INTRODUCTION
Targeted cancer therapy has emerged as a promising approach to revolutionize the field of oncology. It aims to maximize treatment efficacy while minimizing adverse effects commonly associated with conventional cancer therapies [1]. The conventional methods which are included in chemotherapy and radiation therapy, often lack specificity and tend to damage healthy cells along with cancerous ones leading to severe side effects. In contrast, targeted therapy aims to selectively deliver therapeutic agents to cancer cells, leaving healthy cells unharmed [2]. In their pursuit of efficient targeted drug delivery, scientists have thoroughly investigated a variety of nanocarriers, including liposomes, micelles, dendrimers, and inorganic nanoparticles. Due to their distinctive qualities and adaptability, polymer-based nanoparticles have attracted a lot of attention in this industry. Polymers such as poly [lactic-co-glycolic acid] (PLGA), polyethylene glycol (PEG), and poly [lactic acid] make up the majority of these nanoparticles [3]. It is included in biocompatible and biodegradable. These polymers have proven to be highly suitable for targeted cancer therapy, offering numerous advantages. One notable advantage lies in the tunable physicochemical properties of polymer-based nanoparticles. By modifying the composition, molecular weight, and structure of the polymers, researchers can precisely control the size, surface charge, and drug release kinetics of the nanoparticles [4]. This tunability allows for customization to match the specific requirements of different cancer types and therapeutic agents. Another critical aspect is the high drug-loading capacity offered by polymer-based nanoparticles. These nanoparticles have a huge surface region-to-volume proportion and enable efficient encapsulation of a diverse array of therapeutic agents. It includes small molecules, proteins, nucleic acids, and even combination therapies. The high drug-loading capacity ensures optimal delivery of therapeutic payloads, maximizing the concentration of drugs at the tumor site while minimizing systemic exposure and off-target effects.
Polymer-based nanoparticles also offer the advantage of extended circulation time within the bloodstream. A defensive layer known as the “stealth effect” is framed by changing the nanoparticle surface with hydrophilic polymers like PEG This protective layer reduces the identification and removal of nanoparticles by the immune system, resulting in prolonged circulation and enhanced accumulation within the tumor site. Furthermore, polymer-based nanoparticles possess the capability for active targeting. Their surface can be easily tailored by attaching ligands or targeting components that specifically recognize and bind to cancer cell receptors or markers. This active targeting approach significantly improves the selectivity and accumulation of nanoparticles within the tumor, facilitating enhanced drug delivery to cancer cells while minimizing harm to healthy tissues (Fig. 1), thus Nanotechnology improves cancer detection and diagnosis [5].
This review article aims to delve into the wide-ranging application of polymer-based nanoparticles in targeted cancer therapy. It provides a comprehensive overview of the design principles underlying these nanoparticles, including their composition, size, and surface modification strategies [6]. Furthermore, it discusses various fabrication methods employed for the synthesis of polymer-based nanoparticles which are included in nanoprecipitation, emulsion/solvent evaporation, and self-assembly techniques. The review also explores the targeting strategies employed with polymer-based nanoparticles, including ligand-based targeting, pH-responsive targeting, and stimuli-responsive targeting. It discusses the new advances in preclinical and clinical examinations that have showcased the potential of polymer-based nanoparticles in improving cancer treatment outcomes.
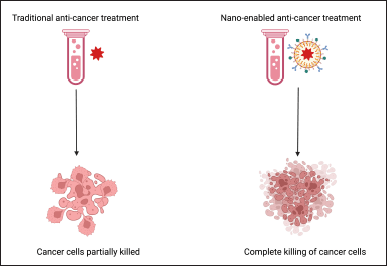 | Figure 1. Nanotechnology improves cancer detection and diagnosis. [Click here to view] |
MECHANISMS OF TARGETED CANCER THERAPY
Targeting tumor heterogeneity
Cancer is a very varied disease because different groups of cells within the tumor exhibit different genetic profiles and behavior behaviors. Combination drugs have recently been created in targeted cancer therapy to address this tumor heterogeneity, to simultaneously target numerous genetic disorders. Researchers are also investigating strategies to target cancer stem cells, a subgroup of tumor cells that cause tumor growth and medication resistance, to produce more significant and durable therapeutic responses [7].
Chimeric antigen receptor T (CAR-T) cells and adoption-based cell therapies
Adoptive cell therapies, in particular CAR-T therapy, have grown into ground-breaking strategies in the treatment of specific cancers. CAR-T cells are designed to detect and target cancer cells that express particular surface antigens [8]. The library of targetable antigens is growing thanks to recent developments in this sector, which additionally improve CAR-T cell durability and persistence and minimize side effects.
Targeting non-coding RNAs
Non-coding long RNAs, in particular microRNAs, are key players in the emergence and spread of cancer. Modern research is identifying many non-coding RNAs as potential therapeutic targets. It is being researched how to target and alter the activity of these non-coding RNAs to stop the growth and spread of malignant cells [9]. These methods include treatments based on RNA interference and antisense oligonucleotides.
Radiolabeled therapeutics
In radiolabeled therapeutics, targeted agents such as antibodies or small compounds are joined with radioactive isotopes. These radiotherapies target and localize the killing of tumor cells by delivering radiation directly to cancer cells that express the target antigen. Modern research in radiolabeled therapeutics is expanding the repertoire of targetable antigens, and radioisotope payloads are being tailored for better therapeutic outcomes [10].
Cancer microenvironment modulation
A patient’s capacity to answer therapy is fundamentally impacted by the cancer microenvironment. Modern research aims to alter the cancer microenvironment to increase the effectiveness of targeted drugs. Among the methods utilized to boost drug absorption and immune cell infiltration are targeting immunosuppressive cells, such as regulatory T-cells and myeloid-derived suppressor cells, as well as reprogramming the cancer stroma [10].
