INTRODUCTION
Traditional medicine is an alternative to conventional therapy, due to the adverse effects they present, there is a tendency for patients to use medicinal plants, as they are readily available and, in many cases, more accessible [1].
Today, the practice of herbal medicine continues to provide health benefits, which is why there is a focus on documenting the ethnobotanical knowledge of each country [2,3]. Ethnobotanical knowledge can disappear if the plant species is threatened with extinction. This serves as an indicator of biodiversity, providing ample scope and opportunities for new research to discover new medicines [4–6]. These therapeutic effects could be explained by the presence of bioactive metabolites, including phenols, flavonoids, carotenoids, and tocopherols, capable of interacting with the human body and reducing the burden of free radicals often associated with chronic diseases [7].
Corymbia citriodora is a species that thrives in low-precipitation areas, and there are plantations that have shown genetic improvements [8]. It is important for its ability to survive, its resistance to endemic diseases and pests, and its high wood production [9]. It is highly profitable and less risky for forest formation and conservation in subtropical areas; C. citriodora stands out from others for the quality of wood it provides to the paper industry [10].
It is a widely distributed species, and its wood is used as raw material for making furniture, poles, fences, houses, and fuel [11]. Similarly, the aerial parts of the tree provide important metabolites for traditional medicine [12] and essential oils that are significantly useful in the chemical and pharmaceutical industry [13].
Within the chemical composition of C. citriodora, important components have been reported, including essential oils (citronellal, citronellol, p-cymene, and spathulenol); phenolic acids (quinic acid, rosmarinic acid, ferulic acid, caffeic acid, gallic acid, ellagic acid, catechol, and 3-O-methylellagic); flavonoids (quercetin, kaempferol, isoquercitrin, isomyricitrin, myricetin, myricitrin, eucalyptin, citriodolic acids, and citrioside A), and glycosides (quercetin-3-O-β-D-galactoside, kaempferol-3-O-β-D-glucoside, quercetin-3-O-β-D-glucuronide, quercetin-3-O-rutinoside, and kaempferol-3-O-α-L-rhamnoside) [14,15].
This species is of great benefit in traditional medicine, as it has been described to be used in the treatment of pain, inflammation, fever, viral respiratory infections, and cancer [15,16]. Furthermore, studies have reported pharmacological and biological properties such as antibacterial, antioxidant, antiproliferative, acaricidal, antifungal, herbicidal, and insecticidal properties [17,18].
Given the information mentioned above, this research aims to describe the ethnobotany for traditional medicine knowledge and quantify the total phenolic and flavonoid compounds in the plant extracts of the species C. citriodora (Hook.) K.D. Hill & L.A.S. Johnson. With this, we aim to reevaluate and endorse the use of the species C. citriodora in traditional medicine. Likewise, we seek to explore how to harness the bioactive compounds present to ensure medicinal properties, thereby considering it the most accessible natural alternative for the treatment of respiratory conditions.
MATERIALS AND METHODS
Botanical material
The species used was C. citriodora (Hook.) K.D. Hill & L.A.S. Johnson, which was acquired from herbalists in the markets located in the city of Trujillo, region La Libertad, Peru. They demonstrated knowledge of the use of medicinal plants in the region. The medicinal plant was collected in Agallpampa, Otuzco, at geographical coordinates (7°57’08’’ S, 78°31’00’’).
Taxonomic identification
A complete specimen was pressed and then prepared according to the standard protocols of the Herbarium Truxillense and taxonomically identified with registration code N° 60829 by the Biologist-Botanist Eric Rodriguez.
Reagents and solvents
Ethanol 96° GL (CKF®), distilled water (Dropaksa®), Folin Ciocalteu reagent (Sigma Aldrich®), sodium carbonate, aluminum chloride, sodium nitrite, and sodium hydroxide (Merck®) were used. Gallic acid (Merck®) and quercetin (Sigma Aldrich®) were used as standards.
