INTRODUCTION
Bioactive compounds and metabolites produced by endophytic fungi, which are isolated from the host plant, might be used for medicine and agriculture purposes [1–3]. Endophytic fungi have an essential role in affecting the quantity and quality of the crude extracts the plant host produces through a certain fungus-host interaction. This shows the importance of understanding how fungi exist in medicinal plants which are implemented traditionally for infection treatment [4]. Endophytic fungi have various secondary metabolites, some of which are bioactive compounds expressed as defensive weapons to protect the host plant against pests and diseases but also as metabolites for specific interactions and communication with the host plant [5]; and enhance the adaptability of both endophytic and host fungi to biotic and abiotic stress [6,7]. Endophytic fungi found in plants in the desert are considered a key source of many natural products [6].
Many fungi have a secondary metabolism that is well developed. A number of fungal species and the diversification of clusters of biosynthetic genes demonstrate an almost infinite capacity for metabolic variation and an untapped opportunity for drug discovery and synthetic biology. In certain cases, plant-related fungi may produce identical bio-compounds as their plant host. The identification of gibberellins in Fusarium fujikuroi and taxol from endophytic fungus connected to Taxus brevifolia supports this hypothesis [8]. The fungus Taxus spp. is the main source of taxol, which is the first anticancer medicine to sell for one billion dollars worldwide.
The fungal taxol will potentially decrease its value and, in some cases, prevent the plant’s extinction. In addition, endophytes have been recognized as an excellent source of novel bioactive natural chemicals, including immunosuppressive, antioxidant, antiviral, anticancer, and antimalarial substances [3,9], as they occupy millions of vascular plants that develop in various uncommon habitats [10]. Moreover, with the therapeutic properties and extraordinary longevity, the plant endures infuriating circumstances since fungal endophytes produce bioactive metabolites that are often harboring [10]. Manganyi and Ateba [3], reported a comprehensive analysis of the untapped potentials of endophytic fungi and the isolation of novel bio-compounds for various applications in the medical, pharmaceutical, food, and agricultural industries. Furthermore, recent investigations showed the discovery of new bioactive compounds such as isocoumarin derivatives, polyketides, azaphilone amide derivatives, chromanones, alkaloids, phenolics, and flavonoids possessing unlimited bioactive properties. Hence, paving unexplored territories in the endophyte-based technologies for the development of new, more efficacious, and cost-effective antimicrobial drugs.
This study also acknowledges and addresses important issues related to host-endophyte interactions, including their ecological significance, potential applications in sustainable agriculture, and the broader implications for the fields of microbiology and biotechnology. By delving into these critical areas, this research seeks to shed light on the complex interplay between host plants and their endophytic microorganisms, offering valuable perspectives on how such interactions can be harnessed for both environmental conservation and innovative scientific advancements. While several investigations have explored the antimicrobial properties of black seeds, few have specifically focused on the host-endophyte interactions and their potential impact on enzyme production and antibacterial activity. In the present study, we explore the bioactivity and enzymatic properties of culturable fungal endophytes isolated from Black seeds (Nigella sativa L.) as a potential source to combat resistant pathogenic bacteria (Fig. 1).
MATERIALS AND METHODS
Isolation and identification of endophytic fungi
A total of one hundred (n = 100) endophytic fungi were aseptically isolated from healthy black cumin seeds collected from Mountain Herb Estates Nursery in Pretoria, South Africa (25°43027.6’’S 27°57054.8’’E) as previously reported by Gopane et al. [11]. Previous findings [11] investigated the identification of the endophytic fungi using polymerase chain reaction targeting the internal transcribed spacer region and distinct morphological characteristics were recorded.
Determination of enzymatic assay
Screening of amylase activity
Amylase activity was evaluated by growing the fungi on Glucose yeast extract peptone agar (GYP: glucose 10.0 g; yeast extract 0.1 g; peptone 0.5 g; soluble starch 2 g; agar 16.0 g per liter, final pH = 6.0). After incubation, the plates were flooded with 1% (v/v) iodine. The zone of inhibition surrounding the colony was considered indicative of the production of amylase [9].