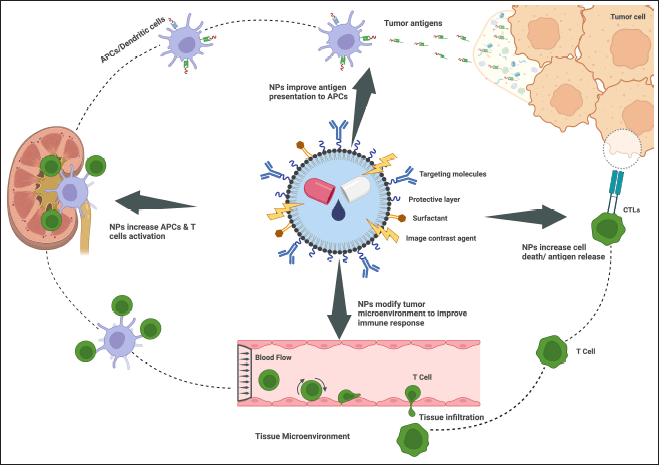 | Figure 2. Schematic diagram of polymer-based nanoparticles in targeted cancer therapy working method. [Click here to view] |
Synthetic lethality and vulnerability screens
Advances in high-throughput screening technologies have made it simpler to identify synthetic lethal interactions in cancer cells. Synthetic lethality is the process of killing cancer cells by simultaneously shutting down two genes, neither of which is required in and of itself to induce cancer (Fig. 2). It could be able to eliminate cancer cells without harming healthy cells by concentrating on synthetic deadly interactions.
CURRENT STRATEGIES IN TARGETED CANCER THERAPY
Small molecule inhibitors
Small molecules are designed to inhibit specific proteins involved in cancer growth and survival [11]. Examples: Imatinib targeting breakpoint cluster region-abelson in chronic myeloid leukemia, vemurafenib targeting mutant v-Raf murine sarcoma viral oncogene homolog B (BRAF) in melanoma.
Monoclonal antibodies
Monoclonal antibodies recognize specific cancer cell surface proteins and interfere with their function or trigger immune-mediated destruction. Examples: Trastuzumab targeting HER2 in breast cancer, rituximab targeting CD20 in B-cell lymphomas.
Immune checkpoint inhibitors
These drugs target molecules that suppress the immune response, allowing the immune system to recognize and attack cancer cells more effectively. Examples: Pembrolizumab targeting PD-1 in various cancers, nivolumab targeting PD-1 in melanoma and lung cancer.
RNA THERAPIES
Spherical nucleic acids (SNAs)
Nanostructures with a spherical arrangement of nucleic acids are called SNAs. Small interfering RNA (siRNA) and other RNA medicines may be delivered to cancer cells using these structures. Treatments for brain cancer based on RNA have the potential to be more effective using this method [12].
siRNA using polymetformin nanoparticles
The potential of polymetformin nanoparticles as vehicles for the delivery of siRNA has been investigated. Treatment accuracy and efficacy may be improved by using these nanoparticles to shield therapeutic RNA from degradation and enhance its targeted delivery to cancer cells.
NANOPARTICLE-BASED DRUG DELIVERY SYSTEMS
Liposomal delivery systems
Encapsulating medications or nucleic acids in lipid nanoparticles called liposomes improves their delivery to cancer cells and protects them from destruction. Several therapeutic medicines have been explored for delivery to brain tumors using this method.
Polymeric nanoparticles
It is possible to immobilize therapeutic payloads, such as RNA treatments, in nanoparticles manufactured from biocompatible polymers. Neurocognitive disorders are notoriously difficult to treat, but these nanoparticles can be made to cross the blood-brain barrier.
PERSONALIZED MEDICINE
Genomic profiling
Recent developments in genetic profiling have made it possible to tailor cancer treatments to individual patients. Customized medicines may be developed to target the distinct features of a patient’s tumor by identifying certain genetic abnormalities or changes in their cancer cells [13].
TARGETING CANCER STEM CELLS
Stem cell-targeted therapies
Because of their significance in tumor development, progression, and recurrence, cancer stem cells are the focus of current research efforts to find new treatments. Preventing cancer recurrence and improving long-term results are the goals of targeting these cells.
COMBINATION THERAPIES
Combinatorial approaches
Combination treatments aim to tackle the cancer cells’ adaptability and heterogeneity by combining several targeted drugs or modalities. The goals of this strategy are to increase reactivity to therapy and decrease resistance.
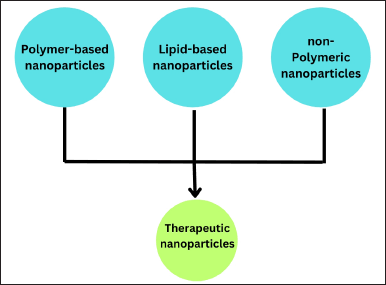 | Figure 3. Representation of the therapeutic nanoparticles. [Click here to view] |
PREPARATION OF POLYMERIC NANOPARTICLE
Polymeric nanoparticle synthesis
Nanoparticle synthesis methods are essential in the development of polymer-based nanoparticles for targeted cancer therapy [14]. These methods enable scientists to create tiny particles. These particles can carry therapeutic drugs to specific sites in the body, such as tumor tissues. There are several techniques commonly used for nanoparticle synthesis.
Emulsion/solvent evaporation
Imagine mixing oil and water in a bowl. In this method, the polymer is dissolved in an organic solvent, such as oil. Then, it is emulsified in an aqueous solution, like water. The emulsion is made by vivaciously blending the two fluids. Afterward, the organic solvent is removed, leaving behind nanoparticles that contain the polymer and any therapeutic drugs [15].
Nanoprecipitation
Picture pouring a colored liquid into clear water. Nanoprecipitation involves mixing a polymer solution with a nonsolvent, which causes the formation of nanoparticles [15]. This happens because the polymer can no longer dissolve in the mixture and forms tiny particles instead. The resulting nanoparticles can encapsulate the therapeutic drugs within their structure [16].
Self-assembly
Think of building blocks fitting together to form a structure. Self-assembly is a method that takes advantage of the natural properties of the polymer to form nanoparticles. The polymer molecules arrange themselves into organized structures, such as micelles [like tiny spheres] or aggregates, which can encapsulate the therapeutic drugs [17].
These synthetic methods allow scientists to control the size, shape, and properties of the nanoparticle [18]. These are important factors for efficient drug delivery. By using these techniques, researchers can create polymer-based nanoparticles that can transport therapeutic drugs to targeted cancer cells, enhancing the effectiveness of cancer treatment.
Encapsulating therapeutic agents
Encapsulating therapeutic agents is a crucial method in using polymer-based nanoparticles for targeted cancer therapy Schematic representations of the therapeutic nanoparticles [19,20]. It involves incorporation of the drugs into the nanoparticles to enable controlled release and targeted delivery to cancer cells. There are different techniques used for encapsulation, including attaching the drugs chemically to the nanoparticles (covalent conjugation) (Fig. 3), trapping them physically within the nanoparticle structure [physical entrapment], or using electrostatic interactions to attract charged drugs to the nanoparticle surface [21].
