INTRODUCTION
Parkinson’s disease (PD), a progressive neurodegenerative disorder affecting the central nervous system, presents a significant burden both for individuals and society at large. This ailment, characterized by a multitude of motor and non-motor symptoms, ranks as the second most prevalent age-related degenerative brain condition and stands as the primary motor-related brain disorder [1]. A hallmark of this disease is the gradual depletion of dopamine-producing cells situated in the substantianigra, a critical brain region responsible for motor control. Despite extensive research, the precise origin of PD remains elusive [2]. At present, it is widely acknowledged that a complex interplay of genetic and environmental factors contributes to the onset and progression of the ailment. The global impact of PD is profound, with millions of individuals grappling with its consequences [3]. According to various sources, the prevalence of this condition varies significantly, contingent on age and geographical location. Remarkably, the incidence of PD rises with advancing age, with the highest rates observed in individuals aged 60 and older [1,3].
The diagnosis of PD predominantly relies on clinical symptoms and neurological examinations, as definitive diagnostic tests do not exist. Imaging modalities, such as MRI and CT scans, are occasionally employed to rule out other conditions that may present with similar symptoms. Nonetheless, the diagnostic process can be intricate due to symptom overlap with other disorders necessitating distinct treatments [4]. While a cure for PD remains elusive, current therapeutic strategies concentrate on symptom management to enhance the quality of life for affected individuals [3]. Medications that augment dopamine levels in the brain provide relief from characteristic symptoms such as tremors, rigidity, and bradykinesia [5]. Complementary therapies, encompassing physical, occupational, and speech therapy, further contribute to symptom alleviation. However, the efficacy of existing therapies is constrained by noteworthy limitations. Medications designed to boost dopamine levels can induce undesirable side effects, including nausea, dizziness, and hallucinations. Over time, their effectiveness may diminish, and some patients may develop motor complications, such as dyskinesias and dystonia [5].
The molecular underpinnings of PD unveil a multifaceted landscape. While the exact causative factors remain uncertain, a convergence of genetic and environmental influences precipitates the disease [3]. Oxidative stress, mitochondrial dysfunction, and inflammation have emerged as pivotal contributors to its pathogenesis. On a molecular scale, the accumulation of misfolded proteins, notably alpha-synuclein, in the brain culminates in the demise of dopamine-producing neurons within the substantianigra. Recent investigations have illuminated a potentially pivotal player in the progression of PD—the brain renin-angiotensin system (RAS). Specifically, the Mas receptor (MasR), an integral component of the RAS, has exhibited neuroprotective attributes in animal models of PD. The RAS system plays a vital role in regulating bodily functions, including electrolyte balance, fluid volumes, and cardiovascular activity [6]. It begins with renin, an enzyme produced in the kidneys, which acts on angiotensinogen (AGT) from the liver to generate angiotensin I (Ang I). Angiotensin-converting enzyme (ACE) is another crucial RAS component, converting Ang I into active angiotensin II (Ang II). While necessary for physiological balance, chronic RAS activation and elevated Ang II levels can have detrimental effects, including inflammation, vasoconstriction, fibrosis, and altered sodium absorption [7]. Beyond the well-established systemic RAS, recent research reveals localized RAS in various tissues, yet our understanding of brain RAS remains limited. Some studies have identified RAS components in the brain, including angiotensin receptors (AT1R and AT2R), but much remains unexplored. Emerging research suggests a role for dysregulated brain RAS in neurodegenerative diseases like PD [8]. Neuroinflammation, oxidative stress, and age-related changes in brain RAS have been linked to this condition. Activation of brain RAS can impact pathological processes, potentially harming neurons. Elevated Ang II levels correlate with cognitive decline, while ACE inhibitors (ACEIs) improve cognition independently of blood pressure. High Ang II levels contribute to oxidative stress and neuroinflammation, while angiotensin receptor blockers (ARBs) mitigate PD risk factors and protect neurons [9]. Nevertheless, the exact roles of the brain RAS and MasR in the disease remain enigmatic, necessitating further exploration and comprehension [6].
Flavonoids, a category of polyphenolic compounds derived from plants, have exhibited notable neuroprotective properties in PD [10,11]. Experimental studies have revealed that the administration of flavonoids or foods rich in flavonoids safeguards dopamine neurons from oxidative damage and mitigates brain inflammation [11]. For example, quercetin, present in various fruits and vegetables, offers a multitude of health benefits, including antioxidative, anti-inflammatory, and neuroprotective effects [12]. Recent investigations propose that flavonoids may also diminish mortality risk in individuals with PD. The mechanism through which flavonoids act in PD involves the activation of endogenous antioxidant enzymes, the suppression of lipid peroxidation, and the inhibition of inflammatory processes [13]. Furthermore, flavonoids exhibit the capacity to enhance neuronal function and promote neurite outgrowth in human dopaminergic neurons [14]. Recent research has highlighted the therapeutic potential of flavonoids concerning the brain’s RAS and the MasR in the context of PD. It has become evident that the brain RAS and MasR contribute to the pathogenesis of PD, and emerging studies indicate that MasR may possess neuroprotective attributes in animal models of PD [14].
While the therapeutic potential of flavonoids in the context of the brain RAS and MasR in PD remains a subject of limited investigation, existing evidence underscores the plausible involvement of these plant-derived secondary metabolites in PD management. Given the paucity of effective therapies for PD, it is imperative to explore additional treatment avenues. Hence, this review aims to elucidate the mechanistic role of RAS and MasR in the pathogenesis of PD and strives to delineate the therapeutic efficacy of flavonoids based on available experimental data.
THE RAS SYSTEM IN PD
The cerebral RAS functions autonomously, encompassing a spectrum of physiological functions and disorders, with PD being a prominent example [15,16]. Comprising critical constituents, this system orchestrates the regulation of blood pressure, fluid homeostasis, and inflammatory processes. Delving into the intricate interactions among these elements in the context of PD assumes paramount significance, as it promises to yield invaluable insights into the underlying mechanisms governing the disease’s progression, thus fostering optimism for enhanced strategies in disease management and treatment [17,18].
THE COMPONENTS OF THE RAS SYSTEM
Brain renin/Pro-renin
Renin, an enzyme tasked with the cleavage of AGT to generate Ang I, functioning as a precursor to various angiotensin peptides, has recently garnered attention for its presence within the brain, albeit in limited quantities. Intriguingly, there is notable co-expression of renin and AGT in diverse brain regions, implying their potential involvement in the pathophysiology of PD [19]. Additionally, it is noteworthy that renin can exist in the brain in its pro-renin form, exhibiting a heightened affinity for pro-renin receptors compared to renin itself. This binding event can activate angiotensin receptors, potentially contributing to cognitive impairment observed in PD [20].
Brain angiotensin-converting enzymes (ACE/ACE2)
The ACE, a pivotal component of the cerebral RAS, exhibits its primary localization in the cerebral vasculature and specific brain regions, including the choroid plexus, organum vasculosum of the lamina terminalis, subfornical organ, and area postrema. Intriguingly, ACE activity has been discerned even within brain areas fortified by the blood-brain barrier (BBB), underscoring its critical role in neurological processes [21]. In PD pathogenesis, ACE has emerged as a factor implicated in cognitive dysfunction. Clinical investigations have furnished evidence that ACEIs possess the capacity to reduce the incidence of dementia in PD patients. Beyond their established antihypertensive effects, ACEIs may exert neuroprotective properties by ameliorating inflammatory processes [22].
ACE2, another indispensable enzyme within the cerebral RAS, boasts widespread expression throughout the brain, particularly in regions governing central blood pressure regulation. ACE2’s enzymatic action involves the conversion of Ang II into Angiotensin (1–7) [Ang (1–7)], which in turn activates the MASR. This axis has garnered attention for its potential neuroprotective effects, counterbalancing the actions of Ang II [23]. The prospect of ACE2 gene therapy has been posited as a promising antihypertensive strategy, holding potential benefits for individuals afflicted with PD.
