. This means that it does not reduce the exertion felt during exercise or mask the subjective perception of alcohol intoxication. It is important to note that a daily caffeine intake of up to 400 mg poses no safety concerns for the average adult [43].
Pregnant women can safely consume up to 200 mg of caffeine per day from all sources without any risks to the fetus. However, when it comes to children and adolescents, there is insufficient information available to determine safe levels of caffeine intake. The panel suggests that the nonconcerning caffeine intake for adults (3 mg/kgBW per day) could serve as a basis for determining a nonconcerning daily caffeine intake for children and adolescents [43].The Panel on Food Additives and Sources of Nutrients Added to Foods (ANS Panel) has concluded that there is no additive interaction between taurine and caffeine in terms of their diuretic effect, based on human intervention studies. In addition, the panel finds it unlikely that taurine has a stimulatory effect on the central nervous system, based on study studies. The second rat, which was used to derive the NOAEL of caffeine at 1,500 mg/kg bw per day, showed behavioral effects [43].
Based on the Indonesian National Agency of Drug and Food Control Regulation Number 24 of 2023 concerning Safety and Quality Requirements for Health Supplements which explains raw materials for health supplements that have the potential to contain contaminants and can pose risks to health, stated that the limits for products containing caffeine and herbs—herbs containing caffeine are 50 mg/serving (maximum 150 mg/day) while the use of coffee as a flavoring is limited to a maximum caffeine level of 10 mg/serving [101].
Nutraceuticals for immunomodulation
The immune system plays a role in maintaining body balance and can be changed by various external and internal factors. Changes in the immune system can impact the development of abnormal health conditions. Therefore, modulating the immune response can be a solution to increase the immune system’s ability to overcome disease-causing agents. Immunomodulation, which involves modifying the immune response, can be used as a method to regulate immune reactions to treat disease.
The application of immunomodulators has become the focus of attention both for the treatment and prevention of various diseases related to abnormal immune responses. The primary function of immunomodulators is to either dampen the immune response for treating autoimmune disorders or boost the immune response in instances of immunodeficiency and infectious diseases. In addition, immunostimulant agents are also used as chemotherapy support for various diseases [102–107]. Diet has an impact on the host’s nutritional and immune conditions, which interact and influence each other, both with symbionts, commensals, and pathogenic microbes. Nutritional feedback can influence host feeding behavior, allowing hosts to adjust their diet to enhance beneficial interactions with microbes and boost their immunity against infections. On the other hand, parasites and pathogens may also alter host feeding behavior to serve their own nutritional needs [108]. Therefore, nutraceuticals are an aspect that can support the performance of the immune system or act as an immunomodulator.
Several bioactive components, especially those from natural sources, which have activity as immunomodulators which modes of action as shown in Figure 6 include ascorbic acid or vitamin C, vitamin D, natural compounds (phytocompounds) such as capsaicin, anthocyanins and curcumin, as well as probiotics, and others [109]. The following are some explanations of these compounds:
Ascorbic acid
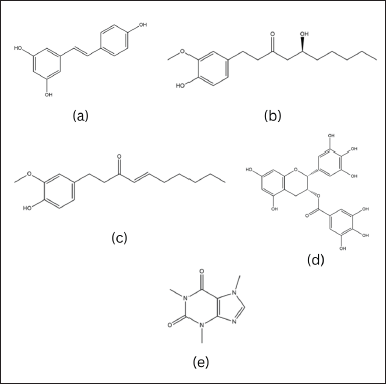
Ascorbic acid or vitamin C as shown in Figure 3 is a compound that was first isolated by the Hungarian biochemist, Albert Szent-Györgyi in 1937. The term “ascorbate” originates from the word scorbutus or scurvy, a condition resulting from a lack of vitamin C in the body [45].
Vitamin C (C6H8O6) has an average molecular weight of 173.12 Da with a boiling point of 552.7°C ± 50.0°C at 760 mmHg, and is soluble in water with logP –2.41 [44].
Ascorbic acid acts as a potent reducing agent and antioxidant, playing a crucial role in combating bacterial infections, detoxification processes, and collagen synthesis in various tissues such as teeth, bones, skin, and capillaries. In addition, it possesses anti-inflammatory properties [110]. Vitamin C enhances adaptive immunity by supporting the differentiation and proliferation of T and B cells, as well as boosting antibody production [109].
Supplementation of vitamin C-rich fruits for 4 weeks was found to enhance microbial killing by boosting neutrophil migration in response to chemotaxis and inhibiting the formation of neutrophil extracellular traps, which are networks of extracellular fibers generated by activated neutrophils [111]. Vitamin C (Vit C) also has the potential to shield against heart damage caused by lipopolysaccharides (LPS) in sepsis scenarios. Pre-treatment with Vit C in endotoxemic mice notably decreased MDA levels, restored SOD activity, reduced inflammatory biomarkers, and minimized cardiomyocyte damage, indicating that Vit C safeguards against endotoxin-induced cardiomyopathy by suppressing oxidative stress cytokines [112].
The two crucial isomer molecules of vitamin C, the reduced form of D-ascorbic acid and the active oxidized form of L-ascorbic acid, naturally exist in equal proportions and interact reciprocally, as illustrated in Figure 3.
Vitamin C can be found in citrus fruits, kiwi, mango, strawberries, papaya, tomatoes, chilies, green leafy vegetables, and broccoli. It is worth noting that most vertebrates can synthesize vitamin C from glucose, but some mammals, including humans, lack the enzyme required for this process. To avoid scurvy, which can have fatal consequences if not treated, it is recommended that humans consume 100–200 mg of vitamin C daily [45].
Vitamin C could be isolated from Australian pine (Casuarina equisetifolia). The procedure involved the addition of oxalic acid and sulfuric acid in the pre-treatment. The resulting mixture is further processed by grinding and filtering, then the presence of ascorbic acid is determined iodometrically in the juice.
After centrifuging the juice, squeezing the residue, macerating with distilled water, and evaporating the solvent, the resulting solution was then alkalized, leading to the precipitation of calcium ascorbate. After filtration and further treatment, the ascorbic acid was recovered and subjected to thorough analysis [113]. Ascorbic acid influences ROS levels from the stage of their formation, mainly originating from mitochondria and specific enzymes such as NADPH oxidase (NOXs) or xanthine oxidase (XO). Ascorbic acid supplementation protects the organism from XO hyperactivity and can directly inhibit XO activity. Research also shows that inhibition of XO activity by ascorbic acid can make a significant contribution to improved gout treatment. Although ascorbic acid does not affect XO activity under normal physiological conditions, treatment with ascorbic acid can inhibit XO hyperactivation in cells subjected to stress, such as due to UV radiation or hydrogen peroxide. Reduction of XO activity by ascorbic acid may also be beneficial in preventing reperfusion injury to stimulated neutrophils and delaying the development of hyperuricemia nephropathy [46].
There is no evidence indicating that vitamin C is carcinogenic, teratogenic, or has adverse effects on reproduction. High vitamin C intakes are generally considered to have low toxicity, with side effects typically reported only after very large doses exceeding 3 g/day. Excess ascorbic acid is unlikely to occur in humans due to saturated intestinal absorption and renal tubular reabsorption. Potential adverse effects of high intakes include digestive disorders, kidney stone formation, pro-oxidant effects, and other health concerns [47].
Vitamin D
The skin produces vitamin D as a secosteroid when the 7-dehydrocholesterol present there interacts with ultraviolet light, as occurs in sunlight. There are two commonly used types of vitamin D supplements: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) as in Figure 3. Vitamin D2 is produced by plants in small amounts, while vitamin D3 is produced by animals and is believed to be more easily converted to 1,25[OH] 2D [48].
Structurally, ergocalciferol differs from cholecalciferol as it has a double bond between C22 and C23 and an additional methyl group at C24. In terms of pharmacological potency, cholecalciferol is considered more powerful than ergocalciferol, making vitamin D3 the preferred choice for medical use [83]. Vitamin D3 can be found in various sources such as cod liver oil (400–1,000 IU), canned tuna (44 IU), and salmon (148 IU) [48].
