INTRODUCTION
Diabetes mellitus is a chronic metabolic health problem with the main characteristic of persistent hyperglycemia. This health disorder causes death and disability throughout the world and attacks the health of many people regardless of gender, age, or country [1–3]. Type 2 diabetes is the most common type of diabetes, accounting for more than 90% of all diabetes cases worldwide. In type 2 diabetes, hyperglycemia is initially a result of the body’s cells being unable to respond optimally to insulin, and this condition is called insulin resistance. With the emergence of insulin resistance, the hormone becomes less effective and encourages increased insulin production so that, ultimately, insulin production is no longer adequate. This occurs due to the failure of pancreatic beta cells to produce insulin [4]. The pathophysiology of type 2 diabetes mellitus is related to insulin resistance and insulin secretion, which results in abnormally high blood glucose levels. In cases of cell dysfunction, there is a lack of insulin secretion, thereby limiting the body’s capacity to maintain physiological glucose levels. Insulin receptors also contribute to increased glucose production in the liver and decreased glucose uptake in muscle, liver, and adipose tissue [5]. The global incidence of diabetes has continued to increase for several decades, and currently, around 537 million cases of diabetes have been reported. This case continues to expand and is estimated to reach 643 million in 2030 [4,6]. Different treatment classes for type 2 diabetes have shown treatment benefits, but incidence continues to increase, encouraging new agent therapy discoveries to treat type 2 diabetes [7].
Currently, dipeptidyl peptidase-4 (DPP-4) inhibitors are used to treat type 2 diabetes mellitus, and this enzyme is one of the active ingredients in treating type 2 diabetes mellitus. DPP-4 promotes the breakdown of the incretin hormones glucagon-like peptide-1 and glucose insulinotropic peptide by dispensing with the N-terminal dipeptide in the form of the hormone [8,9]. Inhibition of DPP-4 protects glucagon-like peptide-1 and glucose insulinotropic peptide from degradation, thereby increasing insulin secretion to help lower blood sugar levels [10]. DPP-4 inhibitors do not cause weight gain, hypoglycemia, or indigestion and can be administered to patients with renal impairment. They are one of the first-line therapies in managing diabetes [11]. Although most synthetic DPP-4 inhibitors are broadly well-tolerated, some side effects have been reported lately, including headaches, nasopharyngitis, and urinary infections [12]. Hence, it is necessary to identify DPP-4 inhibitors from natural products and explore their activity for diabetes mellitus therapy development [13].
One of the potential Borneo medicinal plants is Sungkai (Peronema canescens Jack). This plant has been used for generations as a traditional antidiabetic drug for the Borneo people. The activity of P. canescens fractions as an antidiabetic has never been reported, including scientific data on this plant as a DPP-4 inhibitor. The scientific data related to the antidiabetic activity of Sungkai is an in vivo study [14] that significantly reduces blood sugar levels. With its popular use as an empirical antidiabetic, there is preliminary data about the potential of this plant in lowering blood sugar levels. Still, there is no scientific data on P. canescens as a DPP-4 inhibitor, encouraging research to reveal the activity of this plant as a DPP-4 inhibitor.
In this study, the leaves and stems of P. canescens were extracted by three-graded maceration using n-hexane, ethyl acetate, and methanol as solvents, and each extract was investigated for its inhibitory activity against the enzyme DPP-4. Next, the best extracts in DPP-4 inhibition were fractionated using column chromatography. Fractionation using this method is expected to be able to separate the active part of the extract, so the chromatographic fractions will generally have better activity than the original extract [15]. Compound profiling using ultra-performance liquid chromatography coupled with electrospray ionization/quadrupole-time-of-flight mass spectrometry (UPLC-ESI-QToF-MS/MS) is performed on the most active chromatographic fraction to reveal the compounds contained therein. The interaction between these compounds and DPP-4 will be revealed through molecular docking.
MATERIALS AND METHODS
Plant material
Peronema canescens was collected from Tabalong, South Borneo, Indonesia, during the dry season. The cleaned fresh plant, leaves (5 kg), and stems (3 kg) were dried in an oven at 40°C. The dried leaves and stems are pulverized separately with a grinder, yielding 1.2 kg powdered leaves (24% yield) and 1.1 kg powdered stems (36.67% yield), then refrigerated at 8°C while pending analysis. Plant authenticity was determined, and a voucher specimen was deposited in the faculty of pharmacy, Universitas Indonesia (voucher specimen number 208a/LB/XI/2022).
Chemicals and instrumentation
Chemicals: DPP-4 inhibitor screening assay kit (Cayman Chemicals, Catalog no.700210), analytical and distilled technical grade n-hexane, ethyl acetate, and methanol (Smartlab), Silica Gel 70-230 mesh (Merck), and thin-layer chromatography plate Silica Gel 60 F254 (Merck). Instrumentations: Rotary evaporator (IKA), UV lamp (Camag), Microplate reader (Glomax Promega), UPLC-ESI-QToF-MS/MS (Acquity UPLC H-Class System, Waters USA; Xevo G2-S QToF, Waters USA).