Brain angiotensinogen
The cerebral RAS stands as an autonomous and multifaceted regulatory system, assuming a pivotal role in the orchestration of diverse brain functions and disorders, most notably PD. Comprising several pivotal constituents, this intricate system meticulously governs parameters such as blood pressure, fluid equilibrium, and the inflammatory response within the cerebral domain [24]. Acquiring a comprehensive comprehension of the intricate interactions among these components within the PD context is of paramount importance. Such comprehension furnishes a unique perspective into the fundamental mechanisms steering the progression of this disease [21]. By scrutinizing the interplay of these elements, researchers endeavor to unearth innovative insights that hold the potential to pave the path towards more efficacious strategies for managing and treating PD, with the prospect of enhancing the quality of life for those grappling with this formidable neurodegenerative ailment [25]. The components of the brain RAS system and their relevance in PD are listed in Table 1.
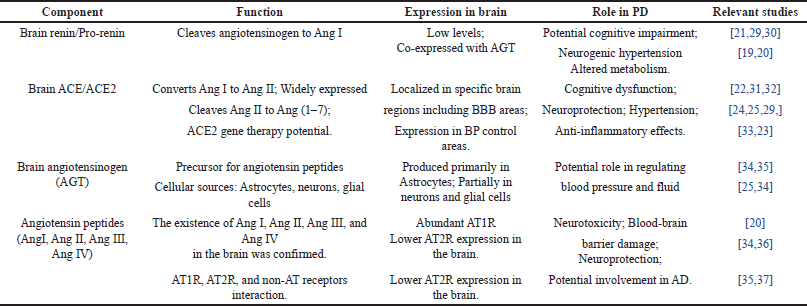 | Table 1. Components of the brain RAS system and their implications in PD. [Click here to view] |
Angiotensin peptides and receptors in PD
Angiotensin peptides, encompassing Ang I and Ang II, have been unequivocally identified within the cerebral milieu, alongside their corresponding receptors, notably AT1R and AT2R, which also find residence in the brain [20]. AT1R manifests abundant presence in the brain and is associated with the detrimental effects attributed to Ang II, whereas AT2R counteracts these actions. The equilibrium between these receptors assumes paramount significance in the preservation of cerebral well-being. In PD, Ang II signaling through AT1R has been implicated in neurotoxicity and damage to the BBB [26]. Conversely, Ang II’s interaction with the AT2R axis has been linked to cerebral growth and the sprouting of neurons, hinting at potential neuroprotective benefits [27]. It is noteworthy that Ang II can undergo conversion to Angiotensin III (Ang III), a significant player in blood pressure regulation. Ang III, in turn, can give rise to Angiotensin IV (Ang IV), which exerts its influence through angiotensin receptor type-4 and holds the promise of conferring protection against neurodegenerative conditions such as PD [28].
The role of angiotensin components in the PD pathogenesis
The RAS revolves around AGT, a precursor protein that gives rise to essential angiotensin peptides. Among these peptides, Ang-II holds prominence, predominantly binding to the AT1 receptor [38]. This interaction has been associated with cognitive dysfunction and neuroinflammation, significant factors in PD. Activation of the AT1 receptor leads to sustained blood pressure elevation, oxidative stress, and neuroinflammation, thereby exacerbating neurodegeneration in PD [39,40]. Within the RAS framework, the AT2 receptor subtype assumes a strikingly different role compared to AT1 in terms of its actions. While AT1 is implicated in neurodegeneration, AT2 appears to possess neuroprotective properties [18,41]. AT2 receptors oversee critical processes such as cell proliferation, differentiation, apoptosis, and regeneration. The intricate interplay between AT1 and AT2 receptors may have implications for neuroprotection in PD, though further research is necessary to elucidate these intricate interactions [42]. Another intriguing aspect of the RAS involves Angiotensin IV (AngIV) and its association with the insulin-regulated aminopeptidase (IRAP) enzyme. AngIV has displayed the potential to enhance memory and cognitive functions, primarily by enhancing various pro-cognitive endogenous peptides. Inhibitors of IRAP, such as LVV-haemorphin7, have shown promise in improving memory and cognitive function in preclinical models [43,44]. These findings suggest that targeting the AngIV /IRAP system could hold therapeutic promise in PD by ameliorating cognitive impairments, which constitute a significant burden in this disease [45]. The Angiotensin (1–7)/ MasR system presents a counterbalancing mechanism to the actions of AngII. This system can counteract inflammation and fibrosis while enhancing glucose utilization and insulin sensitivity. The presence of MasRs in brain regions associated with memory and cognition underscores its relevance to neurodegenerative diseases like PD. Ang (1–7) has been demonstrated to facilitate long-term potentiation in the hippocampus, a crucial process in memory formation. Additionally, this peptide may play a role in protecting against thrombosis, which can impact cerebral perfusion and potentially offer neuroprotection in PD [46].
Evidence for Ras in PD
Excessive oxidative stress is increasingly recognized as a pivotal factor in the pathogenesis of various neurodegenerative disorders, and PD is no exception. The intricate neurobiology has brought the RAS within the brain into focus, revealing its significant role in exacerbating oxidative stress and contributing to the relentless progression of PD. As we investigate the multifaceted mechanisms governing RAS in PD, we acquire valuable insights into oxidative stress, neuroinflammation, apoptosis, neurotrophic factors (NFs), and clinical observations, all contributing to a comprehensive understanding of this intricate interplay in the context of PD.
Oxidative stress
Oxidative stress occupies a central position in the realm of neurodegeneration, and its involvement in PD is particularly notable [47,48]. Within this context, the brain’s RAS with a specific focus on AngII, emerges as a pivotal player in the initiation of oxidative stress. Ang II, through the activation of AT1 receptors, serves as a catalyst for the production of reactive oxygen species (ROS) by NADPH oxidase (NOX) enzymes, widely distributed throughout the brain [47]. This cascade results in an elevated generation of superoxide radicals, which pose a significant threat to dopaminergic neurons [16]. In addition to this, Ang II instigates the NF-kB signaling pathway, further intensifying neuronal vulnerability. Moreover, Ang II exerts an influence on neuronal N-methyl-D-aspartate currents, amplifying ROS production through NOX-2 [49]. The superoxide generated by NOX initiates cascades involving p38 mitogen-activated protein kinase (MAPK) in microglial cells, thereby fostering a state of neuroinflammation. Furthermore, Ang II’s impact extends to nitric oxide (NO) production through AT2 receptor subtypes [16]. This intricate interplay between Ang II and oxidative stress collectively contributes to neuronal damage and cell death, marking a crucial aspect of PD pathogenesis.
Neuroinflammation
Neuroinflammation stands as a pivotal facet of PD pathogenesis, and its dynamics are significantly influenced by the RAS. In the context of disease states, the integrity of the BBB may be compromised, permitting circulatory RAS components to infiltrate the brain [50]. Once within the brain, the local RAS becomes a driver of chronic inflammation, initiating the release of ROS and inflammatory mediators of particular significance is the role of glial cells, which, when activated by RAS, propagate these inflammatory processes, thereby fostering cognitive dysfunction and contributing to neurodegeneration [51,52]. Research has established links between astrocyte-derived Ang II and neuroinflammation, along with neuronal dysfunction. Notably, treatments involving Ang II have been demonstrated to activate microglial pathways, leading to heightened release of proinflammatory cytokines, a phenomenon that significantly contributes to dopaminergic neurodegenerations [53]. Emerging evidence suggests that inhibitors of the RAS, such as ACEIs and ARBs, possess inherent anti-inflammatory properties [54]. These properties translate into a reduction in neurodegenerative processes and an improvement in cognitive function within PD models. Such findings shed light on the potential therapeutic value of targeting the RAS to mitigate neuroinflammation and its detrimental consequences in the context of PD.
