INTRODUCTION
Breast cancers with ER expression and HER2 amplification are still challenging to achieve the best therapy for patients who have been experiencing resistance to drugs or other medications that reduce their efficacy [1]. Namely tamoxifen, the specifically targeted treatment for Estrogen Receptor [2], the usage routinely decreases the sensitivity of cancer cells and causes cancer cells to become resistant [3]. This phenomenon usually happens when the cancer cells change the growth signaling to another pathway rather than ER [4]. The HER2-targeted drugs also show a similar effect that the cancer cells do not respond to the drugs properly due to the use of a different signaling pathway to induce cell division [1,5]. Therefore, the use of generally targeted chemotherapy such as doxorubicin is still the drug of choice.
Doxorubicin is the most common treatment of cancer including luminal breast cancers [6]. However, the use of doxorubicin is limited due to raising the risk for cardiac and heart disease [7]. Doxorubicin is highly disposed of in cardiac cells and induces ROS generation leading to increasing cellular and tissue disruption [8]. This condition should be considered and given special attention when the use of doxorubicin in cancer treatment [9]. The use of ROS-reducing agents will give significant benefits to the co-treatment of doxorubicin in combating cancers [10]. Several natural products have been reported and give promising benefits for this purpose. Hesperidin increases the cytotoxic activity of doxorubicin in several cancer cell lines, such as 4T1 [11], MCF-7, and T47D [12]. The same results have also been achieved by galangal extract [13,14] or rice bran extract [15]. The documentation is still limited and needs to expand more rigorously. In this regard, we challenge the Africa leaf herb (Vernonia amygdalina) to be the co-treatment of doxorubicin on MCF-7 and MCF-7/HER2. MCF-7 is known as an estrogen receptor (ER) positive breast cancer cell line [16], whereas MCF-7/HER2 is HER2 transfected MCF-7 to make transiently high expressed HER2 [17].
Africa leaf (V. amygdalina) also known as bitter leaf, is a plant that contains compounds such as saponins, flavonoids such as luteolin, and steroid glycosides (cardiac glycosides), including vernodalin, vernon amygdalin, vernonioside B1, and vernoniol B1[18,19]. Experimental animal studies have demonstrated various activities of these compounds, including antioxidant, antimutagenic, anticancer, antidiabetic, antibacterial, and analgesic activities [20,21]. The 80% ethanol extract of V. amygdalina showed a cytotoxic effect at a dose of 100 μg/ml on MCF-7 cells and 50 μg/ml on MDA-MB-231 cells, besides the extract resulted in cell cycle arrest in the G1/S phase in MCF cells-7 and triggers apoptosis in both cells, especially at a concentration of 100 μg/ml [22]. The findings indicate a modest potential; hence, it is imperative to undertake further investigation through fractionation to identify specific groups of compounds demonstrating optimal potential, notably flavonoids. These compounds, in general, may also be explored for research purposes as co-chemotherapy agents. Flavonoids present in the Africa leaf exhibit anti-inflammatory [23], antiviral [24], and antioxidant [25]. The cardiac glycosides of V. amygdalina are potential compounds to protect against cardiotoxicity by inhibiting the Na+/K+ pump [26], whereas the ethanolic extract of V. amygdalina which is rich in flavonoid luteolin content and cardiac glycosides show significant inhibitory effect of doxorubicin induced-cardiotoxicity [27]. These facts open the opportunity to use V. amygdalina as a combination chemotherapy with doxorubicin. The active compound of Africa leaf, vernodalinol, has been studied on MCF-7 cell lines, but only in cytotoxic assay with IC50 value of 70–75 μg/ml [28]. This study investigates various fractions of Africa leaves and their physiological impacts. Moreover, an investigation into the potential synergism between these extracts and doxorubicin is conducted. The aim of this study is to provide a novel alternative application of V. amygdalina extract by enhancing the effectiveness of doxorubicin through this combination.
