INTRODUCTION
Chitosan (CS) a natural biodegradable polymer has been extensively explored for diverse pharmaceutical and biomedical application. CS is derived from chitin poly (N-acetyl glucosamine), which is isolated from the shells of the crustaceans, via alkaline deacetylation. CS contains glucosamine and N-acetyl glucosamine units connected together by (1–4) glycosidic links [1]. The structure of CS offers multiple options for chemical modification which can result in a wide range of derivatives possessing unique properties. There are three reactive sites on the CS chain enabling chemical modification: one primary amine and two hydroxyl groups (primary or secondary) (Fig. 1). The primary amine groups present special properties that render CS suitable for pharmaceutical applications. The cationic character of CS contributes to the permeation enhancing, in situ gelling, mucoadhesive, antibacterial, and efflux pump inhibitory functions [1,2]. CS has also demonstrated potential for nucleic acid delivery, tissue engineering, wound healing, and cancer diagnosis. In addition, the property of chitosan nanoparticles (CS NPs) to widen cellular junctions and surface modification has also been well documented. Due to these aforementioned properties, CS is of great interest to researchers in the pharmaceutical and biomedical fields [3,4].
Nevertheless, chemical modification of CS has been prepared and assessed for their different applications. CS conjugates have been developed and proven to be effective. Recently, CS-phenyl boronic acid conjugates have been explored for their various functions.
Boronic acid compounds have a variety of biomedical applications. The majority of the polymeric boronic acids reported in the literature have phenyl boronic acid moieties. Most phenyl boronic acids have pKa values higher than physiological pH. The addition of different substituents on the phenyl ring permits the pKa to be attuned favoring the use of boronic acid-holding polymers at a physiologically appropriate pH range [5]. Phenyl boronic acid and its derivatives can bind reversibly to sugars and 1,2 or 1, 3 diol compounds, hence they could recognize carbohydrates, such as glucose in the blood and sialic acid (SA) in cancer cells [6–9]. This property has been widely explored for application as glucose-responsive polymer complexes, glucose sensors, diagnostic, and various therapeutic applications [10,11]. This review gives an overview of research undertaken with CS-phenyl boronic acid.
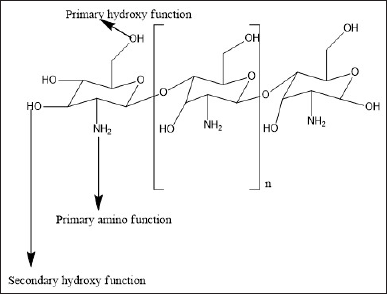 | Figure 1. Structure of CS. [Click here to view] |
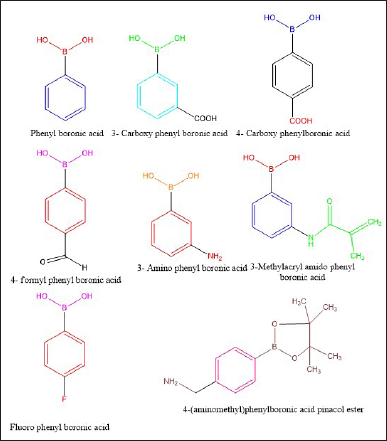 | Figure 2. Chemical structure of various reported PBA moieties conjugated to CS. [Click here to view] |
Various CS-conjugated phenyl boronic acid moieties have been reported in the literature. They include carboxy phenylboronic acid (CPBA), formyl phenylboronic acid (PBA), fluoro PBA, and amino phenylboronic acid (Fig. 2). Each of them has been discussed in this review.
METHODOLOGY
The literature search was done using the scientific databases Scopus, PubMed, and Science direct. The document search tool was employed using the combination of keywords “CS “and PBA. The inclusion criteria were to consider all research articles published from 2000 to 2022. The exclusion criteria was to exclude those articles that did not have biomedical application. The major focus was on the articles which had applications for drug delivery.
RESULTS AND DISCUSSION
The database search using the combination of keywords “CS “and PBA resulted in 74 documents. The articles were screened and sectioned under the following sections pertaining to the type of PBA moiety involved in the conjugation with CS. This review discusses CPBA conjugated CS, formyl PBA conjugated CS, fluoro PBA conjugated CS, aminophenylboronic acid conjugated CS, and acrylamidophenylboronic acid (APBA)-conjugated CS.
CPBA conjugated CS
Nanosized drug delivery systems of chemotherapeutic agents are preferred in cancer therapy due to their selectivity. Although considerable efforts have been made to avoid metastasis, the treatment is challenging as it requires more efficient carriers. Targeted delivery of anticancer medication to tumor cells with the aid of functional carriers is a broadly investigated procedure. The enhanced efficacy is attributable to explicit interactions among ligands and receptors. PBA exhibited high binding affinity to SA residues which are excessively expressed on the outer surface of cancer cells [11].
