INTRODUCTION
Tuberculosis (TB) is a disease caused by Mycobacterium tuberculosis that spreads through the air and increases the risk of catching the Human Immunodeficiency Virus [1,2]. It ranks among the top 10 worldwide causes of death [3]. The death toll from TB reached 1.5 million people in 2020, equal to the COVID-19 death toll of 1.8 million people [4].
Drug resistance is the principal obstacle in TB treatment. It significantly impacts patients’ clinical and financial aspects [5]. The two common types of drug-resistant TB are rifampicin-resistant TB (RR-TB) and multidrug-resistant TB (MDR-TB), of which the latter refers to TB that is resistant to at least the two most effective first-line TB drugs, namely rifampicin and isoniazid (INH) [6]. An estimated 500,000 new cases of RR/MDR-TB are reported globally each year, requiring treatments with second-line TB drugs, which are less effective, pricier, and more toxic than first-line TB drugs [7]. Overcoming drug resistance in TB is an urgent matter in the global fight against antimicrobial resistance [8]. Bedaquiline was the first antituberculosis drug with a novel mode of action against mycobacteria to be approved for DR-TB treatment by the Food and Drug Administration in over four decades [9]. However, this drug has been shown to cause several adverse effects, including gastrointestinal problems, otovestibular dysfunction, vomiting, dizziness, headaches, arthralgia, and prolonged QT interval, which can lead to a fatal cardiac rhythm [10]. These circumstances highlight the importance of developing new antituberculosis drugs to improve and complement existing treatment regimens [11].
INH is one of the most effective first-line TB drugs, possessing a minimum inhibitory concentration (MIC) value of 0.02–0.1 µg/ml [12]. However, its usage has been limited due to resistance. INH primarily inhibits the InhA enzyme, which produces long-chain fatty acids, particularly mycolic acids essential for M. tuberculosis survival [13]. It is worth noting that molecular hybridization of experimentally proven antituberculosis molecules with pharmacophores of several bioactive compounds to create new hybrid molecules that are expected to have greater affinity and efficacy against M. tuberculosis can have the potential to delay the emergence of drug resistance. Several INH-hybrid compounds with antituberculosis activity have been reported in numerous studies [14–17].
On the other hand, cinnamic acid and its derivatives are compounds that are commonly present in plant food and have been reported to exhibit a variety of biological activities, including antituberculosis [18,19], α-glucosidase inhibitor [20,21], and anti-inflammatory [22,23]. Cinnamic acid has been shown to have low toxicity in humans [24]. Furthermore, it has also been reported that hydrazide-hydrazones are a powerful and non-toxic antituberculosis agent with InhA inhibitory activity [25]. In our previous published work, we applied a hybridization strategy to embed the hydrazide-hydrazone structural motif into cinnamic acid to generate cinnamate-amine hybrid molecules [26]. Meanwhile, in this study, we present the antituberculosis activity of cinnamamides based on in vitro and molecular docking experiments.