Extraction
Peronema canescens leaves (1.2 kg) were extracted by three-graded maceration using n-hexane, ethyl acetate, and methanol as solvents (1:20). By increasing the polarity of the solvent, extracts with different polarities were obtained. Maceration was performed twice for each solvent, with the filtrate being concentrated on a rotary evaporator and the solvent removed. Extraction by this method produced n-hexane extract, ethyl acetate extract, and methanol extract from P. canescens leaves. The stems (1.1 kg) were also extracted using the same procedure as the leaves.
Fractionation
Fractionation was performed on selected extracts showing the best activity in inhibiting DPP-4, namely ethyl acetate and methanol extract from the leaves of P. canescens. Fractionation using the column chromatography method with a column length of 50 cm and a diameter of 3.5 cm. A gradient system is applied to this fractionation using a combination of eluents starting with n-hexane-ethyl acetate (9:1, 8:2, and up) to the eluent ethyl acetate-methanol (10:0, 9:1, 8:2, and so on until methanol 100%). Elution results were collected every 100 ml and analyzed for chromatogram patterns using thin-layer chromatography.
DPP-4 inhibition activity
The inhibitory activity of DPP-4 was performed on all extracts and chromatographic fractions of P. canescens using the test protocol specified by the manufacturer [16]. This assay uses sitagliptin as the standard inhibitor of DPP-4 and Gly-Pro-7-Amido-4-methylcoumarin as the substrate. Briefly, this assay has the principle that DPP-4 decomposes the substrate into fluorescent products, namely the free amido-4-methyl coumarin group, which measures its fluorescence with an excitation wavelength of 350–360 and an emission wavelength of 450–465 using a microplate reader (Glomax Discover System). Fluorescence data were obtained by triplication with the calculation of percent inhibition using the following formula determined by the manufacturer.
Compound profiling using UPLC-ESI-QToF-MS/MS
Compound profiling was performed using UPLC (Acquity UPLC®, Waters, USA) and mass spectrometer (Xevo G2-S QToF, Waters, USA). Liquid chromatography separation using a C18 column (Acquity UPLC®, Waters, USA) at column temperature 50°C and room temperature 25°C. The eluent consists of 5 mM ammonium formic water and acetonitrile with 0.05% formic acid using a flow rate of 0.2 ml/minute (step gradient). The mass spectrometry was performed using electrospray ionization (positive mode) and quadrupole time-of-flight (QToF) mass analyzer. The electrospray interface system connected the UPLC separation output system to the mass spectrometer. Compound profiling with the mass spectrometer using conditions: the collision energy 4 Ev (low energy) and high energy collision varied between 25 and 60 V. Cone and desolvation gas flow 0 and 793 l/hour were also used correspondingly, source temperature 100°C and desolvation temperature 350°C. Data acquisition and analysis were processed with Masslynx software.
Molecular docking
The 3-D structures of the compounds glycitein (5317750), pectolinarigenin (5320438), formononetin (5280378), latifoline (5281736), moracin M (185848), loliolide (100332), 3-oxo-alpha-ionol (5370052), and sitagliptin (4369359) were downloaded from the PubChem NCBI database. The 3-D structure of the target protein, DPP-4, was downloaded from the Protein Data Bank database with access code 3G0B [17]. The ligands structure (sitagliptin as a commercial drug, and 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile as a native ligand) were carried out from the PubChem NCBI database as sdf file (3-D form). Both compounds and targeted protein were imported to the Molegro virtual docker version 5.0. The Molegro virtual Docker 5.0 program predicted the protein structure in the cavities with a maximum molecular surface van der Waals as a parameter [18]. The active side of DPP-4 protein was on the protein grid X = 44.79 A; Y = 32.64 A; Z = 18.74 A; radius 11. Those protein grids were used for ligands-protein docking [18]. Docking parameters with Molegro virtual docker are Score Function Moldock Score [Grid]; grid resolution 0.30; algorithm MolDock SE; the number of runs 10, max iteration 1,500; max population size 50; pose generation energy threshold 100, tries 10–30; simplex evolution max steps 300; neighbor distance factor 1.00; multiple poses the number of poses 5; energy threshold 0.00; and cluster similar poses Root Mean Square Deviation (RMSD) threshold 1. Docking data were visualized with the PyMol 2.3 and the Discovery Studio version 21.1.1. Interaction analysis was performed with the Discovery Studio program version 21.1.1.
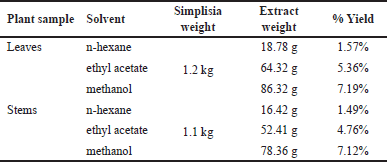 | Table 1. Extracts yield with various solvents. [Click here to view] |
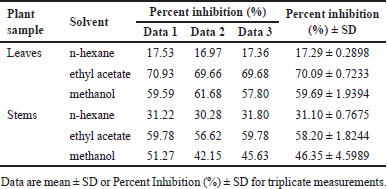 | Table 2. DPP-4 inhibition on various extracts of P. canescens at 100 μg/ml. [Click here to view] |
RESULT
Extraction and DPP-4 inhibition activity of extracts
The extraction was performed on the leaves and stems of P. canescens. Each part of the plant was macerated using a solvent of the increasing polarity of n-hexane, ethyl acetate, and methanol. The yield of the obtained extract is shown in Table 1, and each extract’s activity is shown in Table 2.
