INTRODUCTION
“Ayurveda” is one of the earliest sources of natural medicine, utilized for thousands of years globally to treat diseases and promote recovery [1]. Its usage of botanical preparations aims not only to treat or prevent diseases but also to repair disease-related damage. Artificial drugs can cause a variety of adverse reactions and limit their clinical utility. Due to the generally low toxicity of herbal medicines, they can enhance patient’s quality of life and reduce the need for multiple pharmaceutical actions [2]. Apart from the massive variety of outdated medicinal plants in India, there are about 700 Ayurvedic and Unani plants, as well as 600 Siddha and Aamchi plants [3], that have been used for their therapeutic properties. This rich botanical diversity underlines the deeply rooted tradition of herbal medicine in Indian culture, which offers a plethora of remedies for various diseases and health conditions.
The recent focus by researchers on medicinal herbs such as Berberis aristata, Berberis thunbergia, Berberis vulgaris, Berberis asiatica, Berberis petiolaris, and Berberis aquifolium, highlights their potential in pharmaceutical applications. Several scientists have identified bioactive compounds in the stem, roots, and rhizomes of these plants, with “berberine” emerging as the chief component [4–6]. It has been found that berberine content is present more in the species Berberis aristate that is 5% (Fig. 1 and Fig. 2). A yellowish isoquinoline alkaloid Berberine (Fig. 3) has many pharmacological applications such as antimicrobial, antiviral, antidiabetic, anti-inflammatory, and antidiarrheal [7–9]. Berberine shows significant anti-tumor properties and can be active in the treatment of various cancers such as human colon cancer cells [10] and hepatocellular carcinoma [11]. The anti-inflammatory effect of berberine works by inhibiting activator protein-I, and is a noteworthy factor of transcription involved in the process of inflammation [10].
Berberine also has the capability to treat infections of the intestine by hindering the growth of Helicobacter pylori [12,13]. Furthermore, activation of glycolysis stimulation, adenosine monophosphate active protein kinase, and mitochondrial function inhibition occurs significantly in the case of berberine with regard to type- II diabetes, which subsequently improves both glucose and lipid metabolism [14,15]. Berberine shows its antitumor efficacy by four main mechanisms: inhibiting proliferation, inducing apoptosis, inhibiting angiogenesis, and suppressing metastasis. When the tumor cells increase, berberine acts by inhibiting nuclear feature triggered B-cells (NF-κB) and matrix metalo-proteinases-1, 2, and 9, that reduces cyclooxygenase-2 actions and activates the adenosine monophosphate active protein kinase signaling [16]. Various literature surveys show that berberine component rises arachidonic acid to prostaglandin E-2 ratio in hepatocellular cancer cells which successfully inhibits the arachidonic acid pathway. Also, it works by hindering the expression of cyclooxygenase-2 gene with phospholipase A-2, which averts tumour growth [17].
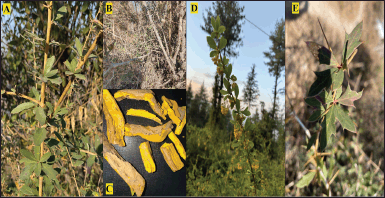 | Figure 1. Plant parts of Berberis aristata: A- Plant; B- Stem; C- Roots; D- Flowers; E- leaves. [Click here to view] |
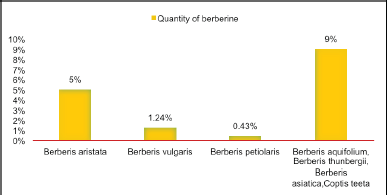 | Figure 2. Quantity of Berberine component in Berberis species [4–6]. [Click here to view] |
Berberine also inhibits the action of a protein so-called focal adhesion kinase in certain cancer cells, such as squamous cell carcinoma and rectal cancer cells. Taking advantage from this, some researchers combined chemotherapy with the administration of berberine to avert tumor degeneration in ovarian cancer [18,19]. As berberine also, has been stated to have useful properties in managing conditions such as hypertension, cardiovascular arrhythmia, and congestive heart failure [13]. Although, berberine offers abundant benefits but its clinical utility is hindered by several limitations, the absorption by the gastrointestinal tract and an unfortunate aqua solubility but ‘absorption by the intestinal tract’ is the foremost among them leads to the low bioavailability (around 5%) of berberine, and classifying it as a class-4 medicine. Thus, this little bioavailability can be attributed to the high binding of berberine to plasma- proteins, resulting in a short-unbound segment available to reach and penetrate target tissues, as well as the first-pass effect that berberine undergoes [13,16].
