INTRODUCTION
Melanin is a pigment that can be found in many kinds of species, including bacteria, fungi, plants, and mammals. The level and distribution of the melanin pigment determine a mammal’s skin and hair color [1]. Melanin functions as a physical barrier to protect the skin from damage when it is exposed to UV radiation and reactive oxygen species (ROS) and contributes to identifying skin phenotypes [2]. For some people, discoloration of their skin caused by high levels of melanin pigment as a result of melanocyte activity induced by the tyrosinase enzyme might be an aesthetic and psychological issue [3]. Skin hyperpigmentation disorder is a description of this condition. The symptoms of skin hyperpigmentation disorders consist of lentigo, melanoma, melasma, and melanosis [4–7].
The treatment of skin hyperpigmentation may employ the administration of skin-lightening agents which act by either directly reducing the activity of the tyrosinase enzyme or by inhibiting the enzyme production process at the transcription or translation stage [8,9]. Tyrosinase, a copper-containing multifunctional metalloenzyme, is also known as polyphenol oxidase. The tyrosinase enzyme is crucial in the synthesis of melanin, due to the melanin biosynthesis pathway [3,4]. Tyrosinase works by catalyzing two initial stages in melanin formation, namely the monophenolase reaction, or hydrolyzing the change in the amino acid L-tyrosine to 3,4 dihydroxyphenylalanine (L-DOPA), and then the diphenolase reaction, or oxidizing L-DOPA to DOPAquinone [5,6].
This approach can be used to remove dark spots on the skin brought on by hormone imbalance, post-inflammation, or UV radiation exposure [7]. The hydroquinone, arbutin, kojic acid, and ascorbic acid categories of lightening substances are some of those available on the market. Each of these substances has the ability to inhibit the synthesis of melanin [4,8,9]. However, the usage of skin-lightening agents may have many side effects, such as the permanent loss of melanocytes caused by hydroquinone when used continuously for an extensive period of time. Hereditary loss of skin color is caused by oxidative damage to lipid membranes, Which causes this disorder [10]. Due to those effects the use of hydroquinone was limited and has been replaced by arbutin and kojic acid, which appears to have no adverse effects on melanocyte cells [11,12]. Nevertheless, arbutin and kojic acid are known to have a lesser in vivo efficacy than hydroquinone, as well as adverse effects such as dermatitis, sensitivity, and erythema [13]. Other natural skin-lightening agents, such as ascorbic acid, tend to be thermolabile and easily broken down, thereby making them less stable when utilized [14]. Therefore, there is an urgent need for bioactive compounds that can inhibit the tyrosinase enzyme’s activity as an anti-melanogenic agent.
Bioactive substances that have the ability to inhibit the tyrosinase enzyme, reduce the synthesis of melanin, and inhibit the expression of the tyrosinase gene have the potential to be developed as anti-melanogenic agents [15]. Compounds from the flavonoid, phenolic, and anthocyanin families are secondary metabolites that have activity as anti-melanogenic agents [16–18]. According to El-Nashar et al. [19], there are many distinct types of flavonoids in nature, including flavones, flavonols, isoflavones, flavan-3-ols, flavanones, and chalcones, which have tyrosinase inhibitory effects using diverse mechanism of action. Several derivatives of resveratrol such as oxyresveratrol and dihydrooxyresveratrol as potential depigmenting agents with IC50 values at 1.7 and 0.3 μM [20].
Natural materials with a variety of metabolites have unique chemical structures and various biological activities that can be found in marine natural resources. In accordance with their representation of 95% of the biosphere, marine resources give new opportunities for research on marine-derived medicines [21–23]. The complexity of marine ecosystems has prevented a thorough study of plants from watery ecosystems. Seagrass, seaweed, and marine sponges are several kinds of natural marine sources that have been investigated for bioactive substances. Marine macroalgae are a group of multicellular plant-like protists that are divided into three groups: brown algae (Phaeophyta), red algae (Rhodophyta), and green algae (Chlorophyta). The only angiosperm plant that flourishes in the marine environment is seagrass. Seagrass and seaweed are widely distributed in the waters of Southeast Asia and have many kinds of biological potential activities, such as antimicrobial, insecticidal, antimalarial, vasoprotective, anti-inflammatory, antioxidant, and anti-melanogenic properties [24–26]. In addition to seagrasses and macroalgae representing the kingdom plantae, and marine sponges representing the marine invertebrate phylum, also have the potential to be developed as anti-melanogenic agents. The existence of metabolites, such as pyrrole compounds, and peptides, which contribute to the inhibition of the tyrosinase enzyme, serves as evidence for this. According to several studies, pyrrole, and peptide compounds that have been identified from many kinds of natural sources have the ability to operate as tyrosinase inhibitors and can lower melanin levels in melanocyte cells [27–34].
The purpose of this review is to summarize the types of seagrasses, seaweeds, and marine sponges that possess anti-melanogenic activity in vitro and in vivo, to identify extracts, fractions, and compounds with potential activity based on IC50 values, and to reveal the most significant functions. An important category of bioactive compounds that have been shown to inhibit the activities of tyrosinase on various substrates. Nevertheless, additional in vivo study is required to support in vitro analysis and establish that bioactive substances effectively enhance the activity and selectivity of specific receptors in the test organism to act systemically. Therefore, it is possible to assess using either of these approaches that it has the potential to be developed specifically as an anti-melanogenic agent.
METHOD
Literature data about marine resources as an anti-melanogenic were extracted from the Scopus, PubMed, and Google Scholar databases. We browsed the database using the following keywords: “seagrass” OR “seaweed” OR “sponge” AND anti-melanogenic* OR “inhibitor tyrosinase” OR reduces melanin synthesis. The browsed scientific literature contained any of these keywords, terms, or phrases in their title, abstract, or keywords. The data included in this review were original articles or conference papers, the use of the English language only, and regarding the study of anti-melanogenic agents of compounds produced from seagrasses, seaweeds, and marine sponges, as shown in Table 1. Scientific literature with the following requirements was excluded, i.e., irrelevant terms, least, and biased information, unavailable full-text, and repetitions. The variables assessed in this review include seagrass species, seaweed species, sponge species, compounds of marine resources, experimental models, tyrosinase inhibition, inhibition of melanin synthesis, and cytotoxic effect.
Extraction and analysis of data
Figure 1 shows the data extraction and analysis of the research scope. The database’s eligible literature was saved and evaluated further.
