INTRODUCTION
There are about 1 billion people with hypertension, and it is estimated to reach 1.5 billion people by 2025 [1]. Hypertension is a global disease burden, the cause of chronic diseases that require other combined treatments such as hyperhomocysteinemia, ambulatory arterial stiffness index, premature ventricular complexes, left ventricular hypertrophy, and so on [2–4]. Among them, hyperuricemia is still a significant disease burden in hypertensive patients. The prevalence of hyperuricemia in adults ranged from 21.4% to 41.4% and currently had a continuing increasing trend [5,6]. Increased serum uric acid often appears in hypertensive patients. Many studies showed that increased serum uric acid increases the risk of developing hypertension within 5 years, which was considered a risk factor independent of others [7]. A linear correlation of serum uric acid with hypertension had been demonstrated with each 1 mg/dl increase in serum uric acid leading to a 13% increase in the risk of hypertension [8].
Treatment of hyperuricemia with abstinence and hypouricemic drugs with allopurinol in elderly patients with hypertension helped reduce complications and contributed to good blood pressure control [9]. A recently introduced drug, febuxotate, has been shown to effectively control uric acid in patients with hypertension [10–12]. However, there were lots of studies that only evaluated allopurinol and febuxostat alone, studies comparing the effects of both drugs in patients with hypertension were minimal [8–12]. In addition, very few similar studies have been conducted in the Vietnamese population. Therefore, the Vietnamese population in particular, and the Asian population in general required a study for optimal treatment selection. Our study aimed to evaluate the therapeutic efficacy of febuxostat compared with allopurinol in hypertension patients with hyperuricemia in Vietnam.
MATERIALS AND METHODS
Study design and population
We conducted a randomized controlled clinical trial with an allocation ratio of 2:1 on 108 outpatients with primary hypertension ≥40 years old treated at the Can Tho University of Medicine and Pharmacy Hospital from May 2020 to May 2021. The study was approved by the Ethics Committee in Biomedical Research of Can Tho University of Medicine and Pharmacy with Decision with Decision No 27/HDDD. All participating patients filled out an informed consent to participate in the study, and the identities of all research subjects were kept confidential.
Including criteria: (1) Hypertension patients diagnosed based on ESC/ESH 2018, with hypertension when systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in two measurements of blood pressure or being treated with antihypertensive drugs [1]; (2) patients with hyperuricemia (elevated serum uric acid was confirmed when serum uric acid concentration for men >420 μmol/l and women >360 μmol, initiate treatment in patients received 3 months of lifestyle changes that were ineffective or the patient was unable to adhere to the lifestyle changes [13]); and (3) patients agree to participate in the study.
Excluding criteria: (1) Secondary hyperuricemia such as kidney failure, hemolysis, G6PD deficiency, after chemotherapy, radiotherapy, acute alcoholism [14]; (2) gout patients; (3) patients suffering from acute or malignant diseases: acute heart failure, acute myocardial infarction, acute respiratory failure, cerebrovascular disease, acute gout, liver cancer, acute leukemia, leukemia prayer, polycythemia; (4) patients who are taking drugs that affect the production and excretion of uric acid for 10 days, such as feburic, probenecid, sulfinpyrazole, salicylate, phenylbutazone, ascorbic acid, ethambutol, pyrazinamide, thiazide diuretics, other cytotoxic drugs; (5) patients with severe chronic kidney disease (estimated glomerular filtration rate < 30 ml/minute); (6) patient intolerant with febuxostat or allopurinol; and (7) patients did not agree to participate in the study.
Sample size
The sample size was calculated based on Becker et al.’s [14] study, the allopurinol group achieving the uric acid target <6.0 mg/dl (<360 μmol/l) was 21.11% (p1 = 0.2111), and the febuxostat group was 53.33% (p2 = 0.5333). We calculate n = 65 with α = 0.01 and β = 0.1 (Z(α/2) = 2.57, Z(β/2) = 1.282). In fact, we conducted the study on 108 patients, more than the sample size to prevent loss of follow-up or nonadherence to treatment.