Berberine containing various nanoparticles (Fig. 4) have been used to improve berberine’s bioavailability, thus this review article explores how these nanoparticles can enhance the beneficial effects and reduce the limitations or side-effects associated with Berberine. The main objectives of this study are (a) To provide an outline of the therapeutic latent and mechanisms action of berberine (b) To highlight the challenges allied with the practice of berberine, such as poor solubility, limited absorption, and low bioavailability (c) To explore the application of various nano-carriers for the delivery of berberine and encapsulation. (d) To assess the efficacy of these nanocarriers in increasing the berberine absorption, solubility, and bioavailability.
NANO-SCALE BERBERINE
Low solubility is a major limitation of the drug Berberine. To overcome this limitation, researchers employed two bottom-up methods, evaporative precipitation of nanosuspension (EPN), and anti-solvent precipitation with a syringe pump (APSP), to produce Brb nanocrystals. The EPN method involved making a saturated ethanol extraction of Brb and quickly adding an anti-solvent that is hexane, followed by solvent evaporation. The APSP method involved injecting a saturated Brb solution in ethanol into deionized H2O as an anti-solvent, via different water-to-solution ratios. The obtained suspensions were then evaporated to form nanocrystals. The mean particle sizes obtained were 71.53 nm for EPN and 102.6 nm for APSP. Dissolution and solubility studies showed that reducing the particle size to the nanoscale effectively resolved the solubility issue of Brb. Formulating drugs as nanosuspensions without carriers is a capable way to enhance the bio-availability and absorption of lipophilic drugs with solubility limitations [20–23]. This limitation can be accomplished by various methods, such as high-speed homogenization, nano-precipitation, sonication, pearl milling, and high-pressure homogenization. Among these methods, the high-pressure homogenization method is highly preferred due to its high productivity and low risk of contamination [24].
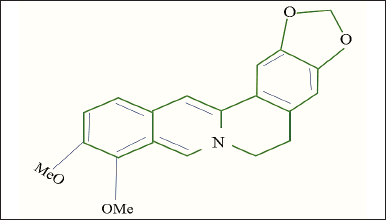 | Figure 3. Chemical structure of berberine compound. [Click here to view] |
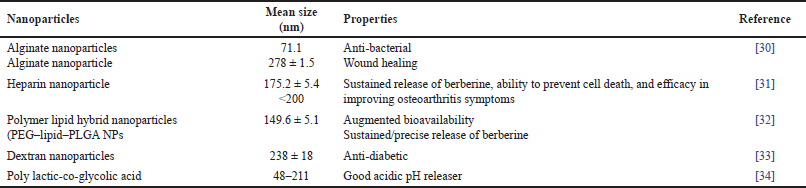 | Table 1. Overview of polymer-derived nanoparticles containing berberine. [Click here to view] |
Wang et al. [25] in their study prepared berberine nanoparticles, i.e., berberine-nanosuspension, by means of a high-pressure homogenization method in which they attained nanoparticles with a particle size < 200 nm. The consequences showed that the solubility and bioavailability of berberine were improved, and the nanoparticles presented an outstanding anti-diabetic activity in diabetic mice models. These nanoparticles confirmed higher hypoglycaemic and reduction of body weight effects compared to berberine alone, and they had very rarer hostile possessions. Xie et al. [26] also examined the effect of berberine nanoparticles on renal ischemia-reperfusion injury, which is the utmost common reason for severe renal failure. The berberine nanoparticles prepared by them were injected into a renal ischemia-reperfusion damage rat model which demonstrated the defensive effects of berberine nanoparticles [27,26,28].