METHOD
Materials and extraction
The extract preparation was conducted through reflux utilizing n-hexane as a solvent, followed by a subsequent step employing methanol. The methanol extract obtained was then fractionated using dichloromethane (DCM), ethyl acetate (EA), and n-butanol (BuOH) as solvents, employing liquid–liquid extraction methods to obtain DCM extract, EA extract, and BuOH extract [21].
Cell culture
The luminal breast cancer cell models, MCF-7 and MCF-7/HER2, were obtained from the cell line collection of Cancer Chemoprevention Research Center, Universitas Gadjah Mada, Indonesia. The cells were maintained using high-glucose DMEM (Gibco) with supplementary of 10% fetal bovine serum (Gibco), 1.5% penicillin–streptomycin (Gibco), and incubated in a CO2 incubator 5% at 37°C.
Cytotoxic MTT assay
Each extract of Africa leaves (V. amygdalina) cytotoxicity was conducted on MCF-7 and MCF-7/HER2 cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were grown at a density of 4×103 cells per well of a 96-well plate and incubated for 24 hours. Subsequently, the cells underwent treatment with varying concentrations of extracts (ranging from 1 to 500 μg/ml) and were incubated for 24 hours. Following the incubation period, the MTT (Sigma) reagent was introduced, and the absorbance was measured at 595 nm using a multi-plate reader (BioRad). Employing Hill’s equation, the absorbance data were transformed into percentage cell viability, facilitating the determination of the IC50 value. Besides, the cytotoxic combination assay was determined using isobologram analysis to acquire the combination index (CI) value. CI values determined using CompuSyn software based on the Chou-Talalay system show the effects of drug combinations.
Cell cycle and apoptosis induction by flow cytometry assay
MCF-7 and MCF-7/HER2 cells were grown in a 6-well approximately 5 × 105 cells/well plate and incubated for 24 hours. The cells were treated with the extract and doxorubicin, either individually or in combination for 24 hours. Cell harvesting was performed using trypsin EDTA, followed by washing with phosphate-buffered saline (PBS) and centrifugation at 500 rpm for 5 minutes. For apoptosis induction, the cells were incubated with annexin-V-FITC and propidium iodide (PI) (BD Pharmingen), and then the analysis was conducted using the BD Accuri C6 Flow cytometer. To assess cell cycle distribution, the cells were fixed with cold 70% ethanol for 30 minutes, washed with PBS, and centrifuged at 500 rpm for 5 minutes. Subsequently, the cells were resuspended in PBS containing 40 μg/ml PI (Sigma), 20 μg/ml RNAse (Roche), and 0.1% TritonX-114 (Sigma). The resuspended cells were then subjected to analysis using the BD Accuri C6 flow cytometer.
RESULT
Cytotoxic activity of several extracts of Daun Afrika
This study intended to the challenge of cytotoxic combination (co-treatment) of Africa leaf extracts and doxorubicin on MCF-7 and MCF-7/HER2. First, we examined the cytotoxic activities of Hex, BuOH, DCM, MeOH, and EA extracts on both cells using MTT Assay. The results show that all of the extracts have low cytotoxicity with IC50 values of more than 200 μg/ml. Among the extracts, DCM extract shows the most cytotoxic potential on MCF-7 and MCF-7/HER2 with an IC50 value of 220 μg/ml (Fig. 1A) and 220 μg/ml (Fig. 1B), respectively. These IC50 values indicate that this extract exhibits weak cytotoxic activity toward both test cells. BuOH has a low IC50 value, but only in MCF-7 cells. Therefore, no further tests were carried out. Whereas, on a vero normal cell, all of the extracts do not show a cytotoxicity effect (Fig. 1C). All these cytotoxic results show in Figure 1D.