Anti-melanogenic activity of seagrasses
Seagrass is one group of aquatic plants that have not been thoroughly studied [35,36]. According to Reynold and Knowlton [37], seagrasses are marine plants that have roots and are capable of producing fruits and flowers (Angiosperms). The only flowering plants that can return to the seabed are seagrasses. Seagrasses also have an important role in supporting ecosystems for millions of marine organisms and helping stabilize sediments [37–39]. In the monocotyledon Alismatales, seagrasses are divided into four families: Cymodoceaceae, Hydrocharitaceae, Posidoniaceae, and Zosteraceae [38,40]. Seagrasses are marine vascular plants derived from higher land plants that later colonized marine habitats, but are often mistaken for algae. The majority of the basic and secondary metabolic characteristics of seagrasses and their relatives Alismatales that inhabit terrestrial and freshwater habitats are very identical [39,62].
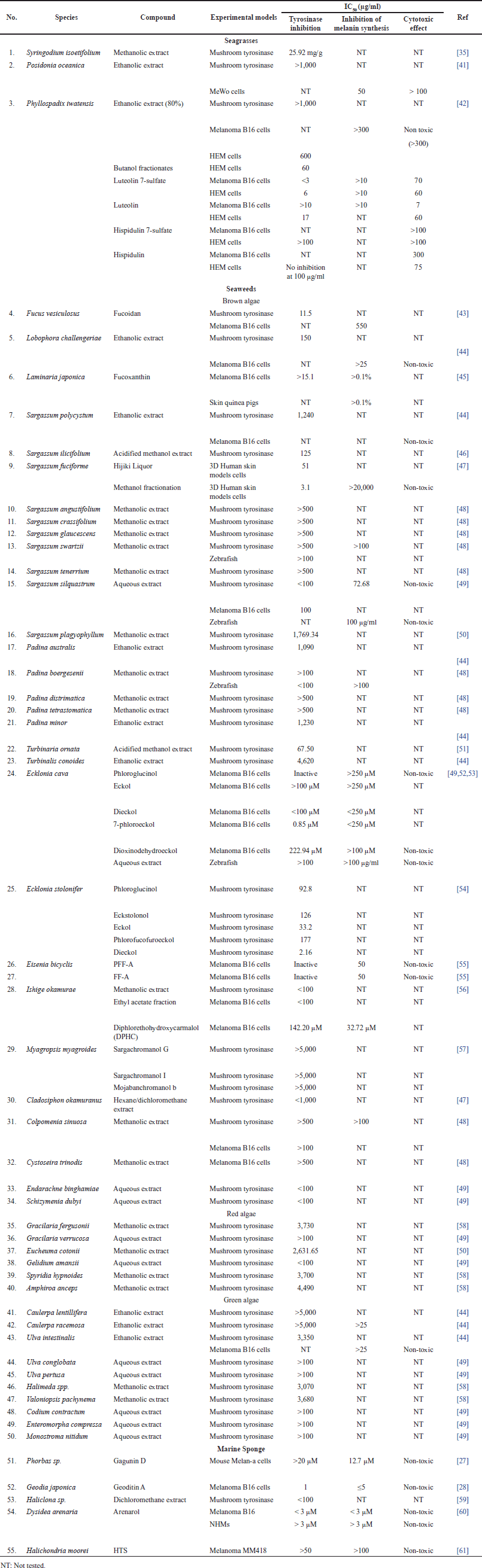 | Table 1. Summarized data of antimelanogenic activity from seagrass, seaweed, and marine sponge. [Click here to view] |
Coastal communities use seagrass biomass as food and for medicinal purposes, such as the treatment of fever, skin diseases, muscles, wounds, stomach aches, anti-pain against stingray stings, and as a potential antimicrobial agent [63]. The fishing communities in the Cuddalore and Nagapattinam areas of Tamil Nadu, South India, employ this Halophila ovalis as a medication to cure various skin conditions, burns, and ulcers [64]. According to Yuvaraj et al. [65], it is also employed as a potent anti-inflammatory and antioxidant. In the Philippines, the seeds of the tropical seagrass Enhalus acoroides are traditionally consumed [66] and are considered to have aphrodisiac and contraceptive properties [67]. Enhalus acoroides has a number of interesting biological activities, including antibacterial, insecticidal, antimalarial, vasoprotective, anti-inflammatory, antioxidant, and antialgal characteristics [24]. However, as far as we can tell from our examination of the literature, there has not been a review that discusses the anti-melanogenic properties of seagrass.
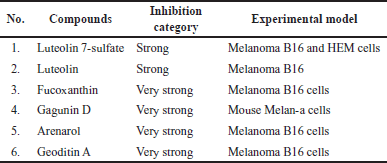
Presently, research on the anti-melanogenesis activities of seagrass metabolites has been relatively limited when compared to seaweeds. Based on a review of the literature published up to April 2023, we include three original articles for review (Table 1). Three species whose anti-melanogenic properties have been studied such as Syringodium isoetifolium, Posidonia oceanica, and Phyllospadix iwatesis. A limited amount of bioactive substances have been identified from seagrasses that are known to inhibit the tyrosinase activity, including caftartic acid, loliolide, and iso loliolide from S. isoetifolium and luteolin 7-sulfate from Phyllospadix iwatensis. Caftartic acid, loliolide, and iso loliolide were able to bind to the active site of the tyrosinase enzyme by in silico study, while S. isoetifolium methanol extract had an IC50 value of 25.92 mg/g when tested in vitro for tyrosinase inhibitory activity [35]. Strong anti-melanogenic activity against murine melanoma B16 cells and human epidermal melanocytes (HEM) is exhibited by luteolin 7-sulfate, which was extracted from the butanol extract of P. iwatensis, without accompanied adverse effects were observed on B16 and HEM cells [42]. The melanin content could be decreased by the ethanol extract of P. oceanica with an IC50 value of 50 μg/ml; however, the toxicity effect on MeWo cells was shown with an IC50 >100 g/ml [41]. Based on these findings, the further investigation is needed for the development of skin-lightening agents, and there are still several potentials to use seagrass as a research subject, considering that there is still an abundance of scientific knowledge on the subject.