POLYMERIC-DERIVED NANOPARTICLE CARRIERS
There are various polymeric systems that have been used for the delivery of drugs, which can be broadly classified into two main groups: natural polymers and artificial polymers. The group of natural polymers includes bio-polymers, i.e., obtained from natural sources, for example, agarose, collagen, albumin, alginate, gelatin, hyaluronic acid, dextran, cyclodextrins, and carrageenan. These are biofriendly and biodegradable polymers, which makes them attractive for drug delivery applications. We have reviewed a few uses of polymers in berberine transportation as shown in Table 1. Furthermore, the synthetic polymer group includes polymers that are chemically created in the laboratories. Examples of synthetic polymers include poly-glycolic acid, poly-lactic acid, polyvinyl pyrrolidone, poly (ε-caprolactone), and polymethacrylates [29]. Both natural and artificial polymers showed an imperative part in the expansion of innovative drug delivery, leveraging their unique properties to achieve efficient and effective drug delivery.
MAGNETIZED MIDPOROUS SILICON-BUILT NANOPARTICLES
The microenvironment of tumor is acidic, which makes the pH-sensitive transporters very effectual and more superior to others for delivering drugs to cancerous cells. Some researchers created pH-sensitive mid-porous nanoparticles composed of iron oxide (Fe3O4) as the head group and silica (SiO2) as the body group. Virtuous super-paramagnetic properties have been shown by these nanoparticles and might transmit a high quantity of the berberine drug and send it to hepatocellular carcinoma (liver cancer) tissues. The berberine-loaded Fe3O4-mSiO2 nanoparticles could weaken the endo/lysosomal membranes in tumor cells, hence enhanced the release of berberine into the cytosol. The researchers developed pH-sensitive nanoparticles that can carry a high amount of the anti-cancer berberine drug and release it inside the hepatocellular cancerous cells. These nanoparticles display effectiveness because they can take advantage of the acidic environment of tumors to deliver the drug exactly to cancer cells [35].
LIPID-BASED NANOPARTICLE TRANSPORTERS
Lipid-based nanoparticles are nanosized carriers composed of lipids, which have gained significant attention in the field of biomedical applications and drug delivery. These nanocarriers offer several benefits, including biocompatibility, biodegradability, and the ability to encapsulate and deliver a wide array of healing mediators, such as small molecules, proteins, nucleic acids, and tomography agents. These lipid nanoparticles are divided into different types on the basis of their composition and structure which includes Liposomes, Solid lipid nanoparticles, nanostructured lipid careers, and micelles. Table 2 summaries the presentation of lipid-derived nanoparticles utilized as berberine transport [36].
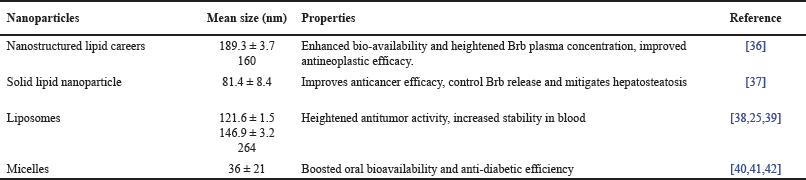 | Table 2. Overview of lipid-derived nanostructures integrating berberine. [Click here to view] |
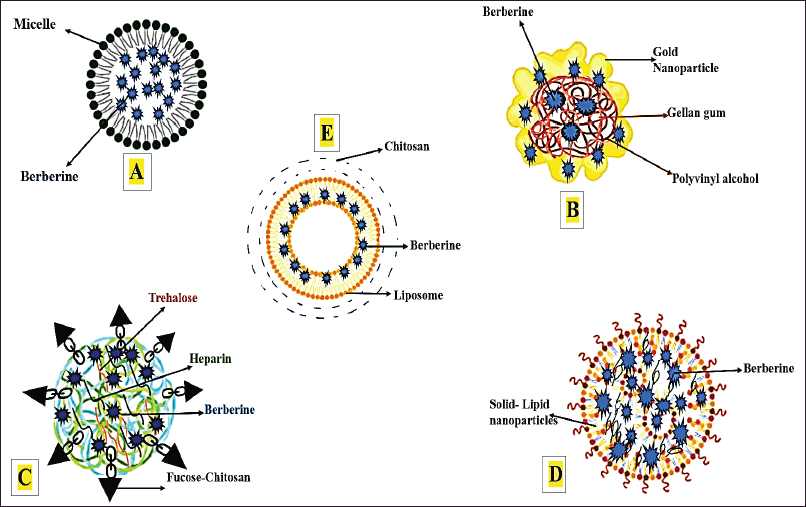 | Figure 4. Various nanoparticles containing berberine: (A) Micelle nanoparticle with loaded berberine, (B) gold nanoparticle with berberine, (C) polymeric nanoparticles containing berberine, (D) solid-lipid nanoparticle (SLN) with loaded berberine, and (E) liposome nanoparticle containing berberine [35,44,46,51]. [Click here to view] |
GOLD AND SILVER-BASED NANOPARTICLES WITH BERBERINE
