INTRODUCTION
Coffee is one of the most common beverages and it accounts for 75% of total global production due to its unique properties and health benefits [1]. Due to this wide consumption around the globe, there has been a noteworthy interest in studying the possible effects of coffee’s bioactive constituents on health [2]. It contains various species, but the main ones are C. arabica (Arabica) and Coffea canephora (Robusta) [3]. Coffee has a very exceptional chemical composition since it contains numerous compounds such as lipids, diterpenes, caffeine, phenolic compounds (e.g., chlorogenic acid—CGA), and many others [4].
Isolation and quantitation of the coffee lipids and diterpenes, and assessment of their health impacts have earned remarkable attention because of their positive health impacts which include anticarcinogenic effect, antioxidant effect, and anti-inflammatory activities [5]. Several experimental procedures have been employed to extract these bioactive components, but the Soxhlet extraction method was proven to be highly efficient for extraction [1]. The extraction of diterpenes has been a very puzzling mission because of the difficulties that accompany isolation and quantification processes where these compounds can be quantified as total or free diterpenes. Choosing the suitable solvent, extraction time, extraction method, and temperature have been considered essential factors that could affect the quantitation of coffee oil and diterpenes to reach maximum efficiency.
Caffeine and CGA are proven to have many health benefits and they can be extracted from coffee beans using various extraction approaches including hot water extraction [6,7] The quantification can be conducted using several techniques, but high-performance liquid chromatography (HPLC) is considered the most commonly utilized method due to its accuracy, simplicity, and reliability. Their levels vary based on the coffee origin, coffee bean type, degree of roasting, and preparation method.
Roasting is a process that causes changes in many physical properties of coffee such as bulk density, tapped density, flowability, sensory, and chemical composition [8]. It was found that the roasting process significantly affects the yield and composition of most of coffee’s bioactive compounds. The yield of the coffee lipid fraction (coffee oil) and diterpenes increases with increasing the roasting temperature due to heat can help remove oil in the material [2,9]. The caffeine content does not change much after roasting, minor losses due to sublimation may occur. CGA showed a noteworthy sensitivity toward temperature and their levels decreased when the roasting process reached higher limits [4].
Despite being worldwide consumed, data concerning the adverse effects of coffee consumption subjected to various coffee roasting degrees is still a research issue [10]. Numerous symptoms including, tachyarrhythmia, palpitation, headache, and dyslipidemia have been linked to the extensive consumption of coffee [11,12]. In the same context, higher habitual coffee consumption is still debated, particularly concerning pathophysiological consequences on diabetic patients [13]. However, it has been reported that people with type 2 diabetes mellitus (T2DM) and other health issues react differently to several natural compounds including caffeine [14].
However, the health effects of coffee consumption, particularly on kidney function in diabetics, remain imprecise and need further clarification [15]. In this manner, some factors such as coffee boiling, degree of roasting, coffee consumption rate, and their link to human health have been studied [2]. The boiling of coffee has been linked to many unwanted effects on human health such as hyperlipidemia [16], poor glycemic control [17], and alteration in kidney function [15]. Converse findings were observed in many studies about caffeine’s effects on glycemic control and insulin sensitivity. Recently, it was pointed out that caffeine may lower insulin sensitivity [18]. However, assessment of coffee roasting degree effects on insulin sensitivity is still scarce and imprecise [19]. Despite that, the relationship between coffee consumption and its roasting degree effect on glycemic control is not yet established. According to a previous study on animal models, the relationship between FBG levels and caffeine dose is unclear [20].
Some reports have linked elevated kidney function parameters such as urea (U) and creatinine (Cr) to the roasting effect of coffee beans in diabetic animals [21]. However, the prior studies did not clearly determine the degree of roasting that has a harmful effect on kidney function parameters. Furthermore, it was reported that the levels of serum creatinine in mice were not affected significantly, which indicates that the high temperature-roasted coffee has no effect on the levels of serum creatinine in mice [22]. Therefore, the current study has taken into consideration the effect of four levels of roasting of coffee samples on renal function in streptozotocin (STZ)-induced diabetic rats. To the best of our knowledge, there were no studies that investigated the extraction and quantification of selected coffee constituents and evaluated the effect of different coffee roasting levels on kidney function parameters in STZ-induced diabetic rats.
MATERIALS AND METHODS
Reagents and chemicals
The caffeine standard, which has a purity of 99.8%, was bought from AZ Chem. Company (Ontario, Canada). CGA standard, which has a purity of 98.0%, was bought from Tokyo Chemical Industry CO. LTD. (Japan). Methyl tert-butyl ether (BME-t) was bought from LOBA Chemie, (Mumbai, India), diethyl ether from Honeywell/Riedel-de Haën, (Germany), and water (HPLC grade) from Techno. Pharm. Chem. (Haryana, India), ethanol HPLC/SPECTRO from FULLTIME, (China), and Anhydrous sodium sulfate were purchased from Sigma-Aldrich (Taufkirchen, Germany). KOH from LOBA Chemie (Mumbai, India), and ethanol were purchased from Gainland Chemical Company (U.K.).
Plant materials
The coffee samples (green coffee, light, medium, and dark-roasted coffee) were purchased from Al-Ameed® Coffee Company (Amman, Jordan) in June 2021.
Instrumentations
An HPLC instrumental setup from (Hitachi Technologies, Tokyo, Japan) was used for analysis. The measurements were done using a Hitachi Elite LaChrom system equipped with a pump, column oven, autosampler, diode array detector (DAD), a (30 cm × 4 mm × 5 μm) LC-18 column (SUPELCO), and an organizer system. The EZ Chrom Elite software, (version 3.3.2) from Agilent (CA, USA), was employed for the analysis of data. All of the injections were employed using a 0.45 μm porosity Nylon syringe filter. The flow rate was 1.0 ml/minute, and the wavelength of absorption was 273.0 and 330.0 nm for caffeine and CGA, respectively.
Mobile phase preparation
The mobile phase mixture was prepared by combining water and methanol (60:40, V/V) for caffeine. While the mobile phase mixture for CGA was prepared by combining 0.1% formic acid with acetonitrile (85:15, V/V) [4].