Covalent conjugation connects the drugs to the nanoparticles through chemical bonds, ensuring a strong and long-lasting attachment. Physical entrapment involves capturing the drugs within the nanoparticles using physical interactions like sticking them together or trapping them in small spaces. Electrostatic interactions occur when the drugs have an electric charge and are drawn to the opposite charge on the nanoparticle surface. Encapsulating the drugs within the nanoparticles brings several benefits for targeted cancer therapy [22]. It shields the drugs from degradation, improves their solubility, and enables precise delivery to the tumor site. By encapsulating the drugs, they can avoid getting eliminated by the body’s natural defense systems and accumulate more effectively at the tumor, while minimizing harm to healthy tissues [23]. Overall, encapsulating therapeutic agents within polymer-based nanoparticles offers a promising approach for targeted cancer therapy. It provides a versatile platform to develop personalized treatments that deliver drugs directly to cancer cells, improving the effectiveness and reducing side effects for cancer patients [24].
SURFACE MODIFICATION TECHNIQUES
Surface modification techniques play a vital role in the application of polymer-based nanoparticles for targeted cancer therapy. These techniques involve modifying the outer surface of nanoparticles to enhance their ability to target cancer cells, improve stability, and optimize drug delivery [24]. Two commonly used surface modification strategies are ligand conjugation and stealth coating.
Ligand conjugation is a method where specific molecules like antibodies or peptides are attached to the nanoparticle surface (Fig. 4). These molecules can recognize and bind to specific markers present on the surface of cancer cells [25]. By attaching these molecules, the nanoparticles can actively target and accumulate at the tumor site. This targeted approach improves the precision of drug delivery to cancer cells, making the therapy more effective while minimizing harm to healthy tissues [26].
Stealth coating, also called PEGylation, is a method where a layer of a polymer called PEG [27] is applied to the surface of nanoparticles. PEG is a safe polymer that attracts water and creates a protective shield around the nanoparticles. This shield helps the nanoparticles to avoid detection and removal by the immune system, allowing them to stay in the bloodstream for a longer time. By escaping the immune system, the nanoparticles can gather at the tumor site by utilizing a process called the enhanced permeability and retention (EPR) effect. This effect takes advantage of the special characteristics of blood vessels in tumors, enabling the nanoparticles to specifically accumulate within the tumor tissues [28].
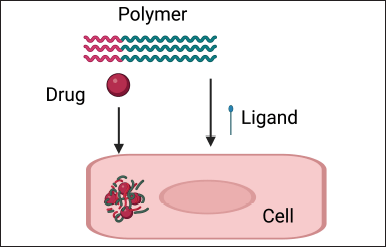 | Figure 4. Ligand conjunction. [Click here to view] |
Surface modification techniques offer several benefits in targeted cancer therapy. They enhance the targeting ability of nanoparticles, ensuring they reach cancer cells more effectively while minimizing damage to healthy cells [29]. Moreover, surface modifications improve the stability of nanoparticles and their circulation in the body, enabling sustained release of drugs and better accumulation at the tumor site [30]. These modifications also provide opportunities for personalized medicine by tailoring the nanoparticles to specific types of cancer or individual patient requirements [31]. In conclusion, surface modification techniques, such as ligand conjugation and stealth coating, are crucial for optimizing the performance of polymer-based nanoparticles in targeted cancer therapy. These modifications improve targeting efficiency, enhance stability, and enable controlled drug delivery, promising more effective and personalized treatments for cancer [32].
CONTROLLED DRUG RELEASE
Controlled drug release is an important method used in the application of polymer-based nanoparticles for targeted cancer therapy. It involves creating nanoparticles that can release therapeutic drugs in a controlled and gradual manner at the tumor site (Fig. 5). This controlled release helps to ensure that the drugs are delivered effectively to the cancer cells, maximizing their effectiveness while minimizing side Drugs [33].
 | Figure 5. Forms of drugs. [Click here to view] |
 | Table 1. Natural and synthetic polymer. [Click here to view] |
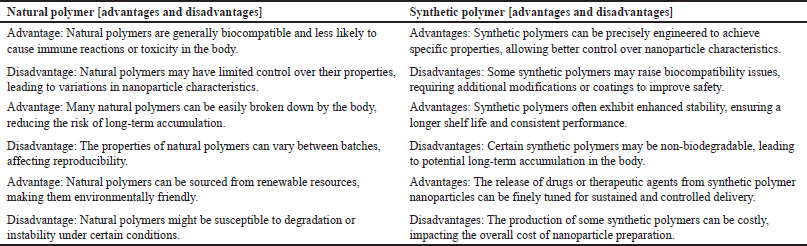 | Table 2. Advantages and disadvantages of natural and synthetic polymers [36] . [Click here to view] |
 | Table 3. Techniques and types of polymeric nanoparticles. [Click here to view] |
There are different ways to achieve controlled drug release from polymer-based nanoparticles. One way is to use special polymers that respond to specific conditions found in tumors, such as changes in pH or temperature. When exposed to these conditions, the polymers change shape and release the drug. Another approach is to use biodegradable polymers that break down over time, slowly releasing the drug [34]. The rate of degradation can be adjusted to control the release of the drug. Nanoparticles with tiny pores or a porous structure can also be used for controlled drug release. These pores are designed to allow the drug to pass through based on its size. By controlling the size of the pores, the release of the drug can be managed. Additionally, external stimuli like light, heat, or magnetic fields can be used to trigger drug release from the nanoparticles. Controlled drug release has many benefits. It allows for precise control over when and how much drug is released, ensuring the best treatment outcomes [31]. It also reduces the exposure of healthy tissues to the drug, minimizing side effects. This method also enables the use of potent drugs that may have limited solubility or stability, as the nanoparticles provide a protective environment for the drug. In conclusion, controlled drug release is a key method in the use of polymer-based nanoparticles for targeted cancer therapy. It involves designing nanoparticles that release drugs in a controlled manner, improving treatment effectiveness and minimizing side effects [35]. This approach offers greater precision, prolonged drug action, and improved outcomes in cancer treatment.
The natural and synthetic polymers are mentioned in Table 1.