Screening of lipase activity
The presence of lipase enzymes was assessed by growing the fungi on sterilized peptone agar (PAM) medium [peptone (10.0 g); NaCl (5.0 g); CaCl2.2H2O (0.1 g); agar (16.0 g); distilled water (1 l); pH = 6.0], supplemented with 1% (v/v) of sterilized tween 20. A clear zone around the colony was recorded as a positive result for lipase production [9].
Screening of laccase activity
All fungal isolates investigated were cultured on potato dextrose agar (PDA), supplemented with 0.04% (v/v), guaiacol (Inqaba, RSA, Pretoria), and 0.01% (w/v) of Chloramphenicol to avoid bacterial growth. The final pH of the medium was adjusted to 5.5. Culture plates were incubated at 28°C for 72 hours and then screened for laccase production which is identified by the formation of reddish–brown zones around the colonies [12].
Proteolytic activity
To determine protease activity, the fungi were cultured on glucose yeast extract peptone agar (GYP) medium glucose (1 g), yeast extract (0.1 g), peptone (0.5 g), agar (16 g), 0.4% (w/v) gelatin at pH 6, and distilled water (1 l). After 3–5 days of colony growth, the plates were treated with saturated ammonium sulfate. The detection of clear zones around the colonies was recorded as positive proteolytic activity [13].
 | Figure 1. Graphical diagram of the overall study. [Click here to view] |
Determination of secondary metabolites
Extraction of secondary metabolites
To extract secondary metabolites, fungal isolates were cultured on PDA agar media and grown at 25°C for 7 days. A plug from the freshly grown mycelia was transferred to 50 ml of Malt broth extract to optimize fungal biomass at 25°C. All liquid cultures were incubated at 25°C for 14 days while shaking at 200 rpm. The fermentation of each fungus was filtered using solvent extraction to separate the filtrates from the mycelia. The resulting extracts were removed and used for analysis. The experiment was performed in triplicates [14].
Screening of antimicrobial properties
The disk diffusion method was used to screen for the bioactive properties and antimicrobial activity of fungi. To achieve this, 18–24 hours standard inoculums were prepared according to the Clinical and Laboratory Standards Institute (CLSI, 2016), plated on Muller Hinton Agar, and incubated at 37°C for 24 hours. Listeria monocytogenes (ATCC 19115), S. aureus (ATCC 25923), E. coli (ATCC 25922), and P. aeruginosa (ATCC 27853) were used for antimicrobial activity. Streptomycin and Fluconazole were used together as positive controls while dimethyl sulfoxide (DMSO) was used as a negative control. Each experiment was carried out in triplicates. The degree of activity was assessed by measuring the diameter (mm) zones of growth inhibition and compared to the positive and negative controls [14].
For statistical analysis, each experiment was carried out in triplicates. The degree of activity was assessed by measuring the diameter (mm) zones of growth inhibition and compared to the positive and negative controls [14]. Statistical analysis of the activity was calculated whereby the formula gives μ = μ1 + μ2/N where μ = mean, μ1and2 = the measured zone of inhibition per plate, and N = the number of plates presenting a particular isolate. The standard deviation per isolate was calculated using the following formula:
where σ represents the standard deviation, N denotes the number of plates, μ is the mean, and x1and2 is the measured zone of inhibition per plate.
RESULTS AND DISCUSSION
The one hundred endophytes previously isolated from black seeds of N. sativa were subjected to extracellular enzyme production in solid media. Out of 100 endophytic fungi, only ten fungal strains are producing extracellular laccase, while the specific activity for amylase was detected for ninety-three endophytic strains. All the fungal strains isolated in this study were tested for their ability to produce enzymes amylase, protease, lipase, and laccase. A large proportion (93%) of the isolates were positive for the production of amylase followed by protease, lipase, and laccase with 72%, 81%, and 10%, respectively (Fig. 2). Out of 100 endophytic fungi, only one of the Alternaria spp. could not produce the amylase. Two out of the 35 strains of Penicillium spp. and 18 strains of Cladosporium spp. were also negative for the production of amylase while all the Fusarium spp. isolated produced amylase (Table 1).