Preparation of standard solutions
The caffeine standard solution of concentration (1000.0 μg/ml) was prepared by dissolving 0.1002 g in 100.0 ml of the mobile phase. The CGA standard solution of concentration (1000.0 μg/ml) was prepared by dissolving 0.1020 g in 100.0 ml of methanol.
Preparation of calibration curve solutions
The caffeine and CGA calibration curve standard solutions were prepared according to the concentration ranges of (10, 50, 100, 200, 500, and 1000 μg/ml), where each peak area was plotted against the concentration.
Sample preparation and extraction
Extraction of coffee oil: Soxhlet extraction
The amounts of coffee oil were assessed according to the Soxhlet extraction procedures that were reported previously [1,5]. The designed procedure was modified and developed for the extraction of the coffee oils. A Soxhlet extractor was used to extract the coffee oil from the coffee samples for further diterpene analysis. A sample of coffee weighing 10.0 g was used and 175.0 ml of BME-t boiling point = 55.2ºC) was added into the thimble, and the extraction was performed at the organic solvent boiling point for 8 hours. A rotary evaporator was used at a temperature of 55ºC to evaporate the BME-t solvent, and the coffee oil was then weighed gravimetrically.
Extraction of coffee diterpenes
The contents of total diterpenes were determined based on the extraction procedure reported previously [23]. The latter procedure was developed and modified in several steps before usage. A saponification method was performed by dissolving 3.9 g of KOH in 35.0 ml of ethanol. Then, the solution was stirred for about 5–10 minutes, the traces in the beaker were washed with 10 ml of ethanol, and then the beaker was incubated in a water bath at a heating temperature of 80ºC for 4 hours. Next, 50.0 ml of distilled water was added, and then 50.0 ml of diethyl ether was added to the same solution. The diterpene compounds were extracted using the liquid–liquid extraction (LLE), and then the organic phase was washed with 15.0 ml of distilled water three times. The organic phase was then treated and dried using anhydrous sodium sulfate. Finally, the organic phase was evaporated using a rotary evaporator and the diterpenes content was weighed gravimetrically.
Sample preparation and extraction of caffeine and CGA from coffee samples
Four types of ground coffee with different roasting degrees were used in this investigation including green, light, medium, and dark-roasted coffee. Triplicates of each type of coffee were extracted using LLE. The green and roasted ground coffee samples were extracted with a hot water extraction technique at 80ºC at a ratio of 1/100 (w/v). One gram of ground coffee was dissolved in 100.0 ml of water, and then the solution was placed in a water bath at 70ºC–80ºC for 20 minutes. Then, ultrasonication was conducted using an Ultrasonic bath (OVAN) for homogenization for around 10 minutes at a temperature of 75ºC. A centrifuge apparatus was used for each sample at 7900 rpm for 15 minutes. Finally, the coffee solutions were filtered, and the extracts were kept at a temperature of −20ºC until the day of analysis.
Animal experimental model
Wistar male laboratory rats (weighing 180–200 g) were used. The rats were acquired from the animals’ house at the Applied Science Private University (Amman, Jordan). The experiments on animals were carried out in accordance with the Declaration of Helsinki and the guidelines were approved by the Institutional Animal Ethical Committee (2022-PHA-13) at the Applied Science Private University. All animals were housed in metabolic cages and kept in typical housing conditions (room temperature of 25°C, humidity of 65%, with 12–12 light: dark cycles). Water and a commercial pellet diet were provided. The experiments were conducted according to streptozotocin-induced diabetes and were performed according to the published experimental model [24] with few modifications.
Streptozotocin-induced diabetic rats
STZ solution was freshly prepared at a concentration of 35.0 mg/kg before injection. To achieve consistent hyperglycemia, diabetes was induced 5–7 days before the beginning of the experiment. STZ is typically given as a single high dosage or a series of low doses. The single high dose is 35–65 mg/kg [25] which might lead to a fast ablation of beta cells and hyperglycemia. However, it should be emphasized that regeneration of the pancreatic islets has been indicated after STZ treatment; consequently, adequate controls should be in place to ensure that any enhancement in glycemia is not due to the spontaneous regeneration of endogenous beta cells [26]. STZ was given to rats in low dosage over a 5-day period to cause insulitis [27]. Doses ranged from 20 to 40 mg/kg per day, which depends on species and strain.
Coffee extracts feeding
Oral Gavage (coffee components will be delivered directly into the stomachs of rats using a procedure known as oral gavage) was used to administer various coffee extracts. The compound was delivered into the stomach using a stainless-steel bulb-tipped gavages needle, or a flexible cannula or tube attached to a syringe.
Coffee dose
Six grams of coffee were dissolved into 120.0 ml of water; each rat was administered 2.0 ml of the solution with a concentration of 100.0 mg of coffee. This study has been achieved in a similar experimental design which has been published previously [28] with few modifications, and it was conducted for 4 weeks (28 days). First-week wash-out was applied before animal scarifying and blood sample collections.
Study design and groups
The rats were divided into two groups: control (normal-glycemic) rats (CRL) and diabetic (DM) rats. CRL and DM were divided into 11 study groups as displayed below and the timetable of the experimental biological part is displayed in (Fig. 1).
Each group then will be divided into subgroups as follows.
A. Normoglycemic rats (Control), CRL = 5 subgroups:
Group 1: (C) = will not receive coffee extract
Group 2: (G) = will receive green (nonroasted) coffee extract only
Group 2: (L) = will receive light-roasted coffee extract only
Group 4: (M) = will receive medium-roasted coffee extract only
Group 5: (D) = will receive dark-roasted coffee extract
B. STZ-induced diabetic rats; DM 6 subgroups (from 6 to 11)
Group 6: (DM) = STZ-diabetic rats will not receive a coffee extract
Group 7: (G-DM) = STZ-diabetic rats will receive green (nonroasted) coffee extract only
Group 8: (L-DM) = STZ-diabetic rats will receive a light-roasted coffee extract
Group 9: (M-DM) = STZ-diabetic rats will receive a medium-roasted coffee extract
Group 10: (D-DM) = STZ-diabetic rats will receive a dark-roasted coffee extract
Group 11: (Met-DM) =STZ-diabetic rats will receive Metformin (Glucophage 500 mg)
Biochemical analysis
The biochemical analysis was undertaken in a quality-controlled registered laboratory at the laboratories of Al-Ahliyya Amman University, (Amman, Jordan). Serum samples were assayed before the start and at the end of experiments (3 weeks). Fasting blood glucose (FBG), creatinine (Cr), and uric acid (UA) were analyzed using liquid-color-specific kits (Human, Germany).