Apoptosis
Apoptosis, a meticulously orchestrated process of programmed cell death, emerges as a significant player in the pathophysiology of PD [55,56]. Within this framework, Ang II, acting primarily via AT1 receptors, takes on a central role as a major contributor to this process. Ang II’s involvement in PD pathogenesis is multifaceted. It initiates oxidative stress and induces the generation of mitochondrial ROS, consequently upregulating pro-apoptotic pathways. Research studies have unveiled that Ang II stimulation leads to the activation of caspase-3, a pivotal step in the cascade of events culminating in dopaminergic neuronal death. [56–58] Furthermore, Ang II exerts influence over autophagy and mitochondrial-mediated cell death pathways, exacerbating the loss of neurons. It is worth noting that the activation of the Ang II/AT1 receptor axis has also been associated with apoptotic signaling cascades involving MAPKs, adding yet another layer of complexity to Ang II’s role in promoting neuronal demise in PD [58,59]. These multifaceted apoptotic mechanisms underscore the significance of Ang II in the context of neuronal cell loss observed in PD.
Neurotrophic factors
NFs play a pivotal role in central nervous system functions, and their dysregulated regulation is closely associated with neurodegenerative diseases, including PD [60,61]. The RAS interfaces with NFs, opening up a promising avenue for the modulation of brain function. Of particular significance is the role of Ang II in regulating the neurotrophin-3 and brain-derived neurotrophic factor cascades, exerting a direct influence on neuronal health [62–65]. A deeper comprehension of this intricate interaction has the potential to unveil novel strategies for enhancing cognitive functions, thereby offering fresh insights into the management of neurodegenerative disorders like PD.
Clinical and pre-clinical insights into ras involvement in PD
In the context of PD, the degeneration of dopaminergic neurons is a prominent feature, often accompanied by oxidative stress, neuroinflammation, and mitochondrial dysfunction. Recent findings have shed light on the involvement of the brain’s RAS in these intricate processes (Table 2). Elevated levels of Ang II have been detected in the cerebrospinal fluid and brain tissues of both PD patients and animal models [66,67]. Notably, glial cells, encompassing microglia and astrocytes, are the primary source of Ang II within the brain. The over-activation of Ang II through its interaction with the AT1 receptor significantly contributes to the progression of PD [9]. The influence of Ang II extends beyond neuroinflammation, impacting mitochondrial function and elevating oxidative stress levels within the brain. The multifaceted interplay of Ang II, mediated by its receptors, presents a promising arena for potential therapeutic intervention aimed at mitigating the loss of dopaminergic neurons and ameliorating the symptoms of PD [57]. Research studies indicate that the blockade of Ang II receptors, particularly AT1, holds the potential to confer neuroprotective effects, thereby reducing the risk of PD. This growing body of evidence underscores the pivotal role of the RAS in the pathogenesis of PD, opening up innovative avenues for the development of treatments targeting this system.
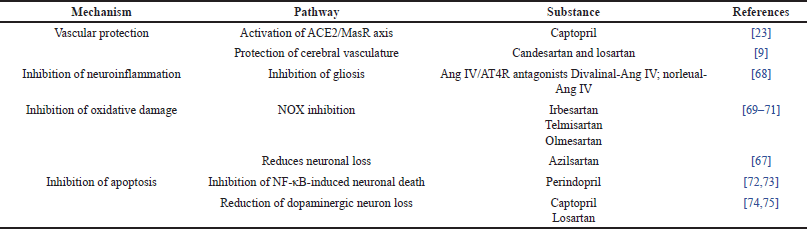 | Table 2. The protective effects of RAS modulators in PD. [Click here to view] |
Natural compounds as aceis
Natural compounds have become a focal point of scientific investigation due to their potential as effective ACEIs [76–80]. This interest is particularly pertinent in PD, as ACEIs have demonstrated promise in managing various aspects of the condition. ACEIs are renowned for their role in regulating the RAS, a complex cascade of enzymatic reactions crucial for controlling blood pressure and fluid balance [81]. These inhibitors comprise peptides, phenolic compounds, and terpenoids found in various biometabolites and plant extracts. The mechanism by which peptides interact with ACE closely resembles that of established ACEIs, rendering them potential candidates for managing conditions like PD [81].
Plant-derived phenolic compounds have emerged as prominent subjects of study in the realm of ACE inhibition [82]. These compounds naturally occur in various plant-based foods, rendering them accessible and potentially beneficial for individuals with PD. Phenolic compounds and terpenoids, on the other hand, exert their ACEIs effects through intricate processes [83]. Specifically, compounds like o-carboxyl cinnamic acids form ionic interactions with divalent zinc ions within the ACE enzyme and establish hydrogen bonds with specific residues. These interactions collectively contribute to ACE inhibition, a focal point of attention in the context of neurodegenerative diseases such as PD [84]. In the pathogenesis of PD, where neuroinflammation and oxidative stress are prevalent, the potential neuroprotective effects of terpenoids hold particular interest [85].
Beyond terrestrial sources, marine organisms have also been explored as potential sources of ACEIs. These marine-derived ACEIs exhibit a mixed-type binding with both the active and non-active sites of the ACE enzyme. This unique mode of interaction induces conformational changes in ACE, resulting in decreased enzymatic activity [86]. However, much research is still needed to fully elucidate the mechanisms behind these marine-derived ACEIs. ACE assumes a pivotal role in the RAS by converting angiotensin I into angiotensin II. The equilibrium of this system carries significant implications for neural function and neuroinflammation. ACEIs, by reducing the production of angiotensin II, have the potential to favorably this balance, thereby contributing to neuroprotection in PD. Research into the bioactive compounds found in these foods and their ACE inhibitory properties holds the potential to yield valuable insights, contributing to the development of natural remedies for managing hypertension, oxidative stress, and neuroinflammation in the context of PD research.
The role of flavonoids in modulatingras system
Flavonoids, a class of polyphenolic compounds, have garnered significant attention due to their potential as ACEIs. These compounds are characterized by a 2-phenyl benzopyrone configuration, typically forming a cyclized oxygen bridge [87,88]. In plants, flavonoids serve multiple functions, acting as protective agents against external factors like UV radiation, parasites, and viruses. Additionally, they regulate enzymes involved in cell metabolism and possess antioxidant properties [88]. The fundamental structure of flavonoids comprises two phenyl rings (A and B rings) linked by a three-carbon chain, which forms a heterocyclic ring (C ring) that closes with benzene ring A. Flavonoids can be categorized into different classes based on the oxidation level of the C ring, including flavanols and anthocyanins (if the C ring is a pyran), or flavonols, flavones, and flavanones (if it is a pyrone). These subclasses can further be distinguished based on the carbon of the C ring to which the B ring is attached [89]. Structural diversity among flavonoids arises from various substitutions, including glycosylation, hydrogenation, hydroxylation, malonylation, methylation, and sulfation. These substitutions, in conjunction with conjugate on patterns, glycosylation, or methylation, contribute to the diverse biological properties and hydrophilicity of flavonoids [90] (Table 3).
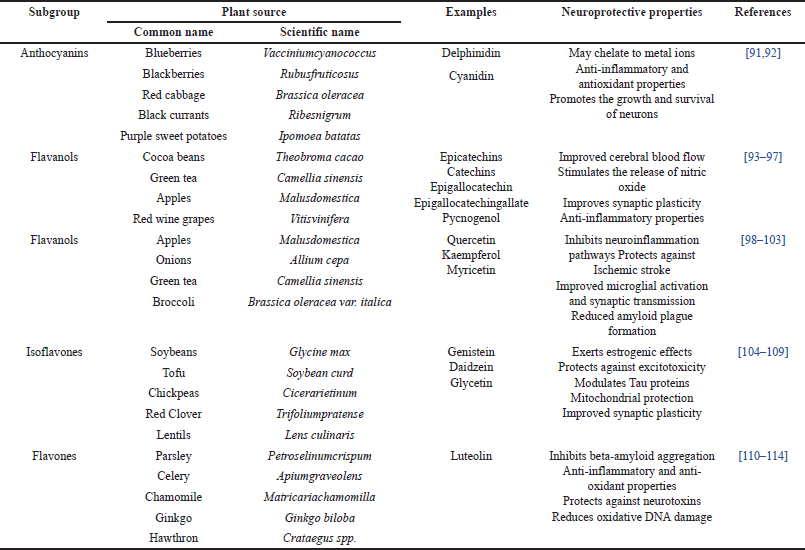 | Table 3. The Neuroprotective properties and natural sources of flavonoids. [Click here to view] |
Significantly, flavonoids have drawn attention for their potential health benefits in humans, including reducing the risk of cardiovascular disease, various cancers, and neurodegenerative conditions [90]. For example, quercetin-3-O-glucoside, a ubiquitous flavonoid compound found in fruits, has demonstrated neuroprotective effects by up-regulating genes involved in lipid and cholesterol synthesis, offering protection against oxidative stress [115]. Furthermore, research has explored the potential of flavonoids as ACEIs to regulate blood pressure, with many flavonoids proving effective in suppressing ACE activity, while specific sub-groups exhibit varying degrees of efficacy in inhibiting ACE.