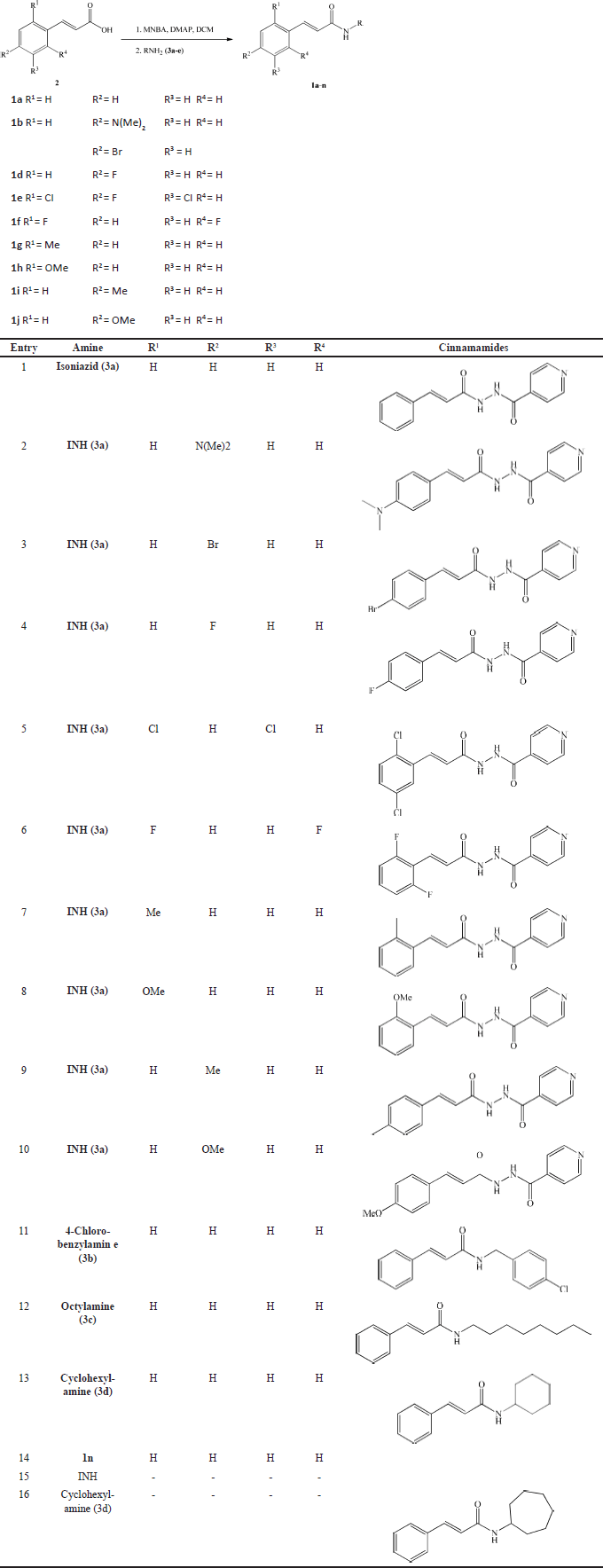 | Table 1. Synthesis of cinnamamides 1a-n. [Click here to view] |
MATERIAL AND METHODS
Antituberculosis study
The antimycobacterial activity of the synthesized compounds 1a-n was investigated by adapting the antimycobacterial activity test method of pyrazinamide [27]. Compounds 1a-n were first prepared as 1,000 µg/ml stock solutions in DMSO 20%. The solutions were then serially diluted to produce solutions with concentrations of 25, 12.5, 6.25, 3.13, 1.56, and 0.78 µg/ml. Each 100 µl of the test solution was put into a 96-well plate and further added with 100 µl of M. tuberculosis H37Rv suspension. The plate was then covered, sealed with plastic clips, and incubated at 37°C for 7 days. Subsequently, 30 µl of 0.01% resazurin was added to the plate and incubated at 37°C for 1 day, after which the color change was observed. Resazurin reduction is indicated by a shift in hue from blue to pink, indicating bacteria growth. Each test compound was subjected to a triple measurement (triplo). INH was used as a standard drug and tested at concentrations of 1.0, 0.5, and 0.25 µg/ml using the same procedure as the test compounds 1a-n.
Molecular docking studies
The molecular docking of cinnamamides on the crystallographic structure of the enoyl-acyl carrier protein reductase (InhA) of M. tuberculosis was performed using Autodock 4.2.6 [28]. The 3D structure of InhA protein (PDB ID: 3FNG) was downloaded from the Protein Data Bank (www.rcsb.org) and then prepared using MGLTools 1.5.6 by eliminating the water and non-protein molecules, adding polar hydrogens, and adding Kollman charges. The 3D structure of cinnamamides was created and minimized using MMFF94 in MarvinSketch 20.18. The docking pocket is located in the center of the active site and is large enough to cover the entire active site. The docking simulation was run using the Lamarckian genetic algorithm. The docking outcomes were visualized using Biovia Discovery Studio 2020. Validation of the docking parameter in this study was carried out by redocking the native ligand, namely 5-(cyclohexylmethyl)-2-(2,4-dichlorophenoxy)phenol (JPL).