Statistical Analysis
The analysis was conducted using a Statistical Package for the Social Sciences (SPSS), version 27.0 (Chicago, USA). The paired T-test was performed to determine any significant differences in each of the trial groups before and after the administration of the extract or metformin. One Way ANOVA test was conducted to evaluate if there are any significant differences in the mean values of each parameter between the different groups of experiment and post hoc comparisons Duncan’s test for comparison of measured parameters.
RESULTS
Quantification of coffee oil and diterpenes
In this study, different types of coffee samples were extracted to obtain the content levels of coffee oil and diterpenes, the coffee samples were analyzed to assess the levels of caffeine and CGA by using the HPLC-DAD instrument. The results also explain the degree of roasting effects on coffee samples including diterpenes, coffee oil, caffeine, and CGA levels. It has been established the association between the area and concentration of caffeine and CGA is shown in the calibration curves. The wavelength and absorbance of caffeine and CGA standards have been figured out by using an ultraviolet-visible (UV-VIS) spectrometer. The purpose of the study is to investigate the effects of roasting degree on selected bioactive compound levels in C. arabica and their associations with glycated hemoglobin levels in STZ-induced diabetic rats. It has been noted that medium-roasted coffee has the highest levels of coffee oil and diterpenes whereas dark coffee contains the lowest level of coffee oil and diterpenes which has an explanation that we will clarify later on in the discussion part. It has been established that caffeine has the highest level in light-roasted coffee. The maximum amount of CGA was found in green coffee.
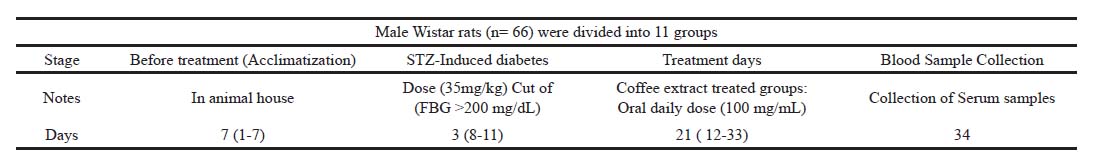 | Figure 1. The timetable of the experimental biological part. [Click here to view] |
Based on one-way ANOVA followed by multiple comparisons in Duncan’s test, there was a significant mean difference in the content of oil and diterpenes as well as their percentages between medium and dark-roasted coffee (Table 1). It also illustrated other significant mean differences between the studied types of coffee samples; green coffee and roasted coffee (light, medium, and dark). The highest content of coffee oil was found in medium-roasted coffee (1.3942 g) which forms around 13.942% of 100.0 g of coffee, whereas the lowest content of coffee oil was found in dark-roasted coffee (1.1664 g) which forms around 11.664% of 100.0 g of coffee. On the other hand, the highest content of diterpenes was found in medium-roasted coffee (0.2797 g) forming around 2.80% of 100.0 g of coffee, while the lowest content of diterpenes was found in dark-roasted coffee (2.2268 g) forming around 2.27% of 100.0 g of coffee.
The correlation between the levels of diterpenes and coffee oil with the degree of roasting for different types of coffee samples is illustrated in Table 1. The levels of diterpenes in coffee oil were affected by the roasting process, but the effect depended on the degree of the roasting temperature. The dark-roasted coffee has the lowest level of diterpenes (0.2268 g), whereas medium-roasted coffee has the highest diterpene level (0.2797 g), it also contains the highest amount of coffee oil (1.3942 g), whereas dark-roasted coffee contained the lowest level of coffee oil (1.1664 g).
Quantification of caffeine and CGA
The development and linearity of the calibration curve for the measurement of the analytes of interest resulted in a high level of accuracy in the determination of caffeine. The HPLC chromatograms for caffeine and CGA standard solutions are illustrated in Figure 2, and their concentrations (μg/ml) and their averages for each coffee sample are illustrated in Figure 3. Light-roasted coffee has the highest average caffeine content (150.39 ± 14.05 μg/ml), while the lowest average caffeine content was found in the green coffee (131.26 ± 7.15 μg/ml). The results of previous studies in regard to the correlation between the caffeine content and degree of roasting temperature were conflicting varying from negative to positive to constant correlation and some of these studies showed no clear correlation where the levels of caffeine changed in a nonsystematic trend. The green coffee produced the highest average of CGA content (357.85 ± 19.73 μg/ml), while the lowest average of CGA content was found in dark-roasted coffee (70.66 ± 3.21 μg/ml). The data showed that CGA highly decreases as the roasting process reaches higher temperatures.
Biological part
The baseline characteristics of the study groups
The baseline characteristics of the nondiabetic (NDM) and STZ-diabetic (DM) animals are shown in Table 2. The mean blood levels of FBG, Cr, and BW were higher in diabetic than nondiabetic rats, whereas mean the U levels were higher in STZ-diabetic groups as well.
Changes in mean serum levels of biochemical parameters of nondiabetic and diabetic study groups at baseline and at the end of the study
Changes in body weight (BW) levels
A significant difference in the means BW was observed in all NDM groups at the baseline and at the end of the study (p-value= 0.025) as shown in Table 3. Except for the light-roasted coffee group, the mean BW was significantly increased in all NDM study groups (p-value <0.05), whereas in DM groups, the mean BW was significantly reduced in all diabetic study groups including coffee-administrated groups (Table 4).