Ethnobotanical study
Field and exploration studies on the C. citriodora species were conducted between April and July. Three major markets in the city of Trujillo, Peru, were selected due to their high distribution of medicinal plants. Sampling was conducted randomly among the herbalists as proposed by Weckerle et al. [19]. The information was collected through semi-structured interviews using a questionnaire from herbalists and verbal informed consent was obtained before the interview. The level of fidelity was not considered because it is the only medicinal plant. The study followed the Code of Ethics of the International Society of Ethnobiology [20], and the protocol was approved by the Ethics Committee of the National University of Trujillo with registration code N° PR003P–2022/CEIFYB.
Preparation and extraction of plant extracts
The acquired C. citriodora species had its leaves selected as the plant material, ensuring they were intact, without inert material or decomposition. The plant material was shade-dried and then dried in a Memmert oven at 40°C for 24 hours, followed by mechanical milling with a Corona mill to obtain particles no smaller than 2 mm. Three extraction systems were prepared, including two aqueous extracts obtained by decoction and infusion, and a hydroethanolic extract of 70% obtained by reflux. The concentration of the extracts was 10% w/v [21].
Quantification of total phenols
This was carried out using the Folin Ciocalteu method. In 10 ml volumetric flasks, 200 μl of the plant extract was taken, 200 μl of 10% v/v Folin Ciocalteu reagent was added, and the mixture was gently mixed. Then, 400 μl of 4% Na2CO3 was added, and the flask was filled with distilled water. After waiting for 90 minutes, the absorbance was measured at 750 nm using a UV-visible spectrophotometer, Peak Instruments. Each measurement was performed in triplicate. Gallic acid was used as the standard for the calibration curve at concentrations of 10–100 μg/ml. The total phenol concentration was expressed as gallic acid equivalents per gram of dry material (mg GAE/g DM) [22,23].
Quantification of total flavonoids
This was conducted using the colorimetric method with aluminum chloride. In 10 ml volumetric flasks, 200 μl of the extract was taken, and 150 μl of 5% w/v NaNO2 was added and gently mixed. Then, 150 μl of 10% w/v AlCl3 was added. After 5 minutes, 1000 μl of 1 M NaOH was added, and the solution was filled with distilled water. After waiting for 30 minutes, the absorbance was measured at 510 nm using a UV-visible spectrophotometer, Peak Instruments. Each measurement was performed in triplicate. Quercetin was used as the standard for the calibration curve at concentrations of 1–100 μg/ml. The total flavonoid concentration was expressed as quercetin equivalents per gram of dry material (mg QCE/g DM) [24,25].
Statistical analysis
All data were obtained in triplicate and expressed as mean and standard deviation (X ± SD). Statistical differences (p < 0.05) were calculated using the analysis of variance (ANOVA) test with Microsoft Office Excel 2019.
RESULTS AND DISCUSSION
The information obtained from herbalists has a significantly positive impact on the use and sustainability of traditional knowledge. Table 1 presents the demographic and ethnobotanical information collected from herbalists in the markets of Trujillo, where a higher percentage of indications for relieving bronchitis (39.1%) and the common cold (21.7%) were found, categorized by the type of ailment (Fig. 1A). In addition, infusion (54.5%) was the predominant method of administration (Fig. 1B).
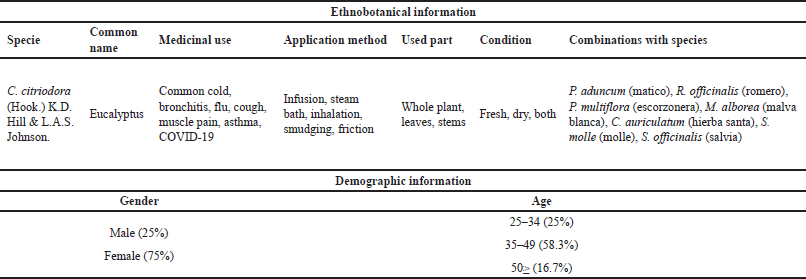 | Table 1. Information on ethnobotanical knowledge of C. citriodora and demographic information of herbalists interviewed in markets. [Click here to view] |
The demographic information of the interviewees indicates that 75% of the respondents were women, and 25% were men. The predominance of women aligns with Mohamadi et al. [26], attributed to the culture of the study area and the greater concern for maternal health [27]. On the other hand, the prevalence in the age group of 35–49 years contrasts with the similarity found in the study by Abba and Dogara [27]. This is due to increased activity and time dedicated to the collection of medicinal plants; furthermore, during this stage, it demonstrates the transmission of traditional medicinal knowledge in the region [28].