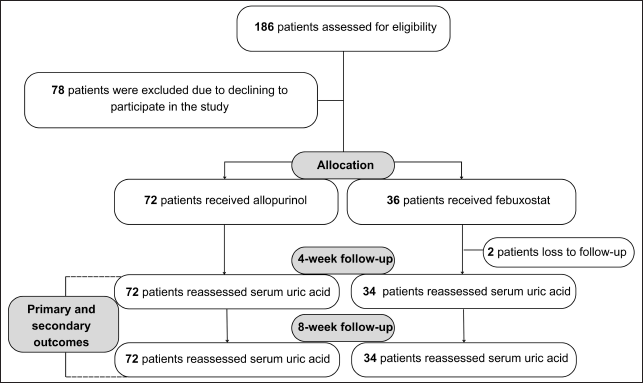 | Figure 1. Flowchart of the study population. [Click here to view] |
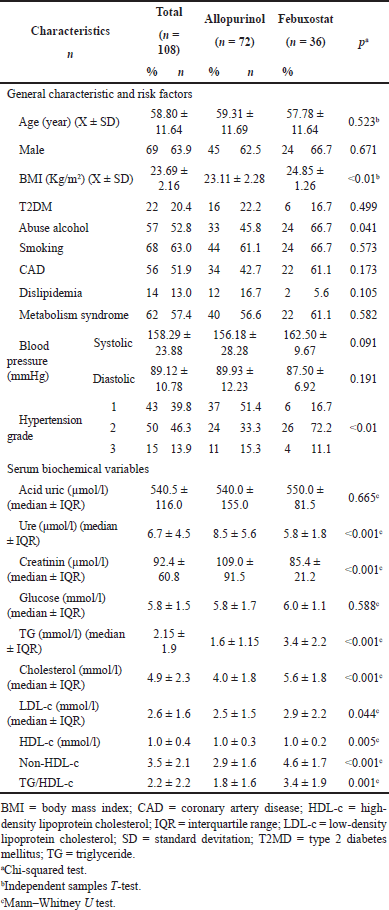 | Table 1. Baseline characteristics of study population. [Click here to view] |
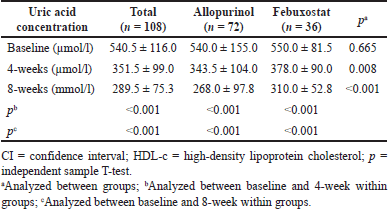 | Table 2. Uric acid concentration before and after treatment. [Click here to view] |
Data collection and intervention methods
All outpatients diagnosed with hypertension and hyperuricemia would have their medical history questioned, and examined, general and clinical characteristics recorded by specialist doctors. Some general characteristics include age, gender, overweight, and obesity (classification based on the WHO Asian body mass index (BMI) classification, overweight and obesity when BMI ≥ 23 kg/m2 medical history of coronary artery disease (CAD) with at least one-time hospitalization, smoking (none if patients have stopped smoking for at least 5 years [15]); type 2 diabetes mellitus (T2DM) (according to American Diabetes Association 2020 guideline [16]), alcohol abuse (drinking ≥2 standard drinks for men (or ≥20 g ethanol/day), and ≥1 standard drink for women (or ≥10 g ethanol/day) [17], metabolic syndrome (according to NCEP-ATP III 2001 [18,19], three out of five criteria of abdominal obesity, high blood triglycerides (TGs), low HDL-c, blood pressure ≥ 130/85 mmHg, blood glucose fasting ≥ 6.1 mmol/l). Quantifies fasting serum uric acid and lipid components in the morning, including baseline, 4 and 8 weeks after intervention. Conduct a blood draw to take 2 ml of venous blood (at least 12 hours before the previous meal) for natural coagulation, serum separation, and quantification of serum lipid components by enzymatic colorimetric method (on the COBAS-C502 machine, HITACHI of Japan). In addition, fasting blood glucose, urea, creatinine were taken.
The intervention was performed in outpatients with high serum uric acid concentration (for men >420 μmol/l and women >360 μmol [13], our research threshold was stricter than previous studies). Group A started treatment with allopurinol 100 mg (once daily), increasing to 300 mg after 4 weeks or 600–900 mg if the treatment goal was not achieved after 8 weeks [1]. Group B used 40 mg febuxostat (once daily), increasing to 80 mg after 4 weeks if the treatment goal was not achieved [1]. All patients participating in the study are numbered from small to large. Equally distributed with a ratio of 1:2, for two groups of patients B and A, respectively. Our study was single-blind, in the study patients were not aware between the two regimens. All participating patients were instructed to use medication, comply with treatment, and make follow-up appointments. Endpoint to control serum uric acid levels <6.0 mg/dl (<360 μmol/l). Record blood uric acid results after 4 and 8 weeks and compare the proportion of controlled serum uric acid concentration between the two groups.