Vitamin D functions as a key regulator of the innate immune system, particularly in the treatment of illnesses triggered by mycobacteria like tuberculosis and leprosy. The active form of Vitamin D, known as 1,25(OH)2D3, boosts the synthesis of defensin β2 and cathelicidin antimicrobial peptide (CAMP) in macrophages and keratinocytes monocytes, thereby strengthening antimicrobial capabilities. In addition, 1,25(OH)2D3 strengthens the innate immune response by enhancing chemotaxis, autophagy, and phagolysosome fusion. Vitamin D also enhances CAMP regulation in various cells within the innate immune system, including keratinocytes, epithelial cells, intestines, lungs, corneas, and placental trophoblasts, which serve as the primary defense barrier [114].
The adaptive immune system serves as the second line of defense against infections. It is characterized by its specificity and ability to acquire immunological memory through exposure to pathogens. While there have been suggestions that 1,25(OH)2D3 could potentially act as an immunomodulator by influencing different cell types, the available data is currently inadequate to establish a definitive role for vitamin D in modulating the adaptive immune system in humans. As a result, there is currently insufficient data support for the therapeutic use of vitamin D and its metabolites in enhancing the response of the adaptive immune system [114].
Carlberg’s team gained insight into the impact of vitamin D3 supplementation on immunity by analyzing the transcriptome of peripheral blood mononuclear cells (PBMCs) stimulated with LPS and β-glucan, both with and without 1,25(OH)2D3. The results showed that the effect of 1,25(OH)2D3 on the immune response was highly dependent on the timing of administration, whether before, during, or after infection in real-life situations. These findings have important implications for medical practitioners, highlighting the significant role of time in considerations regarding vitamin D3 supplementation. In addition, Carlberg and colleagues’ research provides additional evidence of vitamin D3’s role in supporting healthy immunity, providing insight into when and how vitamin D3 can be used to treat or prevent certain diseases by modifying the functional consequences of immune challenges [49].
Vitamin D supplementation can prevent respiratory tract infections [115] and have a good effect on COVID-19 patients [116]. However, based on research Bassatne et al. [117] which conducted a meta-analysis of the effect of vitamin D on COVID-19, more data is still needed because it has not yet proven significant results. Apart from studies on COVID-19, vitamin D supplementation, especially if food intake is inadequate, is needed.
The daily dose requirement for vitamin D based on the IOM, namely 600 IU for children (from 1 year old) and adults, and the NOAEL/UL value ranges between 2,500 IU and 4,000 IU for children to adults. Meanwhile, the need for vitamin D for those who are susceptible to deficiency is even higher [118].
Another proposal of vitamin D requirements is by examining molecular parameters sensitive to vitamin D, they propose classifying individuals as high, moderate, or low vitamin D responders. It is recommended assessing vitamin D status through serum 25(OH)D levels and defining deficiency as 25(OH)D ≤ 20 ng/ml (≤50 nmol/l), insufficiency as 25(OH)D 21–29 ng/ml (52.5–72.5 nmol/l), and sufficiency as 25(OH)D 30–100 ng/ml (75–250 nmol/l) to determine the need of one’s vitamin D [50].
In its application to natural materials, no articles have been found about the direct extraction and isolation of the form of vitamin D3. What was obtained was the extraction of cod liver oil (CLO) as the main source of vitamin D3, then analyzing the vitamin D levels in the CLO, either using High-Performance Liquid Chromatography (HPLC), LC-MS/MS, or conventional measurement methods from the British Pharmacopoeia. Tamil Mani et al. [119] cite the CLO extraction method based on the Moller method created by Peter Moller in 1,850. This method involves mixing the liver into a paste, heating it gradually until the oil rises to the top, then heating and purifying it to produce CLO. Hailisheng Group Co Ltd in 2016 also patented a method for extracting CLO [120].
Extraction of CLO could be conducted using both the traditional hexane method and the eco-friendly supercritical carbon dioxide (SC-CO2) method. The best SC-CO2 extraction conditions were found to be a temperature of 49°C, a pressure of 29.9 MPa, and a CO2 flow rate of 4.97 ml/minute. Time extraction curves indicated CLO recoveries of 68%, 85%, and 89% at 5, 15, and 20 hours, respectively. The SC-CO2 extracted oil exhibited the highest squalene content (≈150 µg/ml), significant levels of vitamins D and K, and minimal heavy metal content. Thermogravimetric analysis revealed that SC-CO2 oil is more prone to thermal degradation due to its high purity [121].
Both conventional and nonconventional methods can be employed for sterol extraction from fungi, either individually or in combination. Nonconventional methods such as UAE and MAE offer the advantage of shorter extraction times compared to conventional methods. Further research is needed to explore the potential of utilizing mushroom extracts enriched with ergosterol and vitamin D2 in the pharmaceutical and food industries [122]. This opens possibilities for the production of nutraceuticals rich in vitamin D from natural sources, considering that the content of natural ingredients varies and can contribute to overall health improvement.
Allicin
Allicin as shown in Figure 3, a naturally occurring compound, possesses a sulfur group in its structural formula, which imparts the distinct smell and taste of garlic, particularly when it is freshly cut or crushed. Its chemical classification is that of a thiosulfinate, and its molecular structure was elucidated by Stoll and Seebeck in 1948. Allicin is synthesized in nature as a result of enzymatic reactions triggered by damage to plant tissue.
The precursor to allicin is a nonproteinogenic amino acid called alliin (S-allyl-l-cysteine sulfoxide). Alliin, along with other S-alkyl-l-cysteine sulfoxides, undergoes hydrolysis catalyzed by the enzyme alliinase. In the case of alliin, this hydrolysis leads to the formation of dehydroalanine and allyl sulfenic acid. Subsequently, two molecules of allyl sulfenic acid spontaneously combine to yield one molecule of allicin. Alliin is present in garlic (Allium sativum) and ramsons (Allium ursinum). Interestingly, red onions (Allium cepa) do not produce alliin but instead synthesize its isomer, isoalliin (trans-(+)-S-(1-propenyl)-l-cysteine sulfoxide) [123].
Garlic extract is commonly used in nutraceutical preparations [124]. Across various cultures, garlic and garlic supplements are known for their immune-boosting properties [51]. The immune-stimulating effects of garlic involve enhancing the total white blood cell count and increasing bone marrow cellularity. Organosulfur compounds found in garlic play a crucial role in counteracting oxidizing agents that hinder fatty acid oxidation, thus preventing the production of pro-inflammatory messengers through interactions with sulfur-containing enzymes. Furthermore, garlic consumption promotes the synthesis of NO and IFN-alpha in humans, potentially offering benefits in viral or proliferative diseases [51].
Allicin can protect the liver from immune response-induced damage by acting as an immunomodulator on T cells and adhesion molecules, as well as by inhibiting NF-kappaB activation [125]. In vitro studies also reveal that allicin can block TNF-alpha-mediated adhesion of T cells to extracellular matrix components and endothelial cells. Allicin plays a role in regulating the immune response by suppressing the TNFα-dependent release of proinflammatory cytokines in the intestinal epithelium. Allicin also hinders phosphatase activity, leading to increased phosphorylation of ERK1/2, a key component in the extracellular to intracellular signaling pathway. Furthermore, allicin inhibits the release of Reactive Nitrogen Species by LPS-stimulated macrophages [123].
Allicin is isolated by grinding the garlic to stimulate the enzymatic reaction of alliin. Subsequently, the crushed garlic is transferred into a beaker containing cold water, which is then covered and vigorously shaken for a brief duration. This step is repeated with the addition of additional cold water. The beaker is held at the top to prevent heat transfer during shaking and then filtered. Several extraction methods could be conducted, including UAE, SFE, MAE, and pressurized extraction. Furthermore, the quantification of allicin is achieved through HPLC and Gas Chromatography techniques [126].
The pressing extraction method yielded water extracts with allicin levels ranging from 42.74 to 50.79 μg/ml. In contrast, the ethanol extract obtained through the same extraction method exhibited lower allicin levels, ranging from 4.39 to 4.56 μg/ml. Furthermore, an alternative study utilizing the Soxhlet method reported remarkably higher allicin levels of 7.068 ppm [127].
Regarding safety, the NOAEL for garlic essential oil was considered greater than 50 mg/kg bw/day orally for 28 days in mice [128]. It is advisable to restrict garlic consumption to a safe daily limit of 250–350 mg/kg to avoid potential toxic effects [52]. The impact of garlic on the liver, kidneys, and heart is dose-dependent. Mice which was administered garlic extract at doses of 500 and 1,000 mg/kg, resulting in toxic effects such as bleeding, nodular edema, and an increase in body and liver weight [129].