Anthocyanins
Anthocyanins, a class of plant compounds, are increasingly intriguing in the realm of health and nutrition, particularly concerning PD. These compounds stem from anthocyanidins, their aglycone form, and transform into anthocyanins upon conjugation with sugars [91]. Recognized for their vibrant pigments, which bestow red, blue, and purple hues upon a variety of fruits and vegetables, anthocyanins have drawn attention to their potential in inhibiting ACE [92]. Studies have illuminated specific anthocyanins possessing ACE inhibitory properties. Notably, delphinidin-3-O-sambubiosides and cyanidin-3-O-sambubiosides, derived from Hibiscus extracts, exhibit dose-dependent ACE inhibition, with IC50 values ranging between 100 and 150 µM. Similarly, cyanidin-3-O-β-glucoside, extracted from rose species, demonstrates in vitro ACE inhibition, while other flavanols from rose extract do not exhibit the same efficacy [116]. Several researchers have explored the potential advantages of anthocyanin-rich extracts from sources such as bilberries, purple corn, purple sweet potatoes, and red radishes. These extracts have undergone investigation for their impact on ACE, and the results indicate that the dietary inclusion of anthocyanin-rich foods may contribute to lower systolic and mean blood pressure, a potentially favorable outcome for individuals dealing with conditions like PD [117].
The mechanisms underlying the blood pressure-lowering effects of anthocyanins are multifaceted, involving antioxidant activity, endothelial NO preservation, and serum lipid oxidation prevention [116,118]. However, it is imperative to acknowledge that while in vitro ACE inhibition is evident, the correlation between this inhibition and its effects in animal models remains intricate and not yet comprehensively elucidated. One hypothesis posits that the metal-chelating ability of flavonoids, particularly those featuring hydroxyl groups at specific positions, contributes to ACE inhibition. Additionally, the planar structure of anthocyanin molecules appears to hold significance in metallopeptidase inhibition. Significantly, anthocyanins have been associated with the downregulation of renin mRNA expression, a pivotal factor in blood pressure regulation. This downregulation may be attributed to their metal-chelating capabilities and their rigid, planar structure, which potentially plays a role in ACE inhibition.
Flavanols
Flavanols, often known as flavan-3-ols or catechins, represent a distinct subgroup within flavonoids. Their hallmark feature is the hydroxyl group positioned at the third carbon of the C ring, distinct from other flavonoids due to the absence of a double bond between the second and third carbons and the presence of a ketone group at the fourth position [88]. This unique chemical arrangement results in flavanols possessing two chiral centers at positions 2 and 3, yielding four potential diastereoisomers. Among these, catechin embodies the trans isomer, while epicatechin takes on the cis configuration. Further differentiations branch into two stereoisomers for each configuration, namely, (+)-catechin, (-)-catechin, (+)-epicatechin, and (-)-epicatechin [89].
Flavanols feature a saturated C-ring bearing a hydroxyl group at the C-3 position and are not typically found in glycosylated forms. They manifest both as individual molecules, commonly referred to as catechins, and as polymers known as procyanidins. Exploration into the relationship between flavanols and ACE inhibition has revealed intriguing connections [89]. Notably, when ACE encountered flavanol-rich sources like chocolate, tea, and wine, a conspicuous correlation emerged between ACE inhibition and the concentrations of procyanidin and epicatechin [94,96,97]. This finding suggests that ACE inhibition attributed to epicatechins, prominently present in cocoa, may elucidate the reported association between dark chocolate consumption and reduced blood pressure. Additionally, major catechins within tea, such as (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin gallate, and (-)-epigallocatechin gallate, have showcased dose-dependent ACE inhibition within human umbilical vein endothelial cell (HUVEC) culture models [90,94,94,96].
Procyanidins, including tetrameric variants like Pycnogenol isolated from French maritime pine, have also emerged as potential regulators of blood pressure, potentially acting through ACE inhibition. Research suggests that flavanols and procyanidins harbor the capability to serve as potent ACEIs in in-vitro settings. Investigations into the structural aspects of flavanols and their interplay with ACE inhibitory properties in vitro. Notably, the number of epicatechin units within procyanidins appears to exert influence over enzyme inhibition, with increased units correlating with heightened inhibition. Interestingly, while monomeric flavanols find absorption in the small intestine, the absorption patterns of higher molecular weight procyanidins remain less transparent [119]. In biological systems, dimers have proven more efficacious when juxtaposed with both tetramers and hexamers.
Flavonols
Flavonols, a specialized subgroup within the realm of flavonoids, boast distinct attributes that render them pertinent in the context of PD [89]. Characterized by a hydroxyl group at position 3 of the C ring, these compounds may also undergo glycosylation, with variations in their properties stemming from disparities in hydroxylation and methylation. Among flavonols, quercetin, kaempferol, and myricetin emerge as the most prevalent constituents in our dietary intake [98–103].
Quercetin, generously distributed across fruits and vegetables, including capers, has garnered attention owing to its antioxidant prowess. Extensive research underscores its capacity to reestablish mitochondrial complex activities and curtail pro-inflammatory markers within the framework of PD [100]. It has displayed remarkable efficacy in ameliorating both the motor and non-motor symptoms associated with PD. Kaempferol, abundant in the stem bark extracts of Cluster Fig, demonstrates in vitro ACE inhibition properties that are contingent upon dosage. Investigations utilizing rat aortic tissues suggest its competence as an ACE inhibitor [120]. An intriguing facet of kaempferol’s ACE inhibitory activity lies in its differentiation from resveratrol, a polyphenol abundantly found in red wine. This disparity may be attributed to the presence of a carbonyl group in kaempferol’s pyran ring. Significantly, the ACE inhibitory potential of flavonols can exhibit substantial variance among distinct plant extracts.
Isoflavones
Isoflavones, a distinct subgroup within the flavonoid family, exhibit a structural similarity to mammalian estrogen hormones, particularly estradiol, and are therefore classified as phytoestrogens [106,107]. These compounds possess the remarkable ability to bind effectively to estrogen receptors, earning them the moniker “phytoestrogens.” Among the prevalent isoflavones found in plants, genistein, daidzein, and glycetin hold prominence. Notably, genistein, the primary isoflavone in soybeans, has garnered significant attention for its potential health benefits. Scientific inquiry has demonstrated the influence of genistein on blood pressure regulation, a pivotal consideration in conditions like PD [104,107]. Experimental evidence, primarily in animal models, has indicated that genistein holds promise in blood pressure reduction. Proposed mechanisms have suggested that genistein’s effects are mediated through estrogen receptors, involving the activation of the ERK1/2 signaling pathway. Rigorous in vitro experimentation has consistently showcased concentration-dependent ACE inhibition by genistein. Interestingly, when isoflavones coexisted with ACE-inhibitory soybean peptide fractions, they did not amplify the inhibitory effect on the enzyme compared to peptide fractions devoid of isoflavones [121]. In hypertensive Wistar rats, pretreatment with a single intravenous injection of genistein at a dose of 25 mg/kg yielded reduced hypertensive responses, accompanied by a significant reduction in ACE activity within rat plasma [122]. While genistein has been the focus of extensive research, exploration of the ACE inhibitory effects of two other soybean isoflavones, daidzein, and glycetin, remains relatively limited.