Fractionation and DPP-4 inhibition activity of fractions
Two extracts with the best inhibitory activity, ethyl acetate and methanol extract from the P. canescens leaves, were further fractionated using column chromatography with a silica gel stationary phase and a gradient system with different solvents to generate increased polarity. The results of the fractionation of ethyl acetate and methanol extract of the leaves part are shown in Tables 3 and 4, which show the eluent of each fraction so that the polarity conditions of each fraction can be described and the mass of each fraction obtained. The fractions were then assayed for inhibitory activity against DPP-4, and the results are shown in Tables 5 and 6. The assay results showed that all fractions had inhibitory activity against DPP-4.
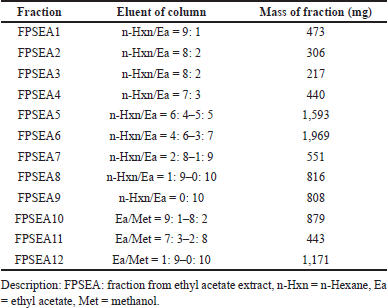 | Table 3. Eluent and mass of ethyl acetate fractions from P. canescens leaves. [Click here to view] |
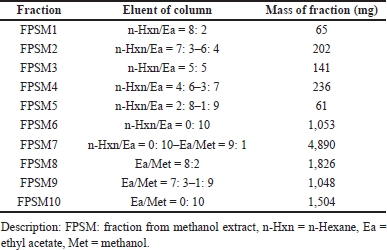 | Table 4. Eluent and mass of methanol fractions from P. canescens leaves. [Click here to view] |
Compound profiling of the most active fraction in DPP-4 inhibition
