INTRODUCTION
Lysiphyllum strychnifolium (W. G. Craib) A. Schmitz (synonym-Bauhinia strychnifolia Craib) belongs to family Fabaceae. L. strychnifolium has been used in traditional remedies for treating fever, stomach disorders, and skin infections [1]. It has several biological properties such as anti-inflammatory [2], antimicrobial properties [3], antihyperglycemic effects [4], antioxidant activity [5,6], and detoxification [7]. Regarding antioxidant activity, it plays an important role in inhibiting or delaying the oxidation mechanisms involving the production of free radicals, which are harmful to human cells [8]. Although the antioxidant activities of L. strychnifolium were examined in the leaves extract [5,6], this activity is rarely referred to in stems, especially with a comparison of different concentrations of extracts. Previous studies reported that phytochemical constituents such as kaempferol, ascorbic acid, α-tocopherol, and cyanidin exert antioxidant compounds by GC-MS, HPLC, and liquid chromatography—mass spectrometry (LC-MS) analyzed methods [9–12].
In this study, it is interesting to clarify the components and antioxidant activities in L. strychnifolium stem extract for further medicinal application. Previous studies of phytochemicals in this plant described some compounds such as gallic acid, quercetin, resveratrol, epicatechin, catechin, and astilbin [2,13,14]. Various compounds in ethanolic (EtOH) extract of L. strychnifolium stem and leaf, as exposed by GC-MS, were reported in previous studies [3]. However, the screening of phytochemicals in methanolic (MeOH) extract blasted with NIST17 libraries was rarely mentioned. Therefore, the present study aimed to investigate the phytochemicals composition in MeOH extract of L. strychnifolium stem by GC-MS. As well, catechin and quercetin in extracts were analyzed by HPLC. Moreover, the antioxidant activities of various stem extracts derived from different solvents used for extraction were determined.
MATERIALS AND METHODS
Plant materials and extracts preparation
The stems with leaves and flowers of L. strychnifolium collected at Yasothon Province, Thailand were identified and the plant reference (BKF No. 197208) was prepared by the Forest and Plant Conservation Research Office, Department of National Parks, Wildlife and Plant Conservation, Thailand.
The stems were collected, washed to remove dust and impurities, dried in a shaded area, cut into small pieces, and then, ground using a grinder to obtain the powder. The extracts were prepared by soaking the stem powder with MeOH, EtOH, or water at a 1:10 ratio (stem powder: solvent), and shaking the mixtures for 3 days. The residues were removed from the extracts by filtration. Consequently, the solvents were evaporated using a vacuum rotary evaporator. All the extracts including MeOH, EtOH, and aqueous extracts, were stored at -20C before preceding the further analysis.
Screening the phytochemical compounds by GC-MS
The phytochemical compounds in L. strychnifolium were initially screened by extracting the stem powder with MeOH and analyzed using GC-MS technique. The MeOH extract of L. strychnifolium was MeOH with MeOH, and the final concentration was adjusted to 10 mg/ml. The extract solution was filtered through nylon membranes before analysis by GC-MS using the following conditions; 70°C 4 min of oven temperature; increasing the temperature 15°C/min to 180°C, 5 min hold; increased 10°C/min to 200°C, 5 min hold; and finally increased 10°C/min to 250°C, 1 min hold; total runtime as 29.33 min. The chemical profiles were compared with the NIST17 library database. The quantities of the detected compounds were compared with the internal standard, heptadecanoic acid methyl ester, and reported as a percentage of content.
Analysis the catechin and quercetin contents in L. strychnifolium stems by HPLC
After primary screening of the phytochemical compounds in the MeOH extract by GC-MS, all the extracts (MeOH, EtOH, and aqueous extracts) were analyzed by HPLC to determine the catechin and quercetin contents. The extracts were diluted with MeOH (HPLC grade) and filtrated through 0.45 μm nylon filter membranes. The HPLC analysis was performed using a WATERS 2690 separation module with a photodiode array detector. The equipment and conditions were set as follows; a reverse-phase C18 column; a mixture of MeOH, acetonitrile, and water (40:15:45, v/v/v) with the addition of 1.0% acetic acid as a mobile phase; detected wavelength at 259 and 279 nm for catechin and quercetin analysis, respectively [15]. The standard stock solutions at 100 mg/ml of catechin (Sigma-Aldrich) and quercetin (Sigma-Aldrich) were prepared by weighing 10 mg of standard powders and dissolving them in 100 μL HPLC-grade MeOH. The stock solutions were then diluted with HPLC-grade MeOH to obtain a final concentration of 0.0625, 0.125, 0.25, and 0.5 mg/ml, which were used for standard curve calculation.