Flavones
Flavones represent a subgroup within the broader category of flavonoids, and they possess distinct structural features [110]. Notably, they feature a double bond between positions 2 and 3 of their chemical structure and a ketone group at position 4 of the C ring. Additionally, a hydroxyl group resides at position 5 of the A ring. Variations within flavones primarily occur at positions 7 of the A ring and positions 3’ and 4’ of the B ring. The glycosylation of flavones tends to occur at positions 5 and 7, while methylation and acylation typically affect the hydroxyl groups within the B ring [111].
While the available information regarding the ACE inhibitory properties of flavones is relatively limited compared to other flavonoid subclasses, some studies have presented promising findings [110,112,112,113]. For example, extracts derived from plants such as Roxb (Ailanthus excelsa) [123], Japanese cedar (Cryptomeria japonica) [124], Hibiscus sabdariffa, [125] and Senecio species [126], which contain flavones, have demonstrated ACE inhibitory properties. Notably, Roxb, recognized for its two major flavones, apigenin, and luteolin, exhibited a dose-dependent inhibition of the ACE enzyme. Interestingly, luteolin-7-O-glucoside exhibited reduced enzyme activity in comparison to luteolin aglycone, potentially attributed to the absence of the hydroxyl group at the 7th position [123].
Japanese cedar, particularly its outer bark ethanol extract rich in flavan-3-ols and flavones, displayed ACE inhibition in vitro, with an IC50 value of 16 µg/ml. This effect is likely due to a synergistic interaction among various compounds present in the extract [127]. Similarly, a hydroalcoholic extract from H. sabdariffa, which was enriched in flavones, exhibited significant ACE inhibition. However, it is noteworthy that this extract did not affect enzymes like elastase, trypsin, and alpha-chymotrypsin [128]. It is important to recognize that these studies primarily investigated the effects of plant extracts containing flavones, leaving open the possibility that other constituents within these extracts contributed to the observed inhibitory effects.
Pharmacological attributes of flavonoid compounds
Metabolism of flavonoids
A comprehensive understanding of flavonoid metabolism is essential when contemplating their potential therapeutic applications, especially concerning diseases such as PD. Flavonoids, commonly present in dietary sources, typically exist as glycosides. Upon ingestion, these glycosidic flavonoids undergo a vital process called deglycosylation in both the small and large intestines [129].
This initial step hinges on the action of an enzyme known as lactase-phlorizin hydrolase (LPH). LPH is responsible for catalyzing the hydrolysis of flavonoid glycosides, such as quercetin 3-O-glucoside and quercetin 4’-O-glucoside, thereby converting them into their respective aglycon forms [130]. Subsequently, these aglycons are absorbed into the intestinal cells through a sodium-glucose co-transporter called SGLT1. Once within the intestinal cells, the aglycons undergo further metabolic transformations facilitated by phase II enzymes [129]. These enzymes encompass uridine-5’-diphosphate-glucuronosyltransferases, sulfotransferases, and catechol-O-methyltransferases. Their role is to convert the aglycons into diverse conjugated metabolites. Following intestinal conjugation, additional conjugation processes, including sulfation and methylation, transpire in the liver. These processes yield various chemical forms of flavonoids that circulate in the bloodstream and eventually get excreted in the urine [131]. It is worth noting that the gut microbiota also participates in the deconjugation of these metabolites, potentially allowing for their reabsorption. The transport of these absorbed flavonoids throughout the body primarily occurs via the lymphatic system. In the bloodstream and various tissues, flavonoids are often encountered in the form of conjugated metabolites, which have been reported to exhibit lower activity compared to their aglycon counterparts [130]. The equilibrium between conjugation and deconjugation processes substantially influences the functional properties of flavonoid metabolites.
Neuronal access of flavonoids
The accessibility of flavonoids to neuronal tissues, particularly in the context of neurodegenerative disorders like PD, is a subject of active investigation. Current understanding is limited, and this knowledge gap poses challenges for the development of flavonoid-based therapies. Epidemiological studies suggest that a daily intake of dietary flavonoids can offer benefits for individuals with neurodegenerative disorders. To bridge this gap in understanding and harness the potential of flavonoids, numerous studies are underway to explore their ability to access neuronal tissues. Research conducted on a human brain endothelial cell line model has yielded valuable insights. Among three tested flavonoids—quercetin, epigallocatechin gallate (EGCG), and cyanidin-3-glucoside (C3G)—EGCG demonstrated a more rapid ability to cross the BBB compared to C3G. Notably, quercetin, on its own, was unable to traverse the BBB. Additionally, an investigation involving eighteen 3-month-old male Sprague-Dawley rats revealed that quercetin could access the BBB when administered alongside α-tocopherol (Vitamin E). These findings hold promise for the potential use of flavonoids in addressing neuronal disorders like PD [132]. The ability of certain flavonoids to cross the BBB means they can reach sites of neuronal damage and potentially exert therapeutic effects. However, it is important to note that while flavonoids show potential, the mechanisms and factors influencing their BBB permeability require further research. Understanding how flavonoids interact with the BBB and their precise impact on neuronal health is essential for developing effective therapies for neurodegenerative conditions.
CONCLUSION
In the pursuit of unraveling the intricate facets of PD pathogenesis and identifying potential therapeutic avenues, the RAS emerges as a compelling player. One key insight gleaned from this exploration is the pivotal role of RAS in oxidative stress, a central player in PD pathophysiology. The activation of Ang II and its receptors within the brain initiates the production of ROS and subsequent oxidative damage, particularly detrimental to dopaminergic neurons. Moreover, Ang II’s impact on neuroinflammation, apoptosis, and NFs further contributes to neuronal vulnerability. Clinical and pre-clinical evidence underscores the significance of RAS modulators, including ACEIs and ARBs, in potentially mitigating neurodegeneration in PD. These findings offer promise for the development of novel treatments that target the RAS to protect dopaminergic neurons and ameliorate PD symptoms.
This review has highlighted the potential of natural compounds, specifically flavonoids, as ACEIs. Anthocyanins, flavanols, flavonols, and isoflavones, subclasses of flavonoids, have demonstrated ACE inhibitory properties, and their consumption through dietary sources could hold potential for individuals with PD. Looking forward, future research endeavors should center on elucidating the precise mechanisms of flavonoid metabolism and their ability to access neuronal tissue. This understanding will facilitate the development of targeted therapies harnessing the potential of flavonoids to protect against neurodegenerative conditions like PD. Additionally, exploring the synergistic effects of flavonoids with other neuroprotective agents may unveil novel and more potent treatment strategies for the future. By amalgamating scientific insights with clinical innovation, we can aspire to enhance the quality of life for PD patients and potentially pave the way toward a future where this debilitating disorder is better managed and ultimately conquered.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health. 2021;9:776847.
2. Klemann CJHM, Martens GJM, Sharma M, Martens MB, Isacson O, Gasser T, et al. Integrated molecular landscape of Parkinson’s disease. Parkinsons Dis. 2017;3(1):14.
3. Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612.
4. Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808.
5. Alves Da Rocha PA, Mcclelland J, Morris ME. Complementary physical therapies for movement disorders in Parkinson’s disease: a systematic review. Eur J PhysRehabil Med. 2015;51(6):693–704.
6. Perez-Lloret S, Otero-Losada M, Toblli JE, Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert OpinInvestig Drugs. 2017;26(10):1163–73.
7. Yang J, Gao Y, Duan Q, Qiu Y, Feng S, Zhan C, et al. Renin-angiotensin system blockers affect cognitive decline in Parkinson’s disease: the PPMI dataset. Parkinsonism RelatDisord. 2022;105:90–5.
8. Goldstein B, Speth RC, Trivedi M. Renin-angiotensin system gene expression and neurodegenerative diseases. J Renin Angiotensin Aldosterone Syst. 2016;17(3):1470320316666750.
9. Farag E, Sessler DI, Ebrahim Z, Kurz A, Morgan J, Ahuja S, et al. The renin angiotensin system and the brain: new developments. J ClinNeurosci. 2017;46:1–8.