Research demonstrates the relationship between popular usage and its biological effects, reinforcing what is documented in traditional medicine. Magalhães et al. [29] report its use as a remedy for fever, flu, cough, sneezing, sinusitis, bronchitis, and asthma. Similarly, Paniagua-Zambrana et al. [30] reported its use in respiratory conditions such as colds, flu, and pneumonia, both as an infusion and in steam baths. On the other hand, Abbass [31] claims that it reduces muscle and stomach pain, cough, and shortness of breath in COVID-19 patients, thereby reducing risk factors for morbidity. In addition, Sharifi-Rad et al. [32] describe its use in the treatment of respiratory conditions, including wheezing, difficulty breathing, cough, chest tightness, and shortness of breath. Among the isolated molecules, tereticornate A and eucliptin have been identified with the potential to reduce the activity of NF-κB/AP-1 and attenuate the secretion of IL-1β [33].
Goodine and Oelgemöller [34] mention it as an antifungal and antibacterial agent. Similarly, Sarma et al. [35] report its potent antimicrobial, anti-inflammatory, and cytotoxic properties, while Tolba et al. [36] describe it as an antioxidant and antimicrobial. Salehi et al. [37] reiterate, highlighting its antimicrobial, antiseptic, antioxidant, and anti-inflammatory properties. The bioactive components serve specific beneficial functions for the human body. Ethnobotanical studies report the use of leaves (48%), with infusions (60%) being the most common preparation method, often directed towards the treatment of respiratory diseases (16%) [38]. Dried leaves and buds are used for inhalation to relieve asthma [39]. Samoisy and Mahomoodally [40] report the use of leaf decoctions for baths. According to Paniagua-Zambrana et al. [30], it is used in the form of infusions and steam baths.
Corymbia citriodora shows that the most frequently used part is the leaves (78.6%), and the predominant plant condition can be both fresh or dry, with 91.7% (Fig. 1C and D). Leaves are commonly used as the predominant plant part in formulations [41]. Afolayan et al. [42] also reported that leaves (34.82%) were the most frequently used, followed by root barks and stems (15.17%). Other studies report that leaves (31.16%) and stems (19.6%) are the most frequently used organs [43]. Bibi et al. [44] reported that the most frequent preparation method was decoction (31%), but infusion (16%) was also commonly used. Magalhães et al. [29] often report consumption in the form of infusions and/or decoctions made from the leaves. The selection of the plant part used often depends on the location of its bioactive metabolites.
They also report that C. citriodora is used in combination with other plants to synergize therapeutic properties, with 35.7% in combination with Piper aduncum and 7.1% in combination with Rosmarinus officinalis, Perezia multiflora, or Malva alborea (Fig. 1E). The combination of C. citriodora with P. aduncum (matico) is mentioned in the studies and is reaffirmed by Mejía and Estela [45]. A list of medicinal plants used in the treatment of respiratory tract diseases and against SARS-CoV-2 was compiled, where P. aduncum was found to be effective in treating bronchitis, chills, colds, cough, tuberculosis, pharyngitis, and pneumonia, as confirmed by Delgado-Paredes et al. [46]. The knowledge base about its medicinal uses is attributed to family members (54%), followed by practical experience (20%), which aligns with the idea that ethnobotanical knowledge is passed down vertically within a family and learned through practice [47,48].
On the other hand, P. aduncum is used for the treatment of other respiratory conditions such as pneumonia, tuberculosis, fever, and the flu in traditional medicine [49,50]. It is also reported that species such as P. multiflora, R. officinalis L., and Salvia officinalis L. [49,51] can address respiratory conditions, and thus, they may synergize with C. citriodora.