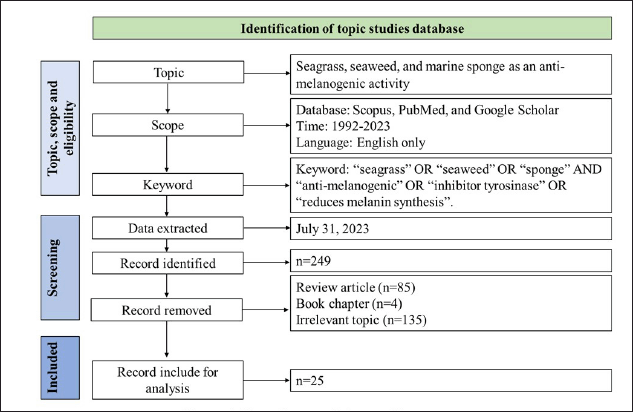 | Figure 1. Flow diagram of the searching strategy. [Click here to view] |
Anti-melanogenic activity of seaweed
Compounds of natural products from marine algae have been well investigated and examined. Algae are eukaryotic photosynthetic organisms that can adapt to diverse environmental situations by generating a variety of secondary metabolites and bioactive constituents, containing phlorotannins and polysaccharides [68,49,56,52]. Numerous studies have been conducted on the functions of bioactive substances and secondary metabolites. These substances exhibit anti-oxidant, anti-inflammatory, anti-melanogenic, anti-proliferative, and anti-aging properties [69–72]. However, limited studies have been conducted to examine the interaction between this compound’s biological activity as a skin-lightening agent and its effectiveness as a depigmenting agent. Three types of marine algae are classified as brown (Phaeophyta), red (Rhodophyta), and green (Chlorophyta). Due to their capacity to adapt to numerous and challenging environmental conditions, these three categories of algae exhibit a wide range of biological activities and are well known for producing several types of bioactive chemicals, including polysaccharides, carotenoids, and flavonoids [73,74]. These bioactive compounds were studied, and it was shown that they had anti-oxidant, anti-inflammatory, photo-protective, and anti-melanogenic activities [15,70,72,75,76]. As a target for skin lightening, this kind of biological activity can be exploited.
In comparison to seagrass, seaweed metabolites are the subject of substantially greater study at the moment. A literature review through April 2023 led to the inclusion of 17 original publications for review (Table 1). We examined 26 different genera that are known to have anti-melanogenic effects and discovered that most research was done on the brown algae genus Sargassum. Although many of these bioactive substances have been found in seaweed [49,56,52,43,45,53,54,57], research on the anti-melanogenic properties of these compounds is still limited.
Anti-melanogenic activity of sponges
Due to reveals, marine sponges are exceptional in terms of identifying active metabolites with the potential to be utilized in medicines. An invertebrate species from the phylum Porifera known as a marine sponge has a sessile way of life, has porous bodies, and collects small particles of food from seawater. They produce metabolites as a form of defense to protect themselves from predators and fouling organisms that tend to attach to their outside surface. Sponges are a major marine resource to produces several types of distinctive and varied bioactive metabolites, as has been explained during the past 30 years. Sponge species consisting of more than 8,000 have been reported and have a wide distribution in the marine environment [77,78]. Due to the many different chemical and physical conditions, nearly every group of marine sponges produces a variety of bioactive compounds with unique structural properties [79,80]. Natural compounds derived from marine sponges have the potential to offer a promising novel treatment option for skin hyperpigmentation disorders [28,61,60]. Bioactive compounds synthesized by marine sponges are chemically diverse and can be grouped as nucleosides, terpenes, sterols, cyclic peptides, and alkaloids [81]. These substances have a strong potential for antioxidant activity, according to previous research. Following the results of previous studies, a compound with antioxidant activity can reduce hyperpigmentation by using a ROS scavenger [82–84].
Existing natural products have been used as lead compounds in the synthesis of derivative compounds having more potential to develop as pharmaceutical medicines [85]. Presently, research on the anti-melanogenesis activities of marine sponge metabolites has been relatively limited when compared to antioxidants, antibacterials, and anticancer activity. Based on a review of the literature published up to April 2023, we include five original articles for review (Table 1). We studied five different genera that have been studied for their anti-melanogenic properties and it has been possible to isolate each of its bioactive ingredients. Even though various bioactive substances from marine sources have been found [27,61,60,28,59], the traceability of their anti-melanogenic activity is still limited.
Figure 2 shows the studies that have been conducted on the anti-melanogenic properties of marine resources such as seagrass, seaweed, and marine sponges, with the number of publications increasing every year. Four article publications made up the majority of those that were released in 2020, with three publications coming in 2019 and 2021. From 1992 until 2018, the number of publications varied and then increased after that year. There are limited studies on anti-melanogenic activity because researchers are more interested in other activities such as antimicrobial, antitumor, and anticancer. They are also interested in chemical compounds produced by symbiotic relationships between sponges and microorganisms. Seaweed metabolites have been established in numerous studies evaluating anti-melanogenic seaweed metabolites to have inhibitory activity against the tyrosinase enzyme and to be able to reduce melanin content in cells. It is also possible to extract prospective metabolites that may eventually be developed as anti-hyperpigmentation therapeutics if further research is conducted, especially from seagrass and sponge materials.
Classification of anti-melanogenic activity of compounds from seagrasses, seaweed, and sponges
In this review, we list the bioactive compounds that have recently been discovered in seagrasses, seaweeds, and marine sponges that have been shown to have anti-melanogenic properties. The units are converted to the same units, or g/ml, to compare IC50 results. Moon et al. [86] defined the classification of all components based on the IC50 value, classifying the activity of the tyrosinase inhibitor component as “very potent inhibitory”: IC50 10 g/ml, “strong inhibitory”: IC50 is 10–100 g/ml, and “moderately inhibitory”: IC50 is 100–500 g/ml. Tyrosinase inhibitors, inhibition of melanin synthesis in cells, and nontoxicity to melanocyte cells are among the characteristics that can be tested to assess a component’s anti-melanogenic activity. The types of test cells used in this study differ in their ability to inhibit the activity of the melanin content, thus it is possible that various IC50 values represent components that are inactive in different test cell melanocyte types. Reviewing the efficacy of inactive substances derived from seagrass, seaweed, and sponges may represent an excellent concept.