For the treatment of tumors, 3-carboxyphenyl boronic acid (3-CPBA) conjugated carboxymethyl CM NPs were prepared.The desolvation method was used for the preparation of CM NPs. 3-CPBA which functioned as a tumor-homing ligand was covered on the outside of CM NPs to facilitate tumor-targeting property on (3-CPBA-NPS). The (3-CPBA-NPs) were prepared by 1-(3-dimethyl aminopropyl)3-ethyl carbodiimide (EDC)-N-hydroxy succinimide (NHS), carbodiimide chemistry (Fig. 3). Doxorubicin (DOX) was incorporated into prepared nanoparticles to acquire CM-DOX NPs and (3-CPBA-DOX NPs). Monolayer cell models and three-dimensional (3-D)cultured multicellular (MCs) spheroids were utilized to assess cellular uptake and cytotoxicity. The in vivo cellular uptake investigation showed that (3-CPBA-DOX NPs) formulation delivered more DOX and retained for a considerable time in lung tissue, compared to non-modified NPs (CM-DOX NPs). In vivo evaluation of anti-tumor efficacy in mice bearing H22 tumor showed that 3-CPBA designed NPs exhibit significant accumulation and penetration potential to the tumor than the non-targeted NPs as evidenced by reduction in the size of the tumor mass. The results of this study proved the potential of 3-CPBA decorated NPs for use in chemotherapy [12].
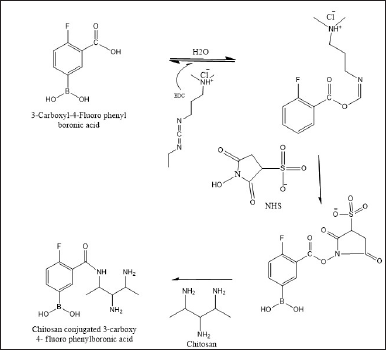 | Figure 3. Schematic diagram of the synthesis of CS conjugated 3-carboxy-4-fluoro phenyl boronic acid. [Click here to view] |
Boronate-conjugated CSs with 4-carboxyphenyl boronic acid (CS-4CPBA) were prepared to investigate urothelial mucoadhesiveness with the intention of prolonging drug residence time in bladder for bladder cancer treatment. Three types of boronate conjugated CS (low, medium, high) derivatives were prepared using NHS and EDC hydrochloride (EDC.HCL) as a coupling reagent, generally employed for covalent amide bond formation. Mucoadhesive properties were investigated by the ex vivo flow-through technique and tensile test. A notable change in the mucoadhesive property of native CS and boronated CS was observed. The findings of the study inferred that mucoadhesive property was highest for the highly boronated CS. The results of the study proved the capability of boronate conjugated CS to function as a drug delivery system endowed with mucoadhesive property and thereby suitable for prolonging the duration of drug action using the mucosal routes of drug administration [13].
An injectable microcapsule incorporated bioadhesive hydrogel of metronidazole (MTZ) was prepared for local delivery in the periodontal pocket. CS-coated MTZ microcapsule (CS-MTZ) were prepared using the dissolution precipitation method. The (CS-MTZ) microcapsule served as a cross-linker along with poly (vinyl alcohol) (PVA) grafted 4CPBA to obtain PVA-CS-MTZ hydrogel. The release behavior, cytotoxicity, and antibacterial activity of the prepared PVA-CS-MTZ hydrogels were evaluated by in vitro and in vivo tests. The prepared PVA-CS-MTZ hydrogels proved to be safe, biocompatible, and exhibited superior in vitro bacteriostasis activity. The anti-inflammatory activity of the prepared gel was evaluated in the rat periodontitis model. The injectable PVA-CS-MTZ hydrogel reduced the inflammation for an extended duration in the periodontal pocket. The findings of this study demonstrate the ability of the hydrogel for enhanced local delivery of MTZ in the periodontal pocket [14].
3-CPBA modified CS conjugate was synthesized for site-specific delivery in Coccidiosis treatment, a parasitic disease in poultry animals. Manifested as low weight gain, diarrhea resulting in the death of chickens. The synthesized CS-3-CPBA could self-assemble in water along with diclazuril (DIC), a cocidiostat to form glucose and pH, dual responsive micelles. DIC was placed into the hydrophobic core of the micelles. The micelles were designed to remain stable in acidic environment and release in intestine, the intended site of action. The drug release profile was evaluated at various pH and glucose concentration. A biphasic release was seen. The rapid release would serve to inhibit the growth of coccidian while the sustained release would aid in sustaining the anti-coccidia effect. DIC/CS-3-CPBA micelles showed good anti-coccidia property, weight gain, and also reduced gastrointestinal damage compared to the commercially available formulation. The remarkable performance of micellar formulation was attributed to the combined effects of DIC, CS, and the micelles. Along with the inhibition of coccidia by DIC, CS exerted antibacterial effect due to available positive charge on CS [15].
CS micelles for delivery of anti-coccidial agents were prepared which were tailored to function with dual stimuli triggered site-specific delivery property. CPBA and SA moieties were conjugated to the CS skeleton through amide reaction using EDC.HCL and NHS. Loading of DIC, anticoccidial agent was facilitated by hydrophobic interaction. The DIC-stacked micelles were designed to stay stable prior to arriving at the disease-ridden cecum site, and the in-center borate bridges were clipped by concentrated glucose under alkaline pH of cecum, creating a free nanostructure and sped up payload discharge. The borate-bridges SPCS/DIC micelles exhibited, biosafety, biostability and adaptable medication discharge pattern for treatment of local infection and delivered superior anti-coccidial efficacy in vivo [16].
PBA can react with SA residues which are overexpressed on malignant tumors resulting in good selectivity and binding efficiency. Grounded on this strategy, Wang et al. [17] prepared DOX-loaded CS NPs and decorated them with 4-CPBA (4-CPBA-CS NPs). The CSNPs were prepared using glutaraldehyde as crosslinker in the presence of ethylene diamine tetra acetic acid. CS NPs were then conjugated with 4-CPBA using EDC.HCL mediated coupling in the presence of NHS.