RESULTS AND DISCUSSION
Antituberculosis activity
Synthesis compounds 1a-n have previously been detailed in prior work [26]. The synthesis of compounds 1a-n is shown in Table 1. Compounds 1a-n were examined for their antimycobacterial activity against M. tuberculosis H37Rv using a redox indicator-based colorimetric method, namely Resazurin Microtiter Assay [29]. A color change identifies drug resistance due to the reduction of resazurin that is directly proportional to the amount of M. tuberculosis living in the medium so that it can be used to determine the MIC of a drug [30]. The obtained MIC values of cinnamamides 1a-n ranged from 3.13 to more than 25 µg/ml, as shown in Table 2. INH was the control standard, with a MIC value of 0.25 µg/ml [31].
Different inhibitory effects were seen on M. tuberculosis by the cinnamamides 1c and 1d with an electron-withdrawing group substituent (F, Br) at the para position in the phenyl ring of the cinnamic skeleton. Cinnamamide 1c with the bromo group inhibited M. tuberculosis at a 3.13 µg/ml concentration. However, cinnamamide 1d with the fluoro group exhibited the same growth-inhibitory effects on M. tuberculosis as cinnamamide 1a (MIC >3.13 µg/ml), which has no substituent group in its phenyl ring. It was discovered that introducing two electron-withdrawing groups (F or Cl) at the ortho or meta positions in cinnamamides 1e and 1f reduced the inhibitory effects. Cinnamamide 1e was able to inhibit M. tuberculosis growth more potently than cinnamamide 1f with a MIC value of 25 µg/ml.
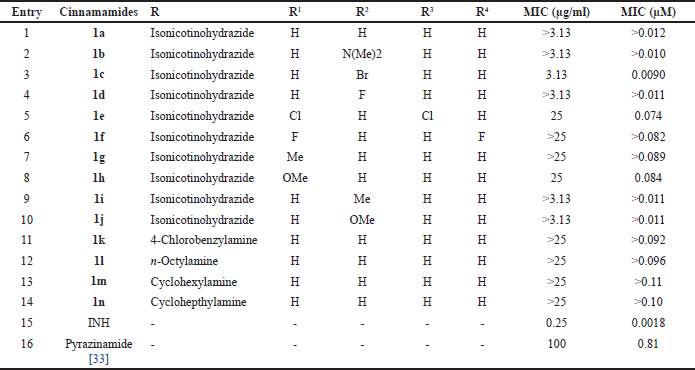 | Table 2. The antituberculosis activity of cinnamamides 1a-n. [Click here to view] |
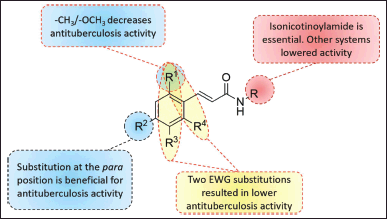 | Figure 1. Structure-activity relationship analysis for the antituberculosis activity of cinnamamides. [Click here to view] |
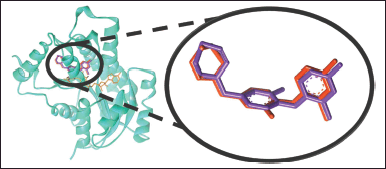 | Figure 2. Binding pose of redocked JPL (red) with an RMSD value of 0.78 Å when superimposed on native JPL (purple) in the active site of InhA protein (PDB ID: 3FNG). Orange color represents the NAD cofactor. [Click here to view] |
Introducing the electron-donor group (Me or OMe) at the para position (compounds 1i and 1j) in the phenyl ring of the cinnamic skeleton was found to be more advantageous for activity than at the ortho position (compounds 1g and 1h), with a MIC value of >3.13 µg/ml, which was the same as cinnamamide 1b. Cinnamamide 1h with substitution of the methoxy group at the ortho position was able to inhibit M. tuberculosis growth with a MIC value of 25 µg/ml. However, cinnamamide 1g with substitution of the methyl group could not inhibit M. tuberculosis growth.