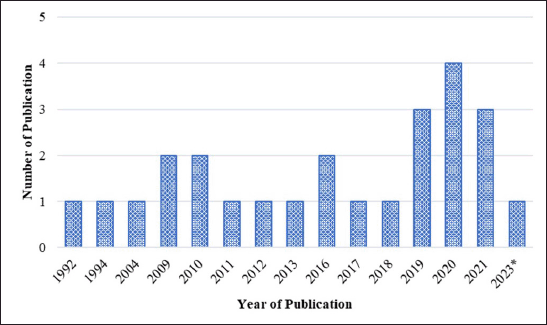 | Figure 2. Distribution of conducted studies about seagrasses, seaweeds, and marine sponges metabolite exploration for anti-melanogenic activity. [Click here to view] |
In this study to determine the inhibition of tyrosinase, five different types of melanocyte cells, whether in melanoma or normal conditions, were used. Murine melanoma B16, HEM, mouse Melan-a, normal human melanocyte (NHM), and melanoma MM418 are the most common types of skin melanocyte cells, which cell models have relevance for the assessment of the anti-melanogenic substance. To investigate new compounds derived from marine resources such as seagrass, seaweed, and marine sponges, many researchers have focused their study on these five types of cells. Potential marine resource compounds are anticipated to be utilized as new drugs for the treatment of hyperpigmentation disorders. Researchers utilized an in vitro study with a cell line and the tyrosine enzyme to facilitate the screening of the anti-melanogenic activity of components obtained from marine resources. In this study, we identified five different types of melanocyte cell lines that were utilized to assess the anti-melanogenic activity of substances derived from marine sources. The use of this tyrosine enzyme and melanocyte cells were also taken into account by other considerations. These characteristics include easy handling and alteration, ideal homogeneity, a high degree of representation of the initial subject, a limitless auto-replicative source, and the ability to reproduce effects given the right conditions. In addition, normal melanocyte cells are also utilized.
Tables 2 and 3 and Figures 3 and 4 show the marine resource-isolated substances classified as very strong and strong tyrosinase inhibitors and synthesis of melanin inhibitors. The flavone or flavonoid luteolin 7-sulfate and luteolin have a yellow crystalline appearance. It is found in a wide variety of plant species, including celery, carrots, olive oil, peppers, and various species of marine macroalga. According to López-Lázaro [87], this metabolite exhibits a variety of biological properties, including antioxidant, anti-inflammatory, antibacterial, and anticancer properties. Phloroglucinol, 7-phloroeckol, dioxinodehydroeckol, phlorofucofuroeckol, eckol, and dieckol are examples of bioactive phlorotannin derivatives that have been identified in the edible brown algae arame (Ecklonia bicyclis) and turuarame (Ecklonia stolonifera) [88]. Tyrosinase inhibitory properties of phlorotanin derivatives present the possibility of being developed as depigmenting agents [52,54,89].
Functional group in potent anti-melanogenic activity
Luteolin and luteolin 7-sulfate (Fig. 5) is a pure yellow crystalline powder representing the category of bioflavonoid. Luteolin is present in many different types of plants, while the luteolin 7-sulfate is only present in a few species of plants, such as P. iwatensis Makino and Zotera marina, a marine algae [90,91]. In a previous study, luteolin 7-sulfate isolated from P. iwatensis was shown to inhibit cellular melanin synthesis. The cAMP-responsive element binding protein and microphthalmia-associated transcription factor (MITF)-mediated signaling pathways are involved in the mechanism of action of luteolin 7-sulfate, which reduces melanin synthesis by weakening the expression of the TYR gene [34,92].
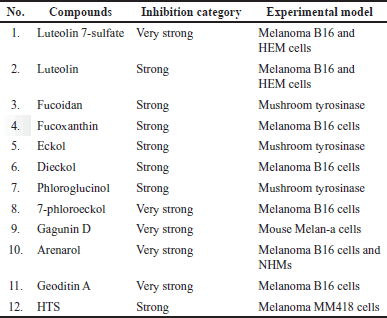 | Table 2. List of compounds with very strong and strong tyrosinase inhibitor activity based on the IC50 value. [Click here to view] |
 | Table 3. List of compounds with very strong and strong biosynthesis melanin inhibitor activity based on the IC50 value. [Click here to view] |
Fucoidan and fucoxanthin (Fig. 5) are two compounds extracted from brown seaweed. Fucus vesiculosus, Cladosiphon okamuranus, Laminaria japonica, Sargassum fusiforme, and Undaria pinnatifida are examples of brown algae that produce fucoidan and fucoxanthin [93]. Both fucoidan and fucoxanthin show anti-melanogenic properties. Fucoidan occurs in the cell walls of brown seaweed, protecting seaweeds from external stress, while fucoxanthin is the pigment responsible for the olive-green color of brown seaweeds. Moreover, the main of fucoxanthin is to harvest light as a part of the process of photosynthesis. Fucoxanthin inhibited tyrosinase activity, melanogenesis in melanoma, and UV-B-induced skin pigmentation. Topical application of fucoxanthin (1%) significantly suppressed mRNA expression of tyrosinase-related protein 1 [45].
Phlorotannins, representing about 5%–12% of the dry mass of marine brown algae (Phaeophyta), are polyphenolic chemicals based on phloroglucinol (13,5-trihydroxy benzene). Phloroglucinol oligomers have been polymerized by polyketides (acetate-malonate) to produce phlorotannins. Phlorotannins with biological activity that support health have been found in several species of marine brown algae, including Hizikia fusiformis, Ecklonia cava, L. japonica, Ecklonia kurome, Ishige okamurae, Sargassum thunbergii, E. bicyclis, U. pinnatifida, and E. stolonifera [94]. Phlorotannins have been found to inhibit tyrosinase, an essential enzyme for the production of melanin. Two phlorotannins, phlorofucofuroeckol-A (PFF-A), and fucofuroeckol-A (FF-A), from the E. bicyclis were recently shown to reduce the biosynthesis of melanin in mouse B16 melanoma cells [55]. The downregulation of TYR and transcription factors associated with microphthalmia by FF-A inhibits melanin production. These results suggest that phlorotannin might regulate melanin to be important for treating hyperpigmentation disorders.