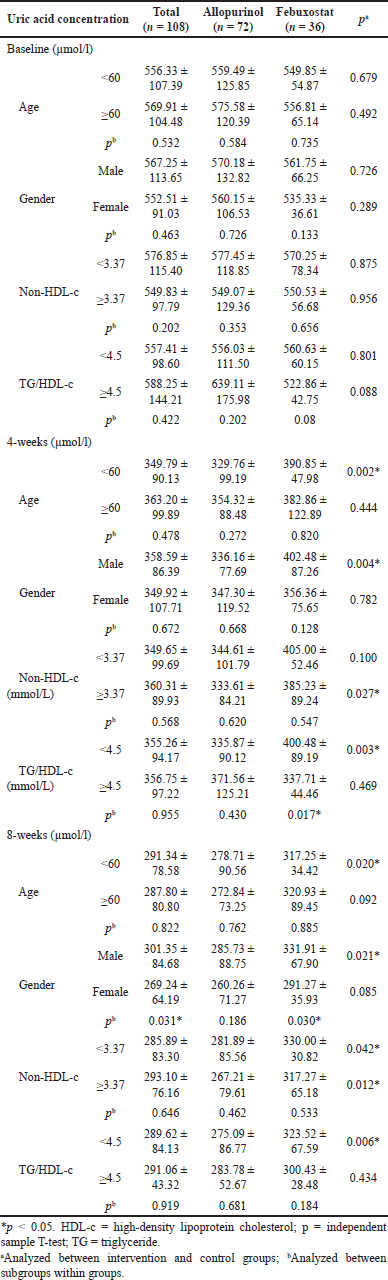 | Table 3. Subgroup analysis of uric acid concentration before and after treatment. [Click here to view] |
Study outcomes
The primary outcome was the effectiveness of serum uric acid control compared between febuxostat versus allopurinol after 4 and 8 weeks. Treatment was effective when serum uric acid concentration <6.0 mg/dl (<360 μmol/l). Evaluate changes in mean serum uric acid concentration between two groups before and after intervention, after 4 and 8 weeks of treatment. The secondary outcomes were subgroup analysis on characteristic groups of age, gender, and serum lipid indices such as non-HDL-c and TG/non-HDL-c ratio to evaluate the impact on treatment outcomes within and between two groups.
Data analysis and bias control methods
All data collected was ensured: Information bias control (clear and specific definition of research variables, information on diagnosis and classification according to uniform standards, data collection through a unified medical record form); selection bias control (following including and excluding criteria, study protocol, imaging tests interpreted by independent radiologists and cardiologists, entered data and statistical processing by an independent statistician, conducted twice to compare the results); clearly explained to the patient before the procedure.
Data were collected and processed by Statistical Package for the Social Sciences 20.0 software. Quantitative variables with normal distribution were described by mean ± SD, and non-normal distribution variables were characterized by the median, maximum, minimum, and interquartile range (IQR). Qualitative variables are represented by rate and percentage. The difference between two qualitative variables described by the chi-squared test, normal distribution quantitative variables by simple t-test (if two groups analyzed) or analysis of variance (if ≥3 groups investigated), quantitative variables with non-normal distribution by Mann–Whitney test (if two groups studied) or Kruskal-Wallis test (if ≥3 groups analyzed), p < 0.05 considered to be statistically significant.