The advantages and disadvantages of natural and synthetic polymers are mentioned in Table 2.
The techniques and types of polymeric nanoparticles in Table 3.
POLYMERIC-BASED NANOPARTICLES IN THE TREATMENT OF CANCER
Polymeric-based nanoparticles stand out in malignant growth treatment because of their extraordinary properties and expected applications. These nanoparticles are regularly made out of biocompatible and biodegradable polymers that can exemplify or form with remedial specialists, taking into consideration focused on and controlled conveyance to malignant growth cells. This is an outline of the way polymeric-based nanoparticles are utilized in the therapy of disease [36].
Drug delivery
Polymeric nanoparticles can embody different sorts of helpful specialists, for example, chemotherapy drugs, little particles, and, surprisingly, natural specialists like siRNA or miRNA. The nanoparticles shield these specialists from debasement in the circulatory system and work with their aggregation in cancer tissues. This designated drug diminishes fundamental poisonousness and upgrades the adequacy of the treatment by concentrating the helpful payload at the cancer site [37].
Targeted therapy
Functionalization of polymeric nanoparticles with a focus on ligands, like antibodies or peptides, empowers the dynamic focusing of disease cells. These ligands perceive explicit markers overexpressed on the outer layer of malignant growth cells, permitting the nanoparticles to tie to and enter these cells specifically. This approach upgrades the particularity of the treatment and limits harm to solid cells [38].
EPR effect
Polymeric nanoparticles can exploit the EPR impact, which is a trademark element of growth vasculature. Cancers will generally have flawed veins and debilitated lymphatic waste, prompting the collection of nanoparticles inside the growth tissue. This detached focus on the procedure considers special amassing of nanoparticles in the growth, further working on the restorative result [39].
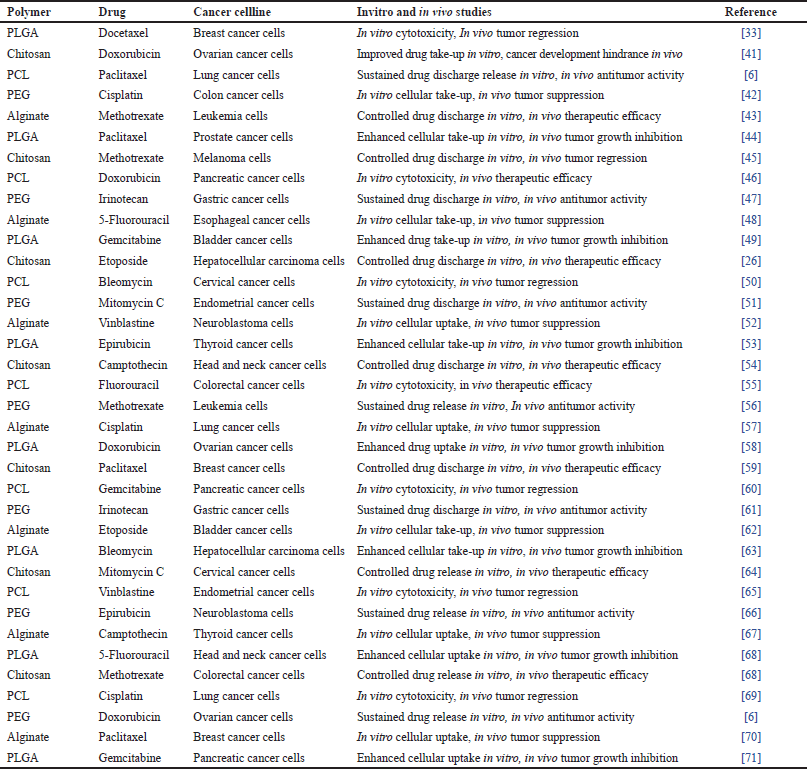 | Table 4. Polymer-based nanoparticles in the treatment of cancer. [Click here to view] |
Combination therapy
Polymeric nanoparticles can convey various remedial specialists, empowering mix treatment draws near. This is particularly valuable for conquering drug opposition or focusing on various pathways associated with disease movement at the same time [40].
Here is the table of polymer-based nanoparticles in the treatment of cancer in Table 4.
CHALLENGES AND FUTURE DIRECTIONS
Polymer-based nanoparticles have emerged as promising drug delivery systems in targeted cancer therapy, offering enhanced drug stability, controlled release, and improved tumor-specific targeting.
Biocompatibility and safety concerns
Guaranteeing the biocompatibility and well-being of polymer-based nanoparticles is a critical challenge in their clinical application. Some polymers may trigger immune responses or cause toxicity limiting their therapeutic use [72]. Advanced research focuses on developing biocompatible polymers and surface modifications. It is used to minimize adverse effects and enhance nanoparticle clearance from the body.
Drug loading and releasing kinetics
Efficient loading of therapeutic agents into polymer-based nanoparticles and controlled release at the tumor site are critical factors influencing treatment efficacy. Advanced research aims to optimize drug loading techniques and tailor releasing kinetics to match the drug’s pharmacokinetics and therapeutic window, ensuring sustained drug exposure at the target site [73].
Biomarker identification
Identifying the reliable biomarkers for patient selection and predicting response to targeted therapies is crucial for maximizing treatment efficacy. Advances in genomic profiling and liquid biopsies are aiding in this area. Examples: Testing for EGFR mutations to predict response to EGFR inhibitors in lung cancer, and HER2 amplification testing to guide HER2-targeted therapy in breast cancer [74].
Personalized nanoparticle design
Each cancer patient’s tumor exhibits unique characteristics, necessitating personalized treatment approaches. Advanced research is moving towards tailoring polymer-based nanoparticles based on patient-specific factors, such as genetic mutations, tumor heterogeneity, and treatment response. Personalized nanoparticle design aims to maximize treatment efficacy while minimizing off-target effects, fostering the development of precision medicine in cancer therapy [75,76].
Combination therapies combining therapies
Combining targeted therapies with other treatment modalities, like chemotherapy, radiation therapy, or immunotherapy, holds promise for improved outcomes. The rational design of combination regimens is an active area of investigation [28]. Examples: Combining BRAF and MEK inhibitors in BRAF-mutated melanoma, combining immune checkpoint inhibitors with other immunomodulatory agents.