Antioxidant activity analysis by ABTS radical scavenging assay
The ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] radical scavenging assay is commonly used to measure antioxidant activity. The ABTS radical solution (0.6–0.7 OD734) was prepared as previously described by Re et al. [16]. The analysis was conducted by pipetting 190 μl of the ABTS radical solution into each well of 96-well plate, then adding 10 μl of each extract to the wells, and incubating in the dark for 5 minutes before measuring the absorbance of the ABTS radical at 734 nm with multichannel micro-plates reader.
Each treatment was tested in triplicate. The Trolox 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as the standard solution. This stock solution, 100 mg/ml, was prepared by weighing 10 mg of standard powder, dissolving with 100 μL gradient grade EtOH, and then diluting to obtain final concentration of 0.0625, 0.125, 0.25, and 0.5 mg/ml. Gradient-grade EtOH, without the extract, was set as a control for all treatments.
Then, the decolorization effect detected in each sample was calculated using the following equation: Decolorization (%) = (Absorbance Control–Absorbance Sample/Absorbance Control) × 100
RESULTS AND DISCUSSIONS
Screening compounds in MeOH stem extract of L. strychnifolium
After analyzing the MeOH extract by GC-MS technique and matching it with NIST17, various compounds were detected and are presented in Table 1. It has been discovered that some bioactive compounds including 1,2-Benzenediol (3.68%); 1,2,3-Benzenetriol (12.16%); 1,3,5-Benzenetriol (24.30%); 13-Docosenamide, (Z)-(3.41%); and D-allose (24.46%), were identified.
The 1,2-benzenediol (pyrocatechol or catechol) has been reported to possess antioxidant property that can decrease the absorbance of 2,2-diphenyl-1-picrylhydrazyl (DPPH). Additionally, it can prevent autoxidation and slow down lipid oxidation by breaking down lipid hydroperoxides [17,18]. The role of the catechol group in antioxidant and neuroprotective effects in rat brain tissue has been determined [19]. The hydroxyl (OH) groups in the molecule of these phenolic compounds are one of the factors contributing to their antioxidant effect. Mechanically, they prevent oxidative stress-induced apoptosis by activating glutathione peroxidase 4 (GPX4) to reduce the accumulation of reactive oxygen species (ROS) [20]. Moreover, it has repelling activity due to the two hydroxyl groups in the ortho-position [21]. Of note, high concentration of 1,2-benzenediol in rats lead to cytotoxicity. The oral route uptake of this compound has a median lethal dose at 300 mg/kg BW [22]
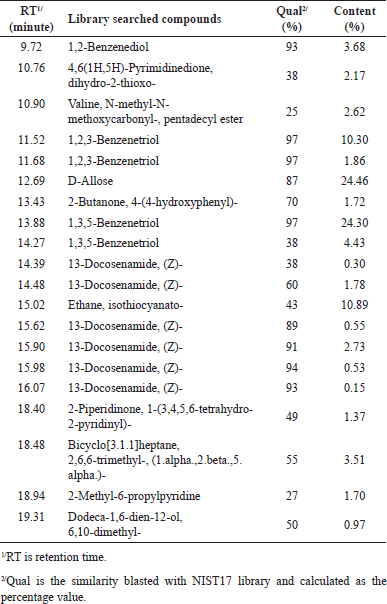 | Table 1. Phytochemical compounds in MeOH extract of L. strychnifolium stems blasted with NIST17 library. [Click here to view] |
Both 1,2,3-Benzenetriol (pyrogallol or pyrophosphoric acid or pyrogallic acid) and 1,3,5-Benzenetriol (phloroglucinol) are isomers of benzenetriol, and have been studied for their potent antioxidant properties. The isolated seed protein derived from Pumpkin (Cucurbita sp.) and its covalent conjugation with 1,2,3-benzenetriol result in an increased number of bound equivalents as the polyphenols concentration increases [23]. This results in the promotion of antioxidant activity. In addition, both compounds have previously been reported to exhibit anti-inflammatory and antimicrobial activities [24,25]. With regard to 1,3,5-Benzenetriol, it is considered as a reducing agent because of its readily donate electrons ability [26]. The antioxidant properties of this compound would have a beneficial effect on cosmetics and skincare because it provides protective activity against oxidative stress and antioxidant activity against DPPH (50% effective concentration values: 12–26 μM) in skin aging [27].