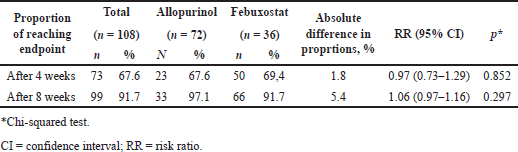 | Table 4. Proportion of uric acid reaching endpoint compared between groups. [Click here to view] |
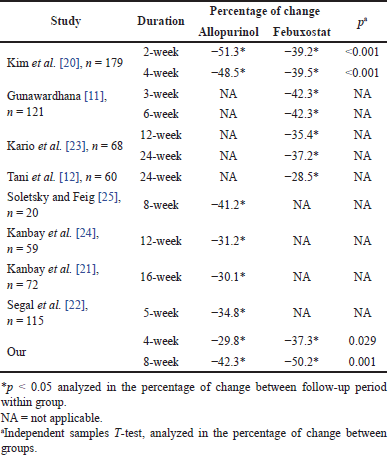 | Table 5. Treatment outcomes comparison with other studies. [Click here to view] |
RESULTS
A total of 186 outpatients who met the sampling criteria were included in the study. One hundred eight patients who agreed to participate in the study were randomly put into groups A and B with an allocation ratio of 2:1, of which two patients were excluded from the study due to loss of follow-up. One hundred six patients were included in the analysis, 72 for the allopurinol group and 34 for the febuxostat group (Fig. 1).
Baseline characteristics of the study population
A total of 108 patients participated, mean age was 58.80 ± 11.68 (years old), and 63.9% were male. Among them, smoking was the risk factor with the highest proportion (70.4%). The highest grade of hypertension was grade 2 (46.3%), and BMI had a difference between the two groups (p < 0.01) (Table 1).
Intervention effectiveness after 4 and 8 weeks
Serum uric acid levels decreased significantly after 4 and 8 weeks in both groups and the difference between the two groups was statistically significant (p ≤ 0.01). The mean uric acid concentration before intervention in both treatment groups with Allopurirol and Febuxobrate was 540.0 ± 155.0 and 550.0 ± 81.5 μmol/l, respectively. After 4 weeks, the mean was 343.5 ± 104.0 and 378.0 ± 90.0 μmol/l, respectively (p = 0.008), and was 268.0 ± 97.8 and 310.0 ± 52.8 μmol/l (p < 0.001) after 8 weeks (Table 2).
Uric acid concentrations were analyzed according to age groups, gender, non-HDL-c, and TG/HDL-c ratio. After the treatment follow-up period, there was a statistically significant difference when compared between groups aged <60 (p after 4 and 8 weeks were 0.002 and 0.020, respectively), male (p after 4 and 8 weeks were 0.004 and 0.021, respectively), non-HDL ≥ 3.37 (p after 4 and 8 weeks were 0.027 and 0.042, respectively), TG/HDL-c < 4.5 (p after 4 and 8 weeks were 0.003 and 0.006, respectively). When compared within the same group, divided by subgroups, the treatment effect of febuxobrate in women was better than in men (p = 0.03) (Table 3).
After 4 weeks of intervention, the proportion of achieving endpoint in the febuxostat and allopurinol groups was 67.6% and 69.4%, respectively (RR = 0.97, 95% CI 0.73–1.29, p = 0.852) (Table 2). After 8 weeks of intervention, the proportion was 97.1% and 91.7%, respectively (RR = 1.06, 95% CI 0.97–1.16, p = 0.297) (Table 4).
DISCUSSION
Our study evaluated the ability to control serum uric acid levels when comparing the febuxostat regimen in group B and allopurinol in group A. Overall, both regimens clearly showed effectiveness in reducing serum uric acid after 4 and 8 weeks of follow-up (p < 0.001) (Table 2). After 8 weeks, the allopurinol regimen showed more effective in the uric acid reduction effect based on mean concentration (p < 0.001) (Table 2). However, the febuxostat regimen showed a higher rate of achieving the endpoint (97.1% vs. 91.7, RR = 1.06, 95% CI: 0.97–1.16, p = 0.297) but was not statistically significant (Table 4). Secondary outcomes were evaluated according to the subgroups analyzed. Allopurinol showed better treatment results in groups with aged <60, male, non-HDL ≥ 3, and TG/HDL-c < 4.5 (p < 0.05) (Table 3).
Our study population had a mean age of 58.8 years old, 63.9% male (Table 1), similar to other studies with ages ranging from 50 to 60 years old and men making up the majority of the population [11,20–22]. The study by Tani et al. [12], Kanbay et al. [24], and Kario et al. [23] recorded a higher age population and the study by Soletsky and Feig [25] was much lower. The mean BMI was 23.69 kg/m2, significantly lower than other studies due to the smaller physical condition of the Vietnamese population compared to other population groups [11,12,20,22–24].