The identification of the compounds in FPSM2 as the most active fraction was done by UPLC-QToF-MS/MS. The Masslynx software processed the data obtained, giving an overview of the compounds contained in FPSM2. The chromatogram of FPSM2 using UPLC-QToF-MS/MS is shown in Figure 1. Table 7 presents UPLC-MS/MS analysis results, including retention time (rt), precursor ion mass (m/z, positive mode), ion fragments formed, and the formula of each compound.
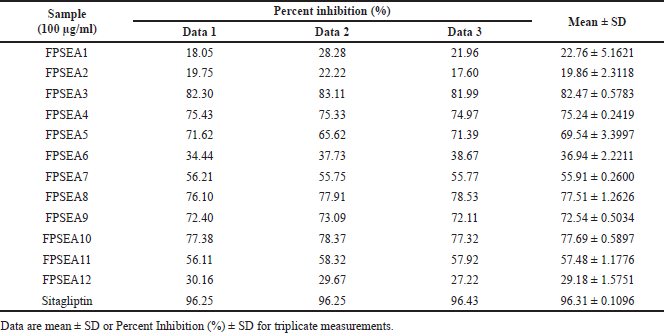 | Table 5. Percent inhibition of ethyl acetate extract’s fractions against DPP-4. [Click here to view] |
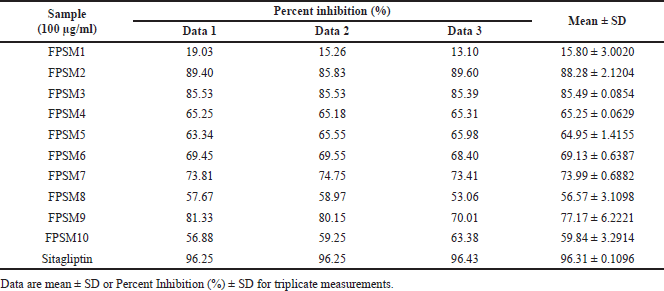 | Table 6. Percent inhibition of methanol extract’s fractions against DPP-4. [Click here to view] |
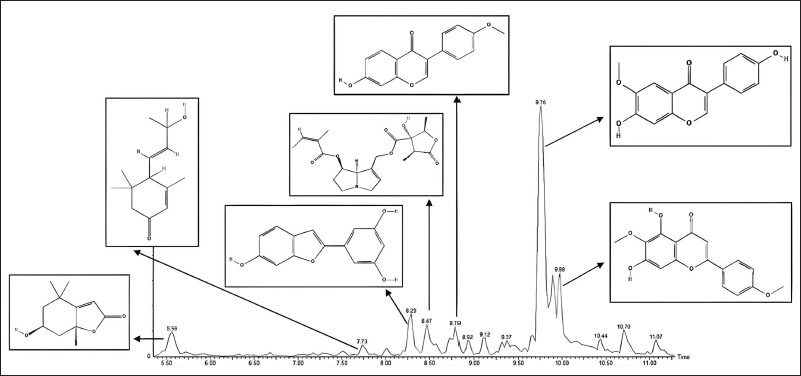 | Figure 1. The chromatogram of FPSM2. [Click here to view] |
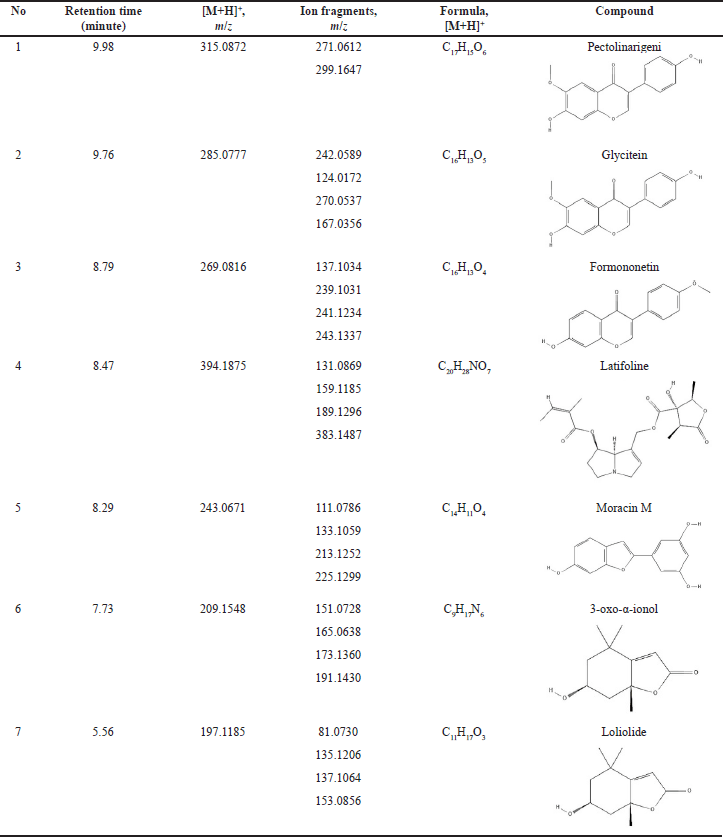 | Table 7. Compounds identified in the most active fraction (FPSM2) from P. canescens leaves. [Click here to view] |
Molecular docking
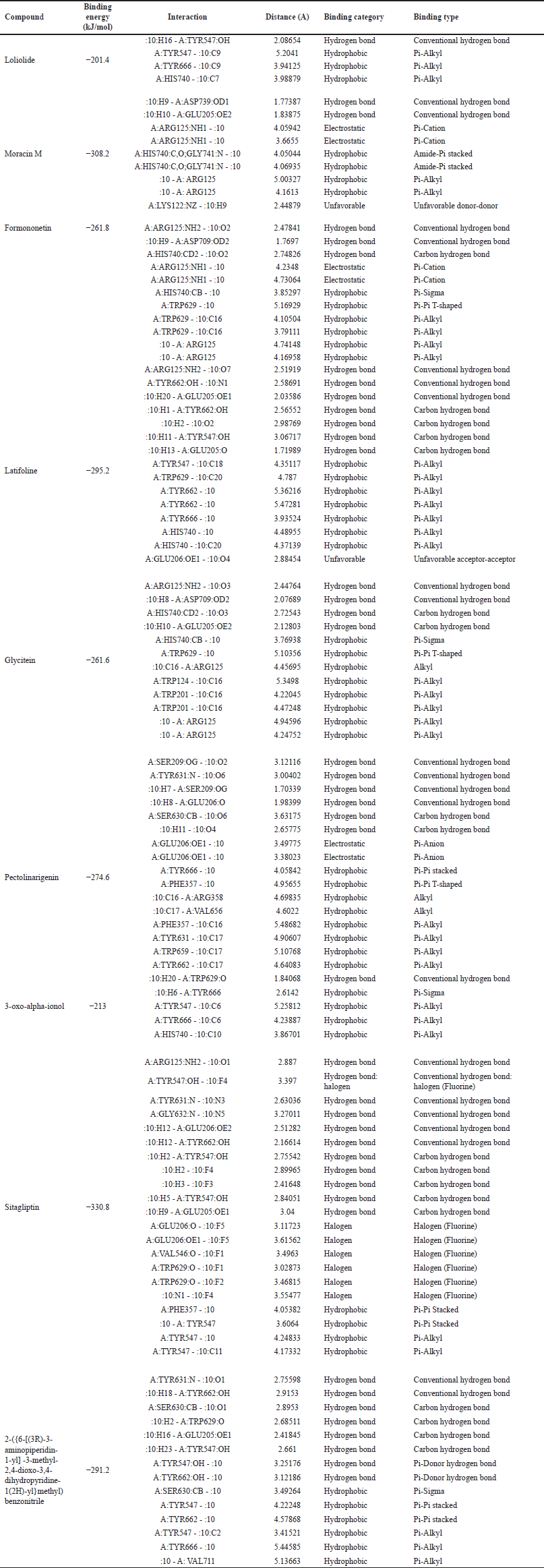
The compounds detected in the profiling of the most active fraction were investigated for the mechanism of interaction with the DPP-4 enzyme protein using molecular docking. Visualization of the interaction between these compounds and the DPP-4 enzyme can be seen in Figures 3–5. The interactions’ details, including binding energy, binding category, and binding type, as well as the amino acid residues that interact, can be seen in Table 8.