Carotenoids
Carotenoids are a diverse group of organic compounds that are produced by plants, bacteria, and fungi, but not by humans. They all have a common backbone structure known as a polyene chain, which consists of a series of conjugated double bonds and two end groups as shown in Figure 3 [130].
Carotenoids, classified as tetraterpenes, are responsible for the vivid yellow, orange, or red pigmentation observed in fruits, leaves, and flowers. Notably, green vegetables contain substantial amounts of carotene and xanthophyll hydrocarbons, contributing to their green coloration. Ripe tomatoes exhibit a lipophilic red pigment called lycopene, while the orange hue of carrots is attributed to β-carotene. Paprika owes its brilliant red pigment to capsanthine, and the pink or red color of crustaceans is a result of astaxanthin.
Generally, carotenoids are hydrophobic molecules with limited solubility in water, and they primarily function in hydrophobic regions within cells. The presence of polar functional groups attached to the polyene chains can alter the polarity of carotenoids, influencing their distribution in biological membranes and their interactions with various molecules [131].
Carotenoids can be categorized into provitamin A compounds such as β-carotene, α-carotene, and β-cryptoxanthin, as well as nonprovitamin A compounds. In addition, carotenoids can be grouped based on their functional groups into xanthophylls (e.g., lutein and zeaxanthin) containing oxygen, and carotenes (e.g., α-carotene, β-carotene, and lycopene) which consist of hydrocarbon chains without functional groups. Furthermore, apocarotenoids such as retinoids, vitamin A, β-ionone, and aromatic volatile compounds α-ionone are produced from carotenoids through oxidative cleavage using carotenoid cleavage dioxygenases [51].
The administration of 2 and 8 mg of astaxanthin, along with inducers like PHA, Con A, and PWM, led to a reduction in CRP levels, enhanced NK-cell activity and lymph proliferation, as well as increased levels of IFN-γ and IL-6. Furthermore, testing astaxanthin stereoisomers at a concentration of 10−8 mol·L−1 on human PBMCs in vitro resulted in elevated levels of IgM, IgG, and IgA when induced by PWM, TNP-KLH, and tetanus toxoid. In addition, administering 40 mg/kgBW of curcumin to mice induced by KLH antigen led to increased IgG production [51].
Oral administration of 150 mg/kg curcumin led to a reduction in leucocyte infiltration, NO, MPO, and TNF-α levels for K. pneumoniae infected mice [55]. In addition, administering 2, 4, and 6 g/kg curcumin to healthy rabbits increased serum IgG and IgM levels. Curcumin was also found to inhibit the immunostimulatory function of dendritic cells and interfere with the maturation of myeloid DCs by suppressing the expression of CD80 and CD86, which are essential for T cell activation, and reducing the production of pro-inflammatory cytokines like IL-12 through the inhibition of MAPK and NF-κB translocation.
Several extraction methods and safety of compounds from the carotenoid group are explained as follows:
Astaxanthin
Astaxanthin is not naturally produced by fish, crustaceans, and other animals; instead, it must be obtained through their diet. In the wild, this compound is primarily created by microorganisms found in aquatic and nonaquatic environments, including bacteria, fungi, yeast, and microalgae. Despite being a natural substance, synthetic astaxanthin is commonly used due to its cost-effectiveness, higher purity, and improved stability [53].
Astaxanthin, a lipophilic compound found in encysted Haematococcus cells, can be extracted using various methods. Various techniques such as solvent, acid, vegetable oil, microwave-assisted, and enzymatic methods were utilized. Hydrochloric acid treatment led to the retrieval of up to 80% of astaxanthin from Haematococcus. A combination of 40% acetone at 80°C for 2 minutes followed by xylase, cellulose, abalone, and acetone powder resulted in a 70% recovery rate. High astaxanthin yields were obtained through hydrochloric acid treatment at different temperatures for 15 and 30 minutes using sonication [54].
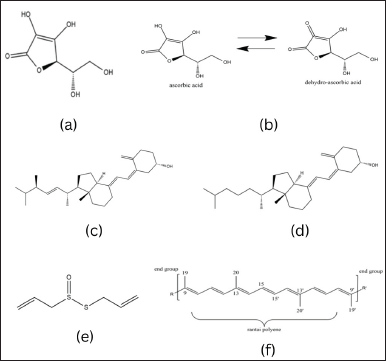 | Figure 3. Chemical structure of (a) ascorbic acid, (b) reversible configuration of hydroxy and dehydroxy ascorbic acid, (c) ergocalciferol, (d) cholecalciferol, (e) allicin, and (f) carotenoid. [Click here to view] |
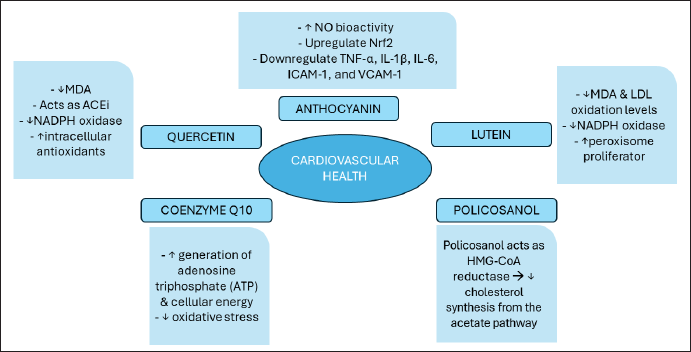 | Figure 4. Representation of cardiovascular nutraceuticals mechanism of action. [Click here to view] |
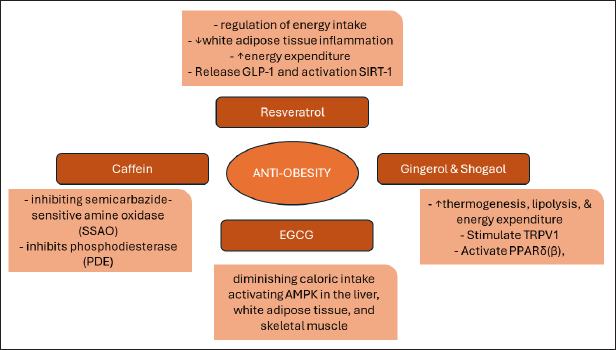 | Figure 5. Representation of anti-obesity nutraceuticals mechanism of action. [Click here to view] |
Astaxanthin is safe to consume with food without significant side effects. Fat-soluble, astaxanthin accumulates in animal tissues without toxic effects in mice. Excessive consumption can cause pigmentation of animal skin, while adding astaxanthin to fish food gives fish a reddish color. It is recommended to consume astaxanthin with omega-3 rich seed oil, and a variety of astaxanthin products at a dose of 2–4 mg/day are available on the market. Studies in adult humans found no side effects at a dose of 6 mg/day for 10 days on blood rheology [54].
Curcumin
Curcumin, also referred to as diferuloymethane, is a hydrophobic polyphenol that is extracted from the rhizomes of various perennial herbal plants belonging to the Curcuma genus, which is a part of the ginger family (Zingiberaceae). Notable species within this genus include Curcuma longa, Curcuma amada, Curcuma zedoaria, Curcuma aromatic, and Curcuma raktakanta. Among these species, Curcuma longa, commonly known as turmeric, holds the highest popularity. Typically, turmeric rhizomes contain approximately 3%–5% of three distinct types of curcuminoid derivatives. These derivatives consist of curcumin (75%), demethoxycurcumin (10%–20%), and bisdemethoxycurcumin (5%). Among these compounds, curcumin stands out as the most significant bioactive compound [56].