DISCUSSION
Targeted cancer therapy using polymer-based nanoparticles has emerged as a potential approach in the field of oncology. This study has covered a wide range of topics related to the use of polymer-based nanoparticles in targeted cancer [77] therapy, which includes design principles, manufacturing processes, targeting strategies, and most recent advancements [78].
The design principles of polymer-based nanoparticles are crucial for their successful application in targeted cancer therapy [79]. By changing the composition, molecular weight, and structural characteristics of polymers, it is possible to precisely control the nanoparticles’ size, surface charge, and drug release kinetics [80]. This tunability is essential to increase drug delivery to cancer cells while avoiding off-target effects [81]. The advantages of polymer-based nanoparticles are their high loading and drug-loading capacity, biocompatibility, and biodegradability. Their capacity to incorporate proteins, nucleic acids, small chemicals, and combination therapies makes them a flexible platform that can support a variety of therapeutic approaches.
While making polymer-based nanoparticles for targeted malignant growth treatment, fabrication techniques are crucial. Techniques that are frequently employed include self-assembly, emulsion/solvent evaporation, and nanoprecipitation. A polymer solution and a nonsolvent are combined in nanoprecipitation, which results in the creation of nanoparticles. A polymer that has been dissolved in an organic solvent must be emulsified with an aqueous solution before the organic solvent is evaporated [82]. Self-assembly creates nanoparticles by arranging polymer molecules into well-organized structures, taking advantage of the intrinsic qualities of polymers. These production techniques offer important control over the nanoparticles’ size, shape, and other characteristics.
Targeting strategies are employed to enhance the specificity and accumulation of polymer-based nanoparticles within tumors while minimizing harm to healthy tissues. Ligand-based targeting involves attaching specific molecules, such as antibodies or peptides, to the nanoparticle surface. These molecules recognize and bind to receptors or markers present on the surface of cancer cells, facilitating active targeting and accumulation at the tumor site [70]. This approach improves the selectivity of drug delivery and reduces the impact on healthy tissues. pH-responsive targeting takes advantage of the acidic tumor microenvironment to trigger drug release from the nanoparticles. Stimuli-responsive targeting utilizes external stimuli, such as light, heat, or magnetic fields, to trigger drug release at specific sites. These targeting strategies enable precise drug delivery, ensuring that therapeutic agents reach the intended location within the tumor [83].
Recent advances in preclinical and clinical studies have demonstrated the potential of polymer-based nanoparticles in targeted cancer therapy. These studies have highlighted the efficacy of polymer-based nanoparticles in delivering therapeutic agents to targeted cancer cells [84]. Stealth coating is accomplished by applying a layer of PEG to the nanoparticle surface and broadens expanding the dissemination season of nanoparticles in the circulation system. It permits in for improved aggregation at the growth site through the EPR impact. It allows enhanced accumulation at the tumor site through the EPR effect. Ligand conjugation improves active targeting and accumulation of nanoparticles within the tumor by specifically recognizing and binding to cancer cell receptors or markers. PH-responsive and stimuli-responsive strategies provide controlled and precise drug release, maximizing treatment effectiveness and minimizing side effects. These advances have shown promising results in preclinical and clinical settings. They are demonstrating the potential of polymer-based nanoparticles to improve cancer treatment outcomes [85].
Combination therapies and personalized medicine are emerging as important areas of research in the field of targeted cancer therapy using polymer-based nanoparticles [86]. Combining targeted therapies with other therapy modalities, like chemotherapy, radiation treatment, or immunotherapy, holds promise for improved outcomes. The rational design of combination regimens aims to enhance treatment effectiveness and overcome resistance mechanisms. Personalized medicine focuses on tailoring nanoparticle-based treatments to specific cancer types or individual patient requirements. This approach considers factors such as biomarkers, genomic profiling, and the unique characteristics of each patient’s tumor, allowing for personalized and optimized treatment strategies.
The application of polymer-based nanoparticles in targeted cancer therapy offers a personalized and effective approach to the treatment of various cancers. The designated principles, fabrication methods, targeting strategies, and recent advancements are discussed in this review emphasizing the potential of polymer-based nanoparticles maximize treatment efficacy while minimizing adverse effects. Further research and clinical studies are necessary to fully explore the capabilities of these nanoparticles and optimize their use in personalized cancer treatments. Continued advancements in the field of targeted cancer therapy using polymer-based nanoparticles hold great promise for improving patient outcomes and revolutionizing the field of oncology.
CONCLUSION