Cytotoxic combination of DCM and EA
In a single cytotoxicity test, DCM has strong potential despite its weak cytotoxicity. Then, we examined the cytotoxic combination with doxorubicin to reveal the synergy against breast cancer cells. The result shows that DCM in combination with doxorubicin has good synergistic properties on MCF-7 (Fig. 2A) and MCF7/HER2 (Fig. 2B) cells in inhibiting cell growth with a CI value of less than 0.3. In addition, we examined EA in MCF-7 cells, even though EA is weakly cytotoxic. On the contrary, EA has an antagonistic effect when combined with doxorubicin against MCF-7/HER2 (data not shown), but EA provides synergistic effect on MCF-7 (Fig. 2C). Therefore, further analysis was carried out to determine the cell cycle profile to see the physiological changes related to the lowering cell viability mediated by DCM alone or in combination with doxorubicin on MCF-7 and MCF-7/HER2.
Cell cycle modulation and apoptosis induction of MCF-7 by DCM and EA in combination with doxorubicin
The synergistic effect on inhibiting cell viability by the combination of DCM, EA, and doxorubicin may be caused by the modulation of cellular physiological processes. To investigate the effect of this alone or combined treatment with doxorubicin on specific cellular physiological processes, we conducted additional investigations into MCF-7 cell cycle progression and apoptosis induction. The total distribution of cells in each phase was measured by flow cytometry with propidium iodide (PI) staining after treatment of cells with half dose (110 μg/ml DCM, 250 μg/ml DCM) and the combination with 250 nM Doxorubicin for 24 hours (Fig. 3A). The results show that a single treatment of DCM accumulated in the S phase, while a single doxorubicin accumulated in the Sub G1 phase. Interestingly, doxorubicin in a single treatment has low accumulation in the G2/M phase, yet in combination with DCM has high accumulation in the G2/M phase (Fig. 3B).
Furthermore, we investigated the apoptosis induction of DCM in combination with doxorubicin to reveal another cellular physiological process (Fig. 4A). The result of EA shows that EA accumulated in the G1 phase, but when its combination with Doxorubicin accumulated in Sub G1 phase, following doxorubicin, it was different from DCM. Hereafter, to find out whether cell death occurred through apoptosis, an apoptosis test was carried out by staining Annexin V-FITC and PI on MCF-7 cells and analyzing by flow cytometry. The single doxorubicin treatment has high levels of apoptosis, both in early and late apoptosis. The high levels of living cells in EA treatment indicate that EA causes cell cycle arrest because the G1 phase is high, however when it is combined with doxorubicin causes early apoptosis (Fig. 3D). The high levels of living cells in DCM treatment also indicates that DCM causes cell cycle arrest in G2/M phase. Therefore, the inhibiting cell of MCF-7 viability is not due to cell death but tends to undergo cell cycle arrest.