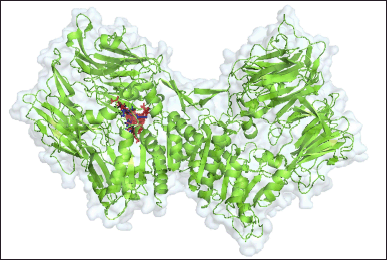 | Figure 2. 3-D structure of the compound-complex with DPP-4 protein, red shows the target compound, blue shows the compound structure of sitagliptin (control), and black shows the compound 2-({6-[(3R)-3- aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile, while green is the DPP4 protein. [Click here to view] |
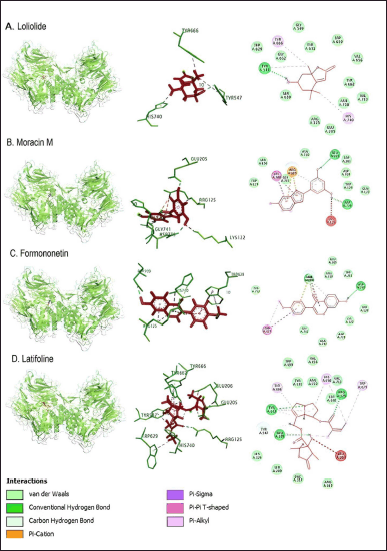 | Figure 3. In the interaction between loliolide, moracin M, formononetin, and latifoline compounds with DPP4 protein, red indicates the target compound, while green is the DPP-4 protein. [Click here to view] |
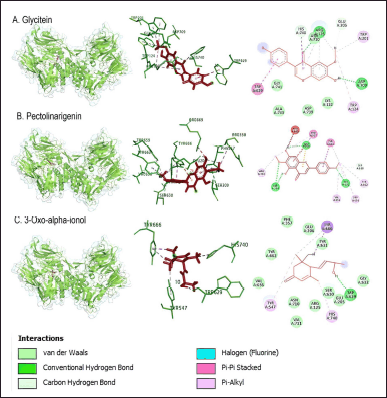 | Figure 4. Interaction of glycitein, pectolinarigenin, and 3-oxo-alpha-ionol compounds with DPP-4 protein. [Click here to view] |
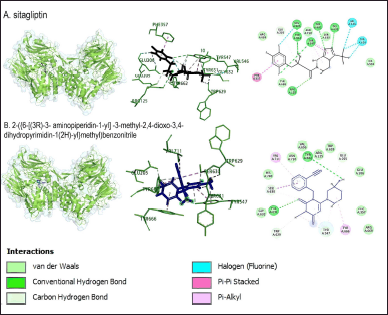 | Figure 5. Interaction of sitagliptin and 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile compounds with DPP-4 protein. Blue shows the compound structure of sitagliptin (control), and black shows the compound 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile, while green is the DPP-4 protein. [Click here to view] |
 | Table 8. Interaction between compound and DPP-4 protein. [Click here to view] |
DISCUSSION
Extraction and DPP-4 inhibition activity of extracts
The extraction showed that the yield of methanol extract was higher from both leaves and stems. This makes it clear that the compounds contained in P. canescens are almost polar. The yield of hexane extract is the lowest because this plant contains fewer non-polar compounds. Several previous studies showed that the content of phytocomponents of this plant, among others, is alkaloids, flavonoids, tannins, phenols, terpenoids, and saponins [19,20]. Phytocomponents, such as alkaloids, flavonoids, tannins, phenols, terpenoids, and saponin, have been widely reported to have inhibitory activity against DPP-4. These phytocomponents or secondary metabolites are potential sources to be developed into new therapeutic agents [21–23].
In general, it can be seen that all extracts have inhibitory activity against DPP-4. Leaf extracts appear to be slightly superior to stem extracts. Ethyl acetate and methanol extract from the leaves showed better activity with percent inhibition of 70.09% ± 0.7233% and 59.69% ± 1.9394% (at 100 μg/ml) compared to other extracts, followed by ethyl acetate extract from the stem with percent inhibition of 58.20% ± 1.8244% (at 100 μg/ml).
Fractionation and DPP-4 inhibition activity of fractions
Fractionation is performed to separate parts of the extract, separating groups of compounds based on polarity differences depending on the eluent used. The presence of fractionation using a chromatographic column using a gradient system with a gradual increase in polarity is expected to be able to separate the active compound group (active fraction) from the less active compound group, leaving the most active part of the extract, which has better activity than the original extract. Several studies have shown that the most active fractions from column chromatography have better activity than the original extract [15,24,25]. However, it cannot be denied that column chromatography has a weakness when polar compounds are bound to a polar stationary phase, so recovery of polar compounds by this method will be poor [26,27].
The most active extracts, namely ethyl acetate and methanol extracts of P. canescens leaves, were fractionated to produce 12 and 10 fractions, respectively. In contrast to the activity of the original extract, the resulting fractions had better inhibition than the original extract. Several fractions (at 100 μg/ml) showed excellent activity, including the FPSEA3 fraction with a percent inhibition of 82.47% ± 0.5783%, the FPSM2 fraction with a percent inhibition of 88.28% ± 2.1204%, and the FPSM3 fraction with a percent inhibition of 85.49% ± 0.0854%. This could be due to the separation of the less active parts of the extract, resulting in fractions with better activity. Sitagliptin as a comparison standard showed better activity than the most active fraction (FPSM2) with a percent inhibition of 96.31% ± 0.1096% (at 100 μg/ml). This could be because sitagliptin is a single compound, while FPSM2 is a fraction that may contain even fewer active compounds. The isolation of FPSM2 in the future will be very promising to produce a DPP-4 inhibitor agent from P. canescens species.