The integration of PBA group bearing negatively charge onto the surface of CSNPs which were positively charged reduced the zeta potential and enhanced the efficacy of nanoparticles to target cancer cells and enhanced the deposition time in tumor sites. The tumor penetration, cellular uptake, biodistribution, and anti-tumor activity were examined in 3-D MCs spheroids models, monolayer cell models, and H22 tumor-bearing mice. Results of the evaluation showed that 4-CPBA-CS NPs had internalized the tumor cells to a greater extent compared to non-decorated nanoparticles.
CPBA conjugated polymers have drawn interest in the area of pH-sensitive drug delivery aimed at tumor targeting. CPBA is reported to improve cellular uptake in tumor cells due to their ability to recognize and interact with SA.
Curcumin-loaded PBA-conjugated CSNPs was prepared for curcumin delivery in the treatment of tumor. Curcumin was successfully loaded due to curcumin-PBA complexation and the hydrogen bonding interactions among nanocarriers and curcumin. PBA-CS was synthesized by an amide reaction between amino groups of CS and the carboxyl group of 3-CPBA. The suppression of the tumor in the presence of curcumin-loaded nanoparticles was additionally assessed utilizing 3D multicellular tumor spheroids (MCTS) as in vitro tumor model. The curcumin loaded PBA-CS NPs showed improved tumor regression in 3-D MCTS than free curcumin. The nanoparticles also demonstrated superior anticancer activity toward HepG2 cells. The study concluded that the nanocarriers of CS conjugated with PBA nanocarriers may perhaps be utilized as a potential strategy for tumor treatment [18].
A hydrogel was prepared by in situ crosslinking of oxidized dextran and PBA-modified CS using EDC/NHS as a coupling agent. The crosslinking was enabled through the imine bond and phenyl boronate ester. Generally, boronate ester formation occurs at pH 8. Hence, the alteration of pH facilitates encapsulation. DOX was encapsulated into the hydrogel through the in-situ gel forming method. The hydrogels were assessed for their injectable property in vitro by injecting a 3-CPBA/CSPBA/DOX solution into PBS pH 7.4. Cytotoxicity of the CSPBA was evaluated in L929 fibroblasts utilizing an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test. Cell viability and cell proliferation of the hydrogels were also examined in L929 fibroblasts. The cells remained viable the cell proliferation was stifled by DOX addition. Hence, the CSPBA/DOX hydrogels framework showed potential as a useful delivery system for anticancer drug delivery [19].
A novel PBA-conjugated CS oligosaccharide-vitamin E copolymer has been reported for improved ocular bioavailability and efficacy in the management of keratitis. Initially, 4-carboxy PBA-CS-VE was prepared with the aid of EDS-NHS following previously reported procedure. The ethanol injection method was used for the preparation of PBA-CS-VE-VRC micelles. The nanomicelles loaded with voriconazole were designed to function as mucoadhesive as their surface was modified with PBA, which is known to interact with SA on the ocular mucin. The PBA-modified micelles demonstrated prolonged ocular retention, superior corneal penetrating ability on a rabbit model of fungal keratitis when compared to the free drug. The results of this study reveal the mucoadhesive potential of the PBA conjugated CS oligosaccharide-vit E copolymer as well as its utility to function as a carrier for topical ocular drug delivery [20].
The various 3-CPBA conjugated CS reported in literature along with their application is displayed in Table 1.
Formyl PBA conjugated CS
Oral delivery of protein and peptide drugs is challenging due to their degradation by enzymes present in the gastrointestinal tract (GIT). One of the approaches to tackle the problem is the use of enzyme inhibitor. This strategy can be made more efficacious if the protease inhibitor is bound covalently to the drug carrier. Boronic acids are reported to form covalent adducts reversibly with the active site of serine proteases. Smoum et al. [21] prepared 4-formylphenylboronic acid (4-FPBA)—CS conjugate potentially to function as oral delivery systems for calcitonin, a proteinaceous drug, as literature reports the capability of 4-FPBA in inhibiting enzymes present in the GIT. Three types of conjugates, 4-FPBA directly conjugated to CS and other two conjugates with spacers pentaglycine and glycylglycine were prepared and evaluated. CS—pentaglycine, PBA conjugate showed good enzyme inhibition when compared with other prepared conjugates attributed to the highest degree of substitution of 4-FPBA. The study concluded that these conjugates proved to be potential agents in protecting calcitonin from intestinal pancreatic serine proteases.
The glucose adsorption sensitivity of 4-FPBA-conjugated chitosan (CS-4-FPBA) and evaluation has been reported. CS was made to react with different amounts of 4-FPBA with the aid of sodium borohydride as a reducing agent. The study was undertaken to assess the relationship between glucose sensitivity and changes in the physicochemical properties of the various conjugates. The findings of the study reported that a higher concentration of 4-FPBA resulted in crystalline conjugate which showed poor access to glucose. The study concluded that the quantity of boronic acid was crucial to illicit glucose adsorption. The study demonstrated the potential of CS-4-FPBA to function as a glucose-sensing polymer [22].