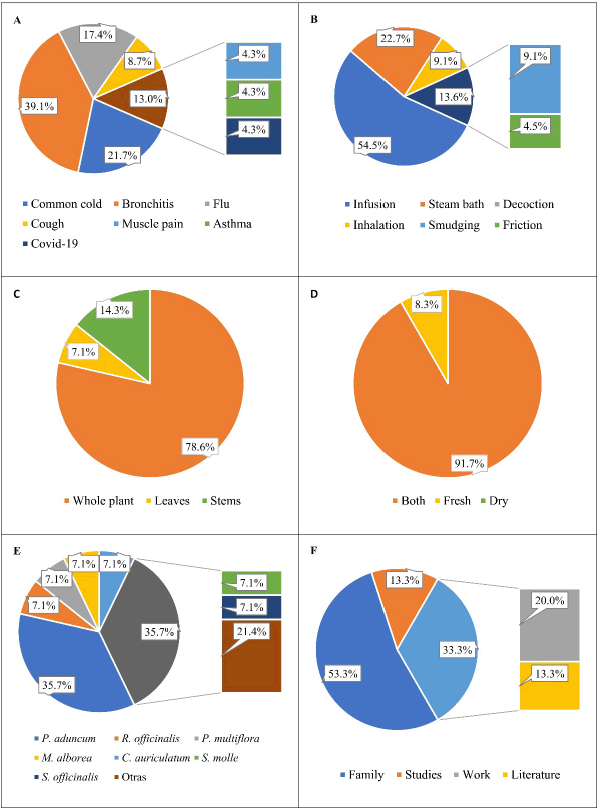 | Figure 1. Ethnobotanical information of C. citriodora collected from herbalists; (A) medicinal use, (B) method of application, (C) used part, (D) condition, (E) combinations with species, and (F) basic of their knowledge. [Click here to view] |
Regarding the total phenol content (Fig. 2A), it was found to be 118.65, 94.44, and 68.31 mg GAE/g DM in hydroethanolic, decoction, and infusion extracts, respectively. The hydroethanolic extract exhibited the highest concentration. The studies indicate that the concentration of C. citriodora is high compared to the 57.02 mg GAE/g DM reported by Bibi et al. [44]. Other authors reported concentrations of 106 g/kg dry matter [52], 58.1 μg GAE/mg of extract [53], and 28.23 mg GAE/g DM [54]. In addition, Toumi et al. [55] reported phenol concentrations ranging from 375 to 400 mg GAE/100 g DM in a 60% ethanol extract, which was influenced by an extraction method involving electric field pulsations.
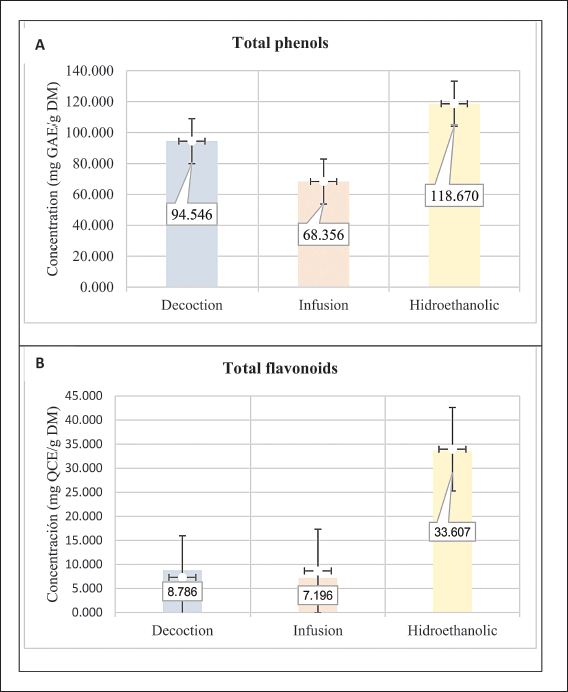 | Figure 2. (A) Total phenols and (B) total flavonoids content in C. citriodora. GAE: gallic acid equivalent, QCE: quercetin equivalent, DM: dry material. Statistic: ANOVA (p < 0.05). [Click here to view] |
Regarding total flavonoids (Fig. 2B), it was reported to be 33.95, 8.66, and 7.31 mg QCE/g DM in hydroethanolic, infusion, and decoction extracts, respectively. It can be observed that the hydroethanolic extract had a higher concentration compared to the study by Gonçalves et al. [54], which reported 4.46 mg EQ/g dry weight. Some authors also refer to concentrations of 4.89 mg/g extract [56] and 50–60 mg EC/100 g DM in a 60% ethanol extract [55], with yield intensified by the extraction method.