According to the research findings, replacing the isonicotinohydrazide moiety with an amine group in a cyclic, acyclic, or aromatic group diminishes the inhibitory effect of cinnamamide. Cinnamamides 1k,l,m,n exhibited inhibitory activity four times lower than cinnamamide 1a, with MIC values greater than 25 µg/ml. The structure-activity relationship of cinnamamides is depicted in Figure 1.
Molecular docking studies
Molecular docking studies were conducted to estimate the binding energy of cinnamamide 1c as it binds to the active site of the InhA protein (PDB ID: 3FNG) and to comprehend its antituberculosis mode of action (Fig. 2) Redocking of JPL at the active site of the InhA protein produces an RMSD value of 0.78 Å (Fig. 3), which is less than 2 Å, meaning that the parameters employed can replicate the native binding pose and interaction of JPL.
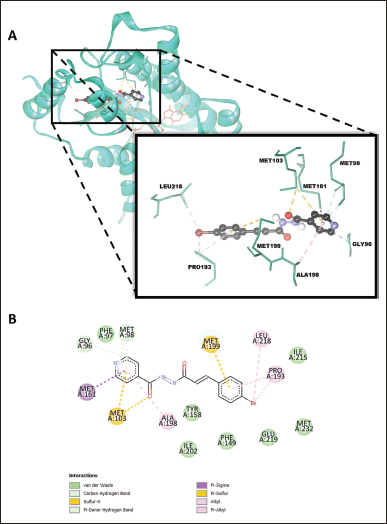 | Figure 3. The binding mode of cinnamamide 1e is shown in (A) a three-dimensional view (note that NAD is intentionally not shown in magnification to clarify visualization) and (B) a two- dimensional view in the active site of the InhA protein (PDB ID: 3FNG). [Click here to view] |
The binding energy of the cinnamamide 1c was −4.13 kcal/mol, as shown in Table 3. This compound is bound to InhA in an extended conformation, where its molecules stretch from the edge of the binding pocket to the sub-binding pocket of the InhA active site, as seen in Figure 3. Most renowned InhA direct inhibitors also bind to the protein in the extended conformation [31]. The phenyl ring of the cinnamoyl skeleton occupied the sub-binding pocket of the InhA active site. It formed hydrophobic π-alkyl and π-sulfur interactions with hydrophobic residues on the substrate binding loop (SBL) region, namely Pro193 and Met199. At the same time, the bromo group at the cinnamoyl skeleton formed hydrophobic alkyl interactions with Leu218 at the sub-binding pocket and Pro193. The isonicotinic moiety of cinnamamide 1c occupied the edge of the binding pocket. The carbonyl group bound to the pyridinyl ring formed a sulfur-X interaction with Met103 at the edge of the binding pocket. The pyridinyl ring formed interactions with several residues in different areas. This ring formed a hydrophobic π-σ with Ala198 on the SBL and a hydrophobic π-alkyl with Met161 at the sub-binding pocket. It also formed two carbon-hydrogen and one π-donor hydrogen bonds with Gly96 and Met98 at the edge of the binding pocket. Notably, cinnamamide 1c did not form any hydrogen bonds with the catalytic residue Tyr158 and only interacted via van der Waals interactions, indicating that its potency does not rely on the conserved interaction network with Tyr158 [32]. Van der Waals contacts were also formed between cinnamamide 1c and the side chain residues Phe97, Phe149, Ile202, Ile215, Glu219, and Met232.