Scaffolds of bioglass 45S5 (BG) composites containing CS-4-FPBA were prepared for bone tissue regeneration. To assess the effect of PBA conjugated CS on the bioactivity of composite, CS/BG and CS-4-FPBA /BG were prepared and assessed. The reason behind adding CS with PBA was to improve cell adhesion capacity, and therefore the bioactivity of the material. The ability to form an apatite layer was evaluated by incubating the prepared composites in simulated body fluid. Cell cytotoxicity assay proved CS-4-FPBA and CS-4-FPBA/BG to be non-harmful compared to samples containing only CS and BG 45S5. The study concluded that incorporated PBAs, CS, and BG 45S5 composite scaffold material show great potential to treat bone tissue degeneration [23].
Insulin-loaded nanoparticles with CS-4-FPBA via polyelectrolyte complexation were prepared for insulin delivery. CS was made to react with different amounts of (4-FPBA) with sodium borohydride as a reducing agent. The insulin release profile as a component of glucose concentration was assessed. The quantity of insulin delivered was significantly higher with higher glucose levels. Yet, at higher concentrations of glucose, release was found to slow down credited to the formation of crosslinks with the boronic acid moieties. The study concluded that the prepared CS-4FPBA conjugate exhibited the potential to function as glucose-dependent insulin delivery system [24].
The preparation of boron-based PBA functionalized CS for use as flame retardant has been reported. The boron-based PBA functionalized CS conjugate (B-CH) was synthesized by Mannich reaction between CS and 4-FPBA. Polylactic acid was added to the Boron-CS conjugate to enhance the fire performance of the composite. The eco-friendly flame retardant demonstrated excellent flame-retardant properties attributed to PBA which improved the char layer through crosslinking. The outcome of this study exhibits the potential of B-CH conjugate to function as a novel bio-based flame retardant [25].
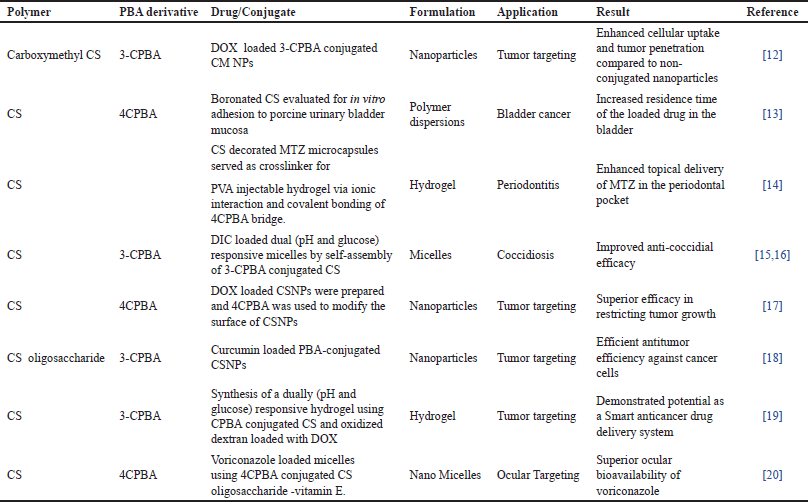 | Table 1. CPBA conjugated CS. [Click here to view] |
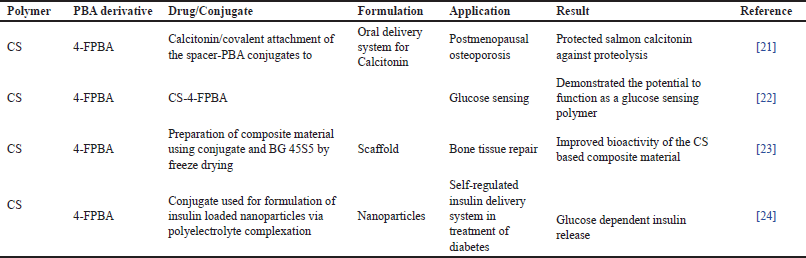 | Table 2. Formyl PBA conjugated CS. [Click here to view] |
The various formyl phenyl boronic acid conjugated CSs reported in literature along with their application are shown in Table 2.
Fluoro PBA conjugated CS
In the management of diabetes subcutaneous injections are repeatedly given. Artificially regulated insulin delivery systems is an attractive strategy to avoid repeated injections. PBA and its derivatives can reversibly bind to sugars hence they could recognize carbohydrates, such as glucose in the blood. This property has been widely explored for application as glucose-responsive material. However, most PBA have pKa values higher than physiological pH. The addition of different substituents on the phenyl ring permits the pKa to be adjusted so that boronic acid-conjugated polymers can be utilized at physiologically appropriate pH range [26].
Insulin-loaded 4-fluorophenyl boronic acid conjugated chitosan (CS-FPBA) was prepared for glucose-responsive release of insulin. The high polarity of fluorine in the FPBA-substituted CS would display lower pKa and would under physiological conditions be more negatively charged. CS-FPBA was synthesized by an amide condensation reaction. FPBA was initially made to react with EDC and NHS, followed by reaction with CS to form (CS-FPBA). For the insulin loading, the prepared CS-FPBA powder was added to insulin solution under stirring at a high speed overnight stirring followed by centrifugation and removal of supernatant. The influence of various factors affecting the preparation of polymer such as solvent type, pH, the molar ratio of raw materials, molecular weight of CS, and degree of deacetylation was investigated. Insulin could be suitably loaded onto CS-FPBA particles enabled by both the electrostatic interactions between CS and insulin, and the dynamic ester bonds between insulin and boronic acid [27]. In contrast to CS-PBA described by Wu et al. [28] the loading capacity of CS-FPBA (23%–27%) could increase to a certain level while the loading capacity of CS-PBA was only 10%–16%, which showed that insulin could be encapsulated at greater extent using a smaller amount of CSFPBA. The study concluded that the CS-FPBA has potential in controlling the release of insulin, and can control the release rate by adjusting the molecular weight of CS.