The main compounds, such as phenols and flavonoids, are influenced and react to certain climatic conditions and soil properties [22]. Variability in polyphenol concentration is attributed to environmental factors such as light intensity, day length, temperature, and nutrient levels [57]. Furthermore, the composition of metabolites in the same species can vary according to geographical location and the different methods used for obtaining phenolic extracts [58].
CONCLUSION
Ethnobotany reported that the species C. citriodora is effective for treating bronchitis, the common cold, and flu, primarily consumed in the form of an infusion made from fresh or dried leaves, sometimes in association with matico (P. aduncum) and escorzonera (P. multiflora). High concentrations of total phenols and total flavonoids were reported at 118.65 mg GAE/g DM and 33.95 mg QCE/g DM in the hydroethanolic extract. The predominant presence of polyphenols implies enhanced anti-inflammatory and antioxidant activity due to their inherent relationship and can positively ensure therapeutic efficacy in traditional use.
ACKNOWLEDGMENTS
The authors are grateful to the technical support staff from the School of Nutrition and Laboratory of Pharmacognosy.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
This work was supported by “Research Support Fund of the César Vallejo University.”
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study followed the Code of Ethics of the International Society of Ethnobiology, and the protocol was approved by the Ethics Committee of the National University of Trujillo with registration code N° PR003P–2022/CEIFYB.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Valdiviezo-Campos JE, Blanco-Olano CM, Olascuaga-Castillo KA, Rubio-Guevara SDR. Uncaria tomentosa (Willd.) DC. (Rubiaceae): native species of Peru, herbal medicine recognized by traditional medicine. Ethnobot Res Appl. 2020;19:1–15. Available from: https://ethnobotanyjournal.org/index.php/era/article/view/1795
2. Ejidike IP, Mtunzi FM, Ledwaba I, Phele MJ, Ejidike OM, Ogunleye O, et al. Combretum species around Africa as alternative medicine: ethnopharmacological and ethnobotanical importance. J App Pharm Sci. 2023;13(8):12–29. CrossRef
3. Souilah N, Miara MD, Bendif H, Medjroubi K, Snorek J. Traditional ethnobotanical knowledge on medicinal plants used by the populations in central Russikada (Northeastern Algeria). J Herbs Spices Med Plants. 2022;28(1):15–35. CrossRef
4. Ganesan K, Xu B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trends Phytochem Res. 2017;1(3):153–68.
5. Kumar A, Kumar S, Komal, Ramchiary N, Singh P. Role of traditional ethnobotanical knowledge and indigenous communities in achieving sustainable development goals. Sustainability. 2021;13(6):3062. CrossRef
6. Turreira-García N, Argyriou D, Phourin C, Srisanga P, Theilade I. Ethnobotanical knowledge of the Kuy and Khmer people in Prey Lang, Cambodia. Cambodian J Nat Hist. 2017;2017:76–101.
7. Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: a review. Eur J Med Chem. 2019;178:687–704. CrossRef
8. Brawner J, Meder R, Dieters M, Lee D. Selection of Corymbia citriodora for pulp productivity. Southern Forests. 2012;74(2):121–31. CrossRef
9. Tambarussi EV, Pereira FB, da Silva PHM, Lee D, Bush D. Are tree breeders properly predicting genetic gain? A case study involving Corymbia species. Euphytica. 2018;214(8):150. CrossRef
10. da Costa MM, de Bittencourt RC, Nogueira TAPC, Silva LS, da Silva WHM, Valverde SR, et al. Technical evaluation of hybrid clones of Corymbia spp. to produce market pulp. Pap Biomater. 2022;7(3):1–6. CrossRef
11. Hung TD, Brawner JT, Lee DJ, Meder R, Dieters MJ. Genetic variation in growth and wood-quality traits of Corymbia citriodora subsp. variegata across three sites in south-east Queensland, Australia. Southern Forests. 2016;78(3):225–39. CrossRef
12. Torres PC. Medicina tradicional china en la COVID-19: análisis bibliométrico. Med Natur. 2021;15(1):27–31. https://dialnet.unirioja.es/servlet/articulo?codigo=7747847
13. Lin L, Chen W, Li C, Cui H. Enhancing stability of Eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind Crops Prod. 2019;140:111615. CrossRef
14. Mohareb ASO, Elashmawy MAA, Nawar MEM, Abdelrahman AK, Ahmed FM, Hassona AEA, et al. Chemical compositions and antifungal activity of Corymbia citriodora, Cupressus macrocarpa, and Syzygium cumini extracts: GC–MS and HPLC analysis of essential oils and phenolic compounds. Biomass Conv Bioref. 2023. CrossRef
15. Perry MJ, Wangchuk P. The ethnopharmacology, phytochemistry and bioactivities of the Corymbia genus (Myrtaceae). Plants. 2023;12(21):3686. Available from: https://www.mdpi.com/2223-7747/12/21/3686
16. García IR, Rodríguez VJ, Lora LMG. Plantas medicinales antivirales: una revisión enfocada en el COVID-19. Med Nat. 2021;15(1):38–45. https://dialnet.unirioja.es/servlet/articulo?codigo=7747849
17. Barbosa LCA, Filomeno CA, Teixeira RR. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules. 2016;21(12):1671. CrossRef
18. Miguel MG, Gago C, Antunes MD, Lagoas S, Faleiro ML, Megías C, et al. Antibacterial, antioxidant, and antiproliferative activities of Corymbia citriodora and the essential oils of eight Eucalyptus species. Medicines. 2018;5(3):61. CrossRef
19. Weckerle CS, de Boer HJ, Puri RK, van Andel T, Bussmann RW, Leonti M. Recommended standards for conducting and reporting ethnopharmacological field studies. J Ethnopharmacol. 2018;210:125–32. CrossRef
20. Bhat MN, Singh B, Surmal O, Singh B, Shivgotra V, Musarella CM. Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology. 2021;10(9):851. CrossRef
21. Venegas CEA, Gómez AAM, Chávez LAN, Valdiviezo CJE, Ormeño LlM, Vásquez CE. Evaluación fitoquímica preliminar del extracto metanólico y etanólico de las flores de Cordia lutea Lam. (Boraginaceae) y su capacidad antioxidante. Arnaldoa. 2019;26(1):359–66. CrossRef
22. Rafi M, Febriany S, Wulandari P, Suparto IH, Ridwan T, Rahayu S, et al. Total phenolics, flavonoids, and anthocyanin contents of six Vireya Rhododendron from Indonesia and evaluation of their antioxidant activities. J App Pharm Sci. 2018;8(9):49–54. CrossRef
23. Rojas-Ocampo E, Torrejón-Valqui L, Muñóz-Astecker LD, Medina-Mendoza M, Mori-Mestanza D, Castro-Alayo EM. Antioxidant capacity, total phenolic content and phenolic compounds of pulp and bagasse of four Peruvian berries. Heliyon 2021;7(8):e07787. CrossRef
24. Chen XQ, Li ZH, Liu LL, Wang H, Yang SH, Zhang JS, et al. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT. 2021;148:111708. CrossRef
25. Hou M, Hu W, Wang A, Xiu Z, Shi Y, Hao K, et al. Ultrasound-assisted extraction of total flavonoids from Pteris cretica L.: process optimization, HPLC analysis, and evaluation of antioxidant activity. Antioxidants. 2019;8(10):425. CrossRef
26. Mohamadi N, Sharififar F, Koohpayeh A, Daneshpajouh M. Traditional and ethnobotanical uses of medicinal plants by ancient populations in Khabr and Rouchon of Iran. J Appl Pharm Sci. 2023;5(11):101–7. CrossRef
27. Abba A, Dogara AM. Ethnomedicinal survey of plants used for management of inflammatory diseases in Ringim Local Government, Jigawa State, Nigeria. Ethnobot Res Appl. 2023;22:1–27. Available from: https://ethnobotanyjournal.org/index.php/era/article/view/3127
28. Abdulrahman MD, Bradosty SW, Hamad SW, Ibrahim MT, Lema AA, Sunusi N, et al. Traditional methods for treatment and management of measles in Northern Nigeria: medicinal plants and their molecular docking. Ethnobot Res Appl. 2022;23:1–18. Available from: https://ethnobotanyjournal.org/index.php/era/article/view/3595
29. Magalhães K do N, Guarniz WAS, Sá KM, Freire AB, Monteiro MP, Nojosa RT, et al. Medicinal plants of the Caatinga, northeastern Brazil: ethnopharmacopeia (1980–1990) of the late professor Francisco José de Abreu Matos. J Ethnopharmacol. 2019;237:314–53. CrossRef
30. Paniagua-Zambrana NY, Bussmann RW, Romero C. Eucalyptus citriodora Hook. Eucalyptus globulus Labill. Myrtaceae. In: Paniagua-Zambrana NY, Bussmann RW, editors. Ethnobotany of the Andes. Cham, Switzerland: Springer International Publishing; 2020. pp 829–36.