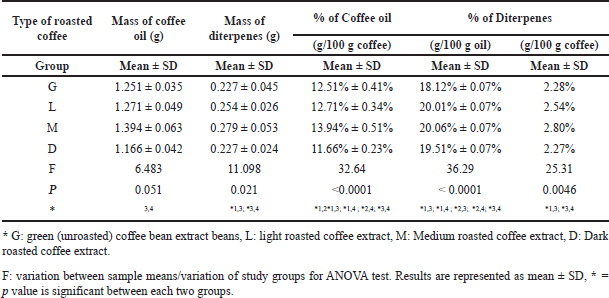 | Table 1. The mass and percentage of coffee oil and diterpenes in coffee samples. [Click here to view] |
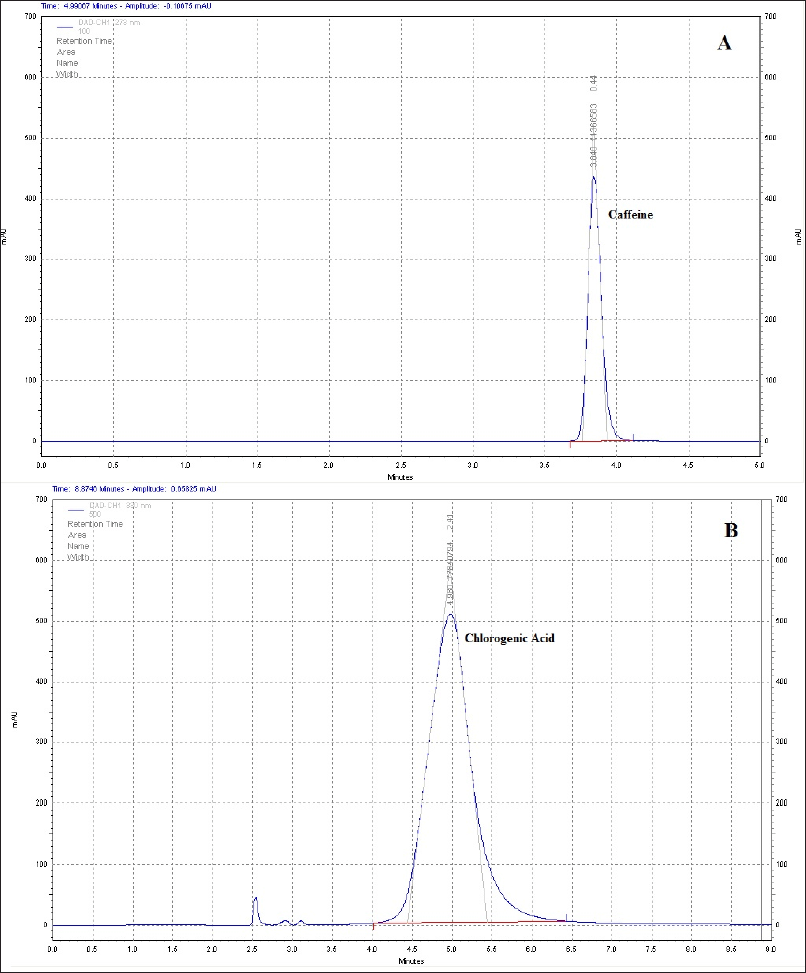 | Figure 2. HPLC chromatogram feature of (A) caffeine standard and (B) chlorogenic acid standard. [Click here to view] |
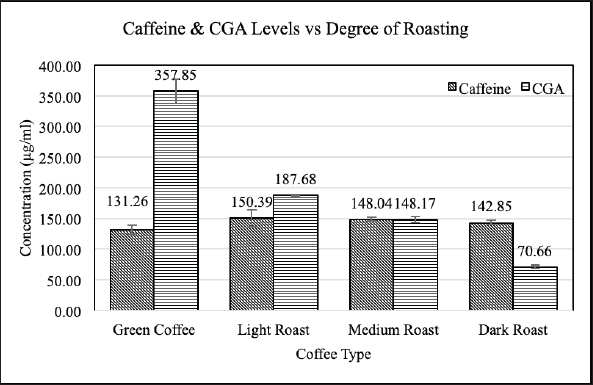 | Figure 3. The caffeine and CGA concentrations in green and roasted coffee. [Click here to view] |
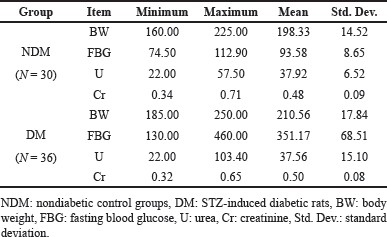 | Table 2. The baseline means and ranges of the biochemical parameters and body weight for nondiabetic (NDM) and STZ-diabetic (DM) rats study groups. [Click here to view] |
The FBG changes
All nondiabetic groups showed normal mean levels at baseline and at the end of the experiment as shown in Table 5. For the diabetic study groups, no significant difference in the mean FBG levels was observed in any coffee-administrated study group. Metformin significantly decreased the mean FBG in the STZ-DM group from 326.0 ± 85.7 to 107.0 ± 14.2 mg/dl with a change of −219 (Pa = 0.002). No significant anti-diabetic effect similar to the metformin effect was observed in any coffee-administrated study group (Table 6).
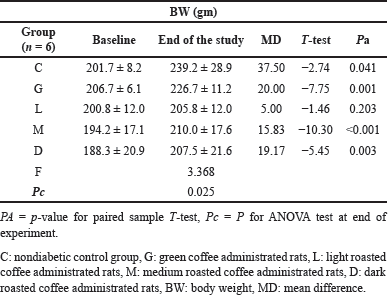 | Table 3. The body weight (BW) changes for nondiabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
The changes in Urea
According to the paired T-test shown in Table 7, the mean U levels in (M and D) groups significantly increased (Pa < 0.05) after coffee administration from 39.8 ± 1.9 to 50.5 ± 5.8 mg/dl and from 30.6 ± 5.2 to 51.1 ± 5.6 mg/dl, with a change of about 10.73 and 20.48 mg/dl, respectively. In diabetic rats, except for the DM group, the mean U level was significantly increased in all diabetic study groups including (Met-DM) group (Table 8). A notable significant elevation in the mean U levels was observed in the D-DM group (Pa = 0.016) after coffee administration from 34.9 ± 4.2 to 132.5 ± 14.3 mg/dl, with a change of about 97.6 mg/dl. The Duncan test of post-hoc multiple comparisons reported that the mean U levels for the group (D-DM) were significantly different from all other groups (DM, Met-DM, G-DM, L-DM, and M-DM), as illustrated in Table 9.