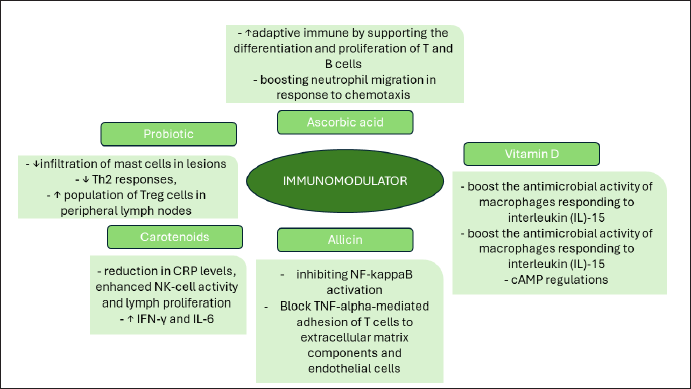 | Figure 6. Representation of immunomodulator nutraceuticals mechanism of action. [Click here to view] |
Curcumin could be extracted by Ionic liquid-based extraction and the use of UAE (IL-UAE). It was found that the yield of curcuminoids in UAE based on [OMIM]Br was 6.14%, higher than the yield of curcuminoids in UAE based on ethanol 85% (4.40%) and hot reflux extraction based on ethanol 85% (5.12%). Quite high yields were also obtained using the EAE extraction method with particle size parameters of 0.180–0.425 mm, 1%–5% (w/w) mixture of α-amylase and amyloglucosidase, incubation 2–8 hours (65°C), 2–24 hours extraction (15–45°C), solid ionic liquid and carbamate ratio 1:10–1:50 (w/w) produced 5.73% curcumin. The use of nonconventional extraction and purification methods could have great potential, especially for the efficiency of curcumin results [132].
Another study optimized the extraction for curcumin from Curcuma longa L. which result was ethanol concentration of 80%, an extraction temperature of 70°C, a liquid to material ratio of 20, and an extraction time of 3 hours, a crude extract with a curcumin yield of 56.8 mg/g could be obtained. To further refine the curcumin extract, the researchers performed isolation and purification of curcuminoids from the crude extract using high-performance counterflow chromatography which purification process achieved a yield of 67% within a timeframe of 70 minutes [133].
Curcumin has a well-established safety profile. For instance, based on reports from JECFA and EFSA, the ADI value for curcumin ranges from 0 to 3 mg/kg body weight. Clinical trials with healthy individuals have shown the safety and effectiveness of curcumin. However, there have been reports of some adverse effects, such as diarrhea, headache, rash, and yellow stools in subjects receiving varying doses of curcumin. In addition, other studies have indicated side effects such as nausea, diarrhea, and changes in certain enzyme levels. The reproductive toxicity study in mice found specific NOAEL values for curcumin intake over two generations [56,57].
Lycopene
Lycopene is classified under the carotenoids group, which is further divided into carotenes and xanthophylls. Carotenes are soluble in nonpolar solvents, while xanthophylls are soluble in water. Lycopene is a natural pigment responsible for the red color in tomatoes, pink guava, watermelon, and other fruits. It is commonly found in fruits and their derivatives, making them significant sources of lycopene in many human diets. With a chemical formula of C40H56, lycopene has 11 conjugated and 2 unconjugated double bonds, along with an open chain structure that grants it antioxidant properties, particularly as a singlet oxygen quencher [58].
Lycopene exhibits beneficial effects on neuroinflammation and depressive-like behavior in animal models [59]. Furthermore, the administration of lycopene has been found to enhance the recovery of neurological function in mice following spinal cord ischemia/reperfusion injury by inhibiting COX-2 [134]. In addition, the antioxidant and anti-inflammatory properties of lycopene have been shown to mitigate cigarette smoke-induced lung damage in mice by reducing ROS, leukocytes, lipid peroxidation, DNA damage, and pro-inflammatory cytokines like TNF-α and INF-γ [60]. In human studies, lycopene has been shown to protect against smoke-induced COPD by reducing neoplastic lesions and preventing cholesterol accumulation in the lungs [135]. Moreover, the combination of lycopene and matrine has been found to protect against LPS-induced acute lung injury in mice [136].
The presence of lycopene in plant chromoplasts is impeded by cell barriers, particularly cell walls, during extraction procedures. To prevent lycopene loss through oxidation or isomerization, it is essential to conduct extraction, storage, and analysis under controlled environmental conditions. Despite the higher bioavailability of cis-lycopene in food, the mechanisms responsible for the isomerization from trans to cis form in vivo are not well understood [58,137].
Extracting all-trans-lycopene from tomatoes using carbon dioxide at 40°C could prevent the degradation of lycopene due to isomerization and oxidation, preserving its health benefits. AC(30) column successfully separated the all-trans-lycopene isomer from its cis counterpart, resulting in an extract with 88% all-trans-lycopene and 12% cis-lycopene [138].While, the supercritical CO2 extraction (SFE-CO2) method to extract lycopene from tomato skin and seeds achieved lycopene levels ranging from 5.79 to 105 g/kg using this extraction technique [131,140]
Lycopene was successfully extracted from tomato skin waste using maceration in refined olive oil. The researchers optimized various parameters including temperature (40–80°C), stirring speed (200–400 rpm), and the ratio of tomato skin to olive oil (2%,5%–5.5%, w/v). The results revealed that a biomass to oil ratio of 2.5% (w/v), a temperature of 80°C, and magnetic stirring at 400 rpm for 45 minutes yielded the highest extraction efficiency, with 99.3% of the initial lycopene content being extracted. This corresponded to a concentration of 1244.50 ± 30.41 mg/kg in dry form, or 35 mg lycopene/kg of olive oil [139]. ,. The study involved administering lycopene produced by recombinant E. coli to mice at varying doses for 28 days. No significant effects were observed in terms of weight gain, clinical signs, or ophthalmoscopy parameters across different treatment groups. In addition, no toxic effects were found in various tests conducted [61].
Probiotics
The concept of probiotics was initiated by Elie Metchnikoff in the early 20th century, who linked the longevity of Bulgarians to their consumption of fermented milk. Meanwhile, French pediatrician Henry Tissier noted that healthy babies had more bifid bacteria in their feces compared to babies with diarrhea. Tissier even recommended the consumption of Bordeaux with specific bacterial cultures to combat diarrhea, highlighting the ancient practice of using fermented milk to treat gastrointestinal issues [141].
Probiotics can consist of a variety of microorganisms, with the most prevalent being bacteria from the Lactobacillus and Bifidobacterium groups. In addition, other bacteria and yeast, like Saccharomyces boulardii, can also serve as probiotics. It is important to note that different types of probiotics can have varying effects. For instance, if a specific strain of Lactobacillus is effective in preventing a disease, it does not guarantee that another strain of Lactobacillus or any Bifidobacterium probiotic will yield the same results [142].
The WHO and FAO expert working group is currently engaged in the task of defining probiotics and establishing the specific characteristics that a bacterial strain must possess to be classified as a probiotic. One of the primary criteria is that the strain must successfully reach its target site of action, typically the intestine, and withstand the various physiological challenges encountered during ingestion, including exposure to gastric acid, intestinal pH, and bile salts. Second, scientific evidence has conclusively demonstrated the beneficial effects of probiotics on the host; the ingestion of probiotics must not pose any risk to the host; and finally, the strain must maintain its distinctive characteristics and remain stable throughout the manufacturing process, as well as be effectively preserved within the matrix in which it is embedded [141].
The International Probiotics Association[143], known as The global voice of probiotics®, has created the probiotic manufacturing guidelines to assist finished product manufacturers and contract manufacturing companies in understanding the unique considerations for probiotic manufacturing. These guidelines offer valuable tips and guidance to ensure high-quality product compliance. The process of producing dry probiotic supplements typically involves a series of fermentations at various scales to generate bacterial biomass using specific growth media and conditions. This is followed by a centrifugation step to eliminate the culture supernatant and concentrate the biomass, a mixing stage where protective agents are added, and finally, a drying stage [144].
In laboratory experiments, the administration of probiotics has been shown to decrease the infiltration of mast cells in lesions, suppress Th2 responses, and increase the population of Treg cells in peripheral lymph nodes. In addition, the application of Lactobacillus plantarum CJLP133 has been found to alleviate skin inflammation in Nc/NgA mice with HDM-induced dermatitis by balancing the Th1/Th2 ratio and activating Tregs. Various findings suggest that probiotics can modulate allergic responses by targeting different immune cells [62].
However, the results from a review of clinical trials, specifically regarding the use of probiotics for eczema prevention, have also been explored. A randomized controlled trial demonstrated that supplementation of L. rhamnosus HN001 (HN001) in mothers starting from 35 weeks of gestation until 6 months of breastfeeding, as well as in infants from birth to 2 years of age, significantly reduced the prevalence of eczema up to 6 years of age. Conversely, Bifidobacterium animalis subsplactis HN019 (HN019) did not yield significant results. A recent meta-analysis of 6 trials, involving 1,955 patients, suggests that early-life probiotic use is likely to prevent AD later in life. However, due to substantial heterogeneity between studies, it is challenging to generalize the effectiveness of probiotics in modulating AD [62].