Gold nanoparticles were first manufactured by Michael Faraday in the nineteenth era, and holds a unique properties like the capacity to bind to aminoalkane and thiol groups, superficial plasmon resonance, and the possible for surface modification. These properties are useful for biomedical applications such as cancer therapy, contrast agents, drug delivery, radiosensitizers, and photochemical agents. Investigators have explored using gold nanoparticles in combination with supplementary particles like polyvinyl alcohol and gellan gum for the enhancement of berberine delivery. These nanoparticle-based formulations have confirmed high drug loading efficacy and controlled release possessions which make them promising drug delivery carriers. Further, the unique thermal and optical properties of gold (Au) nanoparticles, such as heat dissipation and surface plasmon resonance, have been exploited for applications like photo-thermal therapy. In another approach by investigators, biologically modified gold particles have been used as careers for the targeted distribution of berberine to solid tumors. Researchers have attained high berberine at acidic pH (5.8) and 86% drug loading release of berberine, by assigning targeting ligands like folic acid and employing natural resources like tropical fruit peels [43–46].
Silver (Ag) nanoparticles possess some unique properties, like as thermal, optical, and high electric conduction, which make them valuable for innumerable applications, as an anti-bacterial agents, anticancer agents, ophthalmic sensors, and medical device coatings. Some researchers have explored the antibacterial activity of silver-based nanoparticles synthesized by means of the natural isoquinoline alkaloid “berberine”. As Chandra et al. [46] investigated the synergistic effects of berberine-silver nanoparticles with antibiotics, which revealed their potential in combating antibiotic-resistant bacterial infections. One more investigation has been done by Tahan et al. [47] on the antibacterial activity of silver nanoparticles (AgNPs) against multidrug-resistant (MDR) bacterial strains. In their study, they synthesized AgNPs by means of Berberine and evaluated their efficiency against MDR Pseudomonas aeruginosa and Acinetobacter baumannii strains. Biosynthesis of AgNPs has been confirmed using various characterization techniques including XRD, UV-Vis, DLS, FTIR, and zeta potential analysis. Disk diffusion agar and nominal inhibitory concentration tests revealed that the biosynthesized AgNPs proved powerful anti-bacterial activity against the tested MDR strains, inhibiting microbial growth at lower concentrations of AgNPs as compared to conventional antibiotics. Particularly, on combining AgNps with standard antibiotics, a synergetic effect was observed, as established by the checkerboard method. This synergistic action shows that AgNPs can boost the efficacy of present antibiotics against MDR bacterial strains [48]. These studies collectively highlight the promising role of berberine-based silver nanoparticles as substitute anti-microbial agents in varied biomedical applications.
They can also increase the anticancer effects of many medications. In a study by some investigator, they assessed the impact of silver nanoparticles on the feasibility and proliferation of squamous cell carcinoma-25(SSC-25) oral cancer cells, both alone and in a mixture with berberine. The results were found that while silver nanoparticles alone had an effect of anti-proliferative, this outcome was reduced in the presence of Berberine compound. Berberine stimulated the expression of the pro-proliferative Bcl-2 gene and upheld the capability of SCC-25 cancer cells [49]. This non-synergistic phenomenon was attributed to the force of electrostatic between the “+ve” charge of Berberine and the “-ve” charge of silver nanoparticles. Bhanumathi et al. [50] developed novel bio-genic silver nanoparticles as a drug delivery carrier for Berberine. Some in-vitro studies exhibited dose-dependent toxicity of berberine-loaded silver nanoparticles against MDA-MB-231 breast cancer cell lines and Michigan cancer foundation-7, and in-vivo studies confirmed their skill to suppress tumor growth [50,51].