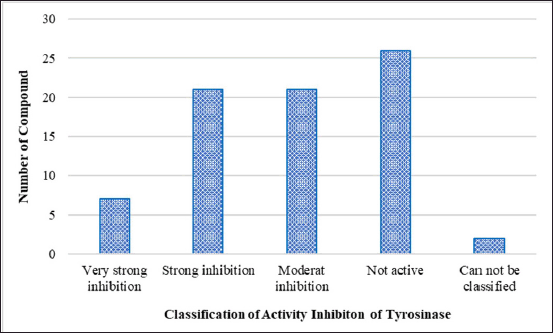 | Figure 3. Classification of compounds with tyrosinase inhibitory activity based on IC50 values. [Click here to view] |
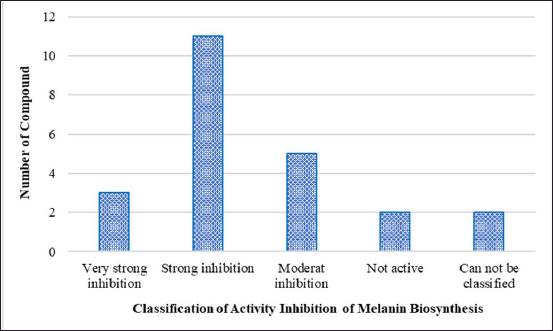 | Figure 4. Classification of compounds with biosynthesis melanin inhibitory activity based on IC50 values. [Click here to view] |
Gagunin D (Fig. 5), a highly oxygenated diterpenoid which is isolated from marine sponge Phorbas sp., has significantly inhibited tyrosinase enzyme activity and decreased the melanin content the melanin without cytotoxicity effect on Melan-a cells at 20 μM for 72 hours. Gagunin D also effectively downregulated the protein level of MITF at 20 μM [27]. Geoditin A an isomalabaricane triterpene which is isolated from the marine sponge Geodia japonica, has significantly inhibited tyrosinase enzyme activity and decreased the melanin content the melanin without cytotoxicity effect on murine melanoma B16 cells at 1 μg/ml. Contribute Geoditin A to melanogenic inhibition was mediated through modulation of the cAMP pathway [28]. Osirisyne derivates are polyacetylenes that are widely distributed in marine sponge Haliclona sp. Dichloromethane extract containing osirisyne derivates has presented significant anti-tyrosinase activity with % inhibition up to 68.9% at 100 μg/ml [59]. Halistanol trisulphate (HTS) caused immediate inhibition of synthesis melanin at 100 μg/ml after 24 hours of treatment. HTS inhibits the maturation of tyrosinase to a form associated with melanin synthesis [61]. Arenarol is a sesquiterpenoids with rearranged drimane skeletons from marine sponge Dysidea arenaria. This compound is potential to be developed into anti-pigmented effect. Inhibitory effect of arenarol on TYR activity at 3.0 μM without cytotoxic after 3 days post-treatment [60].
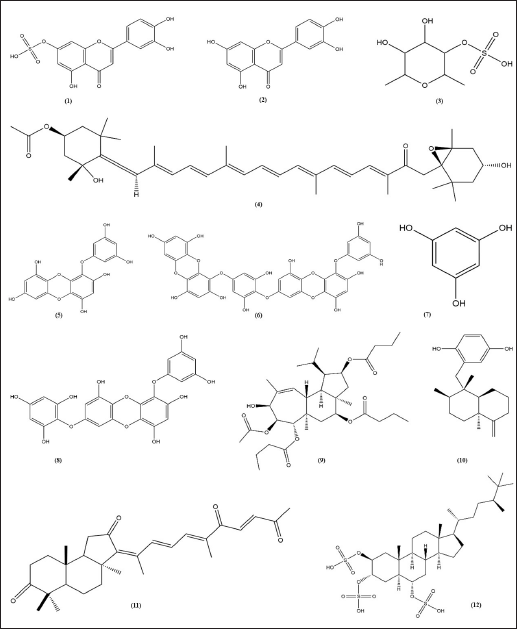 | Figure 5. Structure of anti-melanogenic compounds. Luteolin 7-sulfate (1). Luteolin (2). Fucoidan (3). Fucoxanthin (4). Eckol (5). Dieckol (6). Phloroglucinol (7). 7-phloroeckol (8). Gagunin D (9). Arenarol (10). Geoditin A (11) HTS (12). [Click here to view] |
CONCLUSION
The review’s data showed that the metabolites produced from marine natural resources have the potential to be used as a regulator of melanin biosynthesis. Based on the components that have been summarized, the promising components to be developed as depigmenting agents are derivatives of flavonoids, polysaccharides, and phlorotannin isolated from seaweed, terpenoid derivatives isolated from sponges, while metabolites sourced from seagrass need further exploration because the information about bioactive compounds that have the potential to perform the role of tyrosinase inhibitors is currently very limited. To evaluate the specific mechanism of action of the bioactive components as anti-melanogenic substances, a thorough approach is needed.
ACKNOWLEDGMENTS
The author would like to acknowledge the funding support from UGM number: 5057/UN1.P.II/Dit-Lit/PT.01.01/2023.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve the use of animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16(5):523–31. CrossRef
2. Low E, Alimohammadiha G, Smith LA, Costello LF, Przyborski SA, von Zglinicki T, et al. How good is the evidence that cellular senescence causes skin ageing? Ageing Res Rev. 2021 Nov 1;71:101456. CrossRef
3. Sugumaran M, Barek H. Critical analysis of the melanogenic pathway in insects and higher animals. Int J Mol Sci. 2016 Oct;17(10):1753. CrossRef
4. Rzepka Z, Buszman E, Beberok A, Wrzesniok D. From tyrosine to melanin: signaling pathways and factors regulating melanogenesis. Adv Hyg Exp Med. 2016 Jun 30;70(0):695–708. CrossRef
5. Kurpejovic E, Wendisch VF, Sariyar Akbulut B. Tyrosinase-based production of l-DOPA by Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2021 Dec 1;105(24):9103–11. CrossRef
6. Min K, Park K, Park DH, Yoo YJ. Overview on the biotechnological production of l-DOPA. Appl Microbiol Biotechnol. 2015 [cited 2023 Mar 3];99:575–84. Available from: https://link.springer.com/article/10.1007/s00253-014-6215-4
7. Rathee P, Kumar S, Kumar D, Kumari B, Yadav SS. Skin hyperpigmentation and its treatment with herbs: an alternative method. Futur J Pharm Sci. 2021 [cited 2023 Mar 3];7:132. Available from: https://link.springer.com/article/10.1186/s43094-021-00284-6
8. Srisayam M, Weerapreeyakul N, Kanokmedhakul K. Inhibition of two stages of melanin synthesis by sesamol, sesamin and sesamolin. Asian Pac J Trop Biomed. 2017 Oct 1;7(10):886–95. CrossRef
9. Qian W, Liu W, Zhu D, Cao Y, Tang A, Gong G, et al. Natural skin?whitening compounds for the treatment of melanogenesis (Review). Exp Ther Med. 2020 Jul 1;20(1):173–85. CrossRef
10. Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev [Internet]. 2020 [cited 2023 Mar 3];41(2):1138–66. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/med.21754
11. Solano F, Briganti S, Picardo M, Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19(6):550–71. CrossRef
12. Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16(2):101–10. CrossRef
13. Nautiyal A, Wairkar S. Management of hyperpigmentation: current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021;34(6):1000–14. CrossRef
14. Enaru B, Dretcanu G, Pop TD, Stanila A, Diaconeasa Z. Anthocyanins: factors affecting their stability and degradation. Antioxidants. 2021 Dec;10(12):1967. CrossRef