Probiotic lipid antigens have been found to influence NK cells, also known as natural killer cells. Several specific probiotic strains have been identified for their ability to enhance NK cell activity. For instance, B. polyfermenticus has been shown to increase the population of CD56+ NK cells. In elderly adults, supplementation with Bifidobacterium lactus HN019 has been found to improve NK tumoricidal activity. Another probiotic strain, Lactobacillus casei Shirota (LcS), activates NK cell activity by inducing the surface expression of CD69 and CD25 on CD8+ and CD56+ subsets, even without the presence of other stimuli. In addition to incorporating these specific probiotic strains, consuming yogurt daily can also be beneficial for NK cell function. Overall, these findings suggest that probiotic supplementation and regular consumption of yogurt can have a positive impact on NK cell activity, which plays a crucial role in immune defense against various diseases [145].
Bacteriocins, which are compounds produced by probiotics, have recently been discovered to possess immunomodulatory effects. While the exact mechanisms behind the immunomodulatory properties of bacteriocins are not fully understood, they have been shown to act as signaling peptides that impact host immunity. Recent research has shown that bacteriocins such as nisin A, plantaricin 423, and bacST4SA can pass through endothelial and epithelial cells in vitro without causing harm, and they remain stable in blood plasma. These findings strongly support the idea that bacteriocins can traverse the gut-blood barrier, influencing both local and systemic immunity. Furthermore, it is proposed that bacteriocins may stimulate intestinal epithelial cells to produce antimicrobial substances that help eliminate invasive pathogens [146].
Probiotics are generally considered safe, but there are concerns regarding the generalization of their safety. WHO and FAO at the UN reported in 2002 that probiotics can cause side effects such as systemic infections, deleterious metabolic activity, excessive immune stimulation in susceptible individuals, and gene transfer. A pancreatitis-related clinical trial showed significant mortality in the group treated with probiotics. Although Bifidobacterium and Lactobacillus are considered safe, recent research suggests that some strains of Bifidobacterium may have counterproductive effects on certain disease conditions, such as inflammatory bowel disease and arthritis. It is important not to overlook the safety of probiotics, and the implementation process can be modified to speed up research without significantly increasing costs [62].
NOAEL values for probiotics can vary depending on the specific strain used. For instance, in a 90-day repeated dose toxicity study, the NOAEL of B. breve MCC1274 was found to be greater than 1,000 mg/kg, equivalent to 1.3 × 1011 CFU/kg. Based on this study, the ADI of B. breve MCC1274 was determined to be 1.3 × 109 CFU/kg BW/day. In another study, the lethal dose 50 of R. intestinalis was over 1.9 × 109 CFU/kg, with a NOAEL of 1.32 × 109 CFU/kg/day for 28 days. This research suggests that R. intestinalis could be a promising next-generation probiotic due to its probiotic properties and safety [63].
CHALLENGE
The global nutraceutical market, currently dominated by Europe, US, and Japan, accounts for over 90% of the total market share. It is projected that by 2023, the market will grow to $336 billion from $247 billion in 2019, with a compound annual growth rate (CAGR) of 8% [10]. As the market matures globally, nutraceutical players are now directing their attention toward developing countries, particularly in the Asia Pacific region, with India being a key focus. The Indian market had only a 2% market share of the total global nutraceutical market in 2017. It is estimated to reach $11 billion by 2023, increasing at a CAGR of 21% [147]. However, in several other developing countries, such as Indonesia, for example, nutraceutical products, especially those made from natural ingredients, are still ambiguous in their product registration because the term nutraceuticals is still unfamiliar among the public.
Based on Indonesia Food and Drug Supervisory Agency (BPOM) regulation no. 19 of 2022, Health Supplements are products intended to supplement nutritional needs, maintain, enhance, and/or improve health functions, have nutritional value and/or physiological effects, contain one or more ingredients in the form of vitamins, minerals, amino acids, and/ or other nonplant materials that can be combined with plants. This definition is of course slightly different from nutraceutical products, although some bioactives seem similar, because nutraceuticals, in particular, must be compounds or bioactives that generally come from food, thus excluding plant types that are not in the food category.
Apart from the challenges of public education and regulation regarding nutraceutical products to increase their economic value, the challenges of formulation and maintaining quality, efficacy, and safety are considerations, especially because nutraceutical products are packaged in pharmaceutical preparations such as capsules, tablets, syrup, or others so that they require comprehensive research even though declared safe. In contrast to medicine, traditional medicine, or cosmetics, there are no specific guidelines issued for how to make supplements or how to make good nutraceuticals, perhaps because the perception of medicines and nutraceutical products is still the same. Information regarding ingredients that are safe to use as nutraceutical products in the form of NOAEL or similar is also not abundant for certain natural ingredients so further analysis and study is needed.
CONCLUSION
In this context, this article highlights the potential of various active compounds from natural ingredients as nutraceuticals. Research results show that the use of these compounds can support cardiovascular health, provide anti-obesity effects, and also modulate the immune system. Therefore, further development and clinical research are needed to further explore the health benefits that these natural ingredient-based nutraceuticals can provide. Potential implications of these findings could include the development of new therapeutic products that could improve the quality of life and overall well-being of society.
ACKNOWLEDGEMENTS
We thank the Faculty of Pharmacy, Universitas Indonesia for providing access to the references that are used in this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to this article: Raditya Iswandana contributed to concepts, design, and any revisions or supervision support; Fatimah Azzahra contributed to drafting, admin, technical, or material support; Fatimah Azzahra, Hafizha Fadhila Suleman, and Ranum Wanudya Yunas contributed to data acquisition and analysis/interpretation. All authors agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Kim SK. Marine Nutraceuticals: prospects and perspectives. Marine Nutraceuticals: prospects and perspectives. 1st ed. Boca Raton, FL: CRC Press Taylor & Francis; 2013.
2. WHO. Obesity and overweight. Geneva, Switzerland: WHO; 2024.
3. Badan Pusat Statistik. Prevalensi Obesitas Pada Penduduk Umur > 18 Tahun—Tabel Statistik 2021. [cited 2023 December 22]. Available from: https://www.bps.go.id/id/statistics-table/2/MTQ4MSMy/prevalensi-obesitas-pada-penduduk-umur--18-tahun.html
4. WHO. Cardiovascular diseases (CVDs) 2021. [cited 2023 December 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
5. Wise J. Covid-19: WHO declares end of global health emergency. BMJ. 2023;381:1041. CrossRef
6. Afifa L. Jokowi Declares End of COVID-19 Pandemic Status in Indonesia. Tempo 2023. [cited 2023 October 31]. Available from: https://en.tempo.co/read/1739828/jokowi-declares-end-of-covid-19-pandemic-status-in-indonesia
7. Tarigan M. Perbedaan Tren Konsumsi Suplemen Sebelum dan Pasca Pandemi Covid-19. Tempo 2023. [cited 2023 October 31]. Available from: https://gaya.tempo.co/read/1790169/perbedaan-tren-konsumsi-suplemen-sebelum-dan-pasca-pandemi-covid-19
8. UN News. Post-pandemic world economy still feeling COVID-19’s sting 2023. [cited 2023 October 31]. Available from: https://news.un.org/en/story/2023/05/1136727
9. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46. CrossRef
10. The Business Research Company. Nutraceuticals Market Size, Growth, Trends and Forecast 2024-2033 2024. [cited 2024 January 31]. Available from: https://www.thebusinessresearchcompany.com/report/nutraceuticals-global-market-report
11. Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020;25:3809. CrossRef
12. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. CrossRef
13. Reis JF, Monteiro VS, De Souza Gomes R, Carmo MMD, Da Costa GV, Ribera PC, et al. Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med. 2016;14:315. CrossRef
14. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the re-evaluation of anthocyanins (E 163) as a food additive. EFSA J. 2013;11:3145. CrossRef
15. Krga I, Milenkovic D. Anthocyanins: from sources and bioavailability to cardiovascular-health benefits and molecular mechanisms of action. J Agri Food Chem. 2019;67:1771–83. CrossRef
16. Gammone MA, Riccioni G, D’Orazio N. Marine carotenoids against oxidative stress: effects on human health. Marine Drugs. 2015;13:6226–46. CrossRef
17. Hajizadeh-Sharafabad F, Ghoreishi Z, Maleki V, Tarighat-Esfanjani A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: a systematic review of in vivo, ex vivo and in vitro studies. Pharmacol Res. 2019;149:104477. CrossRef
18. Ravikrishnan R, Rusia S, Ilamurugan G, Salunkhe U, Deshpandé JV, Shankaranarayanan JS, et al. Safety assessment of lutein and zeaxanthin (LutemaxTM 2020): Subchronic toxicity and mutagenicity studies. Food Chem Toxicol. 2011;49:2841–8. CrossRef
19. Sato Y, Joumura T, Nashimoto S, Yokoyama S, Takekuma Y, Yoshida H, et al. Enhancement of lymphatic transport of lutein by oral administration of a solid dispersion and a self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2018;127:171–6. CrossRef
20. Srisaipet A, Phromchan S, Jaipaeng T. Policosanol extraction from beeswax and improvement of the purity. MATEC Web of Conferences. 2017;111:02005. CrossRef