A glucose-responsive system was prepared for the delivery of insulin using two dissimilar copolymers as material to function as pore-blocking agents and PBA-modified mesoporous silica (MS) as a carrier for insulin. 3-fluoro-4-carboxyphenylboronic acid (FCPBA) was made to react with MS to prepare PBA-modified MS-FCPBA and insulin was incorporated into the MS-FCPBA. After that, using a free radical polymerization technique, diol-containing polymer poly(N-isopropyl acrylamide-co-N-acryloyl glucosamine) was prepared. Then to attain the glucose-responsive insulin release system, onto the surface of insulin-loaded MS-FCPBA the polymer was capped. The results indicated that the encapsulation efficiency and loading capacity of insulin could attain 85.9% and 14.7%, respectively. The results of this study confirmed the potential of the system to function as a controlled release system for the delivery of insulin with glucose sensitivity [29].
A controlled release system for the delivery of insulin with glucose-responsive property employing faster degrading MS has been reported. In this study PBA and dendritic MS and were used to formulate a glucose-responsive delivery system for insulin. Briefly, biphase stratification approach was employed to prepare dendritic MS. FCPBA was grafted on the hydroxypropyl CS to obtain PBA-modified hydroxypropyl CS. Insulin was incorporated into the dendritic MS in a weakly acidic environment. The loading capacity was 32.1% while the attained encapsulation efficiency could reach 94.6%. The insulin-loaded dendritic MS was coated with modified hydroxypropyl CS. Another insulin release system was prepared with sodium alginate and calcium ion crosslinking [30]. The results of this study report that both systems proved to be effective in delivering sustained insulin release as shown in Table 3.
Aminophenylboronic acid conjugated CS
Oral delivery of insulin is challenging due to its degradation at gastric pH and degradation by intestinal enzymes. Literature reports the use of various biodegradable and biocompatible materials as well as nanocarriers being explored for the oral administration of proteins and peptides. CS has shown promise as an oral drug carrier owing to its mucoadhesive, biocompatible, nontoxic, and positive charge [2]. Insulin-loaded CS NPs have been prepared by polyelectrolyte complexation with insulin. However, these systems have shown burst release. Hence, to avoid undesired toxicity, the synthesis of a stimuli-responsive drug delivery system is favored. Phenyl boronic acid can function as a glucose-sensitive moiety due to its ability to reversibly bind with 1,2 diols [10]. A schematic diagram presenting the synthesis of CS-conjugated 4-amino phenyl boronic acid is shown in Figure 4.
Insulin-loaded multifunctional nanocarriers based on CS for oral delivery of insulin have been reported. PBA was used to function as a glucose-sensitive unit, CS as nanocarrier backbone, and L-valine to enable absorption in the small intestine. The carboxyl groups of carboxymethyl CS were conjugated to the amino groups of 3amino phenylboronic acid (3-APBA) and valine to synthesize carboxymethyl chitosan (CMCS)-PBA-LV. The insulin-loaded CMCS-PBA-LV nanoparticles were prepared by ionic gelation using sodium tripolyphosphate. The in vitro insulin release under triggered pH and glucose conditions was evaluated. The hypoglycemic effect was evaluated in vivo using streptozotocin-induced diabetic rats. The results of the study inferred that insulin-loaded nanocarriers exhibited low cytotoxicity, excellent stability against proteolytic enzymes and better hypoglycemic effect. The study concluded that insulin-loaded L-valine modified CS-based multifunctional nanocarriers CMCS-PBA-LV show potential as a promising delivery system for oral delivery of insulin [31].
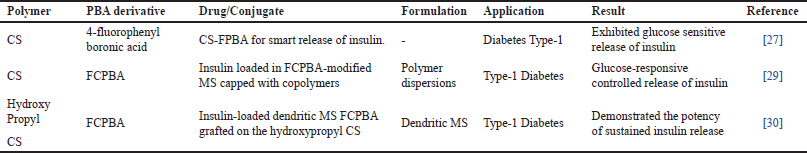 | Table 3. Fluoro PBA conjugated CS. [Click here to view] |
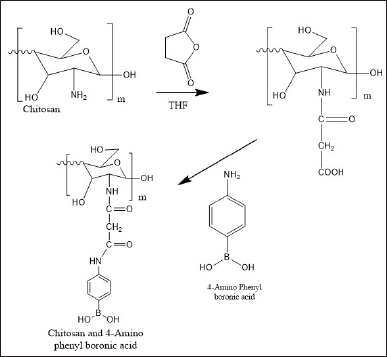 | Figure 4. Schematic diagram of the synthesis of CS conjugated 4-amino phenyl boronic acid. [Click here to view] |
For controlled release of insulin, a glucose-responsive double-layered nanogel was prepared and evaluated. Fluorenylmethyloxycarbonyl chloride protected poly L glutamate was reacted with 3APBA using NHS and EDC to obtain poly L-glutmate-co-N-3-L-glutamylphenylboronic acid. Sodium alginate was made to react with PGGA resulting in SA-PGGA. Insulin-loaded GC/SA-PGGA nanogel was prepared by electrostatic interactions using the isotropic gelation method. Double-layered nanogel showed high biocompatibility. Preclinical studies were conducted in mice and achieved insulin-controlled release at high-level glucose concentrations. Besides, the study reported that GC/SA-PGGA nanogel loaded with insulin-induced blood glucose lowering effect similar to the effect obtained upon administration of free insulin injection twice. These results reveal the potential of nanogels as a platform for superior insulin delivery [32].