Besides the aforementioned compounds, another interesting compound is D-allose-, which is a rare naturally compound occurring monosaccharide belonging to the group of aldohexoses. It has been proposed to play a crucial role in - anti-diabetic properties. In animal studies, it has shown the ability to improve glucose metabolism and enhance insulin sensitivity [28]. Additionally, D-allose has been found to possess antioxidant and anti-inflammatory properties [29–31]. Likewise; 13-Docosenamide, (Z)-; also known as erucamide, is a long-chain unsaturated fatty acid amide. It has been reported for antinociceptive and anti-inflammatory [32].
Catechin and quercetin contents in L. strychnifolium stem extract
Different extracts of L. strychnifolium stems were subjected to determine the quantities of catechin and quercetin using HPLC. The retention times of catechin and quercetin are 2.477 and 4.894 minutes, respectively (Fig. 1). The catechin and quercetin standard curves were plotted, with both R2 values equal to one. As observation in stem extracts, catechin can be found in all samples (Fig. 1 and Table 2), including MeOH (9.55 mg/gDW), EtOH (7.90 mg/gDW), and aqueous (3.46 mg/gDW) extracts. However, quercetin is found in MeOH (0.09 mg/gDW) and aqueous extracts (0.04 mg/gDW), but was not detected in the EtOH extract. In a previous report, quercetin was mentioned in the EtOH stem extract [7], but not in this study. It may be due to the low quercetin content that is out of the limit concentration of LOD used in this study.
Free radical scavenging activity of L. strychnifolium extracts.
The ABTS test was conducted in all extracts; MeOH, EtOH, and aqueous extracts, in order to investigate the effect of different extractive solvents on antioxidant activities. The standard Trolox was used as the reference for antioxidant capacity. The decolorization (%) values of Trolox were plotted versus concentration (mg/ml) ranging from 0 to 0.25 mg/ml. The curve of standard Trolox presents the linear pattern with the equation; y = 297.28x – 0.6755, R2 = 0.9998 (Fig. 2). In contrast, all stem extracts provide the logarithm patterns (Fig. 2). The ABTS radical scavenging assay of different extracts shows a strong inhibitory effect at low concentration as compared to standard Trolox. The Minimal Inhibitory Concentrations requiring for inhibiting the antioxidant activity of 50% (MIC50) of L. strychnifolium extracts were calculated by substituting the 50% value in the equation that obtained from Figure 2. The statistical analysis was performed using a t-test to compare each extract with Trolox. The results show that the radical scavenging activities of all extracts were significantly higher than those of standard Trolox, ranging from 4.95 to 5.26 times (Fig. 3). However, there was no significant difference among the three extracts (data not shown).
Moreover, the relationship of the decolorization of each extract shows logarithm curve, even at the low concentrations (Fig. 2). This implies that the inhibitory potencies of L. strychnifolium extracts are extremely higher than that of standard Trolox. The antioxidant activities observed in this study are possibly the results of 1,2-benzenediol; 1,2,3-Benzenetriol (pyrogallol);1,3,5-Benzenetriol; D-allose; catechin; and quercetin. In a previous report, catechin and quercetin, known as strong antioxidant agents, were identified in L. strychnifolium [33]. In this study, both compounds were also detected in the stem; hence, these greatly exhibited radical scavenging activities are perhaps the results of synergistic effects of catechin, quercetin, and other detected compounds.