10. Jung UJ, Kim SR. Beneficial effects of flavonoids against Parkinson’s disease. J Med Food. 2018;21(5):421–32.
11. Magalingam KB, Radhakrishnan AK, Haleagrahara N. Protective mechanisms of flavonoids in Parkinson’s disease. Oxid Med Cell Longev. 2015;2015:314560.
12. Tamtaji OR, Hadinezhad T, Fallah M, Shahmirzadi AR, Taghizadeh M, Behnam M, et al. The therapeutic potential of quercetin in Parkinson’s disease: insights into its molecular and cellular regulation. Curr Drug Targets. 2020;21(5):509–18.
13. Zhang X, Molsberry SA, Yeh TS, Cassidy A, Schwarzschild MA, Ascherio A, et al. Intake of flavonoids and flavonoid-rich foods and mortality risk among individuals with Parkinson disease: a prospective cohort study. Neurology. 2022;98(10):e1064–76.
14. Bellavite P. Neuroprotective potentials of flavonoids: experimental studies and mechanisms of action. Antioxidants (Basel). 2023;12(2):280.
15. Prusty SK, Sahu PK, Subudhi BB. Angiotensin mediated oxidative stress and neuroprotective potential of antioxidants and AT1 receptor blockers. Mini Rev Med Chem. 2017;17(6):518–28.
16. Labandeira Garcia JL, Rodríguez Perez AI, Garrido Gil P, Rodriguez Pallares J, Lanciego JL, Guerra MJ. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129.
17. Shinohara K, Liu X, Morgan DA, Davis DR, Sequeira-Lopez MLS, Cassell MD, et al. Selective deletion of the brain-specific isoform of renin causes neurogenic hypertension. Hypertension. 2016;68(6):1385–92.
18. Wright JW, Kawas LH, Harding JW. A role for the brain RAS in Alzheimer’s and Parkinson’s diseases. Front Endocrinol (Lausanne). 2013;4:158.
19. van Thiel BS, Góes Martini A, TeRiet L, Severs D, Uijl E, Garrelds IM, et al. Brain renin-angiotensin system: does it exist?: does? Hypertension. 2017;69(6):1136–44.
20. Nakagawa P, Sigmund CD. How is the brain renin-angiotensin system regulated? Hypertension. 2017;70(1):10–8.
21. Bodiga VL, Bodiga S. Renin angiotensin system in cognitive function and dementia. Asian J Neurosci. 2013;2013:1–18.
22. Rygiel K. Can angiotensin-converting enzyme inhibitors impact cognitive decline in early stages of Alzheimer’s disease? an overview of research evidence in the elderly patient population. J Postgrad Med. 2016;62(4):242–8.
23. Jiang T, Gao L, Lu J, Zhang YD. ACE2-Ang-(1-7)-Mas Axis in Brain: a potential target for prevention and treatment of ischemic stroke. CurrNeuropharmacol. 2013;11:209–17. https://doi.org/10.2174/1570159X11311020007.
24. Agassandian K, Grobe JL, Liu X, Agassandian M, Thompson AP, Sigmund CD, et al. Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice. Am J Physiol Regul Integr Comp Physiol. 2017;312:R973–81. doi: https://doi.org/10.1152/ajpregu.00511.2016
25. Uijl E, Ren L, Danser AHJ. Angiotensin generation in the brain: a re-evaluation. Clin Sci (Lond.). 2018;132(8):839–50.
26. Mascolo A, Sessa M, Scavone C, De Angelis A, Vitale C, Berrino L, et al. New and old roles of the peripheral and brain renin–angiotensin–aldosterone system (RAAS): focus on cardiovascular and neurological diseases. Int J Cardiol. 2017;227:734–742.
27. Miners JS, van Helmond Z, Raiker M, Love S, Kehoe PG. ACE variants and association with brain Aβ levels in Alzheimer’s disease. Am J Transl Res. 2010;3(1):73–80.
28. Su G, Dou H, Zhao L, Wang H, Liu G, Huang B, et al. The angiotensin-converting enzyme (ACE) I/D polymorphism in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst. 2015;16(2):428–33.
29. Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda). 2008;23(4):187–93.
30. Shinohara K, Nakagawa P, Gomez J, Morgan DA, Littlejohn NK, Folchert MD, et al. Selective deletion of renin-b in the brain alters drinking and metabolism. Hypertension. 2017;70(5):990–7.
31. Ohishi M, Yamamoto K, Rakugi H. Angiotensin (1–7) and other angiotensin peptides. Curr Pharm Des. 2013;19(17):3060–4.
32. Kamel AS, Abdelkader NF, Abd El-Rahman SS, Emara M, Zaki HF, Khattab MM. Stimulation of ACE2/ANG (1–7)/mas axis by diminazene ameliorates Alzheimer’s disease in the D-galactose-ovariectomized rat model: role of PI3K/Akt pathway. MolNeurobiol. 2018;55(10):8188–202.
33. Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J PhysiolRegulIntegr Comp Physiol. 2007;292(1):R373–81.
34. Abiodun OA, Ola MS. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J Biol Sci. 2020;27(3):905–12.
35. Ren L, Lu X, Danser AHJ. Revisiting the brain renin-angiotensin system-focus on novel therapies. CurrHypertens Rep. 2019;21(4):28.
36. Rodriguez Perez AI, Garrido Gil P, Pedrosa MA, Garcia-Garrote M, Valenzuela R, Navarro G, et al. Angiotensin Type 2 receptors: role in aging and neuroinflammation in the SubstantiaNigra. Brain Behav Immun. 2020;87:256–71.
37. Guo S, Som AT, Arai K, Lo EH, Guo S, Som AT, et al. Effects of angiotensin-II on brain endothelial cell permeability via PPARalpha regulation of para- and trans-cellular pathways. Brain Res. 2019;1722:146353.
38. Mertens B, Vanderheyden P, Michotte Y, Sarre S. The role of the central renin-angiotensin system in Parkinson’s disease. J Renin Angiotensin Aldosterone Syst. 2010;11(1):49–56.
39. Labandeira-Garcia JL, Rodriguez-Pallares J, Rodríguez-Perez AI, Garrido-Gil P, Villar-Cheda B, Valenzuela R, et al. Brain angiotensin and dopaminergic degeneration: relevance to Parkinson’s disease. Am J Neurodegener Dis. 2012;1(3):226–44.
40. Sonsalla PK, Coleman C, Wong LY, Harris SL, Richardson JR, Gadad BS, et al. The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Exp Neurol. 2013;250:376–83.
41. Kaur P, Muthuraman A, Kaur M. The implications of angiotensin-converting enzymes and their modulators in neurodegenerative disorders: current and future perspectives. ACS ChemNeurosci. 2015;6(4):508–21.
42. Mogi M, Horiuchi M. Effect of angiotensin II Type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. GeriatrGerontol Int. 2013;13(1):13–8.
43. De Bundel D, Smolders I, Yang R, Albiston AL, Michotte Y, Chai SY. Angiotensin IV and LVV-Haemorphin 7 enhance spatial working memory in rats: effects on hippocampal glucose levels and blood flow. Neurobiol Learn Mem. 2009;92(1):19–26.
44. Lee J, Albiston AL, Allen AM, Mendelsohn FAO, Ping SE, Barrett GL, et al. Effect of I.C.V. Injection of AT4 receptor ligands, NLE1-angiotensin IV and LVV-Hemorphin 7, on spatial learning in rats. Neuroscience. 2004;124(2):341–9.
45. Rivas-Santisteban R, Lillo J, Muñoz A, Rodríguez-Pérez AI, Labandeira-García JL, Navarro G, et al. Novel interactions involving the mas receptor show potential of the renin-angiotensin system in the regulation of microglia activation: altered expression in parkinsonism and dyskinesia. Neurotherapeutics. 2021;18(2):998–1016.
46. Rabie MA, Abd El Fattah MA, Nassar NN, Abdallah DM, El-Abhar HS. The mas receptor as a future perspective in Parkinson’s disease. J Neurol Neuromedicine. 2018;3(4):65–8.