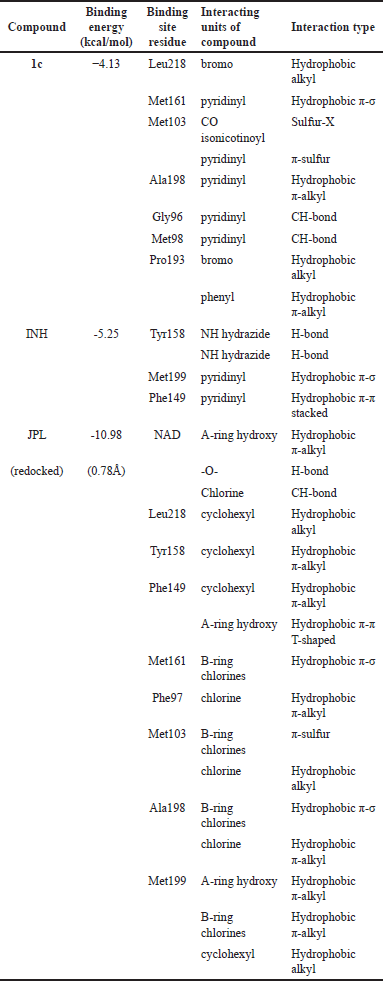 | Table 3. Binding energy and residue interactions of cinnamamide 1c on the active site of InhA protein (PDB ID: 3FNG). [Click here to view] |
CONCLUSION
Studies on the antituberculosis activity of cinnamamide derivatives have been carried out in vitro and in silico. In vitro antituberculosis studies showed that introducing a substituent at the para position of the cinnamoyl moiety in a cinnamate-INH hybrid is more advantageous than substituting at other positions. Cinnamamide 1c with a bromo group at the para position of the cinnamoyl moiety has the greatest inhibitory effect on M. tuberculosis growth, with a MIC value of 3.13 µg/ml. Docking experiments revealed that cinnamamide 1c inhibits the activity of InhA (3FNG) through an extended binding confirmation whose potency is independent of the conserved interaction network with Tyr158. These findings pave the way for developing an InhA inhibitor to help improve TB treatment regimens.
ACKNOWLEDGMENT
The authors acknowledge Ministry of Education and Culture, Research, and Technology of Indonesia Republic for the PFR research grant (1765/PKS/ITS/2024), and Institut Teknologi Sepuluh Nopember for the ORM fund (Indonesia Endowment Fund for Education through Higher Education Endowment Fund Program 2023/2024).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Elhakeem M, Taher A, Abuel-Maaty S. Synthesis and anti-mycobacterial evaluation of some new isonicotinylhydrazide analogues. Bull Fac Pharm Cairo Univ. 2015;53(01):45–52. CrossRef
2. Lohrasbi V, Talebi M, Bialvaei AZ, Fattorini L, Drancourt M, Heidary M, et al. Trends in the discovery of new drugs for Mycobacterium tuberculosis therapy with a glance at resistance. Tuberculosis. 2018;109:17–27. CrossRef
3. Yaghi AR, Shaheed HS, Harun SN, Ali IAH, Khan AH. Survival trend of tuberculosis patients and risk factors associated with mortality and developing drug-resistant tuberculosis in hospital Pulau Pinang, Malaysia: a retrospective study. Adv Respir Med. 2022;90(06):467–82. CrossRef
4. Fernandes GFS, Thompson AM, Castagnolo D, Denny WA, Dos Santos JL. Tuberculosis drug discovery: challenges and new horizons. J Med Chem. 2022;65(11):7489–531. CrossRef
5. Pradipta IS, Idrus LR, Probandari A, Puspitasari IM, Santoso P, Alffenaar JWC, et al. Barriers to optimal tuberculosis treatment services at Community Health Centers: a qualitative study from a high preval from tuberculosis country. Front Pharmacol. 2022;13:857783. CrossRef
6. Chen T, Li Q, Guo L, Yu L, Li Z, Guo H, et al. Lower cytotoxicity, high stability, and long-term antibacterial activity of a Poly(Methacrylic Acid)/Isoniazid/Rifampin nanogel against multidrug-resistant intestinal Mycobacterium tuberculosis. Mater Sci Eng C Mater Biol Appl. 2016;58:659–65. CrossRef
7. Belachew T, Yaheya S, Tilahun N, Gebrie E, Seid R, Nega T, et al. Multidrug-resistant tuberculosis treatment outcome and associated factors at the University of Gondar Comprehensive Specialized Hospital: a ten-year retrospective study. Infect Drug Resist. 2022;15:2891–899. CrossRef