21. Lockwood B. Nutraceuticals: a guide for healthcare professionals. London, UK: Pharmaceutical Press; 2007.
22. Skulas-Ray A, Flock M, Kris-Etherton P. Chapter 29 - The role of diet in the prevention and treatment of cardiovascular disease. In: Coulston AM, Boushey CJ, Ferruzzi MG, editors. Nutrition in the prevention and treatment of disease. 3rd ed. Cambridge, MA: Academic Press; 2013. pp 541–67. CrossRef
23. Arenas-Jal M, Suñé-Negre JM, García-Montoya E. Coenzyme Q10 supplementation: efficacy, safety, and formulation challenges. Compr Rev Food Sci Food Saf. 2020;19:574–94. CrossRef
24. Rabanal-Ruíz Y, Llanos-González E, Alcain FJ. The use of coenzyme Q10 in cardiovascular diseases. Antioxidants. 2021;10:755. CrossRef
25. Hidaka T, Fukui K, Funahashi I, Fukutomi N, Hosoe K. Safety assessment of coenzyme Q10 (CoQ10). BioFactors. 2008;32:199–208. CrossRef
26. Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: from molecular to clinical studies. J Med Food. 2019;22:753–70. CrossRef
27. Kashyap D, Garg VK, Tuli HS, Yerer MB, Sak K, Sharma AK, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9:174. CrossRef
28. Nishimuro H, Ohnishi H, Sato M, Ohnishi-Kameyama M, Matsunaga I, Naito S, et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients. 2015;7:2345–58. CrossRef
29. Kong Y, Tian J, Niu X, Li M, Kong Y, Li R, et al. Effects of dietary quercetin on growth, antioxidant capacity, immune response and immune-related gene expression in snakehead fish, Channa argus. Aquac Rep. 2022;26:101314. CrossRef
30. Kocahan S, Dogan Z, Erdemli E, Taskin E. Protective effect of quercetin against oxidative stress-induced toxicity associated with doxorubicin and cyclophosphamide in rat kidney and liver tissue. PubMed. 2017;11:124–31.
31. Tripathi A, Kumar B, Sagi SSK. Prophylactic efficacy of Quercetin in ameliorating the hypoxia induced vascular leakage in lungs of rats. PLoS One. 2019;14:e0219075. CrossRef
32. Vieira-Frez FC, Sehaber-Sierakowski CC, Perles JVCM, Bossolani GDP, Verri WA, Nascimento R, et al. Anti- and pro-oxidant effects of quercetin stabilized by microencapsulation on interstitial cells of Cajal, nitrergic neurons and M2-like macrophages in the jejunum of diabetic rats. NeuroToxicol. 2020;77:193–204. CrossRef
33. Vrânceanu M, Heghes S-C, Cozma-Petrut A, Banc R, Stroia CM, Raischi V, et al. Plant-derived nutraceuticals involved in body weight control by modulating gene expression. Plants. 2023;12:2273. CrossRef
34. Tian B, Liu J. Resveratrol: a review of plant sources, synthesis, stability, modification and food application. J Sci Food Agri. 2019;100:1392–404. CrossRef
35. Pan M, Wu J, Ho C, Lai CS. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. BioFactors. 2018;44:50–60. CrossRef
36. Aguirre L, Fernández-Quintela A, Arias N, Portillo MP. Resveratrol: anti-obesity mechanisms of action. Molecules. 2014;19:18632–55. CrossRef
37. Edwards JS, Beck M, Riegger C, Bausch J. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida». Ann N Y Acad Sci. 2011;1215:131–7. CrossRef
38. Ahn E, Oh JS. Inhibitory effect of galanolactone isolated from Zingiber officinale roscoe extract on adipogenesis in 3T3-L1 cells. J Korean Soc Appl Biol Chem. 2012;55:63–68. CrossRef
39. James A, Wang K, Wang Y. Therapeutic activity of green tea Epigallocatechin-3-Gallate on metabolic diseases and non-alcoholic fatty liver diseases: the current updates. Nutrients. 2023;15:3022. CrossRef
40. Heckman MA, Weil J, De Mejía EG. Caffeine (1, 3, 7-trimethylxanthine) in Foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010;75:RR77–87. CrossRef
41. Papukashvili D, Rcheulishvili N, Deng Y. Attenuation of weight gain and prevention of associated pathologies by inhibiting SSAO. Nutrients. 2020;12:184. CrossRef
42. Guldiken B, Catalkaya G, Ozkan G, Ceylan FD, Capanoglu E. Toxicological effects of commonly used herbs and spices. In: Patel VB, Preedy VR, editors. Toxicology: oxidative stress and dietary antioxidants, Amsterdam, The Netherlands: Elsevier; 2020. Vol. 1, pp 201–13. CrossRef
43. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the safety of caffeine. EFSA J. 2015;13:4102. CrossRef
44. Royal Society of Chemisry. Ascorbic acid, C6H8O6. Chemspider n.d [cited 2024 Sep 07]. Available from: https://www.chemspider.com/Chemical-Structure.12283687.html
45. Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. (Bp) 2019;9:73–9. CrossRef
46. Gegotek A, Skrzydlewska E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants (Basel) 2022;11:1993. CrossRef
47. Compounds I of M (US) P on DA and R. Vitamin C. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academies Press; 2000.
48. Thiele D, Senti J, Anderson C. Maternal Vitamin D supplementation to meet the needs of the breastfed infant: a systematic review. J Hum Lact?. 2013;29:163–70. CrossRef
49. Aribi M, Mennechet FJD, Touil-Boukoffa C. Editorial: The role of vitamin D as an immunomodulator. Front Immunol. 2023;14:1186635.
50. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. CrossRef
51. Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature’s protection against physiological threats. Crit Rev Food Sci Nutr. 2009;49:538–51. CrossRef
52. Adeola F, Enaibe B, Avwioro G. Evaluating toxicity profile of garlic (Allium sativum) on the liver, kidney and heart using Wistar Rat Model. Int J Trop Dis Health. 2017;26:1–12. CrossRef
53. Molino A, Rimauro J, Casella P, Cerbone A, Larocca V, Chianese S, et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J Biotechnol. 2018;283:51–61. CrossRef
54. Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—A review. Marine Drugs. 2014;12:128–52. CrossRef
55. Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators inspired by nature: a review on curcumin and echinacea. Molecules. 2018;23:2778. CrossRef
56. Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods. 2017;6:92. CrossRef
57. European Medicines Agency. Assessment report on Curcuma longa L., rhizoma. Amsterdam, The Netherlands: European Medicines Agency; 2018.