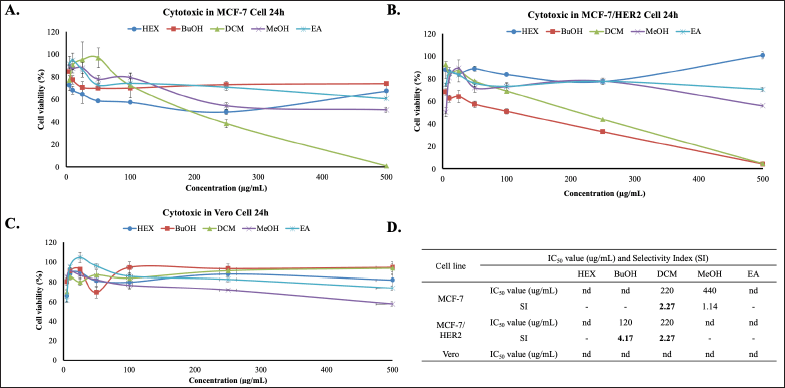 | Figure 1. Cytotoxic effects of Africa Leaves (V. amygdalina) extracts on MCF-7 and MCF-7/HER2 Cells for 24 hours. (A) MCF-7, (B) MCF-7/HER2, (C) Vero cells were cultured in 96-well plates for 24 hours, then exposed to varying concentrations (5–500 μg/ml) of the HEX extract, BuOH extract, DCM, MeOH, and EA extracts. The cell viability was conducted through the MTT assay in triplicate (n = 3). (D) The IC50 value and SI (Selectivity Index) of each extract. The SI is calculated by comparing the IC50 of the extract against Vero cells and MCF-7 nor MCF-7/HER2 cancer cells. [Click here to view] |
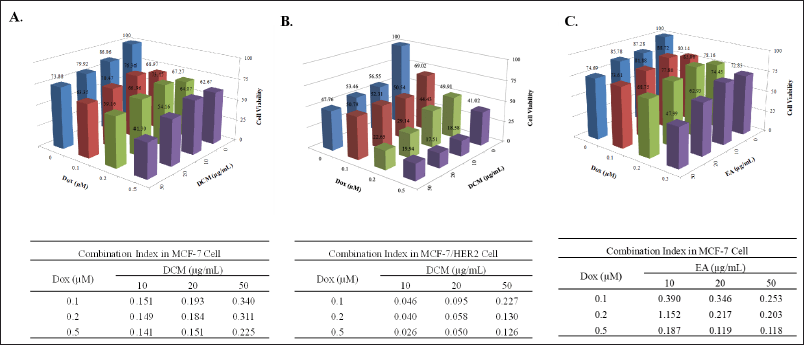 | Figure 2. The combined effect of the DCM extract (A&B) or EA extract (C) from Africa Leaves (V. amygdalina) and doxorubicin (Dox) treatment on MCF-7 (A&C) and MCF-7/HER2 (B) cells. The cells were cultured in 96-well plates for 24 hours and exposed to ¼, and ½ IC50 concentrations of DCM extract and Dox for an additional 24 hours then measured the cell viability (Upper panels). The synergistic effect when the DCM extract is combined with Dox, as indicated by a CI value of less than 0.3 (Lower panels). [Click here to view] |
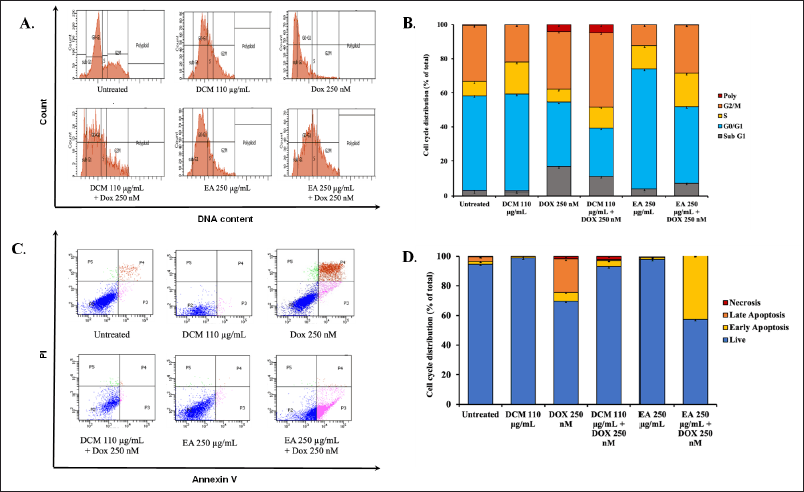 | Figure 3. The cell cycle distribution and apoptotic profile of MCF-7 cells over a 24 hour treatment period. (A) The cell cycle profile with half the IC50 extract (DCM and EA), doxorubicin, and a combination of both compounds in MCF-7 cells for 24 hours incubation. PI solution was added to the cells, and the samples were analyzed using flow cytometry. (B) The distribution of MCF-7 cells across different phases of the cell cycle. (C) The effect of ½ IC50 extracts (DCM and EA), doxorubicin, and a combination of these compounds over 24 hours in MCF-7 cells. The cells were treated for 24 hours, stained with PI reagent, and each sample was analyzed using a flow cytometer. (D) The results of the analysis, illustrate the apoptosis induction for each treatment in MCF-7 cells. [Click here to view] |