47. de Morais SDB, Shanks J, Zucker IH. Integrative physiological aspects of brain RAS in hypertension. CurrHypertens Rep. 2018;20(2):10.
48. Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012;232(1):66–76.
49. Solleiro Villavicencio H, Rivas Arancibia S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front Cell Neurosci. 2018;12:114.
50. Chen M, Lai L, Li X, Zhang X, He X, Liu W, et al. Baicalein attenuates neurological deficits and preserves blood–brain barrier integrity in a rat model of intracerebralhemorrhage. Neurochem Res. 2016;41(11):3095–102.
51. Carvalho C, Moreira PI. Oxidative stress: a major player in cerebrovascular alterations associated to neurodegenerative events. Front Physiol. 2018;9:806.
52. Sun MS, Jin H, Sun X, Huang S, Zhang FL, Guo ZN, et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979.
53. Rodriguez-Perez AI, Sucunza D, Pedrosa MA, Garrido-Gil P, Kulisevsky J, Lanciego JL, et al. Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics. 2018;15(4):1063–81.
54. Torika N, Asraf K, Roasso E, Danon A, Fleisher-Berkovich S. Angiotensin converting enzyme inhibitors ameliorate brain inflammation associated with microglial activation: possible implications for Alzheimer’s disease. J NeuroimmunePharmacol. 2016;11(4):774–85.
55. Zhao HR, Jiang T, Tian YY, Gao Q, Li Z, Pan Y, et al. Angiotensin II triggers apoptosis via enhancement of NADPH oxidase-dependent oxidative stress in a dopaminergic neuronal cell line. Neurochem Res. 2015;40(4):854–63.
56. Kim MS, Lee GH, Kim YM, Lee BW, Nam HY, Sim UC, et al. Angiotensin II causes apoptosis of adult hippocampal neural stem cells and memory impairment through the action on AMPK-PGC1α signaling in heart failure. Stem Cells Transl Med. 2017;6(6):1491–503.
57. Gao Q, Ou Z, Jiang T, Tian YY, Zhou JS, Wu L, et al. Azilsartan ameliorates apoptosis of dopaminergic neurons and rescues characteristic ParkinsonianBehaviors in a rat model of Parkinson’s disease. Oncotarget. 2017;8(15):24099–109.
58. Ou Z, Jiang T, Gao Q, Tian YY, Zhou JS, Wu L, et al. Mitochondrial-dependent mechanisms are involved in angiotensin II-induced apoptosis in dopaminergic neurons. J Renin Angiotensin Aldosterone Syst. 2016;17(4):1470320316672349.
59. Auladell C, de Lemos L, Verdaguer E, Ettcheto M, Busquets O, Lazarowski A, et al. Role of JNK isoforms in the kainic acid experimental model of epilepsy and neurodegeneration. Front Biosci (Landmark Ed). 2017;22(5):795–814.
60. Ledda F, Paratcha G. Assembly of neuronal connectivity by neurotrophic factors and leucine-rich repeat proteins. Front Cell Neurosci. 2016;10:199.
61. Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural Regen Res. 2017;12(4):549–57.
62. Ibáñez CF, Andressoo JO. Biology of GDNF and its receptors—relevance for disorders of the central nervous system. Neurobiol Dis. 2017;97(B):80–9.
63. Lindahl M, Saarma M, Lindholm P. Unconventional neurotrophic factors CDNF and MANF: structure, physiological functions and therapeutic potential. Neurobiol Dis. 2017;97(B):90–102.
64. Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19(3):876.
65. Schaich CL, Wellman TL, Koi B, Erdos B. BDNF acting in the hypothalamus induces acute pressor responses under permissive control of angiotensin II. AutonNeurosci. 2016;197:1–8.
66. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–738.
67. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):17013.
68. Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. ProgNeurobiol. 2015;125:26–46.
69. Rodriguez-Perez AI, Borrajo A, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL. Interaction between NADPH-oxidase and rho-kinase in angiotensin II-induced microglial activation. Glia. 2015;63(3):466–82.
70. Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS. Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res. 2013;38(8):1572–9.
71. Lin HC, Tseng YF, Shen AL, Chao JCJ, Hsu CY, Lin HL. Association of angiotensin receptor blockers with incident Parkinson disease in patients with hypertension: a retrospective cohort study. Am J Med. 2022;135(8):1001–7.
72. Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109(2):656–69.
73. Goel R, Bhat SA, Rajasekar N, Hanif K, Nath C, Shukla R. Hypertension exacerbates predisposition to neurodegeneration and memory impairment in the presence of a neuroinflammatory stimulus: protection by angiotensin converting enzyme inhibition. PharmacolBiochemBehav. 2015;133:132–45.
74. Muñoz A, Rey P, Guerra MJ, Mendez-Alvarez E, Soto-Otero R, Labandeira-Garcia JL. Reduction of dopaminergic degeneration and oxidative stress by inhibition of angiotensin converting enzyme in a MPTP model of parkinsonism. Neuropharmacology. 2006;51(1):112–20.
75. Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, et al. Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in SubstantiaNigra. MolNeurodegener. 2007;2(1):1.
76. Zhang TT, Yang L, Jiang JG. Bioactive comparison of main components from unripe fruits of RubusChingii Hu and identification of the effective component. Food Funct. 2015;6(7):2205–14.
77. Hong F, Ming L, Yi S, Zhanxia L, Yongquan W, Chi L. The antihypertensive effect of peptides: a novel alternative to drugs? Peptides. 2008;29(6):1062–71.
78. Farzamirad V, Aluko RE. Angiotensin-converting enzyme inhibition and free-radical scavenging properties of cationic peptides derived from soybean protein hydrolysates. Int J Food SciNutr. 2008;59(5):428–37.
79. Guang C, Phillips RD. Plant food-derived angiotensin I converting enzyme inhibitory peptides. J Agric Food Chem. 2009;57(12):5113–20.
80. Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, Hufner A, et al. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother Res. 2007;21(1):32–6.
81. Margalef M, Bravo FI, Arola-Arnal A, Muguerza B. Natural angiotensin converting enzyme (ACE) inhibitors with antihypertensive properties. In: Andrade PB, Valentão P, Pereira DM. editors. Natural products targeting clinically relevant enzymes. Hoboken, NJ: John Wiley & Sons; 2017. pp. 45–67. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9783527805921.ch3
82. NileekaBalasuriya BW, Vasantha Rupasinghe HP. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct Foods Health Dis. 2011;1(5):172–88.
83. Zilli AMH, Zilli EM. Review of evidence and perspectives of flavonoids on metabolic syndrome and neurodegenerative disease. Protein PeptLett. 2021;28(7):725–34.
84. Zheng W, Tian E, Liu Z, Zhou C, Yang P, Tian K, et al. Small molecule angiotensin converting enzyme inhibitors: a medicinal chemistry perspective. Front Pharmacol. 2022;13:968104.
85. Vallée A, Lecarpentier Y, Guillevin R, Vallée JN. Circadian rhythms, neuroinflammation and oxidative stress in the story of Parkinson’s disease. Cells. 2020;9(2):314.
86. Wijesekara I, Kim SK. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: prospects in the pharmaceutical industry. Mar Drugs. 2010;8(4):1080–93.
87. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J ClinNutr. 2004;79(5):727–47.
88. Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18(1):75–81.
89. Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26(17):5377.
90. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531.
91. Medina dos Santos N, Berilli Batista P, Batista ÂG, MarósticaJúnior MR. Current evidence on cognitive improvement and neuroprotection promoted by anthocyanins. CurrOpin Food Sci. 2019;26:71–8.
92. Li P, Feng D, Yang D, Li X, Sun J, Wang G, et al. Protective effects of anthocyanins on neurodegenerative diseases. Trends Food Sci Technol. 2021;117:205–17.
93. Caruso G, Torrisi SA, Mogavero MP, Currenti W, Castellano S, Godos J, et al. Polyphenols and neuroprotection: therapeutic implications for cognitive decline. Pharmacol. Ther. 2022;232 (108013):108013. doi: https://doi.org/10.1016/j.pharmthera.2021.108013
94. Socci V, Tempesta D, Desideri G, De Gennaro L, Ferrara M. Enhancing human cognition with cocoa flavonoids. Front Nutr. 2017;4:19.