In conclusion, polymer-based nanoparticles have arisen as a promising tool in targeted cancer therapy. These nanoparticles offer tunable physicochemical properties, high drug-loading capacity, extended circulation time, and active targeting capabilities. Their design principles, fabrication methods, and surface modification techniques contribute to their efficacy in delivering therapeutic agents to cancer cells while minimizing harm to healthy tissues. Controlled drug release mechanisms further enhance their effectiveness by ensuring precise and gradual drug delivery at the tumor site. Recent advances in preclinical and clinical studies have demonstrated the potential of polymer-based nanoparticles in improving cancer treatment outcomes. The combination of targeted therapies, personalized medicine, and optimized treatment strategies holds promise for further advancements in the field. In summary, the application of polymer-based nanoparticles in targeted cancer therapy offers a personalized and effective approach to the treatment of various cancers. These nanoparticles can be precisely engineered to deliver therapeutic agents to specific tumor sites, minimizing side effects and maximizing treatment efficacy. Continued research and clinical studies are needed to fully explore their capabilities and optimize their use in personalized cancer treatments. With further advancements, polymer-based nanoparticles have the potential to revolutionize cancer therapy, providing patients with more effective and tailored treatments.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by Research Excellence and Innovation Grant (Project Code: REIG-FPS-2023/041) under the Centre of Excellence in Research, Value Innovation and Entrepreneurship (CERVIE), UCSI University, Malaysia.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Rizi HAY, Shin DH, Rizi SY. Polymeric nanoparticles in cancer chemotherapy: a narrative review. Iran J Public Health [Internet]. CrossRef
2. Salari N, Faraji F, Torghabeh FM, Faraji F, Mansouri K, Abam F, et al. Polymer-based drug delivery systems for anticancer drugs: a systematic review. Cancer Treat Res Commun [Internet]. 2022 Jan 1;32:100605. CrossRef
3. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev [Internet]. 2003 Feb 1;55(3):329–47. CrossRef
4. Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol [Internet]. 2019 Dec 1;12(1). CrossRef
5. Nicolas S, Bolzinger M, Jordheim LP, Chevalier Y, Fessi H, Almouazen E. Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int J Pharm [Internet]. 2018 Oct 1;550(1–2):170–9. CrossRef
6. Alvi M, Yaqoob A, Rehman K, Shoaib SM, Akash MSH. PLGA-based nanoparticles for the treatment of cancer: current strategies and perspectives. AAPS Open [Internet]. 2022 Aug 1;8(1). CrossRef
7. Jank? F. Tumor heterogeneity in the clinic: is it a real problem? Ther Adv Med Oncol [Internet]. 2013 Dec 20;6(2):43–51. CrossRef
8. Jackson HJ, Rafiq S, Brentjens RJ. Driving CAR T-cells forward. Nat Rev Clin Oncol [Internet]. 2016 Mar 22;13(6):370–83. CrossRef
9. Arun G, Diermeier SD, Spector DL. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol Med [Internet]. 24(3):257–77. CrossRef
10. D’Huyvetter M, Xavier C, Caveliers V, Lahoutte T, Muyldermans S, Devoogdt N. Radiolabeled nanobodies as theranostic tools in targeted radionuclide therapy of cancer. Expert Opin Drug Deliv [Internet]. 2014 Jul 18;11(12):1939–54. CrossRef
11. Chan DA, Giaccia AJ. Harnessing synthetic lethal interactions in anticancer drug discovery. Nat Rev Drug Discov [Internet]. 2011 Apr 29;10(5):351–64. CrossRef
12. Yerpude ST, Potbhare AK, Bhilkar PR, Alok R, Singh RP, Abdala A, et al. Biomedical,clinical and environmental applications of platinum-based nanohybrids: an updated review. Environ Res [Internet]. 2023 Aug 1;231:116148. CrossRef
13. Yerpude ST, Potbhare AK, Bhilkar PR, Thakur P, Khiratkar P, Desimone MF, et al. Computational analysis of nanofluids-based drug delivery system: preparation, current development and applications of nanofluids. Elsevier eBooks [Internet]. 2022. p. 335–64. CrossRef
14. Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol [Internet]. 2015 Apr 1;25(4):198–213. Available from: https://www.cell.com/trends/cell-biology/fulltext/S0962-8924(14)00199-8
15. Alabdali AYM, Kzar MS, Chinnappan S, Mani RR, Selvaraja M, Wen KJ, et al. Application of nanoantibiotics approach against anti-bacterial resistance. Int J Appl Pharm[Internet]. 2022 May 7;34–9. CrossRef
16. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules [Internet]. 2021 Sep 29;26(19):5905. CrossRef
17. Lee JH, Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci [Internet]. 2015 Mar 1;125:75–84. CrossRef
18. Parreidt TS, Müller K, Schmid M. Alginate-based edible films and coatings for food packaging applications. Foods [Internet]. 2018 Oct 17;7(10):170. CrossRef
19. Singh S, Kumar S, Khanna V. A review on surface modification techniques. Mater Today: Proc [Internet]. 2023 Jan 1. CrossRef
20. Xie M, Pan Y, An Z, Huang S, Dong M. Review on surface polishing methods of optical parts. Adv Mater Sci Eng [Internet]. 2022 May 12;2022:1–30. CrossRef
21. Golroudbari HT, Banikarimi SP, Ayati A, Hadizadeh A, Zavareh ZK, Hajikhani K, et al. Advanced micro-/nanotechnologies for exosome encapsulation and targeting in regenerative medicine. Clin Exp Med [Internet]. 2023 Jan 27;23(6):1845–66. CrossRef
22. Boike L, Henning NJ, Nomura DK. Advances in covalent drug discovery. Nat Rev Drug Discov [Internet]. 2022 Aug 25;21(12):881–98. CrossRef
23. Yetisgin AA, Çetinel S, Zuvin M, Ko?ar A, Kutlu Ö. Therapeutic nanoparticles and their targeted delivery applications. Molecules [Internet]. 2020 May 8;25(9):2193. CrossRef
24. El -Hack MEA, Alabdali AYM, Aldhalmi AK, Reda FM, Bassiony SS, Selim S, et al. Impacts of purslane (Portulaca oleracea) extract supplementation on growing Japanese quails’ growth, carcass traits, blood indices, nutrients digestibility and gut microbiota. Poult Sci [Internet]. 2022 Nov 1;101(11):102166. CrossRef
25. Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomed [Internet]. 2014 Jan 1;9:467–483. CrossRef
26. Patra JK, Das G, Fraceto LF, Campos EVR, Del Pilar Rodríguez-Torres M, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol [Internet]. 2018 Sep 19;16(1):71. CrossRef