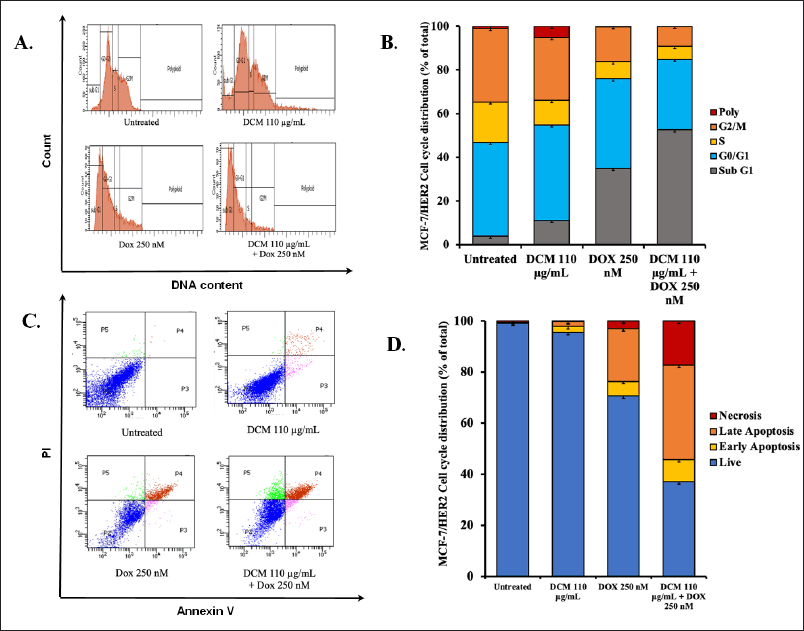 | Figure 4. The cell cycle distribution and apoptotic profile of MCF-7/HER2 cells over a 24 hour treatment period. (A) The cell cycle profile is presented after a 24 hours incubation with DCM and Dox in MCF-7/HER2 cells, with the analysis conducted using PI staining and flow cytometry. (B) The distribution of MCF-7/HER2 cells across different phases of the cell cycle. (C) The effect of ½ IC50 extract (DCM only), doxorubicin, and a combination of these compounds over 24 hours in MCF-7 cells, stained with PI reagent, and subjected to flow cytometry analysis. (D) The results of the analysis, illustrate the apoptosis induction for each treatment in MCF-7/HER2 cells. [Click here to view] |
Cell cycle modulation and apoptosis induction of MCF-7/HER2 by DCM in combination with doxorubicin
We also investigated the physiological processes of DCM treatment whether single or combination with doxorubicin on MCF-7/HER2 cells. The cell cycle of untreated MCF-7 cells shows a normal cell cycle (Fig. 4A), whereas in single DCM treatment shows a slight change in increasing Sub G1 phase (11%). Meanwhile, there was a change in doxorubicin treatment, increasing the level in the Sub G1 phase (35%). Even more surprising, DCM in combination with doxorubicin shows a drastic increase in the Sub G1 phase (52.6%) (Fig. 4B). Therefore, an apoptosis flow cytometry approach using Annexin-V is needed. In this regard, we can see that qualitatively between control and treated cells show a different distribution of cells in each quadrant (Fig. 4C). After counting these distributions, all of the untreated cells still live. DCM treatment is less apoptotic than the single doxorubicin treatment. Interestingly, when combined, the incidence of apoptosis increased significantly by 23% and additional necrosis by 8 % (Fig. 4D). These phenomena indicate a concordance with the increase in the Sub G1 phase of cell cycle assay and apoptosis in Annexin-V assay.
DISCUSSION
Vernonia amygdalina (Africa leaves) are rich in flavonoid luteolin and cardiac glycosides which is a potential source of antioxidant materials and cardiac toxicity protection. This pharmacological characteristic is important as a natural resource to be used as a complementary medicine with some potentially toxic drugs to the cardiovascular system. Doxorubicin is one of the cardiotoxic drugs that should be considered for its application. Since doxorubicin is still the first line in chemotherapy, the use of doxorubicin should be accompanied by some protective agents to attenuate the toxicity to normal cells, including the disruption of cardiac cells. Vernonia amygdalina will give the opportunity to overcome this problem from the perspective of co-chemotherapeutics potential in luminal breast cancers.