95. Khalatbary AR, Khademi E. The green tea Polyphenolic Catechin Epigallocatechin Gallate and neuro protection. Nutr Neuro sci. 2020;23(4):281–94.
96. Singh NA, Mandal AKA, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J. 2016;15(1):60.
97. Yao C, Zhang J, Liu G, Chen F, Lin Y. Neuroprotection by (-)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol Med Rep. 2014;9(1):69–76.
98. Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother. 2021;140:111729.
99. Dajas F, Abin-Carriquiry JA, Arredondo F, Blasina F, Echeverry C, Martínez M, et al. Quercetin in brain diseases: potential and limits. Neurochem Int. 2015;89:140–8.
100. Boyina HK, Geethakhrishnan SL, Panuganti S, Gangarapu K, Devarakonda KP, Bakshi V, et al. In silico and in vivo studies on quercetin as potential anti-Parkinson agent. Adv Exp Med Biol. 2020;1195:1–11.
101. Pan X, Liu X, Zhao H, Wu B, Liu G. Antioxidant, anti-inflammatory and neuroprotective effect of kaempferol on rotenone-induced Parkinson’s disease model of rats and SH-S5Y5 cells by preventing loss of tyrosine hydroxylase. J Funct Foods. 2020;74:104–40.
102. Han X, Zhao S, Song H, Xu T, Fang Q, Hu G, et al. Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: implications in Parkinson’s disease. Redox Biol. 2021;41:(101911).
103. Dhanraj V, Karuppaiah J, Balakrishnan R, Elangovan N. Myricetin attenuates neurodegeneration and cognitive impairment in parkinsonism. Front Biosci (Elite Ed). 2018;10(3):481–94.
104. Wu HC, Hu QL, Zhang SJ, Wang YM, Jin ZK, Lv LF, et al. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen Res. 2018;13(8):1375–83.
105. Siddique YH, Naz F, Jyoti S, Ali F, Rahul. Effect of genistein on the transgenic drosophila model of Parkinson’s disease. J Diet Suppl. 2019;16(5):550–63.
106. Arbabi E, Hamidi G, Talaei SA, Salami M. Estrogen agonist genistein differentially influences the cognitive and motor disorders in an ovariectomized animal model of parkinsonism. Iran J Basic Med Sci. 2016;19(12):1285–90.
107. Wu Q, Wang M, Chen W, Wang K, Wang Y, Exerts D. Daidzein exerts Neuroprotective activity against MPTP-induced Parkinson’s disease in experimental mice and lipopolysaccharide-induced BV2 microglial cells. J BiochemMolToxicol. 2022;36(2):e22949.
108. Goel R, Chaudhary R. Effect of daidzein on Parkinson disease induced by reserpine in rats. Braz J Pharm Sci. 2020;56:e18388.
109. de Rus Jacquet A, Ambaw A, Tambe MA, Ma SY, Timmers M, Grace MH, et al. Neuroprotective mechanisms of red clover and soy isoflavones in Parkinson’s disease models. Food Funct. 2021;12(23):11987–2007.
110. Siddique YH. Role of luteolin in overcoming Parkinson’s disease. BioFactors. 2021;47(2):198–206.
111. Elmazoglu Z, YarSaglam AS, Sonmez C, Karasu C. Luteolin protects microglia against rotenone-induced toxicity in a hormetic manner through targeting oxidative stress response, genes associated with Parkinson’s disease and inflammatory pathways. Drug ChemToxicol. 2020;43(1):96–103.
112. Chen HQ, Jin ZY, Wang XJ, Xu XM, Deng L, Zhao JW. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. NeurosciLett. 2008;448(2):175–9.
113. Siddique YH, Jyoti S, Naz F. Protective effect of luteolin on the transgenic drosophila model of Parkinson’s disease. Braz J Pharm Sci. 2018;54(3):e17760.
114. Kempuraj D, Thangavel R, Kempuraj DD, Ahmed ME, Selvakumar GP, Raikwar SP, et al. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. BioFactors. 2021;47(2):190–7.
115. Warnakulasuriya SN. Antioxidant and cytoprotective properties of long chain fatty acid acylated derivatives of quercetin-3-o-glucoside. Halifax, Canada: Dalhouse University; 2013.
116. Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin Convertin enzyme (ACE) activity by the AnthocyaninsDelphinidin- and Cyanidin-3-O-Sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127(1):7–10.
117. Ma H, Johnson SL, Liu W, DaSilva NA, Meschwitz S, Dain JA, et al. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int J Mol Sci. 2018;19(2):461.
118. Yamane T. Beneficial effects of anthocyanin from natural products on lifestyle-related diseases through inhibition of protease activities. Stud Nat Prod Chem. 2018;58:245–64.
119. Fernández K, Labra J. Simulated digestion of proanthocyanidins in grape skin and seed extracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2013;139(1–4):196–202.
120. Ahmed F, Siddesha JM, Urooj A, Vishwanath BS. Radical scavenging and angiotensin converting enzyme inhibitory activities of standardized extracts of FicusRacemosa stem bark. Phytother Res. 2010;24(12):1839–43.
121. Xu YY, Yang C, Li SN. Effects of genistein on angiotensin-converting enzyme in rats. Life Sci. 2006;79(9):828–37.
122. Montenegro MF, Pessa LR, Tanus-Santos JE. IsoflavoneGenistein inhibits the angiotensin-converting enzyme and alters the vascular responses to angiotensin I and bradykinin. Eur J Pharmacol. 2009;607(1–3):173–7.
123. Loizzo M. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Int J Devoted PharmacolToxicolEval Nat Prod Derivatives. 2007;21:32–6.
124. Chang CI, Chen CC, Wang, SY, Chen JJ, Chen MJ, Wu MD, et al. Two new dimericAbietanoid peroxides with xanthine oxidase and ACE inhibitory activities from the Bark of Cryptomeria Japonica. PhytochemLett. 2020;40:15–20. doi: https://doi.org/10.1016/j.phytol.2020.08.011
125. Salem MA, Michel HE, Ezzat MI, Okba MM, El-Desoky AM, Mohamed SO, et al. Optimization of an extraction solvent for angiotensin-converting enzyme inhibitors from Hibiscus sabdariffa L. based on Its UPLC-MS/MS metabolic profiling. Molecules. 2020;25(10):2307. doi: https://doi.org/10.3390/molecules25102307
126. Loizzo MR, Tundis R, Conforti F, Statti GA, Menichini F. Inhibition of angiotensin converting enzyme activity by five senecio species. Pharm Biol. 2009;47(6):516–20.
127. Tsutsumi Y Shimada A, Miyano A, Nishida T, Mitsunaga T. In vitro screening of angiotensin iconverting enzyme inhibitors from Japanese Cedar (Crptomera Japonica). J Wood Sci. 1997;44(6):463–8.
128. Jonadet M, Bastide J, Bastide P, Boyer B, Carnat AP, Lamaison JL. In vitro enzyme inhibitory and in vivo cardioprotective activities of hibiscus (Hibiscus sabdariffa L.). J Pharm Belg. 1990;45(2):120–4.
129. Yonekura Sakakibara K, Higashi Y, Nakabayashi R. The origin and evolution of plant flavonoid metabolism. Front Plant Sci. 2019;10:943. doi: https://doi.org/10.3389/fpls.2019.00943
130. Murota K, Nakamura Y, Uehara M. Flavonoid metabolism: the interaction of metabolites and gut microbiota. Biosci Biotechnol Biochem. 2018;82(4):600–10. doi: https://doi.org/10.1080/09168451.2018.1444467
131. Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab. 2014;15(1):48–61. doi: https://doi.org/10.2174/138920021501140218125020.
132. Ferri P, Angelino D, Gennari L, Benedetti S, Ambrogini P, Del Grande P, et al. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with Alpha-tocopherol. Food Funct. 2015;6:394–400.