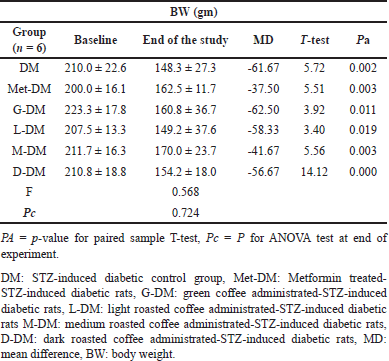 | Table 4. The body weight (BW) changes for diabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
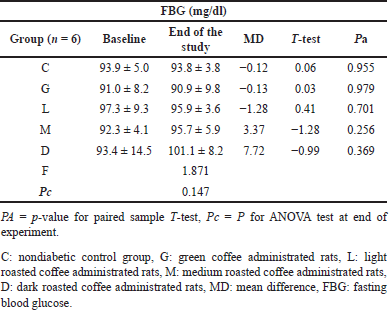 | Table 5. The fasting blood glucose changes for nondiabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
The changes in Creatinine
The mean Cr levels in the nondiabetic group were significantly (Pa = 0.003) increased only in the M study group at the end of the experiment from 0.44 ± 0.02 to 0.58 ± 0.05 mg/dl, with a change of about 0.13 mg/dl (Table 10). In diabetic groups, mean Cr levels were significantly elevated in the DM, L-DM, and D-DM study groups (Table 11).
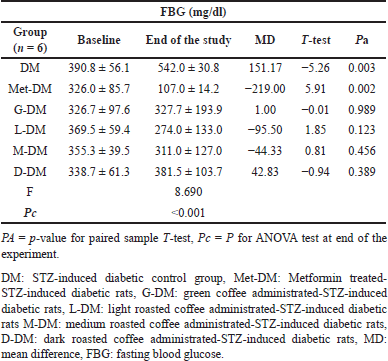 | Table 6. The fasting blood glucose changes for diabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
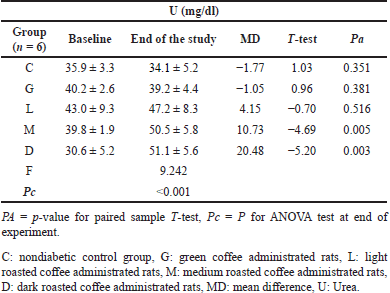 | Table 7. The changes in Urea levels for nondiabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
Stepwise regression analysis
The stepwise regression analysis was conducted to evaluate which independent variables accounted for these inter-associations in both nondiabetic and diabetic study groups. As expected, the multivariate stepwise regression analysis showed a strong relationship and significant effect of the independent variable (FBG) with A1c levels in all nondiabetic study groups in which FBG alone explained approximately more than 95% of the changes observed in A1c levels as shown in Table 12. In the D group, in addition to FBG, the stepwise regression analysis showed a significant relationship and a significant effect on U levels as an ID according to the second multiple linear regression model indicators (R = 0.998, R2 = 0.997, F-test = 440.769, β1 = 0.029, β2 = 0.008, p-value = 0.000).
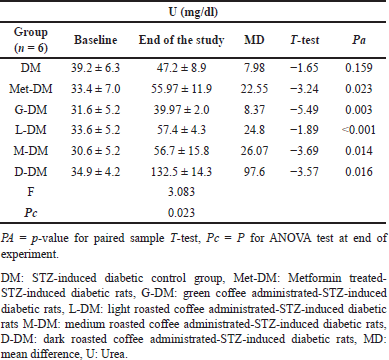 | Table 8. The changes in Urea levels for diabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
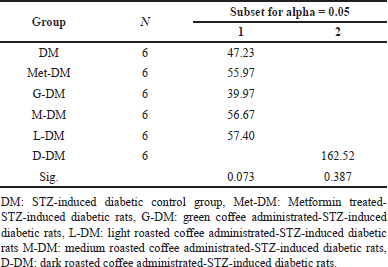 | Table 9. The Duncan test of post-hoc multiple comparisons for determining the differences of total (U) scores at a follow-up level among different DM experimental groups. [Click here to view] |
DISCUSSION
In the current study, diabetic rats administrated with dark coffee extract for two weeks showed significantly higher mean U and Cr levels compared with healthy rats that received the same dose of coffee extract. In addition, there were significant mean U and Cr differences between D-DM rats and other diabetic study groups. These findings were consistent with a previous study that indicated a significant increase in U levels and concluded that the roasting degree of coffee beans may worsen kidney function and reported that dark coffee intake has a significant negative effect on kidney function in diabetics [29]. The remarkable U change in DM rats treated with dark-roasted coffee, which is a novel finding observed in our study, might be due to the significant decline in antioxidant constituents such as CGA and diterpenes. These coffee’s bioactive compounds were reversely associated with the coffee roasting effect as generally mentioned in prior studies [21,30,31].