Fortunately, all the fractionated extracts of V. amygdalina had no cytotoxic effect on normal cell lines. In this regard, we use a vero cell line that represents a normal kidney cell. In contrast, the DCM extract gave low cytotoxic activity against cancer cells, MCF-7 and MCF-7/HER2 but not the other extracts. This result indicates that V. amygdalina has a weak potential as an anticancer agent, especially for luminal breast cancer with ER and HER2 expression. This phenomenon is also the characteristic of herbal extract having a low cytotoxic effect against cancer cells which may be due to the low content of active compounds. Since DCM or EA extracts are relatively semi-polar solvents, these extracts usually contain rich flavonoids and glycoside compounds. We confirmed this fact in our previous study [26]. Luteolin and glycoside forms also do not include the strong cytotoxic agents but they have high antioxidant properties as radical scavengers. Therefore, these extracts could potentially be combined with doxorubicin as a co-chemotherapeutic agent.
The cytotoxicity of the combination treatment is the basic understanding of the potential of co-chemotherapeutics application. In this perspective, DCM extract performed a synergistic effect with doxorubicin on MCF-7 and MCF-7/HER2 cells, whereas EA extract also exhibited a synergistic effect with doxorubicin on MCF-7 cells. These results indicate that both extracts have the potential to be developed as co-chemotherapeutic agents with doxorubicin that need to be explored for further physiological impact. Cell cycle progression and apoptosis evidence will be the main effect due to the cytotoxic activities of the extracts which are closely related to the lowering of cell viability after combination treatments. Cell cycle progression correlates with cell division activity that can be stopped by the treatment, meanwhile, apoptosis can reduce cell viability by losing the living cells. Both physiological processes may go in sequential ways, but they can proceed irrespective of ways that are interesting to be explored.
We found that both cells, MCF-7 and MCF-7/HER2 give different responses regarding cell cycle progression and apoptosis evidence to DCM extract. The MCF-7 cells tend to have an irrespective effect between cell cycle and apoptosis in combination treatment and seem to delay the apoptosis by DCM through cell cycle arrest in the G2/M phase. In contrast, the MCF-7/HER2 shows more sensitivity to undergo apoptosis in combination treatment, meaning that DCM probably has a role in enhancing the apoptosis process by doxorubicin. Nevertheless, the necrotic cells of MCF7/HER2 due to combination treatment could be the effect of accelerating the cell apoptosis evidence in early time and proceeding into necrosis. Both cells are only different in HER2 expression. Therefore, we could speculate that HER2 signaling may play a role in this phenomenon, and inhibition of HER2 signaling will be inhibited by compounds in DCM. This fact will be interesting for further investigation.
Nevertheless, all these limited findings are interesting to give insight into the potential usage of flavonoid and glycoside-rich extract of V. amygdalina in combination with doxorubicin. The future challenges are to explore in more detail the molecular mechanism underlying the disrupting proliferative signaling by flavonoid and its glycoside forms related to the ER and HER2 receptors. The ER and HER2 receptors are common inducers in cell division through MAPK pathways, whereas flavonoids are known to be able to inhibit some kinases. These phenomena could be the coincidence that is important to be elucidated, in vitro and in vivo approaches.
CONCLUSION
In conclusion, DCM synergistically lowers cell viability due to doxorubicin treatment on MCF-7 and MCF-7/HER2. In MCF-7 DCM tends to stop the cell cycle in the G2/M phase with doxorubicin, but DCM enhances the apoptosis caused by doxorubicin in MCF-7/HER2.