Figure 2 demonstrates a representative qualitative interpretation of the presence of enzymes exhibited by the isolated endophytic fungi. The preliminary qualitative analysis of endophytic fungal enzymes on solid-state media is shown by a clear zone appearing around the fungal colony while performing amylase, protease, and lipase. In addition, laccase-positive isolates displayed color change by forming reddish–brown zones around the fungal colonies. Amylase enzyme facilitates the hydrolysis of starch into soluble sugars. Moreover, 93% of the entire fungi isolated showed the potential for production of the enzyme amylase. The enzyme amylase is currently applied in food industries as a flour adjuster, bread softener, and starch hydrolyzer. It is also used for drinking, as a drainage improvement agent in the pulp and paper industry, for the removal of carbohydrate stains, as a fiber-splitting agent in the leather industry, and for bioremediation of vegetable wastes in waste management industries [15,16].
The enzyme protease, which is also produced by 72% of the fungi isolated, has been a useful tool in the removal of dead skin in cosmetics industries, bioremediation of keratinic wastes, removal of biofilm in waste management industries, and improving food quality, by reducing allergenic compounds in food industries [8,16]. Protease enzyme forms the clear zone by catalyzing proteins into simple polypeptides. Hydrolysis of triglycerides or lipids is catalyzed by the presence of lipase enzymes. The triglycerides or lipids are broken down into soluble free fatty acids and glycerol. The Laccase enzyme is responsible for the oxidation of lignin in water. A total of ten fungi in this study displayed the potential to produce the enzyme laccase. Despite the fact that only 10% of isolates displayed laccase activity, it is essential to evaluate the biological significance of these isolates. Do they play a crucial role in a particular ecological niche or have unique properties that make them relevant despite their low prevalence? And to explore whether these isolates have potential applications or unique characteristics that make them valuable for certain industrial or environmental purposes. Laccase is an important enzyme with significant industrial application in the area of bioremediation, decolorization of dye, and detoxification of waste [17].
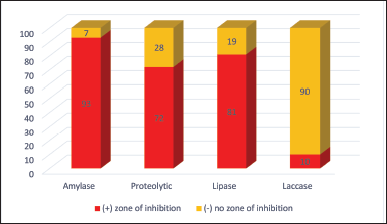 | Figure 2. Enzyme-producing capability of the endophytic fungi. [Click here to view] |
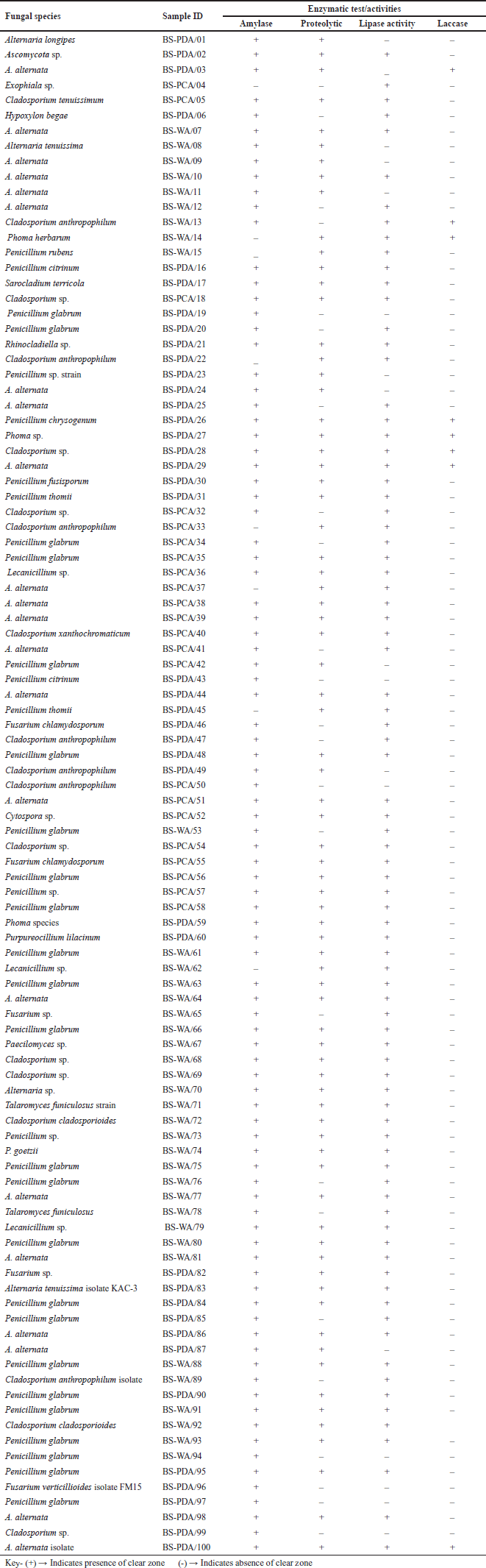 | Table 1. Enzyme-producing capability of endophytic fungi. [Click here to view] |
In addition, the use of enzymes in food, agriculture, chemicals, and pharmaceuticals is gaining increased momentum due to rapid processing time, low input energy requirement, cost effectiveness, non-toxicity, and eco-friendly traits [18]. Furthermore, they find applications in diverse biotechnological processes, including the synthesis of valuable compounds, enzymatic biofuel cells, and the textile and food industries. The presence of laccase-active isolates in the studied population underscores their potential to address environmental challenges and foster innovative industrial processes, warranting further investigation into harnessing their enzymatic capabilities for sustainable and beneficial applications [8]. Fungi function as an excellent source of potential exoenzymes and are essential in the conversion of polysaccharides into soluble products. Fungal enzymes have sparked interest in various processing fields such as pharmaceutical, agricultural, biotechnology, food, and human health. This is due to its stability in high temperatures and extreme pH, its broad substrate specificity is environmentally friendly, and with a broad spectrum of uses [19]. In the manufacturing industry, fungal enzymes aid in simplifying the processing of raw materials in the leather, confectioneries, beverages, and textile sectors [20].