8. Glasauer S, Altmann D, Hauer B, Brodhun B, Haas W, Perumal N. First-line tuberculosis drug resistance patterns and associated risk factors in Germany, 2008-2017. PLoS One. 2019;14(06):e0217597. CrossRef
9. Diaz JMA, Abulfathi AA, te Brake LH, van Ingen J, Kuipers S, Magis-Escurra C, et al. New and repurposed drugs for the treatment of active tuberculosis: an update for clinicians. Respiration. 2023;102(02):83–100. CrossRef
10. Khoshnood S, Goudarzi M, Taki E, Darbandi A, Kouhsari E, Heidary M, et al. Bedaquiline: current status and future perspectives. J Glob Antimicrob Resist. 2021;25:48–59. CrossRef
11. Lagu SB, Yejella RP, Nissankararao S, Bhandare RR, Golla VS, Lokesh BVS, et al. Antitubercular activity assessment of fluorinated chalcones, 2-Aminopyridine-3-Carbonitrile and 2-Amino-4H-Pyran-3-Carbonitrile derivatives: in vitro, molecular docking and in silico drug likeliness studies. PLoS One. 2022;17(06):e0265068. CrossRef
12. de Faria CF, Moreira T, Lopes P, Costa H, Krewall JR, Barton CM, et al. Designing new antitubercular isoniazid derivatives with improved reactivity and membrane trafficking abilities. Biomed Pharmacother. 2021;144:112362. CrossRef
13. Aslan EK, Han M?, Krishna VS, Tamhaev R, Dengiz C, Do?an ?D, et al. Isoniazid linked to sulfonate esters via hydrazone functionality: design, synthesis, and evaluation of antitubercular activity. Pharmaceuticals. 2022;15(10):1301. CrossRef
14. Beteck RM, Seldon R, Jordaan A, Warner DF, Hoppe HC, Laming D, et al. Quinolone-isoniazid hybrids: synthesis and preliminary in vitro cytotoxicity and anti-tuberculosis evaluation. Medchemcomm. 2019;10(02):326–31. CrossRef
15. Oliveira JRS, Shiguemoto CYK, das Neves AR, Moreira FMF, Gomes GB, Perdomo RT, et al. Design, synthesis and antitubercular activity of novel isoniazid-cyclic-amine-azachalcones hybrids. J Braz Chem Soc. 2020;31(06):1284–295. CrossRef
16. Santoso M, Fahmi MRG, Kurniawan, YS, Ersam T, Fatmawati S, Martak F, et al. Isoniazid-Isatin hydrazone derivatives: synthesis, antitubercular activity and molecular docking studies. Trends Sci. 2021;18(21):39. CrossRef
17. Panda SS, Girgis AS, Mishra BB, Elagawany M, Devarapalli V, Littlefield WF, et al. Synthesis, computational studies, antimycobacterial and antibacterial properties of pyrazinoic acid–isoniazid hybrid conjugates. RSC Adv. 2019;9:20450–62. CrossRef
18. Assaleh MH, Bjelogrlic SK, Prlainovic N, Cvijetic I, Bozic A, Arandjelovic I, et al. Antimycobacterial and anticancer activity of newly designed cinnamic acid hydrazides with favorable toxicity profile. Arab J Chem. 2022;15:103532. CrossRef
19. Bairwa R, Kakwani M, Tawari NR, Lalchandani J, Ray MK, Rajan MGR, et al. Novel molecular hybrids of cinnamic acids and guanylhydrazones as potential antitubercular agents. Bioorg Med Chem Lett. 2010;20(05):1623–625. CrossRef
20. Ernawati T, Mun’im A, Hanafi M, Yanuar A. Synthesis of cinnamamide derivatives and their α-Glucosidase inhibitory activities. JSM. 2020;49(02):315–22. CrossRef