58. Guerra AS, Hoyos CG, Molina-Ramírez C, Velásquez-Cock J, Vélez L, Gañán P, et al. Extraction and preservation of lycopene: a review of the advancements offered by the value chain of nanotechnology. Trends Food Sci Technol. 2021;116:1120–40. CrossRef
59. Zhang F, Fu Y, Zhou X, Pan W, Shi Y, Wang M, et al. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J Neuroimmunol. 2016;298:1–8. CrossRef
60. Campos KKD, Araújo GR, Martins TL, Bandeira ACB, Costa G de P, Talvani A, et al. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J Nutr Biochem. 2017;48:9–20. CrossRef
61. Jian WC, Chiou MH, Chen YT, Lin CN, Wu MC, Du CJ, et al. Twenty-eight-day oral toxicity study of lycopene from recombinant Escherichia coli in rats. Regul Toxicol Pharmacol. 2008;52:163–8. CrossRef
62. Sharma G, Im SH. Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: current status and future prospects. Allergy Asthma Immunol Res. 2018;10:575–90. CrossRef
63. Zhang C, Ma K, Nie K, Deng M, Luo W, Wu X, et al. Assessment of the safety and probiotic properties of Roseburia intestinalis: a potential “Next Generation Probiotic.” Front Microbiol. 2022;13:973046. CrossRef
64. Frak W, Wojtasinska A, Lisinska W, Mlynarska E, Franczyk B, Rysz J. Pathophysiology of cardiovascular diseases: new insights into molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines. 2022;10:1938. CrossRef
65. Sosnowska B, Penson PE, Banach M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc Diagn Ther. 2017;67:S21–31. CrossRef
66. Juliani HR, Welch C, Wu Q, Diouf B, Malainy D, Simon JE. Chemistry and quality of Hibiscus (Hibiscus sabdariffa) for developing the natural-product industry in Senegal. J Food Sci. 2009;74:S113–21. CrossRef
67. Sapian S, Taib IS, Katas H, Latip J, Zainalabidin S, Hamid ZA, et al. The role of anthocyanin in modulating diabetic cardiovascular disease and its potential to be developed as a nutraceutical. Pharmaceuticals. 2022;15:1344. CrossRef
68. Bars-Cortina D, Ali S, Piñol-Felis C, Motilva M. Chemopreventive effects of anthocyanins on colorectal and breast cancer: a review. Semin Cancer Biol. 2022;81:241–58. CrossRef
69. Bhat I, Yathisha UG, Karunasagar I, Mamatha BS. Nutraceutical approach to enhance lutein bioavailability via nanodelivery systems. Nutr Rev. 2020;78:709–24. CrossRef
70. Indrawati R, Kurniawan JM, Wibowo AA, Juliana J, Gunawan IA, Heriyanto H, et al. Integrated solvent-free extraction and encapsulation of lutein from marigold petals and its application. Cyta J Food. 2019;17:121–7. CrossRef
71. Janikula M. Policosanol: a new treatment for cardiovascular disease? Altern Med Rev. 2002;7:203–17.
72. Shen J, Luo F, Lin Q. Policosanol: extraction and biological functions. J Funct Foods. 2019;57:351–60.
73. Raizner AE, Quiñones MÁ. Coenzyme Q10 for patients with cardiovascular disease. J Am Coll Cardiol. 2021;77:609–19. CrossRef
74. Vaghari H, Vaghari R, Jafarizadeh-Malmiri H, Berenjian A. Coenzyme Q10 and its effective sources. Am J Biochem Biotechnol. 2016;12:214–9. CrossRef
75. Rujiralai T, Raekasin N, Cheewasedtham W, Cheewasedtham C. Development of an effective extraction process for coenzyme Q10 from Artemia. Chem Pap. 2014;68:1041–8. CrossRef
76. Sambandam B, Devasena T, Arivarasan A, Raman P. Extraction and isolation of flavonoid quercetin from the leaves of trigonella foenum-graecum and their anti-oxidant activity. Int J Pharm Pharm Sci. 2016;8:120–4.
77. Papakyriakopoulou P, Velidakis N, Khattab E, Valsami G, Korakianitis I, Kadoglou NPE. Potential pharmaceutical applications of quercetin in cardiovascular diseases. Pharmaceuticals. 2022;15:1019. CrossRef
78. Abbot V, Sharma P. Effect of quercetin on micellization behaviour of Tween-20 in hydro-ethanolic solvent system: an electrolyte induced thermodynamic study. J Phys Conf Ser. 2020;1451:012013. CrossRef
79. Patil K, Karri S, Hatware K, Sharma S. Natural anti-obesity agents and their therapeutic role in management of obesity: a future trend perspective. Biomed Pharmacother. 2019;110:224–38. CrossRef
80. Panuganti KK, Nguyen M, Kshirsagar RK. Obesity. Treasure Island, FL: StatPearls Publishing; 2024.
81. Cuciureanu M, Caratasu C-C, Gabrielian L, Frasinariu O, Checherita LE, Trandafir LM, et al. 360-degree perspectives on obesity. Medicina-Lithuania. 2023;59:1119. CrossRef
82. PubChem [Internet]. PubChem compound summary for CID 445154, Resveratrol. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2024 July 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Resveratrol
83. Khattar S, Khan SA, Zaidi SAA, Darvishikolour M. Resveratrol from dietary supplement to a drug candidate: an assessment of potential. Pharmaceuticals 2022;15(8):957.
84. Wang DG, Liu W, Chen G. A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J Pharm Anal. 2013;3:241–7. CrossRef
85. Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, et al. Potential adverse effects of resveratrol: a literature review. Int J Mol Sci. 2020;21:2084. CrossRef
86. PubChem [Internet]. PubChem compound summary for CID 442793, Gingerol. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004; [cited 2024 July 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Gingerol
87. PubChem [Internet]. PubChem compound summary for CID 5281794, Shogaol. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2024 July 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Shogaol
88. Wei CK, Tsai Y, Korinek M, Hung PH, El-Shazly M, Cheng YB, et al. 6-Paradol and 6-Shogaol, the pungent compounds of ginger, promote glucose utilization in adipocytes and myotubes, and 6-Paradol reduces blood glucose in High-Fat Diet-Fed mice. Int J Mol Sci. 2017;18:168. CrossRef
89. Arachchilage DVG, Young DJ, Choo WS. Extraction, purification, food applications, and recent advances for enhancing the bioavailability of 6-gingerol from ginger – a review. Qual Assur Saf Crops Foods. 2022;14:67–83. CrossRef
90. Mele MA. Bioactive compounds and biological activity of ginger. J Multidiscip Sci. 2019;1:1–7. CrossRef
91. Ishfaq M, Hu W, Hu Z, Guan Y, Zhang R. A review of nutritional implications of bioactive compounds of Ginger (Zingiber officinale Roscoe), their biological activities and nano-formulations. 2022;34:1–12. CrossRef
92. Attari VE, Mahdavi AM, Javadivala Z, Mahluji S, Vahed SZ, Ostadrahimi A. A systematic review of the anti-obesity and weight lowering effect of ginger (Zingiber officinale Roscoe) and its mechanisms of action. Phytother Res. 2017;32:577–85. CrossRef
93. PubChem [Internet]. PubChem compound summary for CID 65064, Epigallocatechin Gallate. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2024 July 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Epigallocatechin-Gallate
94. Xu FW, Lv YL, Zhong Y, Xue Y, Wang Y, Zhang L, et al. Beneficial effects of green tea EGCG on skin wound healing: a comprehensive review. Molecules. 2021;26:6123. CrossRef
95. Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64:87–99. CrossRef
96. Vuong QV, Stathopoulos CE, Nguyen MH, Golding JM, Roach PD. Isolation of green tea catechins and their utilization in the food industry. Food Rev Int. 2011;27:227–47. CrossRef
97. Hu J, Webster D, Cao J, Shao A. The safety of green tea and green tea extract consumption in adults—results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–33. CrossRef
98. PubChem [Internet]. PubChem compound summary for CID 2519, Caffeine. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2024 July 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Caffeine.