Compound profiling of the most active fraction in DPP-4 inhibition
The strategy for identifying compounds with UPLC-QToF-MS/MS is based on peak analysis at a specific retention time and subsequent comparison with the database [28]. In various compound profiling analyses using UPLC-ESI-QToF-MS/MS, this method has been reported to be more sensitive and selective compared to other chromatographic methods [29]. Mass spectrometry analysis with QToF as a mass analyzer has the ability to detect various compounds. QToF, as a mass analyzer, can reveal mass information of compounds contained in the sample, making the structure identification of compounds more accurate and precise [30].
Seven peaks have been identified in the most active fraction (FPSM2), where the compounds detected are the flavonoids (glycitein, pectolinarigenin, and formononetin), an alkaloid (latifoline), phenol (moracin M), and terpenoids (3-oxo-α-ionol, loliolide). Compound profiling has never been done on this species before, and the compounds detected using UPLC-ESI-QToF-MS/MS are the first compounds identified in this species. Flavonoids contained in the most active fraction have a role in inhibiting the DPP-4 enzyme. Several studies have reported the potential of the flavonoids glycitein and formononetin in treating diabetes mellitus [31,32]. While glycitein has never been reported as a DPP-4 inhibitor, formononetin is reported to have inhibition against DPP-4 [33]. Sewidan et al. [34] reported the DPP-4 inhibitory activity of pectolinarigenin at a concentration of 100 mM with a percent inhibition of 34.7% ± 0.59%. Moracin M has been reported to have the potential for treating diabetes because of its ability to lower blood sugar through in vivo assays, inhibit enzymes associated with diabetes mellitus (α-Glucosidase, Protein Tyrosine Phosphatase 1B), and inhibit the formation of advanced glycation end products. Still, data on this compound related to DPP-4 inhibition has never been done [35–37]. Loliolide has been reported to have a role in antidiabetic activity [38,39], and its activity in inhibiting α-Amylase and α-Glucosidase has been reported [40]. Still, its inhibition of DPP-4 has never been reported before. The terpenoid compound 3-oxo-α-ionol and the alkaloid compound latifoline are reported for the first time to be contained in the active fraction of the DPP-4 inhibitor from the species P. canescens. Disclosure of these compounds shows the potential of this species in inhibiting the DPP-4, where compounds in the class of flavonoids, phenols, alkaloids, and terpenoids have been widely published as inhibitors of the DPP-4 [34,41–45].
Molecular docking
The control compounds used are two, sitagliptin as a comparator control, and the compound 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile as an inhibitor control and docking validation control. Sitagliptin, as a control, showed the lowest binding energy, which was −330.8 kJ/mol. The bond energy of the complexes showed a range of bond energy values of −201.4–−330.8 kJ/mol, with the types of bonds detected in the complexes being hydrogen bonds, hydrophobic, and unfavorable bonds (Table 8). Based on the 3-D display of control compound complexes and DPP-4 protein, all compounds bind in the same region as the inhibitor control (Fig. 2). The interaction of all compounds around the validation control compound indicates validated docking.
Arg125 residues with sitagliptin were also shown on the active side of moracin M, formononetin, latifoline, and glycitein compounds. Tyr547 residues were identified on the active side of loliolide, latifoline, 3-oxo-alpha-ionol, sitagliptin, and 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile. Pectolinarigenin compound showed the same active side as the control, sitagliptin, and 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile, namely at residue Tyr631. Residue Glu206, the active side of sitagliptin, was also detected as the active side of latifoline and pectolinarigenin. Residue Glu205 identified two control compounds, moracin M, latifoline, and glycetin. The compounds formononetin, latifoline, glycitein, and 3-oxo-alpha-ionol have an active side of Trp629, which was also identified in both control compounds (Figs. 3–5; Table 8).
CONCLUSION
Peronema canescens has been shown to have inhibitory activity against DPP-4, with the inhibitory activity of the most active fraction (FPSM2) reaching 88.28% ± 2.1204% at 100 μg/ml, suggesting that this plant has excellent potential to be developed as a drug for diabetes mellitus, especially as a DPP-4 inhibitor. Compound profiling has successfully revealed the compounds in FPSM2 as the most active fraction of P. canescens, including pectolinarigenin, glycitein, formononetin, latifoline, 3-oxo-alpha-ionol, moracin M, and loliolide. This study proves that the potential of P. canescens as an antidiabetic is limited to the inhibition activity of DPP-4. Therefore, further research is needed to confirm its various other antidiabetic mechanisms to reveal the complete mechanism of this plant as an antidiabetic.
ACKNOWLEDGMENT
The authors thank the Drug Development and Pharmacognosy-Phytochemistry Laboratory, Faculty of Pharmacy, Universitas Indonesia, and the Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universiti Malaya, for the support and research facilities provided. The author is also grateful to the Universitas Indonesia for the PUTI-Q2 grant that has been given.
AUTHOR CONTRIBUTIONS
The authors made significant contributions to the research conception and design, including to data collection or the analysis and interpretation of data; participated in the article drafting or the critical revision of the article about all intellectual content; consented to submission in the current journal; gave final approval for the version to be published; and agree to be responsible for all aspects of the work. The authors are eligible to be authors according to the guidelines of the International Committee of Medical Journal Editors (ICMJEs).
FINANCIAL SUPPORT
This project was supported by the PUTI-Q2 Grant (NKB-1172/UN2.RST/HKP.05.00/2022), Universitas Indonesia.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This research does not involve human subjects or animals in the experiments.