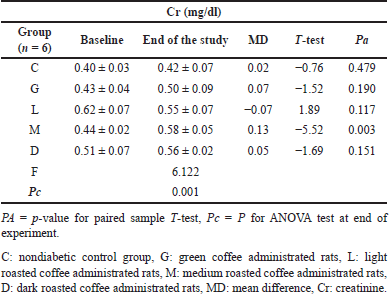 | Table 10. The changes in creatinine levels for nondiabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
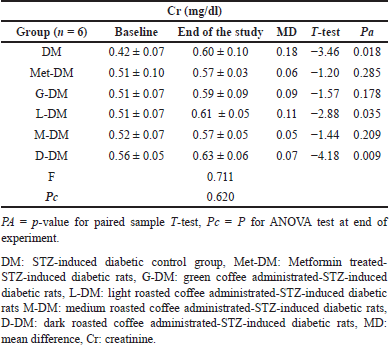 | Table 11. The changes in creatinine levels for diabetic rats at baseline and at the end of the experiment in all study groups. [Click here to view] |
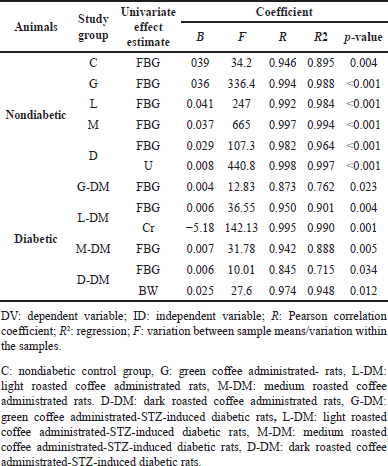 | Table 12. The multivariate associations between the experiment FBG (independent variable) and A1c levels (dependent variable) using stepwise regression at the end of the experiment. [Click here to view] |
In this manner, it was shown that green coffee extract contains significant levels of many antioxidant compounds such as glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) as well as a reduction in lipid peroxides [30]. However, the health effects of coffee consumption, particularly on kidney function in diabetics, remain imprecise and need further clarification [15]. In this manner, some factors such as coffee boiling, roasting, degree of roasting, coffee consumption rate, and their link to human health have been studied [11]. While the effect of the degree of roasting has not yet been decided, the boiling of coffee has been linked to many unwanted effects on human health such as hyperlipidemia [16], poor glycemic control [17], and alteration in kidney function [15]. At the end of the current experiment which lasted for 3 weeks, the study has shown that the glycemic and kidney function parameters levels were differentially affected by boiled coffee intake due to differences in roasting degrees which agrees with what was indicated previously [32].
Concerning glycemic control, a potential adverse side effect of coffee consumption on glycemic control has considerably been linked to the variability in the coffee roasting degrees [33]. The current study has shown that FBG is insignificantly positively associated with the coffee roasting degree which showed a good agreement with the reported findings on animal models, where the consumption of CGA reduced FBG, increased sensitivity to insulin, and slowed the appearance of glucose in circulation after the glucose load [13]. It is well known that the levels of caffeine, diterpene, and CGA in coffee beans are critically influenced by coffee roasting degrees [4]. Their levels were assessed accordingly in the current experiment to clarify the potentially harmful effect of dark coffee extracts on kidney function parameters in diabetic rats by phytochemistry analysis of the different coffee extracts.
Effect of degree of roasting temperature on the levels of coffee oil and diterpenes
This investigation displayed an increase in the coffee oil and coffee diterpenes contents in light and medium-roasted coffee as the degree of roasting temperature increased while their levels declined in dark-roasted coffee. The roasting process influenced the level of diterpenes in coffee, although the effect varied depending on how strong the roasting process was. Coffee oil yields range from 5.7% to 16.0%, with the average being 11.25% [9]. The larger yield of coffee oil was produced at 195°C roasting temperature, with an average yield of 15.3%. The yield value of coffee oil showed that the greater the roasting temperature, the higher the yield of coffee oil. Heat can lower water content while breaking down oil cells in the substance. The higher the roasting temperatures, the higher the yield of coffee oils. These results supported our findings with regard to the levels of coffee diterpenes and their relationship with the degree of roasting temperature [2,9]. On the other hand, if the degree of roasting is conducted at a higher temperature (>220ºC), the content of coffee oil would be lower. Roasting coffee beans using higher temperatures degrees can lead to the evaporation of any volatile compounds and the moisture content (water content) that are contained in the beans when compared to roasting coffee at lower temperatures [4]. A parallel correlation was found between the degree of roasting and coffee oil levels. The higher content of diterpenes was found in light and medium-roasted coffee but at a higher limit, as in dark-roasted coffee, the levels of diterpenes declined [2]. The latter studies showed a good agreement with our findings where the degree of roasting had a significant and positive correlation with the levels of coffee oil and diterpenes in coffee except in dark-roasted coffee, a negative correlation, and a decline in the coffee oils and diterpenes levels was observed.
Effect of degree of roasting temperature on the levels of caffeine
The degree of roasting has a considerable impact on the amount of caffeine produced whereas the previous investigations illustrated that the caffeine levels are dependent on the degree of roasting temperature. A recent study indicated that light-roasted coffee had the maximum caffeine concentration, which showed good agreement with our findings [34]. Furthermore, dark-roasted coffee contains less caffeine than light-roasted coffee because the roasting process reduces the caffeine amount of the bean [4]. It was indicated that increasing the temperature will lessen the water amount in the coffee beans. The caffeine levels in dark-roasted coffee were significantly lower after increasing the temperature to higher limits compared to the light and medium-roasted coffee. This trend was demonstrated in a previous study whereat 240ºC, the caffeine levels reached a maximum of 29.89 mg/g and then dropped to 29.24 mg/g at 250ºC [35]. On the other hand, the caffeine levels remained stable under certain conditions; however, it showed that roasting can degrade caffeine and this requires much harsher roasting conditions [36]. Furthermore, the highest concentration of caffeine in most coffee samples of different geographical origins was determined in medium-roasted coffee [4]. While in some specific origins (e.g., Columbian coffee), light-roasted coffee showed the maximum concentration of caffeine which could be attributed to several parameters such as the altitude, harvesting time, and temperature [4].
Effect of degree of roasting temperature on the levels of CGA
It has been reported on many occasions that the levels of CGAs decrease as the degree of roasting reaches higher limits since this CGA is proven to undergo thermal breakdown as the degree of roasting keeps rising [4,35]. This work demonstrated that green coffee beans have the highest concentration of CGA, which displayed a good agreement with the majority of the literature cited. Our results demonstrated a negative correlation between the levels of CGA and the degree of roasting temperature where the highest average concentration of CGA was noted in green coffee and the lowest average concentration was in dark-roasted coffee. It was reported that when the temperature is too high (230ºC for even 15 minutes of roasting), the carbon–carbon bonds in CGA are broken, resulting in isomerization and degradation, longer periods of roasting resulted in a loss of total CGA [37].