15. Ferreira AM, de Souza AA, Koga RD, Sena ID, Matos MD, Tomazi R, et al. Anti-melanogenic potential of natural and synthetic substances: application in Zebrafish model. Molecules. 2023 Jan;28(3):1053. CrossRef
16. Kalaivani D, Arun V. Anti-melanogenic potential of Acalypha indica ethyl acetate fraction on Zebra fish embryos. Curr Trends Biotechnol Pharm. 2022 Feb 11;16(1):23–34. CrossRef
17. Jin S, Hyun TK. Ectopic expression of production of anthocyanin pigment 1 (PAP1) improves the antioxidant and anti-melanogenic properties of ginseng (Panax ginseng C.A. Meyer) Hairy roots. Antioxidants. 2020 Oct;9(10):922. CrossRef
18. Vijayakumar R, Raja S. Secondary metabolites: trends and reviews [Internet]. London, UK: IntechOpen; 2022. 312 p. CrossRef
19. El-Nashar HAS, El-Din MIG, Hritcu L, Eldahshan OA. Insights on the inhibitory power of flavonoids on tyrosinase activity: a survey from 2016 to 2021. Molecules. 2021 Jan;26(24):7546. CrossRef
20. Chaita E, Lambrinidis G, Cheimonidi C, Agalou A, Beis D, Trougakos I, et al. Anti-melanogenic properties of greek plants. A novel depigmenting agent from Morus alba Wood. Molecules. 2017 Apr;22(4):514. CrossRef
21. Blunt J, Copp B, Keyzers R, Munro MH, Prinsep M. Marine natural products. Nat Prod Rep [Internet]. 2015 [cited 2024 Jan 3];32(2):116–211. CrossRef
22. Jimeno J, Faircloth G, Sousa-Faro JF, Scheuer P, Rinehart K. New marine derived anticancer therapeutics—a journey from the sea to clinical trials. Mar Drugs. 2004 Mar;2(1):14–29. CrossRef
23. Samirana PO, Jenie RI, Murti YB, Setyowati EP Application of metabolomics on marine sponges and sponge-associated microorganisms: a review. J Appl Pharm Sci. 2022;12:18–33. CrossRef
24. Vincenti LD, Glasenapp Y, Cattò C, Villa F, Cappitelli F, Papenbrock J. Hindering the formation and promoting the dispersion of medical biofilms: non-lethal effects of seagrass extracts. BMC Complement Med Ther. 2018 [cited 2023 Jul 2];18(1):1–7. CrossRef
25. Kim DH, Mahomoodally MF, Sadeer NB, Seok PG, Zengin G, Palaniveloo K, et al. Nutritional and bioactive potential of seagrasses: a review. S Afr J Bot. 2021 Mar 1;137:216–27. CrossRef
26. Ali MS, Ravikumar S, Beula JM. Bioactivity of seagrass against the dengue fever mosquito Aedes aegypti larvae. Asian Pac J Trop Biomed. 2012 Jul 1;2(7):570–3. CrossRef
27. Lee HY, Jang EJ, Bae SY, Jeon J, Park HJ, Shin J, et al. Anti-melanogenic activity of Gagunin D, a highly oxygenated diterpenoid from the marine sponge Phorbas sp., via modulating tyrosinase expression and degradation. Mar Drugs. 2016 Nov;14(11):212. CrossRef
28. Cheung FWK, Guo J, Ling YH, Che CT, Liu WK. Anti-melanogenic property of Geoditin A in murine B16 melanoma cells. Mar Drugs. 2012 Feb;10(2):465–76. CrossRef
29. Hu YG, Gao ZP, Zheng YY, Hu CM, Lin J, Wu XZ, et al. Synthesis and biological activity evaluation of 2-cyanopyrrole derivatives as potential tyrosinase inhibitors. Front Chem. 2022 Jun 17;10:914944. CrossRef
30. Kim CS, Noh SG, Park Y, Kang D, Chun P, Chung HY, et al. A potent tyrosinase inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with anti-melanogenesis properties in α-MSH and IBMX-induced B16F10 melanoma cells. Molecules. 2018 Oct;23(10):2725. CrossRef
31. Alizadeh N, Hossein Sayahi M, Iraji A, Yazzaf R, Moazzam A, Mobaraki K, et al. Evaluating the effects of disubstituted 3-hydroxy-1H-pyrrol-2(5H)-one analog as novel tyrosinase inhibitors. Bioorg Chem. 2022 Sep 1;126:105876. CrossRef
32. Park SY, Kim YH, Kim YH, Park GT, Lee SJ. Beta-carboline alkaloids harmaline and harmalol induce melanogenesis through p38 mitogen-activated protein kinase in B16F10 mouse melanoma cells. BMB Rep. 2010 Dec 31;43(12):824–9. CrossRef
33. Ryu IY, Choi I, Jung HJ, Ullah S, Choi H, Al-Amin M, et al. In vitro anti-melanogenic effects of chimeric compounds, 2-(substituted benzylidene)-1,3-indanedione derivatives with a β-phenyl-α, β -unsaturated dicarbonyl scaffold. Bioorg Chem. 2021 Apr 1;109:104688. CrossRef
34. Lee J, Jeong Y, Jin Jung H, Ullah S, Ko J, Young Kim G, et al. Anti-tyrosinase flavone derivatives and their anti-melanogenic activities: importance of the β-phenyl-α,β-unsaturated carbonyl scaffold. Bioorg Chem. 2023 Jun 1;135:106504. CrossRef
35. Rengasamy KRR, Sadeer NB, Zengin G, Mahomoodally MF, Cziáky Z, Jeko J, et al. Biopharmaceutical potential, chemical profile and in silico study of the seagrass—Syringodium isoetifolium (Asch.) Dandy. S Afr J Bot. 2019;127:167–75. CrossRef
36. Papenbrock J. Highlights in seagrasses’ phylogeny, physiology, and metabolism: what makes them special? ISRN Bot. 2012 Dec 10;2012:1–15. CrossRef
37. Reynolds PL, Knowlton N. Seagrass and seagrass beds. Smithsonian Ocean Portal. 2018 Mar 22; 2018:116. Available from: http://ocean.si.edu/seagrass-and-seagrass-beds
38. Hartog C den, Kuo J. Taxonomy and biogeography of seagrasses. In: Larkum AWD, Orth RJ, Duarte CM, editors. Seagrasses: biology, ecologyand conservation [Internet]. Dordrecht, The Netherlands: Springer; 2006 [cited 2023 Jun 26]. pp 1–23. CrossRef
39. Cullen-Unsworth L, Unsworth R. Seagrass meadows, ecosystem services, and sustainability. Environ Sci Policy Sustain Dev. 2013 May 1;55(3):14–28. CrossRef
40. Li X, Zhou Z. Phylogenetic studies of the core Alismatales inferred from morphology and rbcL sequences. Prog Nat Sci. 2009 Aug 10;19(8):931–45. CrossRef
41. Cornara L, Pastorino G, Borghesi B, Salis A, Clericuzio M, Marchetti C, et al. Posidonia oceanica (L.) Delile ethanolic extract modulates cell activities with skin health applications. Mar Drugs. 2018 Jan 10;16(1):21. CrossRef