DATA AVAILABILITY
All generated and analyzed research data are included in this article
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Widyawati T, Yusoff NA, Bello I, Asmawi MZ, Ahmad M. Bioactivity-guided fractionation and identification of antidiabetic compound of Syzygium polyanthum (Wight.)’s leaf extract in streptozotocin-induced diabetic rat model. Molecules. 2022;27(20):6814.
2. Manalo RAM, Arollado EC, Heralde FM. Anti-hyperglycemic fraction from Alternanthera sessilis L. leaves gets elucidated following bioassay-guided isolation and mass spectrometry. Braz J Pharm Sci. 2023;59:e21283.
3. Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
4. Boyko EJ, Esztergalyos B, Gautam S, Helman B, Pinkepank M, Randi A, et al., editors. IDF diabetes atlas, Brussels, Belgium. 10th ed. IDF; 2021. vol. 64, 665–76 pp.
5. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):1–34.
6. Zhou H, Liao J, Ou J, Lin J, Zheng J, Li Y, et al. Bioassay-guided isolation of Fenghuang Dancong tea constituents with α-glucosidase inhibition activities. Front Nutr. 2022;9(4):1050614.
7. Wu M, Yang Q, Wu Y, Ouyang J. Inhibitory effects of acorn (Quercus variabilis Blume) kernel-derived polyphenols on the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase IV. Food Biosci [Internet]. 2021;43(April):101224. CrossRef
8. Shaikh S, Lee E, Ahmad K, Ahmad S, Lim J, Choi I. A comprehensive review and perspective on natural sources as dipeptidyl peptidase-4 inhibitors for management of diabetes. Pharmaceuticals. 2021;14(591):1–18.
9. Chalichem NSS, Jupudi S, Yasam VR, Basavan D. Dipeptidyl peptidase-IV inhibitory action of Calebin A: an in silico and in vitro analysis. J Ayurveda Integr Med [Internet]. 2021;12(4):663–72. CrossRef
10. Kato E, Uenishi Y, Inagaki Y, Kurokawa M, Kawabata J. Isolation of rugosin A, B and related compounds as dipeptidyl peptidase-IV inhibitors from rose bud extract powder. Biosci Biotechnol Biochem [Internet]. 2016;80(11):2087–92. CrossRef
11. Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of clinical endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract [Internet]. 2020;26(1):107–39. CrossRef
12. Jao CL, Hung CC, Tung YS, Lin PY, Chen MC, Hsu KC. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. BioMedicine. 2015;5(3):9–15.
13. Cian RE, Nardo AE, Garzón AG, Añon MC, Drago SR. Identification and in silico study of a novel dipeptidyl peptidase IV inhibitory peptide derived from green seaweed Ulva spp. hydrolysates. Lwt. 2022;154:112738.
14. Latief M, Sari PM, Fatwa LT, Tarigan IL, Rupasinghe HPV. Antidiabetic activity of Sungkai (Peronema canescens Jack) leaves ethanol extract on the male mice induced alloxan monohydrate. Pharmacol Clin Pharm Res. 2021;6(2):64–74.
15. Triadisti N, Sauriasari R, Elya B. Antioxidant activity of fractions from Garcinia hombroniana Pierre leaves extracts. Pharmacogn J. 2018;10(4):682–5.
16. Cayman Chemical Company. DPP (IV) inhibitor screening assay Kit, Michigan, United States. Cayman Chemical Company; 2017.
17. Zhang Z, Wallace MB, Feng J, Stafford JA, Skene RJ, Shi L, et al. Design and synthesis of pyrimidinone and pyrimidinedione inhibitors of dipeptidyl peptidase IV. J Med Chem. 2011;54(2):510–24.
18. Bitencourt-Ferreira G, de Azevedo WF. Docking screens for drug discovery. 1st ed. In: de Azevedo WF, editor. Molegro Virtual Docker for Docking. Molegro Virtual Docker for Docking. New York, NY: Humana New York NY; 2019. vol. XVII, 286 p.
19. Maigoda T, Judiono J, Purkon DB, Haerussana ANEM, Mulyo GPE. Evaluation of Peronema canescens leaves extract: fourier transform infrared analysis, total phenolic and flavonoid content, antioxidant capacity, and radical scavenger activity. Open Access Maced J Med Sci. 2022;10(A):117–24.
20. Dillasamola D, Aldi Y, Wahyuni FS, Rita RS, Dachriyanus, Umar S, et al. Study of Sungkai (Peronema canescens, Jack) leaf extract activity as an immunostimulators with in vivo and in vitro methods. Pharmacogn J. 2021;13(6):1397–407.
21. Sarian MN, Ahmed QU, Mat So’Ad SZ, Alhassan AM, Murugesu S, Perumal V, et al. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study. Biomed Res Int. 2017;2017:1–14.
22. Tran N, Pham B, Le L. Bioactive compounds in anti-diabetic plants?: from herbal medicine to modern drug discovery. Biology (Basel). 2020;9(252):1–31.
23. Salleh NH, Zulkipli IN, Yasin HM, Ja F, Ahmad N, Amir W, et al. Systematic review of medicinal plants used for treatment of diabetes in human clinical trials?: an ASEAN perspective. Hindawi—Evid Based Complement Altern Med. 2021;2021(Article ID 5570939):1–10.