Current technological advances universally utilize Fusarium, Aspergillus, Humicola, Trichoderma, and Penicillium in various industrial applications [21]. Cellulase and amylase produced by Trichoderma sp. and Aspergillus spp., are currently being utilized for bioethanol, textiles, and detergent productions. While fungal proteases and keratinases are manufactured for food, detergent, pharmaceutical, leather, and waste management applications. Furthermore, fungal acidic pectinases reduce the cloudiness and bitterness of fruit juices, whereas fungal phytases enhance the nutritive value of poultry diets [22]. In 2020, the industrial enzyme market was estimated at US$ 5.9 billion; in addition, the growth projection between 2020 and 2026 was 6.5% reaching US$ 8.7 billion at the end of this period as reported by Industrial Enzymes Market by Type [23].
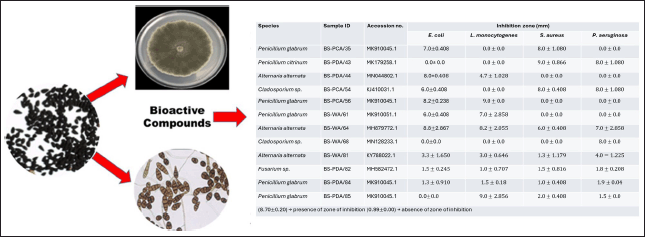
Endophytes are rich sources of bioactive metabolites and extracellular enzymes of important applications [24]. Enzymes of microbial origin have equally become of interest among researchers because of their wide range of medical and industrial applications, due to certain inherent features, such as stability, catalytic activity, ease of production, and optimization compared to those of plant and animal origin; and ultimately, they can be manipulated genetically for flexibility regarding industrial applications [17]. The fermentation broths of 100 endophytic fungi isolated from 100 black cumin seeds were screened for antibacterial properties against pathogenic L. monocytogenes (ATCC 19115), S. aureus (ATCC 25923), E. coli (ATCC 25922), and P. aeruginosa (ATCC 27853). Our results show that nineteen (n = 19%) endophytic fungal extracts produced bioactive secondary metabolites which were active against the tested pathogens and showed a broad spectrum. Of 19, endophytic fungi exhibiting antibacterial activity, 9 (47%) belonged to Penicillium genus, 5 (26%) were previously identified as Alternaria, 3 (16%) belonged to Cladosporium and 1 (5%) was Fusarium genus, as displayed in Figure 3.
Understanding the host plant specificity could have provided crucial insights into the potential role of host-endophyte interactions in enzyme production and antibacterial activity. Host plants and their associated endophytic microorganisms have co-evolved over millennia, leading to intricate relationships that influence the biochemistry of both partners. Investigating the specificity of these interactions would have allowed us to decipher the underlying mechanisms governing the synthesis of enzymes and the expression of antibacterial compounds. Such insights could have far-reaching implications, not only for advancing our understanding of plant-microbe interactions but also for harnessing these interactions in various fields, including agriculture and biotechnology. By unraveling the mysteries of host specificity, we could have unlocked the potential to engineer or optimize these partnerships for enhanced enzyme production and natural antibacterial defenses, ultimately benefiting both plant health and human applications [25].