42. Kwak JY, Seok JK, Suh HJ, Choi YH, Hong SS, Kim DS, et al. Antimelanogenic effects of luteolin 7-sulfate isolated from Phyllospadix iwatensis Makino. Br J Dermatol. 2016 Sep 1;175(3):501–11. CrossRef
43. Wang ZJ, Xu W, Liang JW, Wang CS, Kang Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr J Tradit Complement Altern Med. 2017 Aug 4;14(4):149–55. CrossRef
44. Choosuwan P, Praiboon J, Boonpisuttinant K, Klomjit A, Muangmai N, Ruangchuay R, et al. Inhibitory effects of Caulerpa racemosa, Ulva intestinalis, and Lobophora challengeriae on tyrosinase activity and α-MSH-induced melanogenesis in B16F10 melanoma cells. Life. 2023 Apr;13(4):934. CrossRef
45. Shimoda H, Tanaka J, Shan SJ, Maoka T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J Pharm Pharmacol. 2010 Sep 1;62(9):1137–45. CrossRef
46. Arguelles EDLR. Evaluation of antioxidant capacity, tyrosinase inhibition, and antibacterial activities of brown seaweed, Sargassum ilicifolium (Turner) C. Agardh 1820 for cosmeceutical application. J Fish Environ. 2021 Feb 1;45(1):64–77. Available from: https://li01.tci-thaijo.org/index.php/JFE/article/view/242157
47. Takashi H. Cosmetic potential of boiled water of Hijiki (Sargassum fusiforme) grown in the ocean in Okinawa, Japan [Internet]. Preprints. 2021 [cited 2023 Sep 5]. CrossRef
48. Namjooyan F, Farasat M, Alishahi M, Jahangiri A, Mousavi H. The anti-melanogenesis activities of some selected brown macroalgae from Northern Coasts of the Persian Gulf. Braz Arch Biol Technol. 2019 Jun 13;62:e19180198. CrossRef
49. Cha SH, Ko SC, Kim D, Jeon YJ. Screening of marine algae for potential tyrosinase inhibitor: those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J Dermatol. 2011;38(4):354–63. CrossRef
50. Dolorosa MT, Nurjanah, Purwaningsih S, Anwar E, Hidayat T. Tyrosinase inhibitory activity of Sargassum plagyophyllum and Eucheuma cottonii methanol extracts. IOP Conf Ser Earth Environ Sci. 2019 May;278(1):012020. CrossRef
51. Arguelles E, Sapin A. Bioprospecting of Turbinaria ornata (Fucales, phaeophyceae) for cosmetic application: antioxidant, tyrosinase inhibition and antibacterial activities. J Int Soc Southeast Asian Agric Sci. 2020 Dec 1;26:30–41.
52. Yoon NY, Eom TK, Kim MM, Kim SK. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J Agric Food Chem. 2009 May 27;57(10):4124–9. CrossRef
53. Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, Choi YU, et al. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol In Vitro. 2009 Sep 1;23(6):1123–30. CrossRef
54. Kang HS, Kim HR, Byun DS, Son BW, Nam TJ, Choi JS. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch Pharm Res. 2004 Dec 1;27(12):1226–32. CrossRef
55. Ohno Y, Kondo S, Tajima K, Shibata T, Itoh T. Effect of phlorotannins isolated from Eisenia bicyclis on melanogenesis in mouse B16 melanoma cells. Nat Prod Commun. 2021 May 1;16(5):1934578X211019264. CrossRef
56. Heo SJ, Ko SC, Kang SM, Cha SH, Lee SH, Kang DH, et al. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem Toxicol. 2010 May 1;48(5):1355–61. CrossRef
57. Kim KBWR, Jeong SM, Kim MJ, Ahn DH. Tyrosinase inhibitory effects of sargachromanol G, sargachromanol I and mojabanchromanol b isolated from Myagropsis myagroides. Indian J Pharm Sci. 2020 Feb 1;82(1):171–4. CrossRef
58. Mahomoodally MF, Bibi Sadeer N, Zengin G, Cziáky Z, Jeko J, Diuzheva A, et al. In vitro enzyme inhibitory properties, secondary metabolite profiles and multivariate analysis of five seaweeds. Mar Drugs. 2020 Apr;18(4):198. CrossRef
59. Campos PE, Herbette G, Chendo C, Clerc P, Tintillier F, de Voogd NJ, et al. Osirisynes G-I, new long-chain highly oxygenated polyacetylenes from the mayotte marine sponge Haliclona sp. Mar Drugs. 2020 Jul 3;18(7):350. CrossRef
60. Choi BK, Cha BY, Fujiwara T, Kanamoto A, Woo JT, Ojika M, et al. Arenarol isolated from a marine sponge abrogates endothelin-1-stimulated melanogenesis by interrupting MEK phosphorylation in normal human melanocytes. Cytotechnology. 2013 Dec;65(6):915–26. CrossRef
61. Townsend E, Moni R, Quinn R, Parsons PG. Reversible depigmentation of human melanoma cells by halistanol trisulphate, a novel marine sterol. Melanoma Res. 1992;1(5–6):349–57. CrossRef
62. Zidorn C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): chemical diversity, bioactivity, and ecological function. Phytochemistry. 2016 Apr 1;124:5–28. CrossRef
63. de la Torre-Castro M, Rönnbäck P. Links between humans and seagrasses—an example from tropical East Africa. Ocean Coast Manag. 2004 Jan 1;47(7):361–87. CrossRef
64. Zulkifli L, Muksin YD, Hartanto P, Desimarlina Y, Idrus AA, Syukur A. Phytochemical profiles and ethnomedicine preliminary studies on seagrass species in the Southern Coast of Lombok Island Indonesia. IOP Conf Ser Earth Environ Sci. 2021 Nov;913(1):012102. CrossRef
65. Yuvaraj N, Kanmani P, Satishkumar R, Paari A, Pattukumar V, Arul V. Seagrass as a potential source of natural antioxidant and anti-inflammatory agents. Pharm Biol. 2012 Apr;50(4):458–67. CrossRef
66. Klangprapun S, Buranrat B, Caichompoo W, Nualkaew S. Pharmacognostical and physicochemical studies of Enhalus acoroides (L.F.) Royle (Rhizome). Pharmacogn J. 2018;10(6s):s89–94. CrossRef
67. Alino PM, Cajipe GJB, Ganzon-Fortes ET, Licuanan WRY, Montano NE, Tupas LM. The use of marine organisms in folk medicine and horticulture: a preliminary study. SICEN Leafl 1 Suppl SICEN Newsl Univ Philipp. 1990;298:8.