24. Triadisti N, Rahayu S, Zamzani I. Metabolite profiling of Ficus deltoidea’s most active fraction as anti-Candida albicans using UPLC-QToF-MS/MS. J Young Pharm. 2021;13(1):58–62.
25. Mahayasih PGMW, Elya B, Hanafi M. Alpha-glucosidase inhibitory activity of Garcinia lateriflora Blume leaves. J Appl Pharm Sci. 2017;7(10):100–4.
26. Zhang S, Ma Y, Ma R, Wang Q, Dang J. Combination of medium- and high-pressure liquid chromatography for isolation of L-tryptophan (Q-marker ) from Medicago sativa extract. Separations. 2022;9(9):1–11.
27. Nikoletta. A short notes on column chromatography. J Chromatogr Res [Internet]. 2021;4(1):1. Available from: https://www.scitechnol.com/peer-review/a-short-notes-on-column--chromatography-vUfW.php?article_id=18544
28. Wolfender JL, Marti G, Thomas A, Bertrand S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J Chromatogr A. 2015;1382(February 2015):136–64.
29. Grata E, Guillarme D, Glauser G, Boccard J, Carrupt PA, Veuthey JL, et al. Metabolite profiling of plant extracts by ultra-high-pressure liquid chromatography at elevated temperature coupled to time-of-flight mass spectrometry. J Chromatogr A. 2009;1216(30):5660–8.
30. Li SL, Song JZ, Choi FFK, Qiao CF, Zhou Y, Han QB, et al. Chemical profiling of radix paeoniae evaluated by ultra-performance liquid chromatography/photo-diode-array/quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2009;49(2):253–66.
31. Kurylowicz A. The role of isoflavones in type 2 diabetes prevention and treatment—a narrative review. Int J Mol Sci. 2021;22(1):1–31.
32. Ahmed QU, Ali AHM, Mukhtar S, Alsharif MA, Parveen H, Sabere ASM, et al. Medicinal potential of isoflavonoids: polyphenols that may cure diabetes. Molecules. 2020;25(23):1–19.
33. Pan J, Zhang Q, Zhang C, Yang W, Liu H, Lv Z, et al. Inhibition of dipeptidyl peptidase-4 by flavonoids: structure–activity relationship, kinetics and interaction mechanism. Front Nutr. 2022;9(May):1–17.
34. Sewidan N, Khalaf RA, Mohammad H, Hammad W. In-vitro studies on selected Jordanian plants as dipeptidyl peptidase-IV inhibitors for management of diabetes mellitus. Iran J Pharm Res. 2020;19(4):95–102.
35. Kwon RH, Thaku N, Timalsina B, Park SE, Choi JS, Jung HA. Inhibition mechanism of components isolated from Morus alba branches on diabetes and diabetic complications via experimental and molecular docking analyses. Antioxidants. 2022;11(2):1–22.
36. Zhang M, Chen M, Zhang HQ, Sun S, Xia B, Wu FH. In vivo hypoglycemic effects of phenolics from the root bark of Morus alba. Fitoterapia [Internet]. 2009;80(8):475–7. CrossRef
37. Jung M, Park M, Lee H, Kang YH, Kang E, Kim S. Antidiabetic agents from medicinal plants. Curr Med Chem. 2006;13(10):1203–18.
38. Grabarczyk M, Winska K, Maczka W, Potaniec B, Aniol M. Loliolide–the most ubiquitous lactone. Folia Biol Oecol. 2015;11:1–8.
39. Song JH, Lee D, Lee SR, Yu JS, Jang TS, Nam JW, et al. Identification of bioactive heterocyclic compounds from mulberry and their protective effect against streptozotocin-induced apoptosis in INS-1 cells. Mol Med Rep. 2018;17(4):5982–7.
40. Thissera B, Visvanathan R, Khanfar MA, Qader MM, Hassan MHA, Hassan HM, et al. Sesbania grandiflora L. Poir leaves: a dietary supplement to alleviate type 2 diabetes through metabolic enzymes inhibition. S Afr J Bot [Internet]. 2020;130(February):282–99. CrossRef
41. Sharma D, Kumar S, Kumar S, Kumar D. DPP-IV inhibitors from natural sources: an alternative approach for treatment and management of diabetes. Indian J Nat Prod Resour. 2019;10(4):227–37.
42. Beidokhti MN, Lobbens ES, Rasoavaivo P, Staerk D, Jäger AK. Investigation of medicinal plants from Madagascar against DPP-IV linked to type 2 diabetes. S Afr J Bot [Internet]. 2018;115:113–9. CrossRef
43. Akhtar N, Jafri L, Green BD, Kalsoom S, Mirza B. A multi-mode bioactive agent isolated from Ficus microcarpa L. Fill. With therapeutic potential for type 2 diabetes mellitus. Front Pharmacol. 2018;9(NOV):1–12.
44. Gu L, Tian T, Xia L, Chou G, Wang Z. Rapid isolation of a dipeptidyl peptidase IV inhibitor from Fritillaria cirrhosa by thin-layer chromatography-bioautography and mass spectrometry-directed autopurification system. J Planar Chromatogr—Mod TLC. 2019;32(6):447–51.
45. Morikawa T, Ninomiya K, Akaki J, Kakihara N, Kuramoto H, Matsumoto Y, et al. Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium. J Nat Med. 2015;69(4):494–506.