A total of eight-one (n = 81%) of the fungal extracts showed no activity against the investigated pathogens. Despite the overall 19% endophytic fungal extracts, only seven (n = 7%) were able to inhibit all the bacterial pathogens. Alternaria alternata (6–9 mm) and Penicillium goetzii (7–9 mm), with sample identities BS-WA/64 and BS-WA/74, respectively, produced metabolites with commendable growth inhibition against L. monocytogenes (ATCC 19115), S. aureus (ATTC 25923), E. coli (ATCC 25922), and P. aeruginosa (ATCC 27853). Therefore, A. alternata and P. goetzii showed the highest activities in this study. Bioactivity screening against bacterial human pathogen strains showed that S. aureus (ATTC 25923) was resistant, followed by E. coli (ATCC 25922) and E. coli (ATCC 25922). In contrast, P. aeruginosa (ATCC 27853) was found to be most sensitive and was inhibited by fifteen (n = 15) endophytic fungal extracts as shown in Table 2.
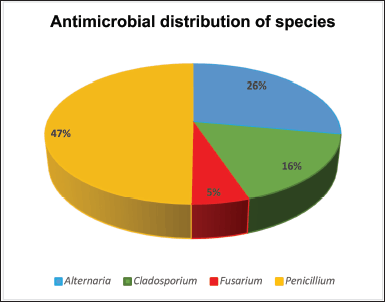 | Figure 3. Genus distribution of fungal extracts displaying antibacterial activity. [Click here to view] |
These pathogens, apart from being etiological agents for life-threatening infections, are known for the rapid acquisition of multidrug-resistant genes against conventional or synthetic antimicrobials. Thus, becoming a life-threatening foodborne pathogen in hospitalized Individuals and a serious crisis in the healthcare system [26]. Having understood that antimicrobial resistance is a serious global health concern, which requires immediate solutions to resolve this imminent problem [27–30]. Health issues caused by pathogenic bacteria are increasing on a daily basis and endophytic fungi thus, provide an important source of bioactive secondary metabolites against these infectious pathogens [31]. Endophytic fungi serve as a sustainable source of novel bioactive natural products [32]. Chutulo and Chalannavar [33] established that the endophytes group is an under-studied class of microorganisms, despite the fact that they have an untapped wealth of bioactive and chemically novel compounds with tremendous applications in several medical, pharmaceutical, industrial, and agricultural sectors [3,33–35]. Endophytic fungi have the potential to produce different useful chemical substances, such as antibiotics, industrial enzymes, and natural pigments [36]. Therefore, this will equally contribute a great deal to the on-going fight against the daily increase in multidrug-resistant pathogens, which is a combined effort toward the eradication of infectious diseases. Increasing resistance by clinically important pathogens, coupled with undesirable side-effects of synthetic antimicrobial agents, indicates an urgent need for novel and effective bioactive compounds of natural origin, possibly with unique modes of action.