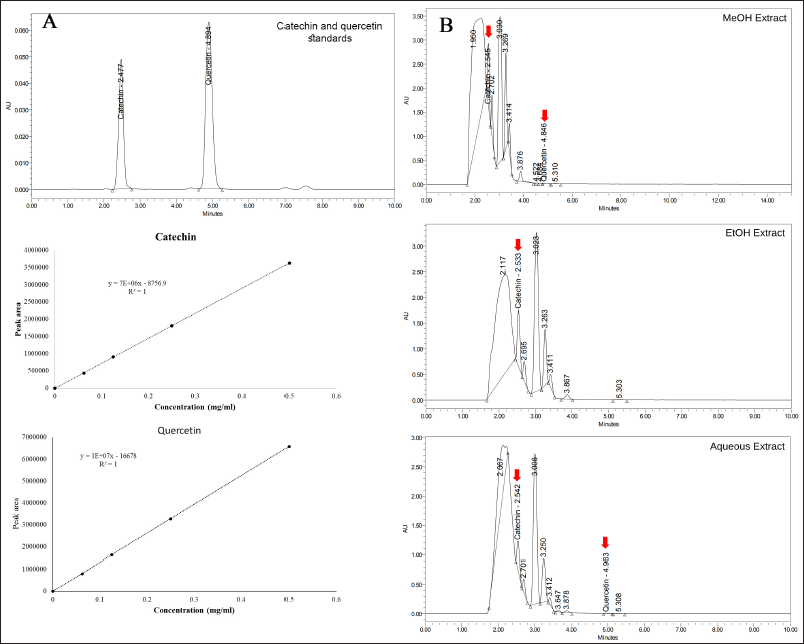 | Figure 1. HPLC chromatogram and standard curves of catechin and quercetin (A) and L. strychnifolium MeOH, EtOH, and aqueous extracts (B). [Click here to view] |
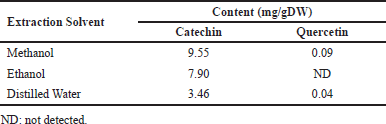 | Table 2. Catechin and quercetin contents in L. strychnifolium stem extracted with different solvents. [Click here to view] |
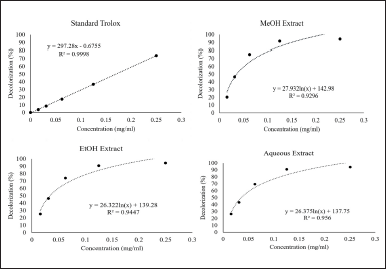 | Figure 2. ABTS radical scavenging assays of standard Trolox and L. strychnifolium stem MeOH, EtOH, and aqueous extracts. [Click here to view] |
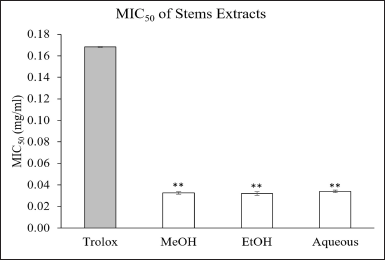 | Figure 3. Minimal inhibitory concentration required to inhibit the antioxidant activity of 50% (MIC50) of different L. srychnifolium extracts by ABTS radical scavenging assays. The statistical analysis, t-test, was compared between each of stem extracts and Trolox at p value < 0.01. [Click here to view] |
CONCLUSION
Based on the results obtained in the present study, it can be concluded that L. strychnifolium stem extracts exhibit remarkable antioxidant activities. This is attributed to the presence of several antioxidant agents, including catechin, quercetin, 1,2-Benzenediol, 1,2,3-Benzenetriol, 1,3,5-Benzenetriol, 13-Docosenamide (Z-), and D-allose. These findings support previous research on the use of L. strychnifolium in treating diseases, particularly its detoxification properties. Furthermore, these findings provide strong evidence for the potential application of L. strychnifolium stems as a source of bioactive compounds.
ACKNOWLEDGEMENT
The plant was kindly identified and the reference prepared by the Forest and Plant Conservation Research Office, Department of National Parks, Wildlife and Plant Conservation, Thailand. GC-MS equipment was supported by the Central Instrument Facility of Mahidol University (Kanchanaburi Campus). The manuscript ws kindly proofread by Dr. Noel Pabalan.
AUTHOR CONTRIBUTIONS
NK conducted the conceptualization, methodology; resources; complete the experiments; data collections and analysis; interpret the data; and writing the original draft and review manuscript. KP managed the conceptualization; funding recruitment; resources preparation; statistical analysis; interpret the data; writing the original draft; proof, review and editing, and finalization the manuscript; and project administration.
FINANCIAL SUPPORT
This study was financial supported by Integrated Research and Innovation budget, Year 2019, National Research Council of Thailand (NRCT), Thailand, [Contract Number 39/2562].
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sampaopan Y, Kitprapiumpon N, Kongkiatpaiboon S, Duangdee N, Wongyai S. Isolation and HPLC Analysis of Astilbin in Lysiphyllum strychnifolium (syn. Bauhinia strychnifolia) Stems. Sci Technology Asia. 2021; 26(1): 208–15.