The degree of roasting temperature had a considerable impact on the CGA content in C. arabica brews, which explains some of the disparities in CGA content among various samples. In the dark roasted, the CGA losses are the greatest [4], others have related this to the conversion of CGA isomers to precursors in integrated melanoidins [38]. Moreover, it was reported that green coffee had the highest CGA level, which decreased as the roasting degree and temperature increased [34]. Interestingly, these antioxidant effects of green coffee are consistent with an early hypothesis attempting to explain the anti-hyperglycemic and anti-oxidant properties associated with green coffee consumption [39]. Therefore, and despite that the current study was designed on a small animal sample, it provides insight into the combined effect of the various coffee roasting degrees on glycemic control and kidney function in normal and diabetic rats. Notwithstanding these limitations, the study suggests that green coffee, as well as light-roasted coffee, have a lower negative effect on glycemic control and kidney function, in particular the U levels, in STZ-induced diabetic rats. This evidence might be related to the significant changes in the concentrations of diterpenes and CGA assayed phytochemically and accordingly compared in the four coffee types subjected to various roasting degrees.
CONCLUSION
An efficient extraction technique of green and roasted coffee samples was performed to assess the levels of coffee oil, diterpenes, caffeine, and chlorogenic acid. The BME-t and diethyl ether are proven to be efficient solvents for extracting the coffee oil and diterpenes from coffee samples. The designed HPLC method was suitable for the quantitation of caffeine and chlorogenic acid. The present study indicated that the roasting process could affect the levels of the studied coffee bioactive compounds in the green coffee and roasted coffee samples. The results of this study showed a positive relationship between the degree of roasting temperature and the levels of coffee oil and diterpenes, but it slightly decreased in dark-roasted coffee. The results unsurprisingly indicated how the levels of chlorogenic acid decreased dramatically as the roasting temperature increased. It also demonstrated a negative correlation between the caffeine levels and the degree of roasting temperature; hence, it showed that the highest concentration of caffeine was found in light-roasted coffee which showed a good agreement with some of the previous studies.
Based on phytochemical analysis of the coffee samples subjected to different roasting degrees, the current study showed a significant elevation in the mean urea levels in diabetic rats treated with dark-roasted coffee extract. This may exacerbate kidney nephropathy due to the potential oxidative effects of dark-roasted coffee extract due to deep roasting compared with a green coffee extract that contains higher concentrations of chlorogenic acid. In conclusion, green coffee bean extract, which contains higher levels of antioxidants, appears to have a less negative effect on diabetic kidneys than dark-roasted coffee bean extract, which appears to dramatically exacerbate diabetic nephropathy.
ACKNOWLEDGMENT
The authors are thankful to the Applied Science Private University (Amman, Jordan), for fully supporting this research. The authors are also very thankful to Salem Al-Shawabkah and Fawzi Elerini for their technical support and assistance in animal samples handling, and Rahmeh Kherfan for her assistance in the biological samples analysis.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest to disclose.
ETHICAL APPROVALS
The experiments on animals were carried out in accordance with the Declaration of Helsinki and the guidelines were approved by the Institutional Animal Ethical Committee (2022-PHA-13) at the Applied Science Private University.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Speer K, Kölling-Speer I. The lipid fraction of the coffee bean. Brazilian J Plant Physiol. 2006;18(01):201–16. CrossRef
2. Awwad S, Abu-Zaiton A, Issa R, Said R, Sundookah A, Habash M, et al. The effect of excessive coffee consumption, in relation to diterpenes levels of mediumroasted coffee, on non-high-density lipoprotein cholesterol level in healthy men. Pharmacia. 2023;70(01):49–59. CrossRef
3. Yepes Y, Uribe D, Röthlisberger S. A review of the chemopreventive effects of the main bioactive compounds in coffee in colorectal cancer. J Appl Pharm Sci. 2021;11(07):046–54. CrossRef
4. Awwad S, Issa R, Alnsour L, Albals D, Al-momani I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules. 2021;26(24):7502. doi:https://doi.org/10.3390/molecules26247502
5. Dias RCE, De Faria AF, Mercadante AZ, Bragagnolo N, De Benassi MT. Comparison of extraction methods for kahweol and cafestol analysis in roasted coffee. J Braz Chem Soc. 2013;24(03):492–9.CrossRef
6. Hasoun LZ, Khader HA, Abu-Taha MI, Mohammad BA, Abu-Samak MS. A cross-sectional study on the combined effect of body weight and coffee consumption on serum levels of leptin, vitamin b12, and folic acid in healthy young adult males. J Multidiscip Healthc. 2021;14:639–50. CrossRef
7. Ahmad I, Pertiwi AS, Kembaren YH, Rahman A, Mun’im A. Application of natural deep eutectic solvent-based ultrasonic assisted extraction of total polyphenolic and caffeine content from coffe beans (Coffea beans L.) for instant food products. J Appl Pharm Sci. 2018; (08) 8:138–43. CrossRef
8. Nakilcioglu-Tas E, Ötles S. Physical characterization of arabica ground coffee with different roasting degrees. Acad Bras Cienc. 2019;91(02):1–15. CrossRef
9. Ariga SR, Aisyah Y, Patria A, Arpi N, Yunita D. Physicochemical characterization of oil from roasted coffee. Proceeding 8th AIC Heal Life Sci. 2018;94–102.