For self-regulated insulin delivery, a biocompatible based glucose-sensitive polymer with phenyl boronic acid was prepared. CS conjugated 3-APBA (3APBA-CS) was synthesized using EDC.HCL acid and hydroxy benzotriazole. Insulin loaded glucose sensitive nanoparticles were prepared by self-assembly technique. Insulin-loaded 3-APBA-CS NPs displayed the high EE (53.6%). Insulin release profile indicated that the release of insulin was in glucose concentration-reliant manner. The outcome of the study reveals that the copolymer could control insulin release and was responsive to glucose [28].
PBA conjugated polymers have attracted interest in the area of tumor treatment using pH-sensitive drug delivery. PBA is reported to improve cellular uptake in tumor cells due to their ability to recognize and interact with SA.
PBA-modified F127-CS conjugate was prepared for use of the polymer conjugate to formulate drug-loaded micelles with DOX as a model drug. 3-APBA and succinic anhydride were dissolved in anhydrous tetrahydrofuran. The mixture was evaporated and the residue dissolved in ethyl acetate to obtain carboxylated-PBA. The carboxylated-PBA was made to react with amino group in CS-F127 under activation of NHS and EDC to obtain PBA-CS-F127. PBA and its derivatives can reversibly bind to 1, 3 diol compounds. Encapsulation with DOX was facilitated due to the interaction with the 1,3 diol group in DOX.
The targeting ability of the PBA portion in the conjugate was evaluated by comparing with DOX micelles prepared with CS-F127. The in vitro cytotoxic study demonstrated that the introduction of PBA could increase targeting ability of DOX-PBA-M to B16 cells when related to PBA unmodified DOX-M. Results of the study show the potential of PBA -CS-F127 conjugates to function as a drug carrier for chemotherapeutic drugs in the treatment of cancer [33]. Table 4 shows the various amino PBA conjugates reported in the literature along with their application.
APBA conjugated CS
Efficient delivery of chemotherapeutic drugs to the tumor is challenging due to meager active tumor targeting and scarce internalization by tumor cells. To address these challenges, active tumor-targeted ligands have been used to alter nanoparticle carriers. Phenyl boronic acid can recognize overexpressed SA in cancer cells. Folic acid can also function as a tumor-targeting ligand due to its affinity to bind to folate receptors overexpressed on cancer cells. Cao et al. [34] prepared tumor targeting nanoparticles using folic acid-modified CMCS(FA/CMCS) polymerized with N-3-APBA. APBA was also polymerized with non-conjugated CMCS to form CMCS-PAPBA NPs. DOX was then loaded into prepared nanoparticles. The distribution and cellular uptake of DOX-loaded CMCS-PAPBA and FA/CMCS-PAPBA NPs were investigated in tumor cells cultured as 3D MCs spheroid mimicking the tumor environment. The penetration ability of drug-loaded nanocarriers and accumulation was studied in H22 tumor-bearing mice. The results exhibited the ability of FA-modified nanoparticles to accumulate and penetrate in 2-D and 3-D cell models. The enhanced drug accumulation in tumor was responsible for increased anti-tumor efficiency in H22 tumor-bearing mice.
CS NPs containing boronic acid were prepared by the polymerization of N-3- APBA polymer and CS to form (CS-APBA NPs). Incorporation of the APBA group in the nanoparticles would aid in the recognition of tumor by the reaction with SA groups present on the tumor surface. The CS-PAPBA NPs were conjugated with tumor penetrating peptide (iRGD) and loaded with DOX. The cellular uptake, biodistribution, tumor penetration, and tumor activity were studied using 3-D MCs in vitro model and in vivo in mice bearing H22 tumor.
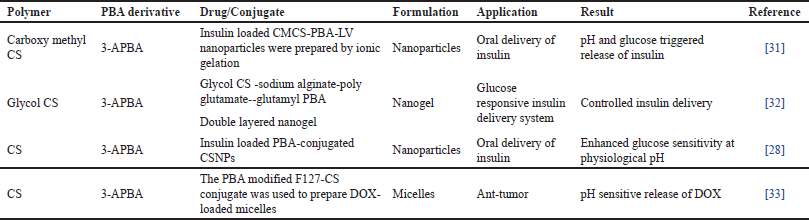 | Table 4. Amino phenyl boronic acid conjugated CS. [Click here to view] |
 | Table 5. APBA conjugated CS. [Click here to view] |
DOX-loaded iRGD conjugated nanoparticles exhibited much better penetration ability than free DOX and non-conjugated nanoparticles [35]. These findings were further supported by in vivo tumor examinations, biodistribution analysis, and observation of survival rate.
Phenylboronic acid pinacol (PBAP) ester conjugated ChitoPEG copolymer
Photosensitizer-based photodynamic therapy is a promising strategy for cancer therapy. Polymeric nanoparticulate carriers are being explored to deliver photosensitizers for targeting cancers. Jeong et al. [36] prepared photosensitizer-incorporated CS-polyethyleneglycol-PBAP ester nanoparticles for cancer targeting.