31. Abbass H. Eucalyptus essential oil; an off-label use to protect the world from COVID-19 pandemic: review-based hypotheses. Univers J Pharm Res. 2020;5:57–60. CrossRef
32. Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-Rad M, Valussi M, et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules. 2017;22(1):70. CrossRef
33. Brezáni V, Leláková V, Hassan STS, Berchová-Bímová K, Nový P, Kloucek P, et al. Anti-infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus Labill. Viruses. 2018;10(7):360. CrossRef
34. Goodine T, Oelgemöller M. Corymbia citriodora: a valuable resource from Australian flora for the production of fragrances, repellents, and bioactive compounds. ChemBioEng Rev. 2020;7(6):170–92. CrossRef
35. Sarma N, Gogoi R, Loying R, Begum T, Munda S, Pandey S, et al. Phytochemical composition and biological activities of essential oils extracted from leaves and flower parts of Corymbia citriodora (Hook.) J Environ Biol. 2021;42:552–562. CrossRef
36. Tolba H, Moghrani H, Aboun A, Maachi R. Essential oil of Algerian Eucalyptus citriodora: chemical composition, antioxidant and antimicrobial activities. Rev Nat Technol. 2018;10(01):105–11. CrossRef
37. Salehi B, Sharifi-Rad J, Quispe C, Llaique H, Villalobos M, Smeriglio A, et al. Insights into Eucalyptus genus chemical constituents, biological activities and health-promoting effects. Trends Food Sci Technol. 2019;91:609–24. CrossRef
38. Bermúdez del Sol A, Gallegos A, Sánchez MJG, Andi GDD, Bravo SLR. Etnobotánica cuantitativa de las plantas medicinales en el cantón Penipe, provincia de Chimborazo, Ecuador. La Técnica. 2022;12(2):42–50. https://dialnet.unirioja.es/servlet/articulo?codigo=8736650
39. Mani JS, Johnson JB, Hosking H, Ashwath N, Walsh KB, Neilsen PM, et al. Antioxidative and therapeutic potential of selected Australian plants: a review. J Ethnopharmacol. 2021;268:113580. CrossRef
40. Samoisy AK, Mahomoodally F. Ethnopharmacological appraisal of culturally important medicinal plants and polyherbal formulas used against communicable diseases in Rodrigues Island. J Ethnopharmacol. 2016;194:803–18. CrossRef
41. Gbekley HE, Katawa G, Karou SD, Anani K, Tchadjobo T, Ameyapoh Y, et al. Ethnobotanical study of plants used to treat asthma in the maritime region in Togo. Afr J Tradit Complement Altern Med. 2017;14(1):196–212. CrossRef
42. Afolayan F, Sulaiman K, Okunade W, Garrido G. Ethnobotanical survey of plants used in cancer therapy in Iwo and Ibadan, South-Western of Nigeria. J Pharm Pharmacogn Res. 2020;8:346–67.