Subgroup analysis showed that the effectiveness of allopurinol was better in ages <60 years (p after 4 and 8 weeks were 0.002 and 0.020, respectively), and male gender (p were 0.004 and 0.021, respectively), non-HDL ≥ 3.37 (p were 0.027 and 0.042, respectively), TG/HDL-c < 4.5 (p were 0.003 and 0.006, respectively) (Table 3). When compared within the same group, divided by subgroups, the treatment effect of febuxostat in women was better than in men (p = 0.03) (Table 3). Other studies such as Gunawardhana [11] reported that the treatment effect of febuxostat was better in the eGRF group from 60 to 90 ml/phút/1.73 m2.
After the follow-up period, the febuxostat group had a lower rate of achieving the endpoint at 4 weeks of evaluation and higher at 8 weeks compared with the allopurinol group (p > 0.05) (Table 4). The mean concentrations of uric acid in the allopurinol group were statistically significantly lower compared to the febuxostat group (Table 2) after 4 weeks (p = 0.008) and 8 weeks (p < 0.001). The uric acid reduction effect when analyzed through the percentage change between periods was lower in the allopurinol group and higher in the febuxostat group when compared with the randomized controlled trials of Kim et al. [20]. Our study was different from other studies, which showed that febuxostat provided better treatment results when compared with allopurinol (Table 5) [20]. The difference could be due to differences in populations including ethnicity and BMI. Therefore, in the Vietnamese population, there was no difference in treatment options for both drug groups.
Both regimens showed a high ability to reduce serum uric acid and achieved the endpoint after the follow-up period. Specifically, after 8 weeks of follow-up, >90% of patients achieved the endpoint (Table 4). Similar to other studies, there were good improvement results of either or both regimens over the treatment period [11,12,20–24]. Therefore, the treatment effectiveness of both regimens could be highly recommended for application in the clinical practice of treating hyperuricemia. In addition, our study recorded higher treatment results than other studies. Specifically, after 8 weeks of treatment in the febuxostat group, the percentage change was significantly higher than in previous studies with longer follow-up periods (−50.2% vs. 28.5%–42.3%) (Table 5) [11,12,20,23]. However, other populations had much higher BMI than ours, which could lead to bias, but we deliberately selected studies with the most similar populations possible (including hypertension and hyperuricemia). The allopurinol treatment group also showed no different results compared to other studies (42.3% vs. 29.8%–51.3%) (Table 5) [20–22,24,25]. Therefore, hyperuricemia in Vietnamese hypertension patients treated with allopurinol and febuxostat was more optimal than compared to other population groups and could be applied in clinical practice.
Strengths and weaknesses of the study
Our study’s threshold for intervention in serum uric acid concentration was smaller and stricter than previous studies, so the study brings novelty to the population. The intervention results of the study can contribute to understanding the effectiveness of two types of regimens in controlling serum uric acid. Therefore, our research results could be as medical evidence to choose treatment regimens in the Vietnamese population. In addition, our study has a sample collection process with clearly designed including and excluding criteria. All patients in the study volunteered and had benefited from the study. The intervention research design closely follows the CONSORT 2010 checklist. Research methods and methods were clearly described and reproducible.
However, our study was evaluated in one hospital, which could lead to bias in general and clinical characteristics. Therefore, a study with similar or larger sample sizes in multiple centers in different regions is needed to better represent the characteristics of the population. Our study has the longest treatment period of 8 weeks. In clinical practice, patients use the drug longer than the evaluation period. In addition, our study was conducted with a single-blind design, which was another reason leading to bias. However, our study was designed to minimize this limitation through bias control methods in the method section. Therefore, a double-blind clinical trial with a longer evaluation period is needed to better evaluate the effectiveness and adverse effects of the two regimens in long-term use.
CONCLUSION
In hypertensive patients with hyperuricemia, treatment with febuxostat and allopurinol has been shown to have a high ability to reduce serum uric acid and achieve endpoint. Therefore, both regimens could be applied in clinical practice in the Vietnamese population. There was no difference in treatment outcomes between the two regimens, preference among the two regimens was not necessary.