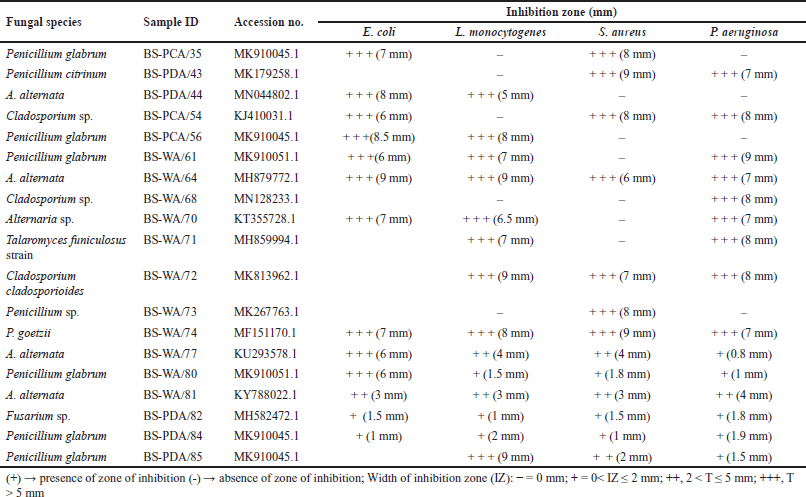 | Table 2. Sensitivity test of antimicrobials producing endophytic fungi against selected pathogens. [Click here to view] |
CONCLUSION
In conclusion, most culturable endophytic fungi inhibiting tissues of black seeds possess certain important traits with useful industrial applications. Some of them produce bioactive secondary metabolites, which are active against clinically important pathogens and could be further developed as broad-spectrum antimicrobial agents of natural origin. Others produce pigments and/or enzymes with potential diverse applications in different industries. These are, in addition to their ability to resist environmental stresses, which may be due to temperature, acidity, and salinity conditions. Ultimately, forthcoming research in this area promises to provide a deeper understanding of the symbiotic relationship between N. sativa and its endophytic fungi, uncover novel bioactive compounds and enzymes, and explore practical applications in fields ranging from medicine to agriculture and biotechnology. Considering the foregoing, black seeds could be a good source of pharmaceutical and industrially important endophytic fungi. Further studies are required to elucidate the complete characterization and other possible metabolites’ bioactive effects.
ACKNOWLEDGMENTS
The authors would like to thank the North-West University (NWU), Mafikeng Campus (specifically the Department of Microbiology).
AUTHOR CONTRIBUTIONS
BG and AS conducted and analyzed the laboratory research and statistical analysis and wrote the initial manuscript. C-DKT, TR, CNA, and MCM constructed the concept, planned the experiments, and edited the manuscript. All authors have read and approved the final manuscript.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
All authors declare that there is no conflict of interest regarding the publication of this paper.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. De Carvalho CR, Ferreira MC, Amorim SS, da Silva Florindo RHD, Assis JCSD, Zani CL, et al. Bioactive compounds of endophytic fungi associated with medicinal plants. In: Yadav AN, Singh S, Mishra S, Gupta A, editors. Recent advancement in white biotechnology through fungi. New York, NY: Springer, Cham. 2019;303–61. CrossRef
2. Lee C, Shim SH. Endophytic Fungi inhabiting medicinal plants and their bioactive secondary metabolites. Nat Prod Sci. 2020;26:10–27. CrossRef
3. Manganyi MC, Ateba CN. Untapped potentials of endophytic fungi: a review of novel bioactive compounds with biological applications. Microorganisms 2020;8:1934. CrossRef
4. Manganyi MC, Regnier T, Kumar A, Bezuidenhout CC, Ateba CN. Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. S Afr J Bot. 2018;116:192–99. CrossRef
5. Lugtenberg BJ, Caradus JR, Johnson LJ. Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol. 2016;92:12. CrossRef
6. Ali AH, Abdelrahman M, Radwan U, Ali AH, El-Zayat S, El-Sayed MA. Effect of thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl Soil Ecol. 2018;124:155–162. CrossRef
7. Jain P, Pundir RK. Potential role of endophytes in sustainable agriculture-recent developments and future prospects. In Endophytes: biology and biotechnology. New York, NY: Springer, Cham. 2017;145–169. CrossRef
8. Souza PMD, Bittencourt MLDA, Caprara CC, Freitas MD, Almeida RP, Silveira D, et al. A biotechnology perspective of fungal proteases. Braz J Microbiol. 2015;46:337–46. CrossRef
9. Toghueo RMK, Ejiya IE, Sahal D, Yazdani SS, Boyom FF. Production of cellulolytic enzymes by endophytic fungi isolated from cameroonian medicinal plants. Int J Curr Microbiol Appl Sci. 2017;6:1264–71. CrossRef
10. Strobel G, Daisy B, Castillo U, Harper J. Natural products from endophytic microorganisms. J Nat Prod. 2004;67:257–68. CrossRef
11. Gopane B, Kaptchouang Tchatchouang C-D, Regnier T, Ateba CN, Manganyi MC. Community diversity and stress tolerance of culturable endophytic fungi from black seed (Nigella sativa L.). S Afr J Bot. 2021;137:272–7. CrossRef
12. Kalra KCR, Shavez M, Sachdeva S. Isolation of laccase producing Trichoderma Spp. and effect of pH and temperature on its activity. Int J Chemtech Res. 2013;5:2229–35.