2. Sampaopan Y, Suksaeree J. Formulation Development and pharmaceutical evaluation of lysiphyllum strychnifolium topical patches for their anti-inflammatory potential. AAPS Pharm Sci Tech. 2022;23(5):116. CrossRef
3. Sukprasert S, Pansuksan K, Sriyakul K. Lysiphyllum strychnifolium (Craib) A. Schmitz extract, a novel neuraminidase inhibitor of avian influenza virus subtype H5N1. J Herb Med, 2020a;20:100330. CrossRef
4. Goli AS, Sato VH, Sato H, Chewchinda S, Leanpolchareanchai J, Nontakham J, Yahuafai J, Thilavech T, Meesawatsom P, Maitree M. Antihyperglycemic effects of Lysiphyllum strychnifolium leaf extract in vitro and in vivo. Pharm Biol. 2023;61(1):189–200. CrossRef
5. Sato V, Chewchinda S, Nuamnaichati N, Mangmool S, Bunleu S, Lertsatitthanakorn P, Ohta S, Sato H. Pharmacological mechanisms of the water leaves extract of Lysiphyllum strychnifolium for its anti-inflammatory and anti-hyperuricemic actions for gout treatment. Pharmacogn Mag, 2019;15(60):98–106. 10.4103/pm.pm_14_18
6. Noonong K, Pranweerapaiboon K, Chaithirayanon K, Rurayarn K, Ditracha P, Changklungmoa N, Kueakhai P, Hiransai1 P, Bunluepuech K. Antidiabetic potential of Lysiphyllum strychnifolium (Craib) A. Schmitz compounds in human intestinal epithelial Caco-2 cells and molecular docking-based approaches. BMC Complement Med Ther, 202222:235. CrossRef
7. Kuendee N, Naladta A, Kulsirirat T, Yimsoo T, Yingmema W, Pansuksan K, Sathirakul K, Sukprasert S. Lysiphyllum strychnifolium (Craib) A. Schmitz extracts moderate the expression of drug-metabolizing enzymes: In Vivo study to clinical propose. Pharmaceuticals. 2023;16(2):237. CrossRef
8. Ayoka TO, Ezema1 BO, Eze CN, Nnadi CO. Antioxidants for the prevention and treatment of non-communicable diseases. J Explor Res Pharmacol. 2022;7(3):178–88.
9. Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: from chemistry to biology. Molecules, 2009;14(6):2202–11. 10.3390/molecules14062202
10. Rizvi S, Raza ST, Ahmed F, Ahmad A, Abbas S, Mahdi F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ Med J. 2014;14(2):e157–65. Epub 2014 Apr 7.
11. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–46. 10.3390/nu2121231. Epub 2010 Dec 10.
12. Michalak M. Plant-derived antioxidants: significance in skin health and the ageing process. Int J Mol Sci, 2022;23(2):585. 10.3390/ijms23020585.
13. Sukprasert S, Deenonpoe R, Yimsoo T, Yingmema W, Prasopdee S, Krajang A, Kornthong N, Pattaraarchachai J, Daduang S. Antidote activity and protective effects of Lysiphyllum strychnifolium (Craib) A. Schmitz extract against organophosphate pesticide in omethoate-treated rats. J Tradit Complement Med. 2020b;11(3):189–96. 10.1016/j.jtcme.2020.03.001.
14. Praparatana R, Maliyam P, Barrows LR, Puttarak P. Flavonoids and phenols, the potential anti-diabetic compounds from Bauhinia strychnifolia Craib. Stem. Molecules. 2022;27(8):2393. 10.3390/molecules27082393.
15. Yuangang Zu, Chunying Li, Yujie Fu, Chunjian Zhao. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J Pharm Biomed Anal, 2006; 41(3): 714–19.
16. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 1999; 26(9–10): 1231–37. 10.1016/s0891-5849(98)00315-3.
17. Chong YM, Chang SK, Sia WCM, Yim HS. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci, 2015;12:18–25.
18. Hashemi SMB, Khaneghah AM, Koubaa M, Lopez-Cervantes J, Yousefabad SHA, Hosseini SF, Karimi M, Motazedian A, Asadifard S. Novel edible oil sources: microwave heating and chemical properties. Food Res Int. 2017;92:147–53.