ACKNOWLEDGMENT
The authors thank the “Riset Kolaborasi Indonesia (RKI)” project under Universitas Gadjah Mada in collaboration with Universitas Sumatera Utara, Indonesia, 2023 with contract number 2675/UN1/DIT/Dit-Lit/PT.01.03/2023
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental protocol for this study was approved by the Ethics Committee of Universitas Gadjah Mada, Indonesia (No. KE/FK/1004/EC/2023).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Novitasari D, Jenie RI, Kato JY, Meiyanto E. Chemoprevention curcumin analog 1.1 promotes metaphase arrest and enhances intracellular reactive oxygen species levels on TNBC MDA-MB-231 and HER2-positive HCC1954 cells. Res Pharm Sci. 2023 Jul 1;18(4):358–70. CrossRef
2. Hermawan A, Putri H. Identification of potential gene associated with berberine in overcoming tamoxifen resistance by functional network analysis. J Appl Pharm Sci. 2020 Jun 30;10(7):009-18.
3. Zhang Z, Park JW, Ahn IS, Diamante G, Sivakumar N, Arneson D, et al. Estrogen receptor alpha in the brain mediates tamoxifen-induced changes in physiology in mice. Elife. 2021 Mar 1;10:e63333. CrossRef
4. Hermawan A, Ikawati M, Khumaira A, Putri H, Jenie RI, Angraini SM, et al. Bioinformatics and in vitro studies reveal the importance of p53, PPARG and notch signaling pathway in inhibition of breast cancer stem cells by hesperetin. Adv Pharm Bull. 2021 Feb;11(2):351. CrossRef
5. Handayani S, Susidarti RA, Utomo RY, Meiyanto E, Jenie RI. Synergistic cytotoxic and antimigratory effect of brazilein and doxorubicin on HER2-overexpressing cells. Asian Pac J Cancer Prev. 2022 Aug;23(8):2623. CrossRef
6. Nurhayati IP, Khumaira A, Ilmawati GP, Meiyanto E, Hermawan A. Cytotoxic and antimetastatic activity of hesperetin and doxorubicin combination toward Her2 expressing breast cancer cells. Asian Pac J Cancer Prev. 2020 May;21(5):1259. CrossRef
7. Wulandari F, Novitasari D, Kirihata M, Kato JY, Meiyanto E. New curcumin analog, CCA-1.1, synergistically improves the antiproliferative effect of doxorubicin against T47D breast cancer cells. Indones J Pharm. 2020;31:244–56. CrossRef
8. Ikawati M, Jenie RI, Utomo RY, Amalina ND, Ilmawati GP, Kawaichi M, et al. Genistein enhances cytotoxic and antimigratory activities of doxorubicin on 4T1 breast cancer cells through cell cycle arrest and ROS generation. J Appl Pharm Sci. 2020 Oct 5;10(10):095–104.
9. Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021 Jul 1;139:111708. CrossRef
10. Salsabila IA, Nugraheni N, Ahlina FN, Haryanti S, Meiyanto E. Synergistic cotreatment potential of soursop (Annona muricata L.) leaves extract with Doxorubicin on 4T1 cells with antisenescence and anti-reactive-oxygen-species properties. Iran J Pharm Res. 2021;20(2):57.
11. Amalina ND, Salsabila IA, Zulfin UM, Jenie RI, Meiyanto E. In vitro synergistic effect of hesperidin and doxorubicin downregulates epithelial-mesenchymal transition in highly metastatic breast cancer cells. J Egypt Natl Cancer Inst. 2023 Dec;35(1):6. CrossRef
12. Hermawan A, Putri H. Targets and molecular mechanisms of a citrus flavonoid, hesperidin, against luminal breast cancer cells: an integrative bioinformatics analysis. Asian Pac J Trop Biomed. 2019 Dec 1;9(12):531–8. CrossRef
13. Ahlina FN, Nugraheni N, Salsabila IA, Haryanti S, Da’i M, Meiyanto E. Revealing the reversal effect of galangal (Alpinia galanga L.) extract against oxidative stress in metastatic breast cancer cells and normal fibroblast cells intended as a co-chemotherapeutic and anti-ageing agent. Asian Pac J Cancer Prev. 2020 Jan 1;21(1):107–17. CrossRef
14. Alif I, Utomo RY, Ahlina FN, Nugraheni N, Hermansyah D, Putra A, et al. Immunopotentiation of galangal (Alpinia galanga L.) when combined with T-cells against metastatic triple-negative breast cancer, MDA-MB 231. J Appl Pharm Sci. 2021 Jul 18;11(11):053–61. CrossRef
15. Zulfin UM, Rahman A, Hanifa M, Utomo RY, Haryanti S, Meiyanto E. Reactive oxygen species and senescence modulatory effects of rice bran extract on 4T1 and NIH-3T3 cells co-treatment with doxorubicin. Asian Pac J Trop Biomed. 2021 Apr 1;11(4):174–82. CrossRef