99. Vuong QV, Roach PD. Caffeine in green tea: its removal and isolation. Sep Purif Rev. 2013;43:155–74. CrossRef
100. Tabrizi R, Saneei P, Lankarani KB, Akbari M, Kolahdooz F, Esmaillzadeh A, et al. The effects of caffeine intake on weight loss: a systematic review and dos-response meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2018;59:2688–96. CrossRef
101. Health Ministry of Indonesia. Peraturan BPOM No. 24 Tahun 2023 Tentang Persyaratan Keamanan Dan Mutu Suplemen Kesehatan. Database Peraturan Perundang-Undangan Indonesia - [PERATURANGOID] n.d. [cite 2024 Jul 09]. Available from: https://peraturan.go.id/id/peraturan-bpom-no-24-tahun-2023
102. Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci. 2015;6:655. doi: https://doi.org/10.3389/fpls.2015.00655
103. Razali FN, Sinniah SK, Hussin H, Zainal Abidin N, Shuib AS. Tumor suppression effect of Solanum nigrum polysaccharide fraction on Breast cancer via immunomodulation. Int J Biol Macromol. 2016;92:185–93. CrossRef
104. Rasheed HMF, Rasheed F, Qureshi AW, Jabeen Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J Ethnopharmacol. 2016;186:244–50. CrossRef
105. Sharma KR. Immunomodulatory studies on triterpenoids from Scoparia dulcis Linn. Biochem Pharmacol. 2015;04. CrossRef
106. Ilyas U, Katare DP, Aeri V, Naseef PP. A review on hepatoprotective and immunomodulatory herbal plants. Phcog Rev. 2016;10:66. CrossRef
107. Pujol JL, Vansteenkiste J, De Pas TM, Atanackovic D, Reck M, Thomeer M, et al. Safety and immunogenicity of MAGE-A3 cancer immunotherapeutic with or without adjuvant chemotherapy in patients with resected stage IB to III MAGE-A3-positive non-small-cell lung cancer. J Thorac Oncol. 2015;10:1458–67. CrossRef
108. Cotter SC, Simpson SJ, Raubenheimer D, Wilson K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct Ecol. 2010;25:186–98. CrossRef
109. Medoro A, Davinelli S, Colletti A, Di Micoli V, Grandi E, Fogacci F, et al. Nutraceuticals as modulators of immune function: a review of potential therapeutic effects. Prev Nutr Food Sci. 2023;28:89–107. CrossRef
110. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid—PMC n.d. [cited 2023 December 17]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9598715/
111. Bozonet SM, Carr AC, Pullar JM, Vissers MCM. Enhanced human neutrophil vitamin C status, chemotaxis and oxidant generation following dietary supplementation with vitamin C-Rich SunGold Kiwifruit. Nutrients. 2015;7:2574–88. CrossRef
112. Shati AA, Zaki MSA, Alqahtani YA, Al-Qahtani SM, Haidara MA, Dawood AF, et al. Antioxidant activity of vitamin C against LPS-induced septic cardiomyopathy by down-regulation of oxidative stress and inflammation. Curr Issues Mol Biol. 2022;44:2387–400. CrossRef
113. Wapwera JA, Lori JA. Extraction and isolation of ascorbic acid from australian pine (casuarina equisetifolia) needles grown in jos and environs. Bull Chem Soc Niger. 2021;46. CrossRef
114. Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10:1656. CrossRef
115. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. CrossRef
116. Ashique S, Gupta K, Gupta G, Mishra N, Singh SK, Wadhwa S, et al. Vitamin D—A prominent immunomodulator to prevent COVID-19 infection. Int J Rheum Dis. 2023;26:13–30. CrossRef
117. Bassatne A, Basbous M, Chakhtoura M, Zein OE, Rahme M, Fuleihan GE-H. The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metabol Clin Exp. 2021;119:154753. CrossRef
118. Meza-Meza M, Ruiz Ballesteros A, De la Cruz-Mosso U. Functional effects of vitamin D: from nutrient to immunomodulator. Crit Rev Food Sci Nutr. 2020;62. CrossRef
119. Mani T, Binesh A, Kaliyamurthi V. Extraction of cod liver oil. Vigyanvarta. 2023 [cited 2024 Sep 07]. Available from: https://vigyanvarta.com/ArticleDetails.aspx?id=1082
120. Haihui Z, Chen Wenbo, Qinghao J, Yufeng C, Lesheng, Ruijuan D. Cod liver oil extract and preparation method thereof. CN105985859A. 2016.
121. Supercritical carbon dioxide extraction of squalene rich cod liver oil: optimization, characterization and functional properties. J Supercrit Fluids. 2022;188:105693. CrossRef
122. Papoutsis K, Grasso S, Menon A, Brunton NP, Lyng JG, Jacquier JC, et al. Recovery of ergosterol and vitamin D2 from mushroom waste—potential valorization by food and pharmaceutical industries. Trends Food Sci Technol. 2020;99:351–66. CrossRef
123. Borlinghaus J, Albrecht F, Gruhlke MCH, Nwachukwu ID, Slusarenko AJ. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–618. CrossRef
124. Nguyen BT, Hong HT, O’Hare TJ, Wehr JB, Menzies NW, Harper SM. A rapid and simplified methodology for the extraction and quantification of allicin in garlic. J Food Compos Anal. 2021;104:104114. CrossRef
125. Bruck R, Aeed H, Brazovsky E, Noor T, Hershkoviz R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin a-induced hepatic injury in mice. Liver Int. 2005;25:613–21. CrossRef
126. Dhwani S, Poojitha P, Gurumoorthi P. A review on different extraction and quantification methods of allicin from garlic. J Xidian Univ. 2021;15:183–96. CrossRef
127. Bar M, Binduga UE, Szychowski KA. Methods of isolation of active substances from garlic (Allium sativum L.) and its impact on the composition and biological properties of garlic extracts. Antioxidants. 2022;11:1345. CrossRef
128. Lin YE, Lin MH, Yeh TY, Lai YS, Lu KH, Huang HS, et al. Genotoxicity and 28-day repeated dose oral toxicity study of garlic essential oil in mice. J Tradit Complement Med. 2022;12:536–44. CrossRef
129. Siddique A, Iqbal J, Sheikh MA. Effects of garlic (Allium Sativum) on the Weights of Liver in Albino Rats. PJMHS. 2015;9:1051–4.
130. Khalil A, Tazeddinova D, Aljoumaa K, Kazhmukhanbetkyzy ZA, Orazov A, Toshev AD. Carotenoids: therapeutic strategy in the battle against viral emerging diseases, COVID-19: an overview. Prev Nutr Food Sci. 2021;26:241–61. CrossRef
131. Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174:1290–324. https://doi.org/10.1111/bph.13625
132. Jiang T, Ghosh R, Charcosset C. Extraction, purification and applications of curcumin from plant materials-a comprehensive review. Trends Food SciTechnol. 2021;112:419–30. CrossRef
133. Pan Y, Ju R, Cao X, Pei H, Zheng T, Wang W. Optimization extraction and purification of biological activity curcumin from Curcuma longa L by high-performance counter-current chromatography. J Sep Sci. 2020;43:1586–92. CrossRef
134. Hua Y, Xu N, Ma T, Liu Y, Xu H, Lu Y. Anti-inflammatory effect of lycopene on experimental spinal cord ischemia injury via Cyclooxygenase-2 Suppression. Neuroimmunomodulation. 2019;26:84–92. CrossRef
135. Mustra Rakic J, Liu C, Veeramachaneni S, Wu D, Paul L, Chen CYO, et al. Lycopene inhibits smoke-induced chronic obstructive pulmonary disease and lung carcinogenesis by modulating reverse cholesterol transport in ferrets. Cancer Prev Res (Phila) 2019;12:421–32. CrossRef
136. Li WW, Wang TY, Cao B, Liu B, Rong YM, Wang JJ, et al. Synergistic protection of matrine and lycopene against lipopolysaccharideinduced acute lung injury in mice. Mol Med Rep. 2019;20:455–62. CrossRef
137. Choksi PM, Joshi VY. A Review on lycopene—extraction, purification, stability and applications. Int J Food Prop. 2007;10:289–98. CrossRef
138. Gómez-Prieto MS, Caja MM, Herraiz M, Santa-María G. Supercritical fluid extraction of all-trans-lycopene from tomato. J Agric Food Chem. 2003;51:3–7. CrossRef
139. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod Process. 2019;117:321–8. CrossRef
140. Development of a tomato pomace biorefinery based on a CO2-supercritical extraction process for the production of a high value lycopene product, bioenergy and digestate. J Clean Prod. 2020;243:118650. CrossRef
141. Butel MJ. Probiotics, gut microbiota and health. Méd Mal Infect. 2014;44:1–8. CrossRef
142. NCCIH. Probiotics: what you need to know. NCCIH 2019. [cited 2023 December 22]. Available from: https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know
143. International Probiotics Association. Probiotic manufacturing guidelines. Vol. 1, The Global Voice of Probiotics; 2019.
144. Duboux S, Van Wijchen M, Kleerebezem M. The possible link between manufacturing and probiotic efficacy; a molecular point of view on Bifidobacterium. Front Microbiol. 2021;12:812536. CrossRef
145. Yahfoufi N, Mallet J, Graham E, Matar C. Role of probiotics and prebiotics in immunomodulation. Curr Opin Food Sci. 2018;20:82–91. CrossRef
146. Thoda C, Touraki M. Immunomodulatory properties of probiotics and their derived bioactive compounds. Appl Sci. 2023;13:4726. CrossRef
147. Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K, et al. A comprehensive review on nutraceuticals: therapy support and formulation challenges. Nutrients. 2022;14:4637. CrossRef