CONCLUSION AND FUTURE SCOPES
This review revealed that although berberine (Brb) consumes many valuable belongings, but it faces certain confines in its dissolution, absorption, and bio distribution. Nanotechnology is proposed as a useful approach to overcome these limitations. Several studies have demonstrated improved pharmacological effects of Brb when it is encapsulated in different nanocarriers. The major findings were polymeric nanostructures made of alginate, dextran, and PLGA were used for drug delivery and controlled discharge of Berberine, exhibiting therapeutic effects against osteoarthritis, diabetes, and microbial infections. Lipid-built nanomaterial carriers, such as solid lipid nanoparticles, nanostructured lipid carriers, micelles, and liposomes, encapsulating Brb showed antidiabetic and antitumor activities. Combinations of Berberine with nanocarriers like dendrimers, gold nanoparticles (AuNPs), and silver nanoparticles were found to be useful in cancer therapy. AuNP-Brb conjugates were investigated for thermal treatment, and carbon dots berberine demonstrated bio-imaging capabilities. The following are some future scopes:
- By leading more wide investigation on the biodistribution, toxicity, and biochemicals of Berberine-loaded nanocarriers will ensure their scientific conversion.
- By the exploration of the potential of Brb-nanocarrier for targeted drug delivery to specific tissues or organs, we can improve therapeutic efficacy and reducing side effects.
- We can further investigate the synergetic properties of Berberine in grouping with another therapeutic nanocarriers for improved therapeutic consequences.
- Conduct clinical trials to assess the berberine-nanocarrier formulation efficacy and protection of auspicious in anthropoid subjects.
Therefore, in general, while nanotechnology has shown hopeful results in improving the therapeutic potential and delivery of berberine, further examination is necessary to interpret these nanoparticle carriers into clinical applications.
ACKNOWLEDGEMENT
The authors are highly thankful to Dean and Professor Dr. Ajay Singh, School of Applied and Life Sciences (SALS), Uttaranchal University, Dehradun, for providing necessary facilities during the study period.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the sources of data provided in this manuscript have duly been referred in the references which are freely available in public domain.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Dias DA, Urban S, Roessner U. “A historical overview of natural products in drug discovery.” Metabolites 2012 Apr 16;2(2):303–36.
2. Mangin D, Bahat G, Golomb BA, Mallery LH, Moorhouse P, Onder G, et al. International group for reducing inappropriate medication use & polypharmacy (IGRIMUP): position statement and 10 recommendations for action. Drugs Aging. 2018 Jul;35(7):575–87. CrossRef
3. Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med. 2017;7:234–44. CrossRef
4. Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009–2012). Expert Opin Ther Pat. 2013 Feb;23(2):215–31. CrossRef
5. Tabeshpour J, Imenshahidi M, Hosseinzadeh H, A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome, Iran J Basic Med Sci. 2017;20(5):557. CrossRef
6. Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008 Aug;22(8):999–1012. CrossRef
7. Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969 Sep;15(9):1067–76.
8. Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S, Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–4.
9. Babaeenezhad E, Rashidipour M, Jangravi Z, Sarabi MM, Shahriary A. Cytotoxic and epigenetic effects of berberine-loaded chitosan/pectin nanoparticles on AGS gastric cancer cells: Role of the miR-185-5p/KLF7 axis, DNMTs, and global DNA methylation. Int J Biol Macromol. 2024;260:129618.