27. Kim JU. Block copolymer thin films on patterned substrates [Internet]. 2010. Available from: https://scholarworks.unist.ac.kr/handle/201301/51633
28. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov [Internet]. 2020 Dec 4;20(2):101–24. CrossRef
29. Liu L, Pan D, Chen S, Martikainen MV, Kårlund A, Jin K, et al. Systematic design of cell membrane coating to improve tumor targeting of nanoparticles. Nat Commun [Internet]. 2022 Oct 19;13(1):6181. CrossRef
30. Niculescu AG, Grumezescu AM. Novel tumor-targeting nanoparticles for cancer treatment—a review. Int J Mol Sci [Internet]. 2022 May 8;23(9):5253. CrossRef
31. Sun L, Li H, Ye Y, Yang L, Islam R, Tan S, et al. Smart nanoparticles for cancer therapy. Signal Transduct Target Ther [Internet]. 2023 Nov 3;8(1). CrossRef
32. Abdelkawi A, Slim A, Zinoune Z, Pathak Y. Surface modification of metallic nanoparticles for targeting drugs. Coatings [Internet]. 2023 Sep 21;13(9):1660. CrossRef
33. Zieli?ska A, Carreiró F, Oliveira AMM, Neves A, Pires BA, Venkatesh DN, et al. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules [Internet]. 2020 Aug 15;25(16):3731. CrossRef
34. Alabdali AYM, Khalid R, Kzar M, Ezzat MO, Huei GM, Hsia TW, et al. Design, synthesis, in silico and antibacterial evaluation of curcumin derivatives loaded nanofiber as potential wound healing agents. J King Saud Univ Sci [Internet]. 2022 Oct 1;34(7):102205. CrossRef
35. Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. Drug release study of the chitosan-based nanoparticles. Heliyon [Internet]. 2022 Jan 1;8(1):e08674. doi: https://www.cell.com/heliyon/pdf/S2405-8440(21)02777-8.pdf
36. Jose S, Cinu TA, Sebastian R, Shoja MH, Aleykutty N, Durazzo A, et al. Transferrin-conjugated docetaxel–PLGA nanoparticles for tumor targeting: influence on MCF-7 cell cycle. Polymers [Internet]. 2019 Nov 19;11(11):1905. CrossRef
37. Sharma S, Pathania AR. Biodegradable polymers green synthesis of nanoparticle – an overview. Mater Today: Proc [Internet]. 2022 Jan 1;62:3827–31. CrossRef
38. Lee C, Kim TI, Oh DW, Bae SM, Ryu J, Kong H, et al. In vivo and in vitro anticancer activity of doxorubicin-loaded DNA-AUNP nanocarrier for the ovarian cancer treatment. Cancers [Internet]. 2020 Mar 9;12(3):634. CrossRef
39. Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol [Internet]. 2013 Jan 1;04(02):1000164. CrossRef
40. Li W, Sun Y, Chen J, Jiang Z, Yang J. PEGylated cisplatin nanoparticles for treating colorectal cancer in a PH-responsive manner. J Immunol Res [Internet]. 2022 Aug 5;2022:1–11. CrossRef
41. Gawande MB, Goswami A, Felpin F, Asefa T, Huang X, Silva R, et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev [Internet]. 2016 Mar 3;116(6):3722–811. CrossRef
42. Nogueira DR, Tavano L, Mitjans M, Pérez L, Infante MR, Vinardell MP. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials [Internet]. 2013 Apr 1;34(11):2758–72. CrossRef
43. Kciuk M, Marciniak B, Kontek R. Irinotecan—still an important player in cancer chemotherapy: a comprehensive overview. Int J Mol Sci [Internet]. 2020 Jul 12;21(14):4919. CrossRef
44. Cheng M, Dai D. Inhibitory of active dual cancer targeting 5-fluorouracil nanoparticles on liver cancer in vitro and in vivo. Front Oncol [Internet]. 2022 Aug 5;12:971475. CrossRef
45. Nair PR. Delivering combination chemotherapies and targeting oncogenic pathways via polymeric drug delivery systems. Polymers [Internet]. 2019 Apr 5;11(4):630. CrossRef
46. Bonferoni MC, Gavini E, Rassu G, Maestri M, Giunchedi P. Chitosan nanoparticles for therapy and theranostics of hepatocellular carcinoma (HCC) and liver-targeting. Nanomaterials [Internet]. 2020 Apr 30;10(5):870. CrossRef
47. ?api?ska Z, Szwedowicz U, Choroma?ska A, Saczko J. Electroporation and electrochemotherapy in gynecological and breast cancer treatment. Molecules [Internet]. 2022 Apr 12;27(8):2476. CrossRef
48. Nocito MC, De Luca A, Prestia F, Avena P, La Padula D, Zavaglia L, et al. Antitumoral activities of curcumin and recent advances to improve its oral bioavailability. Biomedicines [Internet]. 2010 Oct 14;9(10):1476. Available from: https://www.mdpi.com/2227-9059/9/10/1476
49. Moammeri A, Abbaspour K, Zafarian A, Jamshidifar E, Motasadizadeh H, Moghaddam FD, et al. PH-responsive, adorned nanoniosomes for codelivery of cisplatin and epirubicin: synergistic treatment of breast cancer. ACS Appl Bio Mat [Internet]. 2022 Feb 7;5(2):675–90. CrossRef
50. Entezar-Almahdi E, Mohammadi-Samani S, Tayebi L, Farjadian F. Recent advances in designing 5-fluorouracil delivery systems: a stepping stone in the safe treatment of colorectal cancer. Int J Nanomed [Internet]. 2020 Jul 1;Volume 15:5445–58. CrossRef
51. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther [Internet]. 2018 Mar 16;3(1):7. CrossRef
52. Wang H, Zhao Y, Wu Y, Hu YL, Nan K, Nie G, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials [Internet]. 2011 Nov 1;32(32):8281–90. CrossRef
53. Niu S, Williams GR, Wu J, Wu J, Zhang X, Chen X, et al. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J Nanobiotechnol [Internet]. 2019 Sep 10;17(1). CrossRef
54. Sloat BR, Sandoval MA, Dong L, Chung WG, Lansakara-P DSP, Proteau P, et al. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int J Pharm [Internet]. 2011 May 1;409(1–2):278–88. CrossRef
55. Wang X, Wang Y, Chen ZG, Shin DM. Advances of cancer therapy by nanotechnology. Cancer Res Treat [Internet]. 2009 Jan 1;41(1):1–11. CrossRef
56. Son KH, Hong JH, Lee JW. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed [Internet]. 2016 Oct 1;Volume 11:5163–85. CrossRef
57. Sun Y, Wen M, Yang Y, He M, Li A, Bai L, et al. Cancer nanotechnology: enhancing tumor cell response to chemotherapy for hepatocellular carcinoma therapy. Asian J Pharm Sci [Internet]. 2019 Nov 1;14(6):581–94. CrossRef