16. Comsa S, Cimpean AM, Raica M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015 Jun 1;35(6):3147–54.
17. Zhao S, Ohara S, Kanno Y, Midorikawa Y, Nakayama M, Makimura M, et al. HER2 overexpression-mediated inflammatory signaling enhances mammosphere formation through up-regulation of aryl hydrocarbon receptor transcription. Cancer Lett. 2013 Mar 1;330(1):41–8. CrossRef
18. Nowak J, Kiss AK, Wambebe C, Katuura E, Kuzma L. Phytochemical analysis of polyphenols in leaf extract from Vernonia amygdalina Delile plant growing in Uganda. Appl Sci. 2022 Jan 17;12(2):912. CrossRef
19. Ondua M, Njoya EM, Abdalla MA, McGaw LJ. Anti-inflammatory and antioxidant properties of leaf extracts of eleven South African medicinal plants used traditionally to treat inflammation. J Ethnopharmacol. 2019 Apr 24;234:27–35. CrossRef
20. Okello D, Chung Y, Kim H, Lee J, Rahmat E, Komakech R, et al. Antioxidant activity, polyphenolic content, and FT-NIR analysis of different Aspilia africana medicinal plant tissues. Evid Based Complement Altern Med. 2021 Sep 15;2021:e9917810. CrossRef
21. Hasibuan PA, Harahap U, Sitorus P, Satria D. The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon. 2020 Jul 1;6(7):e04449. CrossRef
22. Wong FC, Woo CC, Hsu A, Tan BK. The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One. 2013 Oct 24;8(10):e78021. CrossRef
23. Owor RO, Bedane KG, Openda YI, Zühlke S, Derese S, Ong’amo G, et al. Synergistic anti-inflammatory activities of a new flavone and other flavonoids from Tephrosia hildebrandtii Vatke. Nat Prod Res. 2021 Nov 18;35(22):4486–93. CrossRef
24. Kim CH, Kim JE, Song YJ. Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules. 2020 May 20;25(10):2379. CrossRef
25. Sugiura Y, Katsuzaki H, Imai K, Amano H. The anti-allergic and anti-inflammatory effects of phlorotannins from the edible brown algae, Ecklonia sp. and Eisenia sp. Nat Prod Commun. 2021 Nov;16(12):1934578X211060924. CrossRef
26. Harahap U, Dalimunthe A, Haro G, Syahputra RA, Utomo DH, Satria D. In-silico analysis of cardiac glycosides from vernonia amygdalina delile. leaves as cardiotonic through inhibition of NA+/K+ ATPase ion transport. Rasayan J Chem. 2021 Jan 1;14(1):161–5.
27. Syahputra RA, Harahap U, Harahap Y, Gani AP, Dalimunthe A, Ahmed A, et al. Vernonia amygdalina ethanol extract protects against doxorubicin-induced cardiotoxicity via TGFβ, cytochrome c, and apoptosis. Molecules. 2023 May 24;28(11):4305. CrossRef
28. Luo X, Jiang Y, Fronczek FR, Lin C, Izevbigie EB, Lee KS. Isolation and structure determination of a sesquiterpene lactone (vernodalinol) from Vernonia amygdalina extracts. Pharm Biol. 2011 May 1;49(5):464–70. CrossRef