10. Khemani M, Sharon M, Sharon M, encapsulation of berberine in nano-sized PLGA synthesized by emulsification method. ISRN Nanotechnol. 2012. CrossRef
11. Wang Z, Wang YS, Chang ZM, Li L, Zhang Y, Lu MM, et al. Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem Biol Drug Des. 2017 Mar;89(3):464–9. CrossRef
12. Lin YH, Lin JH, Chou SC, Chang SJ, Chung CC, Chen YS, et al. Berberine-loaded targeted nanoparticles as specific Helicobacter pylori eradication therapy: in vitro and in vivo study. Nanomedicine (Lond). 2015 Jan;10(1):57–71. CrossRef
13. Xue M, Zhang L, Yang MX, Zhang W, Li XM, Ou ZM, et al. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int J Nanomedicine. 2015 Aug 5;10:5049–57. CrossRef
14. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states, Diabetes. 2006 Aug;55(8):2256–64. CrossRef
15. Shen R, Kim JJ, Yao M, Elbayoumi TA. Development and evaluation of vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int J Nanomedicine. 2016 Apr 26;11:1687–700. CrossRef
16. Li J, Li O, Kan M, Zhang M, Shao D, Pan Y, et al. Berberine induces apoptosis by suppressing the arachidonic acid metabolic pathway in hepatocellular carcinoma. Mol Med Rep. 2015 Sep;12(3):4572–7. CrossRef
17. Park JJ, Seo SM, Kim EJ, Lee YJ, Ko YG, Ha J. Berberine inhibits human colon cancer cell migration via AMP-activated protein kinase-mediated downregulation of integrin β1 signaling. Biochem Biophys Res Commun. 2012 Oct 5;426(4):461–7.CrossRef
18. Zhao Y, Cui L, Pan Y, Shao D, Zheng X, Zhang F, et al. Berberine inhibits the chemotherapy-induced repopulation by suppressing the arachidonic acid metabolic pathway and phosphorylation of FAK in ovarian cancer. Cell Prolif. 2017 Dec;50(6):e12393. CrossRef
19. Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos. 2006 Dec;34(12):2064–72.
20. Sahibzada MUK, Sadiq A, Faidah HS, Khurram M, Amin MU, Haseeb A, et al. Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des Devel Ther. 2018 Feb 14;12:303–12. CrossRef
21. Kakran M, Sahoo NG, Tan IL, Li L, Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J Nanopart Res. 2012;14:757. CrossRef
22. Jacobs C, Kayser O, Müller RH. Nanosuspensions as a new approach for the formulation for the poorly soluble drug tarazepide. Int J Pharm. 2000;196:161–4. CrossRef
23. Mehra M, Sheorain J, Bakshi J, Thakur R, Grewal S, Dhingra D, et al. Synthesis and evaluation of berberine loaded chitosan nanocarrier for enhanced in-vitro antioxidant and anti-inflammatory potential. Carbohydr Polym Technol Appl. 2024 Jun 1;7:100474. CrossRef
24. Wang Z, Wu J, Zhou Q, Wang Y, Chen T. Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. Evid Based Complement Alternat Med. 2015;2015:239749.CrossRef
25. Wang T, Wang N, Song H, Xi X, Wang J, Hao A, et al. Preparation of an anhydrous reverse micelle delivery system to enhance oral bioavailability and anti-diabetic efficacy of berberine. Eur J Pharm Sci. 2011 Sep 18;44(1-2):127–35. CrossRef
26. Xie D, Xu Y, Jing W, Juxiang Z, Hailun L, Yu H. Berberine nanoparticles protects tubular epithelial cells from renal ischemia-reperfusion injury. Oncotarget. 2017 Apr 11;8(15):24154–62. CrossRef
27. Rouschop K, Leemans J. Ischemia–reperfusion treatment: opportunities point to modulation of the inflammatory response. Kidney Int. 2008;73(12):1333–5. CrossRef
28. Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol. 2001;5(4):447–51. CrossRef
29. Zhou Y, Liu SQ, Peng H, Yu L, He B, Zhao Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int Immunopharmacol. 2015 Sep;28(1):34–43. CrossRef
30. Mehra M, Sheorain J, Kumari S. Synthesis of berberine loaded polymeric nanoparticles by central composite design. InAIP Conference Proceedings 2016 Apr 13 (Vol. 1724, No. 1). AIP Publishing.CrossRef