PBAP was conjugated with ChitoPEG copolymer to obtain ChitoPEG-PBAP followed by incorporation of nanophotosensitizer for photodynamic treatment with reactive oxygen species (ROS)-specific feature in the management of various cancers.
Ce6 incorporated nanophotosensitizers showed sensitivity counter to ROS and quick Ce6 release. The ChitoPEG-PBAP nanophotosensitizers showed more efficacy and were internalized into cancer cells compared to Ce6 alone. Ce6 incorporated nano photosensitizers inhibited the tumor growth and reduced viability of cancer cells with greater efficacy in comparison to Ce6 alone. Moreover, ChitoPEG-PBAP nanophotosensitizers were competently carried to irradiated tumor tissues, demonstrating that ChitoPEG-PBAP nanophotosensitizers can be successfully transported to the tumor in a ROS-sensitive manner [36]. The Ce6 incorporated ChitoPEG-PBAP nanophotosensitizer has shown promise for photodynamic therapy of cancer as shown in Table 5.
PATENTS
Glucose-sensitive nanoparticle for cancer diagnosis and therapy. Application KR1020140078399A. This patent relates to a glucose-sensitive nanoparticle that comprises a PBA derivative and a biocompatible polymer and is formed via amphiphilic conjugation of the PBA derivative and the biocompatible polymer which are chemically bonded with each other.
CONCLUSION
The conjugation of CS with PBA has been shown to exhibit a plethora of properties that can be exploited for a variety of applications. In this review, an effort was made to compile different PBA derivatives conjugated to CS and showcase their potential. Phenyl boronic acid-conjugated CS were formulated as nanoparticles, micelles, and hydrogel functioning as drug carriers for diverse therapeutic applications. Many studies have reported enhanced tumor targeting with phenylboronic acid-conjugated CS. Another important application of these conjugates is their ability to function as glucose sensitive polymer which enables self-regulated insulin release in the treatment of diabetes besides functioning as a diagnostic agent. Though considerable research has been done with CS-PBA conjugates demonstrating potential in various biomedical applications, there aren’t clinical studies reported to facilitate transition into clinics. We hope that this review will provide insight into the novel applications of CS-PBA conjugates to researchers to further explore their potential.
ACKNOWLEDGMENTS
The authours are grateful to Indian Council for Medical Research (ICMR), Government of india for granting juniour research fellow to Brahmam Bheemisetty and Manipal College of Pharmaceutical Sciences (MCOPS) and Manipal Academy of Higher Education for their support in carrying out the literature search and access to databases.
AUTHOR CONTRIBUTIONS
Brahmam Bheemisetty made substantial contribution to acquisition of data and drafting the article. Shaila Lewis, contributed to the conception and design, drafting and revising. The authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by the Indian Council Medical Research (ICMR) Government of India [Grant number: 5/4/5-5/ Diab.19-NCD-111] provided to the corresponding author, Lewis SA, and the Junior Research Fellowship to the first author, Bheemisetty Brahmam.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Jayakumar R, Menon D, Manzoor K, Nair SV, Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym. 2010 Sep 5;82(2):227–32.
2. Ramya R, Venkatesan J, Kim SK, Sudha PN. Biomedical applications of chitosan: an overview. J Biomater Tissue Eng. 2012 Jun 1;2(2):100–11.
3. Bernkop-Schnürch A, Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012 Aug 1;81(3):463–9.
4. Mohammed MA, Syeda JT, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017 Nov 20;9(4):53.
5. Brooks WL, Deng CC, Sumerlin BS. Structure–reactivity relationships in boronic acid–diol complexation. ACS Omega. 2018 Dec 19;3(12):17863–70.
6. Lan T, Guo Q. Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application. Nanotechnol Rev. 2019 Dec 31;8(1):548–61.
7. Huang Q, Wang L, Yu H, Ur-Rahman K. Advances in phenylboronic acid-based closed-loop smart drug delivery system for diabetic therapy. J Control Release. 2019 Jul 10;305:50–64.
8. Cheng F, Jäkle F. Boron-containing polymers as versatile building blocks for functional nanostructured materials. Polym Chem. 2011;2(10):2122–32.
9. Chai Z, Ma L, Wang Y, Ren X. Phenylboronic acid as a glucose-responsive trigger to tune the insulin release of glycopolymer nanoparticles. J Biomater Sci Polym Ed. 2016 May 2;27(7):599–610.
10. Ma R, Shi L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: synthesis and applications in drug delivery. Polym Chem. 2014;5(5):1503–18.
11. Wang J, Wu W, Jiang X. Nanoscaled boron-containing delivery systems and therapeutic agents for cancer treatment. Nanomedicine. 2015 Apr;10(7):1149–63.
12. Wang X, Wei B, Cheng X, Wang J, Tang R. 3-Carboxyphenylboronic acid-modified carboxymethyl chitosan nanoparticles for improved tumor targeting and inhibitory. Eur J Pharm Biopharm. 2017 Apr 1;113:168–77.
13. Kolawole OM, Lau WM, Khutoryanskiy VV. Synthesis and evaluation of boronated chitosan as a mucoadhesive polymer for intravesical drug delivery. J Pharm Sci. 2019 Sep 1;108(9):3046–53.
14. Dong Z, Sun Y, Chen Y, Liu Y, Tang C, Qu X. Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment. ACS Appl Bio Mater. 2019 Nov 1;2(12):5985–94.