58. Jia M, Yang L, Yang X, Huang Y, Wu H, Huang Y, et al. Development of both methotrexate and mitomycin C loaded PEGylated chitosan nanoparticles for targeted drug codelivery and synergistic anticancer effect. ACS Appl Mater Interfaces [Internet]. 2014 Jul 10;6(14):11413–23. CrossRef
59. Tomko AM, Whynot EG, O’Leary L, Dupré DJ. Anti-cancer potential of cannabis terpenes in a taxol-resistant model of breast cancer. Can J Physiol Pharmacol [Internet]. 2022 Aug 1;100(8):806–17. CrossRef
60. Meng L, Wang Z, Hou Z, Wang H, Zhang X, Zhang X, et al. Study of epirubicin sustained–release chemoablation in tumor suppression and tumor microenvironment remodeling. Front Immunol [Internet]. 2022 Dec 20;13:1064047. CrossRef
61. Samimi H, Sohi AN, Irani S, Arefian E, Mahdiannasser M, Fallah P, et al. Alginate-based 3D cell culture technique to evaluate the half-maximal inhibitory concentration: an in vitro model of anticancer drug study for anaplastic thyroid carcinoma. Thyroid Res [Internet]. 2021 Dec 3;14(1):27. CrossRef
62. Haider M, Elsherbeny A, Jagal J, Hubatová-Vacková A, Ahmed IS. Optimization and evaluation of poly(lactide-co-glycolide) nanoparticles for enhanced cellular uptake and efficacy of paclitaxel in the treatment of head and neck cancer. Pharmaceutics [Internet]. 2020 Aug 30;12(9):828. CrossRef
63. Al-Nemrawi NK, Hameedat F, Al-Husein B, Nimrawi S. Photolytic controlled release formulation of methotrexate loaded in Chitosan/TIO2 nanoparticles for breast cancer. Pharmaceuticals [Internet]. 2022 Jan 26;15(2):149. CrossRef
64. Duan X, He C, Kron SJ, Lin W. Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol [Internet]. 2016 Feb 5;8(5):776–91. CrossRef
65. Ye W, Du J, Zhang B, Ren N, Song Y, Mei Q, et al. Cellular uptake and antitumor activity of DOX-Hyd-PEG-FA nanoparticles. PLoS one [Internet]. 2014 May 14;9(5):e97358. CrossRef
66. Joseph TM, Mahapatra DK, Esmaeili A, Piszczyk ?, Hasanin MS, Kattali M, et al. Nanoparticles: taking a unique position in medicine. Nanomaterials [Internet]. 2023 Jan 31;13(3):574. CrossRef
67. Gahtani RM, Alqahtani A, Alqahtani T, Asiri SA, Mohamed JMM, Prabhu S, et al. 5-Fluorouracil-Loaded PLGA nanoparticles: formulation, physicochemical characterisation, and in VitroAnti-Cancer activity. Bioinorg Chem Appl [Internet]. 2023 Apr 17;2023:1–11. CrossRef
68. Novio F. Design of targeted nanostructured coordination polymers (NCPS) for cancer therapy. Molecules [Internet]. 2020 Jul 29;25(15):3449. CrossRef
69. Verma J, Warsame C, Seenivasagam RK, Katiyar NK, Aleem E, Goel S. Nanoparticle-mediated cancer cell therapy: basic science to clinical applications. Cancer Metastasis Rev [Internet]. 2023 Feb 24;42(3):601–27. CrossRef
70. Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Convergence [Internet]. 2019 Jul 15;6(1). CrossRef
71. Fernández-Lázaro D, Hernández JLG, García AC, Martínez AC, Mielgo-Ayuso J, Hernández JJC. Liquid biopsy as novel tool in precision medicine: origins, properties, identification and clinical perspective of cancer’s biomarkers. Diagnostics [Internet]. 2020 Apr 13;10(4):215. CrossRef
72. Zhang X, Xu X, Bertrand N, Pridgen EM, Swami A, Farokhzad OC. Interactions of nanomaterials and biological systems: implications to personalized nanomedicine. Adv Drug Deliv Rev [Internet]. 2012 Oct 1;64(13):1363–84. CrossRef
73. Bennet D, Kim S. Polymer nanoparticles for smart drug delivery. In: Sezer AD, editor. Application of nanotechnology in drug delivery [Internet].
74. Shen Z, Nieh M, Li Y. Decorating nanoparticle surface for targeted drug delivery: opportunities and challenges. Polymers [Internet]. 2016 Mar 17;8(3):83. CrossRef
75. Thangam R, Patel KD, Kang H, Paulmurugan R. Advances in engineered polymer nanoparticle tracking platforms towards cancer immunotherapy—current status and future perspectives. Vaccines [Internet]. 2021 Aug 23;9(8):935. CrossRef
76. Muthwill MS, Kong P, Dinu IA, Necula D, John C, Palivan CG. Tailoring polymer-based nanoassemblies for stimuli-responsive theranostic applications. Macromol Biosci [Internet]. 2022 Sep 26;22(11). CrossRef
77. Ofridam F, Tarhini M, Lebaz N, Gagnière É, Mangin D, Ela??Ssari A. pH-sensitive polymers: classification and some fine potential applications. Polym Adv Technol [Internet]. 2021 Feb 3;32(4):1455–84. CrossRef
78. Bao Y, Maeki M, Ishida A, Tani H, Tokeshi M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. PLoS one [Internet]. 2022 Aug 4;17(8):e0271050. CrossRef
79. Plucinski A, Lyu Z, Schmidt BVKJ. Polysaccharide nanoparticles: from fabrication to applications. J Mater Chem B [Internet]. 2021 Jan 1;9(35):7030–62. CrossRef
80. Martínez-Muñoz OI, Ospina-Giraldo LF, Mora-Huertas CE. Nanoprecipitation: applications for entrapping active molecules of interest in pharmaceutics. In: Abu-Thabit N, editor. Nano- and microencapsulation [Internet].
81. Xu L, Wang X, Liu Y, Yang G, Falconer RJ, Zhao C. Lipid nanoparticles for drug delivery. Advanced NanoBiomed Res [Internet]. 2021 Nov 25;2(2). CrossRef
82. Pulingam T, Foroozandeh P, Chuah J, Sudesh K. Exploring various techniques for the chemical and biological synthesis of polymeric nanoparticles. Nanomaterials [Internet]. 2022 Feb 8;12(3):576. CrossRef
83. Macchione MA, Strumia MC. Stimuli-responsive nanosystems as smart nanotheranostics. Elsevier eBooks [Internet]. 2023. p. 363–96. CrossRef
84. Rasel MdSI, Mohona FA, Akter W, Kabir S, Chowdhury AA, Chowdhury JA, et al. Exploration of site-specific drug targeting—a review on EPR-, stimuli-, chemical-, and receptor-based approaches as potential drug targeting methods in cancer treatment. J Oncol [Internet]. 2022 Sep 29;2022:1–26. CrossRef
85. Bandyopadhyay A, Das T, Nandy S, Sahib S, Preetam S, Gopalakrishnan AV, et al. Ligand-based active targeting strategies for cancer theranostics. Naunyn-Schmiedeberg’s Arch Pharmacol [Internet]. 2023 Jul 19;396(12):3417–41. CrossRef
86. Atkinson SP, Andreu Z, Vicent MJ. Polymer therapeutics: biomarkers and new approaches for personalized cancer treatment. J Pers Med [Internet]. 2018 Jan 23;8(1):6. CrossRef