43. de Lobo RA AM, Lobo ACBNM, de Oliveira AFM, de Andrade LHC. La etnobotánica como parámetro para el estudio del mimetismo cultural entre el pueblo gitano. Bol Latinoam Caribe Plantas Med Aromáticas. 2022;21(4):530–47. CrossRef
44. Bibi F, Abbas Z, Harun N, Perveen B, Bussmann RW. Indigenous knowledge and quantitative ethnobotany of the Tanawal area, Lesser Western Himalayas, Pakistan. PLoS One. 2022;17(2):e0263604. CrossRef
45. Mejía GSY, Estela GAM. Uso empírico de Eucalyptus globulus y Piper aduncum para tratar la COVID-19 en los pobladores de Barrios Altos. Available from: http://hdl.handle.net/20.500.14140/1148
46. Delgado-Paredes EG, Delgado-Rojas RP, Rojas-Idrogo C. Peruvian medicinal plants and cosmopolitan plants with potential use in the treatment of respiratory diseases and COVID-19. Int J Plant Anim Environ Sci. 2021;11(02):295–321. CrossRef
47. Caballero-Serrano V, McLaren B, Carrasco JC, Alday JG, Fiallos L, Amigo J, et al. Traditional ecological knowledge and medicinal plant diversity in Ecuadorian Amazon home gardens. Glob Ecol Conserv. 2019;17:e00524. CrossRef
48. Lozada M, Ladio A, Weigandt M. Cultural transmission of ethnobotanical knowledge in a rural community of northwestern Patagonia, Argentina. Econ Bot. 2006;60(4):374–85. CrossRef
49. Cárdenas JAL, López JMM, Saldarriaga JY, Mamani CMC, Manrique RFC, Soto FGC, et al. Use of medicinal herbs to relieve symptoms of respiratory conditions and COVID-19. Vive Rev Salud. 2022;5(15):738–49. CrossRef
50. Mgbeahuruike EE, Yrjönen T, Vuorela H, Holm Y. Bioactive compounds from medicinal plants: focus on Piper species. South Afr J Bot. 2017;112:54–69. CrossRef
51. Villena-Tejada M, Vera-Ferchau I, Cardona-Rivero A, Zamalloa-Cornejo R, Quispe-Florez M, Frisancho-Triveño Z, et al. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: a cross-sectional survey. PLoS One. 2021;16(9):e0257165. CrossRef
52. Hassan A, Abu Hafsa SH, Elghandour MMY, Kanth Reddy PR, Salem MZM, Anele UY, et al. Influence of Corymbia citriodora leaf extract on growth performance, ruminal fermentation, nutrient digestibility, plasma antioxidant activity and faecal bacteria in young calves. Anim Feed Sci Technol. 2020;261:114394. CrossRef
53. Idris MM, Yelwa AM, Muhammad A. Phytochemical screening, cytotoxicity and antioxidant activities of leaves extracts from Eucalyptus citriodora. J Trop Pharm Chem. 2021;5(3):165–73. CrossRef
54. Gonçalves JPZ, Seraglio J, Macuvele DLP, Padoin N, Soares C, Riella HG. Green synthesis of manganese based nanoparticles mediated by Eucalyptus robusta and Corymbia citriodora for agricultural applications. Colloids Surf Physicochem Eng Asp. 2022;636:128180. CrossRef
55. Toumi MN, Benyamina A, Bouzidi MA, Semmak A, Bellebna Y, Toumi F, et al. Intensification of the extraction yield of Eucalyptus globulus phenolic compounds with pulsed electric field. Appl Sci. 2022;12(19):9455. CrossRef
56. Dey B, Mitra A, Katakam P, Singla RK. Exploration of natural enzyme inhibitors with hypoglycemic potentials amongst Eucalyptus Spp. by in vitro assays. World J Diabetes. 2014;5(2):209–18. CrossRef
57. Rienth M, Vigneron N, Darriet P, Sweetman C, Burbidge C, Bonghi C, et al. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—a review. Front Plant Sci. 2021;12:643258. CrossRef
58. Das PR, Darwish AG, Ismail A, Haikal AM, Gajjar P, Balasubramani SP, et al. Diversity in blueberry genotypes and developmental stages enables discrepancy in the bioactive compounds, metabolites, and cytotoxicity. Food Chem. 2022;374:131632. CrossRef