31. Xu H, Yuan XD, Shen BD, Han J, Lv QY, Dai L, et al. Development of poly (N-isopropylacrylamide)/alginate copolymer hydrogel-grafted fabrics embedding of berberine nanosuspension for the infected wound treatment. J Biomater Appl (North America). 2014 May;28(9):1376–85. CrossRef
32. Kapoor R, Singh S, Tripathi M, Bhatnagar P, Kakkar P, Gupta KC. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS One. 2014 Feb 20;9(2):e89124.CrossRef
33. Yu F, Ao M, Zheng X, Li N, Xia J, Li Y, et al. PEG-lipid-PLGA hybrid nanoparticles loaded with berberine-phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017 Nov;24(1):825–33. CrossRef
34. Wang L, Li H, Wang S, Liu R, Wu Z, Wang C, et al. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech. 2014 Aug;15(4):834–44. CrossRef
35. Wang L, Li H, Wang S, Liu R, Wu Z, Wang C, et al. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. Aaps Pharmscitech. 2014;15:834–44. CrossRef
36. Wang ZP, Wu J, Chen TS, Zhou Q, Wang YF. In vitro and in vivo antitumor efficacy of berberine-nanostructured lipid carriers against H22 tumor. In: Chen WR, editor. Biophotonics and Immune Responses X. SPIE; 2015 Mar 9. Vol. 9324, pp. 112–9. CrossRef
37. Yin J, Hou Y, Yin Y, Song X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int J Nanomedicine. 2017 Dec 6;12:8671–80. CrossRef
38. Behl T, Singh S, Sharma N, Zahoor I, Albarrati A, Albratty M, et al. Expatiating the Pharmacological and Nanotechnological Aspects of the Alkaloidal Drug Berberine: Current and Future Trends. Molecules. 2022 Jun 9;27(12):3705. CrossRef
39. Luo X, Li J, Guo L, Cheng X, Zhang T, Deng Y, Preparation of berberine hydrochloride long-circulating liposomes by ionophore A23187-mediated ZnSO4 gradient method. Asian J Pharm. 2013;8(4):261–6. CrossRef
40. Lin YC, Kuo JY, Hsu CC, Tsai WC, Li WC, Yu MC, et al. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int J Pharm. 2013 Jan 30;441(1-2):381–8. CrossRef
41. Nguyen TX, Huang L, Liu L, Abdalla AME, Gauthier M, Yang G. Chitosan coated nano-liposomes for the oral delivery of berberine hydrochloride. J Mater Chem B. 2014;2(41):7149–59. CrossRef
42. Faraday M. The Bakerian lecture: experimental relations of gold (and other metals) to light. Philos Trans Soc Lond 1857;147:145–81.
43. Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012 Feb;85(1010):101–13. CrossRef
44. Souza CR, Oliveira HR, Pinheiro WM, Biswaro LS, Azevedo RB, Gomes AJ, et al. Gold nanoparticle and berberine entrapped into hydrogel matrix as drug delivery system. J Biomater Nanobiotechnol. 2015;6(01):53.
45. Pandey S, Mewada A, Thakur M, Shah R, Oza G, Sharon M. Biogenic gold nanoparticles as fotillas to fire berberine hydrochloride using folic acid as molecular road map. Materials Science and Engineering: C. 2013 Oct 1;33(7):3716–22.CrossRef
46. Chandra H, Patel D, Kumari P, Jangwan JS, Yadav S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mat Sci Eng: C. 2019;102:212–20. CrossRef
47. Tahan M, Zeraatkar S, Neshani A, Marouzi P, Behmadi M, Alavi SJ, et al. Antibacterial potential of biosynthesized silver nanoparticles using Berberine extract against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Indian J Microbiol. 2024;64(1):125–32. CrossRef
48. Mirhadi E, Rezaee M, Malaekeh-Nikouei B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed Pharmacother. 2018 Aug 1;104:465–73. CrossRef
49. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. CrossRef
50. Dziedzic R, Kubina RJ, Bu?dak M, Skonieczna K. Cholewa, silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of Berberine. Molecules. 2016;21(3):365. CrossRef
51. Bhanumathi R, Vimala K, Shanthi K, Thangaraj R, Kannan S, Bioformulation of silver nanoparticles as berberine carrier cum anticancer agent against breast cancer. New J Chem. 2017;41(23):14466–77. CrossRef