19. De La Cruz JP, Ruiz-Moreno MI, Guerrero A, López-Villodres JA, Reyes JJ, Espartero JL, Labajos MT, González-Correa JA. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J Nutr Biochem. 2015;26(5):549–55. 10.1016/j.jnutbio.2014.12.013. Epub 2015 Feb 14.
20. Xie X, Wu F, Tian J, Liu Z, He H, Bao D, Li G, Li H, Chen J, Lai Y, Chen Z, Fan J, Chen G, Lai C. Pyrocatechol alleviates cisplatin-induced acute kidney injury by inhibiting ROS production. Oxid Med Cell Longev. 2022;2022:2158644. 10.1155/2022/2158644.
21. Choe E. Roles and action mechanisms of herbs added to the emulsion on its lipid oxidation. Food Sci Biotechnol. 2020;29:1165–79. CrossRef
22. OECD (2003a). SIDS Initial Assessment Profile (SIAP) on 1,2-Dihydroxybenzene (pyrocatechol, catechol) (120-80-9). Accessed September 2023. http://webnet.oecd.org/Hpv/UI/handler.axd?id=0ecbfe38-1c21-4562-aefd-220c345516a3
23. Yang C, Wang B, Wang J, Xia S, Wu Y. Effect of pyrogallic acid (1,2,3-benzenetriol) polyphenol-protein covalent conjugation reaction degree on structure and antioxidant properties of pumpkin (Cucurbita sp.) seed protein isolate. LWT—Food Sci Technol. 2019;109:443–49. CrossRef
24. Baharuddin N. Potential use of Quercus infectoria gall extracts against urinary tract pathogenic bacteria. Int J Res Pharmacol Pharmacother. 2014;3:184–91.
25. Zhang Y, Yang S, Qiu Z, Huang L, Huang L, Liang Y, Liu X, Wang M, Zhou B. Pyrogallol enhances therapeutic effect of human umbilical cord mesenchymal stem cells against LPS-mediated inflammation and lung injury via activation of Nrf2/HO-1 signaling. Free Radic Biol Med, 2022;191:66–81. 10.1016/j.freeradbiomed.2022.08.030. Epub 2022 Aug 24.
26. Finewax Z, de Gouw J, Ziemann P. Products and secondary organic aerosol yields from the OH and NO3 radical-initiated oxidation of resorcinol. ACS Earth Space Chem. 2019;3:1248–59. 10.1021/acsearthspacechem.9b00112.
27. Jayawardhana HHACK, Jayawardena TU, Sanjeewa KKA, Liyanage NM, Nagahawatta DP, Lee H-G, Kim J-I, Jeon Y-J. Marine algal polyphenols as skin protective agents: current status and future prospectives. Mar Drugs. 2023;21(5):285. CrossRef
28. Kishida K, Iida T, Yamada T, Toyoda Y. d-Allose is absorbed via sodium-dependent glucose cotransporter 1 (SGLT1) in the rat small intestine. Metabol Open. 2021;11:100112. 10.1016/j.metop.2021.100112.
29. Ishihara Y, Katayama K, Sakabe M, Kitamura M, Aizawa M, Takara M, Itoh K. Antioxidant properties of rare sugar D-allose: effects on mitochondrial reactive oxygen species production in Neuro2A cells. J Biosci Bioeng. 2011;112(6):638–42. ISSN 1389-1723. CrossRef
30. Ju J, Hou R, Zhang P. D-allose alleviates ischemia/reperfusion (I/R) injury in skin flap via MKP-1. Mol Med. 2020;26(1):21. 10.1186/s10020-020-0138-6.
31. Tohi Y, Taoka R, Zhang X, Matsuoka Y, Yoshihara A, Ibuki E, Haba R, Akimitsu K, Izumori K, Kakehi Y, Sugimoto M. Antitumor effects of orally administered rare sugar D-Allose in bladder cancer. Int J Mol Sci. 2022;23(12):6771. 10.3390/ijms23126771.
32. Shareef H, Haidar J, Hussein H, Hameed I. Antibacterial effect of ginger (Zingiber officinale) Roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Orient J Chem. 2016;(32):817–37. 10.13005/ojc/320207
33. Kalender Y, Kaya S, Durak D, Uzun FG, Demir F. Protective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male rats. Environ Toxicol Pharmacol. 2012;33(2):141–148. CrossRef