21. Hu CM, Wang WJ, Ye YN, Kang Y, Lin J, Wu PP, et al. Novel cinnamic acid magnolol derivatives as potent α-Glucosidase and α-Amylase inhibitors: synthesis, in vitro and in silico studies. Bioorg Chem. 2021;116:105291. CrossRef
22. Chen P, Xu Z, Wang X, He J, Yang J, Wang J, et al. Discovery of new cinnamic derivatives as anti-inflammatory agents for treating acute lung injury in mice. Arch Pharm (Weinheim). 2023;356(02):e2200191. CrossRef
23. Ernawati T, Yuliani T, Minarti M, Dewijanti ID. Anti-inflammatory activities of methyl trans-cinnamate derivatives on carrageenan-induced Paw Edema in Male Sprague-Dawley rats. Proceedings of International Summit on Science Technology and Humanity; 2019 December 3-4; Surakarta, Indonesia. Surakarta: Universitas Muhammadiyah Surakarta; 2020. p. 557–563. Available from: https://proceedings.ums.ac.id/index.php/iseth/article/view/1427.
24. De P, Bedos-Belval F, Vanucci-Bacqué C, Baltas M. Cinnamic acid derivatives in tuberculosis, malaria and cardiovascular diseases—a review. Curr Org Chem. 2012;16(06):747–68. CrossRef
25. Teneva Y, Simeonova R, Valcheva V, Angelova VT. Recent advances in anti-tuberculosis drug discovery based on hydrazide–hydrazone and thiadiazole derivatives targeting InhA. Pharmaceuticals (Basel). 2023;16(04):484. CrossRef
26. Aijijiyah NP, Wati FA, Rahayu R, Srilistiani A, Mahzumi F, Aulia T, et al. Synthesis, α-glucosidase inhibitory activity, and molecular docking of cinnamamides. Med Chem Res. 2023;32(04):723–35. CrossRef
27. Wati FA, Adyarini PU, Fatmawati S, Santoso M. Synthesis of pyrazinamide analogues and their antitubercular bioactivity. Med Chem Res. 2020;29(12):2157–163. CrossRef
28. Abuelizz HA, Dib RE, Marzouk M, Anouar E-H, Maklad YA, Attia HN, et al. Molecular docking and anticonvulsant activity of newly synthesized quinazoline derivatives. Molecules. 2017;22(07):E1094. CrossRef
29. Palomino JC, Martin A, Portaels F. Rapid drug resistance detection in Mycobacterium tuberculosis: a review of colourimetric methods. Clin Microbiol Infect. 2007;13(08):754–62. CrossRef
30. Chakansin C, Yostaworakul J, Warin C, Kulthong K, Boonrungsiman S. Resazurin rapid screening for antibacterial activities of organic and inorganic nanoparticles: potential, limitations and precautions. Anal Biochem. 2022;637:114449. CrossRef
31. Zhang Q, Han J, Zhu Y, Yu F, Hu X, Tong HHY, et al. Discovery of novel and potent InhA direct inhibitors by ensemble docking-based virtual screening and biological assays. J Comput Aided Mol Des. 2023;37:695–706. CrossRef
32. Martínez-Hoyos M, Perez-Herran E, Gulten G, Encinas L, Álvarez-Gómez D, Alvarez E, et al. Antitubercular drugs for an old target: GSK693 as a promising InhA direct inhibitor. eBioMedicine. 2016;8:291–301. CrossRef
33. Campanerut PAZ, Ghiraldi LD, Spositto FLE, Sato DN, Leite CQF, Hirata MH, et al. Rapid detection of resistance to pyrazinamide in Mycobacterium tuberculosis using the resazurin microtitre assay. J Antimicrob Chemother. 2011;66:1044–046. CrossRef