10. Godos J, Pluchinotta FR, Marventano S, Buscemi S, Volti GL, Galvano F, et al. Coffee components and cardiovascular risk: beneficial and detrimental effects. Int J Food Sci Nutr. 2014;65(08):925–36. CrossRef
11. Habash M, Al-shakhshir S, Abusamak M, Mohammad MY, AbuSamak M. The association of coffee consumption rate with serum 25-hydroxyvitamin D, non-HDL levels, and TC/HDL ratio in females with vitamin D deficiency. Women’s Heal. 2022; 18:1–10. CrossRef
12. Abu-Taha M, Dagash R, Mohammad BA, Basheiti I, Abu-Samak MS. Combined effect of coffee consumption and cigarette smoking on serum levels of vitamin B12, folic acid, and lipid profile in young male: a cross-sectional study. Int J Gen Med. 2019;12:421–32. CrossRef
13. Reis CEG, Dórea JG, da Costa THM. Effects of coffee consumption on glucose metabolism: a systematic review of clinical trials. J Tradit Complement Med. 2019; 9(03):184–91. CrossRef
14. Barham A, Mohammad B, Hasoun L, Awwad S, Mosleh I, Aljaberi A, et al. The combination of omega−3 fatty acids with high doses of vitamin D3 elevate A1c levels: a randomized clinical trial in people with vitamin D deficiency. Int J Clin Pract. 2021;75(11):1–9. CrossRef
15. Boonphang O, Ontawong A, Pasachan T, Phatsara M, Duangjai A, Amornlerdpison D, et al. Antidiabetic and renoprotective effects of Coffea arabica pulp aqueous extract through preserving organic cation transport system mediated oxidative stress pathway in experimental type 2 diabetic rats. Molecules. 2021;26(07):1–15. CrossRef
16. Buscemi S, Marventano S, Antoci M, Cagnetti A, Castorina G, Galvano F, et al. Coffee and metabolic impairment: an updated review of epidemiological studies. NFS J. 2016; 3:1–7. CrossRef
17. Ranheim T, Halvorsen B. Coffee consumption and human health—beneficial or detrimental?—Mechanisms for effects of coffee consumption on different risk factors for cardiovascular disease and type 2 diabetes mellitus. Mol Nutr Food Res. 2005;49(03):274–84. CrossRef
18. Dansinger M, Williams PT, Superko HR, Asztalos BF, Schaefer EJ. Effects of weight change on HDL-cholesterol and its subfractions in over 28,000 men and women. J Clin Lipidol. 2019;13(02):308–16. CrossRef
19. Lee JH, Oh MK, Lim JT, Kim HG, Lee WJ. Effect of coffee consumption on the progression of type 2 diabetes mellitus among prediabetic individuals. Korean J Fam Med. 2016;37(01):7–13. CrossRef
20. Urzúa Z, Trujillo X, Huerta M, Trujillo-Hernández B, Ríos-Silva M, Onetti C, et al. Effects of chronic caffeine administration on blood glucose levels and on glucose tolerance in healthy and diabetic rats. J Int Med Res. 2012;40(06):2220–30. CrossRef
21. Silvério A dos SD, Pereira RGFA, da Silveira Duarte SM, Figueiredo SA, de Araújo Paula FB, Araújo TH, et al. Coffee intake (Coffea arabica L.) reduces advanced glycation end product (AGEs) formation and platelet aggregation in diabetic rats. Rev Ciências Farm Básica Apl. 2021;42:1–14. CrossRef
22. Widodo IP, Syamsurizal S, Utami DT. The effect of high temperature roasted Coffea liberica on physiological and histological structure of mice’s kidney. Indones J Cancer Chemoprevention. 2019;10(01):30. CrossRef
23. Guercia E, Berti F, Navarini L, Demitri N, Forzato C. Isolation and characterization of major diterpenes from C. canephora roasted coffee oil. Tetrahedron Asymmetry. 2016;27(14–15):649–56. CrossRef
24. Zhang FL, Ye C, Li G, Ding W, Zhou W, Zhu H, et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp Anim. 2003;52(05):401–7. CrossRef
25. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview K. Indian J Med Res. 2012;136(01):451–72.
26. Grossman EJ, Lee DD, Tao J, Wilson RA, Park SY, Bell GI, et al. Glycemic control promotes pancreatic beta-cell regeneration in streptozotocin-induced diabetic mice. PLoS One. 2010;5(01):1–6. CrossRef
27. Lukic ML, Stošic-Grujicic S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol. 1998;6(1–2):119–28. doi: https://10.1155/1998/92198.
28. Rustenbeck I, Lier-Glaubitz V, Willenborg M, Eggert F, Engelhardt U, Jörns A. Effect of chronic coffee consumption on weight gain and glycaemia in a mouse model of obesity and type 2 diabetes. Nutr Diabetes. 2014;4:1–9. CrossRef
29. Naglaa M, Rezq A. Effect of green and roasted coffee beans extracts on some physiological parameters and histological structures in rats. African J Biol Sci. 2009;5(02):181–95.
30. Bhattacharyya S, Kumar R, Sengupta A, Hazra AK, Sur TK. Chlorogenic acid enriched green coffee ameliorated renal injury in rats. Mymensingh Med J. 2020;29(04):991–1000
31. Alnsour L, Issa R, Awwad S, Albals D, Al-Momani I. Quantification of total phenols and antioxidants in coffee samples of different origins and evaluation of the effect of degree of roasting on their levels. Molecules. 2022;27(05). CrossRef
32. Hubert K, Stephan M, Kerstin K. Coffee and lower risk of type 2 diabetes: arguments for a causal relationship. Nutrients. 2012;13:1–17. CrossRef
33. Rakvaag E, Dragsted LO. Acute effects of light and dark roasted coffee on glucose tolerance: a randomized, controlled crossover trial in healthy volunteers. Eur J Nutr. 2016;55(07):2221–30. CrossRef
34. Król K, Gantner M, Tatarak A, Hallmann E. The content of polyphenols in coffee beans as roasting, origin and storage effect. Eur Food Res Technol. 2020;246(01):33–9. CrossRef
35. Van Cuong T, Hong Ling L, Kang Quan G, Jin S, Shu Jie S, Le Linh T, et al. Effect of roasting conditions on concentration in elements of Vietnam Robusta coffee. Food Technol. 2014; 18(02):19–34. CrossRef
36. Ludwig IA, Mena P, Calani L, Cid C, Del Rio D, Lean MEJ, et al. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014;5(08):1718–26. CrossRef
37. Farah A, de Paulis T, Trugo LC, Martin PR. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J Agric Food Chem. 2005;53(05):1505–13. CrossRef
38. Perrone D, Farah A, Donangelo CM. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J Agric Food Chem. 2012;60(17):4265–75. CrossRef
39. Ahmed GM, El-Ghamery HE, Samy MF. Effect of green and degree of roasted arabic coffee on hyperlipidemia and antioxidant status in diabetic rats. Adv J Food Sci Technol. 2013;5(05):619–26. CrossRef