15. Zhang X, Xu G, Gadora K, Cheng H, Peng J, Ma Y, et al. Dual-sensitive chitosan derivative micelles for site-specific drug release in the treatment of chicken coccidiosis. RSC Adv. 2018;8(26):14515–26.
16. Cheng H, Zhang H, Xu G, Peng J, Wang Z, Sun B, et al. A combinative assembly strategy inspired reversibly borate-bridged polymeric micelles for lesion-specific rapid release of anti-coccidial drugs. Nano-Micro Lett. 2020 Dec;12:1–9.
17. Wang X, Tang H, Wang C, Zhang J, Wu W, Jiang X. Phenylboronic acid-mediated tumor targeting of chitosan nanoparticles. Theranostics. 2016;6(9):1378.
18. Wang J, Liu LG, Jiao WQ, Yang H, Liu J, Liu D. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr Polym. 2021 Mar 15;256:117497.
19. Li J, Hu W, Zhang Y, Tan H, Yan X, Zhao L, et al. pH and glucose dually responsive injectable hydrogel prepared by in situ crosslinking of phenylboronic modified chitosan and oxidized dextran. J Polym Sci Part A Polym Chem. 2015 May 15;53(10):1235–44.
20. Sun X, Sheng Y, Li K, Sai S, Feng J, Li Y, et al. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022 Jan 15;138:193–207.
21. Smoum R, Rubinstein A, Srebnik M. Chitosan−pentaglycine−phenylboronic acid conjugate: a potential colon-specific platform for calcitonin. Bioconjug Chem. 2006 Jul 19;17(4):1000–7.
22. Asantewaa Y, Aylott J, Burley JC, Billa N, Roberts CJ. Correlating physicochemical properties of boronic acid-chitosan conjugates to glucose adsorption sensitivity. Pharmaceutics. 2012 Dec 27;5(1):69–80.
23. Thibault MH, Comeau C, Vienneau G, Robichaud J, Brown D, Bruening R, et al. Assessing the potential of boronic acid/chitosan/bioglass composite materials for tissue engineering applications. Mater Sci Eng C. 2020 May 1;110:110674.
24. Siddiqui NA, Billa N, Roberts CJ, Asantewaa Osei Y. Cross-linked dependency of boronic acid-conjugated chitosan nanoparticles by diols for sustained insulin release. Pharmaceutics. 2016 Oct 8;8(4):30.
25. Cui X, Wu Q, Sun J, Gu X, Li H, Zhang S. Preparation of 4-formylphenylboronic modified chitosan and its effects on the flame retardancy of poly (lactic acid). Polym Degrad Stab. 2022 Aug 1;202:110037.
26. Shen D, Yu H, Wang L, Khan A, Haq F, Chen X, et al. Recent progress in design and preparation of glucose-responsive insulin delivery systems. J Control Release. 2020 May 10;321:236–58.
27. Luo L, Song R, Chen J, Zhou B, Mao X, Tang S. Fluorophenylboronic acid substituted chitosan for insulin loading and release. React Funct Polym. 2020 Jan 1;146:104435.
28. Wu Z, Zhang S, Zhang X, Shu S, Chu T, Yu D. Phenylboronic acid grafted chitosan as a glucose-sensitive vehicle for controlled insulin release. J Pharm Sci. 2011 Jun 1;100(6):2278–86.
29. Huang Q, Yu H, Wang L, Shen D, Chen X, Wang N. Preparation of dendritic mesoporous silica/phenylboronic acid-modified hydroxypropyl chitosan and its glucose-responsive performance. Polym Sci Series A. 2021 Nov;63:757–68.
30. Huang Q, Yu H, Wang L, Shen D, Chen X, Wang N. Synthesis and testing of polymer grafted mesoporous silica as glucose-responsive insulin release drug delivery systems. Eur Polym J. 2021 Aug 15;157:110651.
31. Li L, Jiang G, Yu W, Liu D, Chen H, Liu Y, et al. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater Sci Eng C. 2017 Jan 1;70:278–86.
32. Lee D, Choe K, Jeong Y, Yoo J, Lee SM, Park JH, et al. Establishment of a controlled insulin delivery system using a glucose-responsive double-layered nanogel. RSC Adv. 2015;5(19):14482–91.
33. Feng R, Wang W, Zhu L, Xu H, Chen S, Song Z. Phenylboronic acid-functionalized F127-oligochitosan conjugate micelles for doxorubicin encapsulation. J Biomed Mater Res Part B Appl Biomater. 2020 Nov;108(8):3345–55.
34. Cao Z, Wang X, Cheng X, Wang J, Tang R. In vitro and in vivo antitumor study of folic acid-conjugated carboxymethyl chitosan and phenylboronic acid–based nanoparticles. Int J Polym Mater Polym Biomater. 2017 Jul 3;66(10):495–506.
35. Wang X, Zhen X, Wang J, Zhang J, Wu W, Jiang X. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles. Biomaterials. 2013 Jun 1;34(19):4667–79.
36. Jeong YI, Kim T, Hwang EJ, Kim SW, Sonntag KC, Kim DH, et al. Reactive oxygen species-sensitive nanophotosensitizers of aminophenyl boronic acid pinacol ester conjugated chitosan-g-methoxy poly (ethylene glycol) copolymer for photodynamic treatment of cancer. Biomed Mater. 2020 Aug 28;15(5):055034.