13. Sunitha VHND, Srinivas C. Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agric Res. 2013;9:1–9.
14. Sharma D, Pramanik A, Agrawal PK. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. 3 Biotech. 2016;6:210. CrossRef
15. Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzyme Res. 2011;2011:Article ID 280696. doi: https://doi.org/10.4061/2011/280696 CrossRef
16. Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174. CrossRef
17. El Monssef RAA, Hassan EA, Ramadan EM. Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann Agric Sci. 2016;61:145–54. CrossRef
18. Choi JM, Han SS, Kim HS. Industrial applications of enzyme biocatalysis: current status and future aspects. Biotechnol Adv. 2015;33:1443–54. CrossRef
19. Ramadiyanti M, Djali M, Mardawati E, Andoyo R. Production of laccase enzyme by Marasmius sp. from the bark of cocoa beans. Sys Rev Pharm. 2020;11(3):405–409. E-ISSN 0976-2779.
20. Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agr Sci Tech. 2005;1:67–80.
21. Monteiro MCP, Tavares DG, Nery EM, Queiroz MV, Pereira OL, Cardoso PG. Enzyme production by Induratia spp. isolated from coffee plants in Brazil. Braz Arch Biol Technol. 2020;63:1–9 e20180673. CrossRef
22. Kango N, Jana UK, Choukade R. Fungal enzymes: sources and biotechnological applications. In: Satyanaayana T, Deshmukh S, Deshpande M, editors. Advancing frontiers in mycology and mycotechnology. Singapore: Springer. 2019. CrossRef
23. da Câmara Rocha J, da Silva Araújo J, de Paiva WKV, Ribeiro ES, de Araújo Padilha CE, de Assis CF, et al. Yellow mombin pulp residue valorization for pectinases production by Aspergillus niger IOC 4003 and its application in juice clarification. Biocatal Agric Biotechnol. 2020;30:101876. CrossRef
24. Pavithra N, Sathish L, Ananda K. Antimicrobial and enzyme activity of endophytic fungi isolated from Tulsi. J Pharm Biome. 2012;16:2014.
25. Pathak P, Rai VK, Can H, Singh SK, Kumar D, Bhardwaj N, et al. Plant-endophyte interaction during biotic stress management. Plants. 2022;11(17):2203. CrossRef
26. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–18. CrossRef
27. Abdallah EM. Black seed (Nigella sativa) as antimicrobial drug: a mini-review. Nov Appro Drug Des Dev. 2017;3:1–5.
28. Gupta PD, Birdi TJ. Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med. 2017;8:266–75. CrossRef
29. Serra R, Grande R, Butrico L, Rossi A, Settimio UF, Caroleo B, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev Anti Infect Ther. 2015;13:605–613. CrossRef
30. Zhang L, Hou L, Zhang S, Kou X, Li R, Wang S. Mechanism of S. aureus ATCC 25923 in response to heat stress under different water activity and heating rates. Food Control. 2020;108:106837. CrossRef
31. Farhat H, Urooj F, Tariq A, Sultana V, Ansari M, Ahmad VU, et al. Evaluation of antimicrobial potential of endophytic fungi as sociated with healthy plants and characterization of compounds produced by endophytic Cephalosporium and Fusarium solani. Biocatal Agric Biotechnol. 2019;18:101043. CrossRef
32. Khare E, Mishra J, Arora NK. Multifaceted Interactions between endophytes and plant: developments and prospects. Front Microbiol. 2018;9:2732. CrossRef
33. Chutulo EC, Chalannavar RK. Endophytic mycoflora and their bioactive compounds from Azadirachta indica: a comprehensive review. J Fungi. 2018;4:42. CrossRef
34. Gouda S, Das G, Sen SK, Shin HS, Patra JK. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol. 2016;7:1538. CrossRef
35. Sishuba A, Leboko J, Ateba CN, Manganyi MC. First report: diversity of endophytic fungi possessing antifungal activity isolated from native Kougoed (Sceletium tortuosum L.). Mycobiology. 2021;49:89–94. CrossRef
36. Venkatachalam M, Magalon H, Dufossé L, Fouillaud M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: new resources for natural red-colored metabolites. J Food Compost Anal. 2018;70:35–48. CrossRef