ACKNOWLEDGMENTS
The authors would like to thank the Rectorate Board of Can Tho University of Medicine and Pharmacy and the Board of Directors of Can Tho University of Medicine Hospital for creating favorable conditions for this study to be carried out.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
Toan Hoang Ngo was funded by the Master, PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), code VINIF. 2023.TS.132.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Can Tho University of Medicine and Pharmacy Hospital from May 2020 to May 2021. The study was approved by the Ethics Committee in Biomedical Research of Can Tho University of Medicine and Pharmacy with Decision with Decision No 27/HDDD. All participating patients filled out an informed consent to participate in the study, and the identities of all research subjects were kept confidential.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Williams, B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
2. Kim Tran S, Ngo TH, Phuong Nguyen AT, Ngoc Huynh HT, Tan Vo C, Bao Nguyen NN, et al. The effect of bisoprolol on premature ventricular complex in Vietnamese patients with hypertension and left ventricular hypertrophy. Curr Hypertens Rev. 2023;19(1):42–51.
3. Tran SK, Huynh HTN, Ngo TH, Nguyen ATP, Vo CT, Nguyen T, et al. Effectiveness of combination of perindopril and indapamide on ambulatory arterial stiffness index in Vietnamese patients with primary hypertension. Pharm Sci Asia. 2022;49:478–85.
4. Tran SK, Ngo TH, Nguyen PH, Truong AB, Truong GK, Tran KDD, et al. Hyperhomocysteinemia in patients with newly diagnosed primary hypertension in Can Tho city, Vietnam. Healthcare. 2023;11(2):234.
5. Stewart DJ, Langlois V, Noone D. Hyperuricemia and hypertension: links and risks. Integr Blood Press Control. 2019;12:43–62.
6. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64(10):1431–46.
7. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–21.
8. Palazzuoli A, Hashemi H, Jameson LC, McCullough PA. Hyperuricemia and cardiovascular disease. Rev Cardiovasc Med. 2017;18(4):134–45.
9. Singh JA, Ramachandaran R, Yu S, Curtis JR, Allopurinol use and the risk of acute cardiovascular events in patients with gout and diabetes. BMC Cardiovasc Disord. 2017;17(1):76.
10. Akimoto T, Morishita Y, Ito C, Iimura O, Tsunematsu S, Watanabe Y, et al. Febuxostat for hyperuricemia in patients with advanced chronic kidney disease. Drug Target Insights. 2014 Aug 13;8:39–43
11. Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, et al. Effect of febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 randomized placebo-controlled study. J Am Heart Assoc. 2017;6(11):e006683.
12. Tani S, Nagao K, Hirayama A. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin Drug Investig. 2015;35:823–31.
13. Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, Harrison’s principles of internal medicine. New York, NY: McGraw-Hill Education; 2015.
14. Becker MA, Schumacher HR, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353(23):2450–61.
15. Thompson B, Lichtenstein E, Corbett K, Nettekoven L, Feng Z. Durability of tobacco control efforts in the 22 Community Intervention Trial for Smoking Cessation (COMMIT) communities 2 years after the end of intervention. Health Educ Res. 2000;15(3):353–66.
16. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–31.
17. Beghin I, Cap M, Dujardin B, World Health Organization. A guide to nutritional assessment. Geneva: World Health Organization; 1988.
18. Program TNCE. Executive Summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97.
19. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421.
20. Kim HA, Seo YI, Song YW. Four-week effects of allopurinol and febuxostat treatments on blood pressure and serum creatinine level in gouty men. J Korean Med Sci. 2014;29(8):1077–81.
21. Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, et al. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6(8):1887–94.
22. Segal MS, Srinivas TR, Mohandas R, Shuster JJ, Wen X, Whidden E, et al. The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J Am Soc Hypertens. 2015;9(8):610–9.e1.
23. Kario K, Nishizawa M, Kiuchi M, Kiyosue A, Tomita F, Ohtani H, et al. Comparative effects of topiroxostat and febuxostat on arterial properties in hypertensive patients with hyperuricemia. J Clin Hypertens (Greenwich). 2021 Feb;23(2):334-344.
24. Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227–33.
25. Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5);1148–56.