68. Ibañez E, Cifuentes A. Benefits of using algae as natural sources of functional ingredients. J Sci Food Agric. 2013;93(4):703–9. CrossRef
69. Kalasariya HS, Patel AK, Suthar RJ, Pereira L. Exploring the skin cosmetic benefits of phenolic compounds and pigments from marine macroalgae: a novel green approach for sustainable beauty solutions [Internet]. Preprints. 2023 [cited 2023 Sep 5]. CrossRef
70. Mhadhebi L, Mhadhebi A, Robert J, Bouraoui A. Antioxidant, anti-inflammatory and antiproliferative effects of aqueous extracts of three mediterranean brown seaweeds of the genus Cystoseira. Iran J Pharm Res IJPR. 2014;13(1):207–20.
71. Mirata S, Asnaghi V, Chiantore M, Salis A, Benvenuti M, Damonte G, et al. Photoprotective and anti-aging properties of the apical frond extracts from the mediterranean seaweed Ericaria amentacea. Mar Drugs. 2023 May;21(5):306. CrossRef
72. Kim MJ, Hyun KH, Hyun JH, Im S, Sim J, Lee NH. Anti-melanogenic activities of Sargassum muticum via MITF downregulation. Orient J Chem. 2017 Aug 10;33(4):1589–94. CrossRef
73. Thiyagarasaiyar K, Mahendra CK, Goh BH, Gew LT, Yow YY. UVB radiation protective effect of brown alga Padina australis: a potential cosmeceutical application of Malaysian seaweed. Cosmetics. 2021 Sep;8(3):58. CrossRef
74. Mohy El-Din SM, El-Ahwany AMD. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J Taibah Univ Sci. 2016 Jul 1;10(4):471–84. CrossRef
75. Sangeetha J, Thangadurai D. Algal genetic resources: cosmeceuticals, nutraceuticals, and pharmaceuticals from algae. Boca Raton, FL: CRC Press; 2022. 398 p. Available from: http://192.168.9.248:8080/jspui/handle/123456789/430
76. Poulose N, Sajayan A, Ravindran A, Sreechithra T, Vardhan V, Selvin J, et al. Photoprotective effect of nanomelanin-seaweed concentrate in formulated cosmetic cream: with improved antioxidant and wound healing properties. J Photochem Photobiol B. 2020 Apr 1;205:111816. CrossRef
77. Soest RWMV, Boury-Esnault N, Vacelet J, Dohrmann M, Erpenbeck D, Voogd NJD, et al. Global diversity of sponges (Porifera). PLoS One. 2012 Apr 27;7(4):e35105. CrossRef
78. Maldonado M, Aguilar R, Bannister R, Bell J, Conway J, Dayton P, et al. Sponge grounds as key marine habitats: a synthetic review of types, structure, functional roles, and conservation concerns [Internet]. Cham, Switzerland: Springer International Publishing; 2017 [cited 2023 Sep 5]. CrossRef
79. Bergé JP, Barnathan G. Fatty acids from lipids of marine organisms: molecular biodiversity, rolesas biomarkers, biologically active compounds, and economical aspects. In: Ulber R, Le Gal Y, editors. Marine biotechnology I [Internet]. Berlin, Heidelberg, Germany: Springer; 2005 [cited 2023 Sep 5]. pp 49–125. CrossRef
80. Ghosh S, Sarkar T, Pati S, Kari ZA, Edinur HA, Chakraborty R. Novel bioactive compounds from marine sources as a tool for functional food development. Front Mar Sci [Internet]. 2022 [cited 2023 Sep 5];9:832957. Available from: https://www.frontiersin.org/articles/10.3389/fmars.2022.832957
81. Laport MS, Santos OCS, Muricy G. Marine sponges: potential sources of new antimicrobial drugs. Curr Pharm Biotechnol. 2009 Jan 1;10(1):86–105. CrossRef
82. Baek SH, Cao L, Jeong SJ, Kim HR, Nam TJ, Lee SG. The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean Sargassum species. J Food Qual. 2021 Jan 18;2021:e6640789. CrossRef
83. Mapoung S, Semmarath W, Arjsri P, Umsumarng S, Srisawad K, Thippraphan P, et al. Determination of phenolic content, antioxidant activity, and tyrosinase inhibitory effects of functional cosmetic creams available on the Thailand market. Plants. 2021 Jul;10(7):1383. CrossRef
84. Yener I, Kocakaya SO, Ertas A, Erhan B, Kaplaner E, Oral EV, et al. Selective in vitro and in silico enzymes inhibitory activities of phenolic acids and flavonoids of food plants: relations with oxidative stress. Food Chem. 2020 Oct 15;327:127045. CrossRef
85. Harvey AL. Natural products in drug discovery. Drug Discov Today. 2008 Oct 1;13(19):894–901. CrossRef
86. Moon JY, Yim EY, Song G, Lee NH, Hyun CG. Screening of elastase and tyrosinase inhibitory activity from Jeju Island plants: Jeju Adasi Bitkilerinde Elastaz ve Tirosinaz Inhibitör Aktivitesinin Incelenmesi Özet. EurAsian J Biosci. 2010 Dec;4:41–53. CrossRef
87. López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009 Jan;9(1):31–59. CrossRef
88. Bak SS, Sung YK, Kim SK. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2014 Aug 1;387(8):789–93. CrossRef
89. Manandhar B, Wagle A, Seong SH, Paudel P, Kim HR, Jung HA, et al. Phlorotannins with potential anti-tyrosinase and antioxidant activity isolated from the marine seaweed Ecklonia stolonifera. Antioxidants. 2019 Aug;8(8):240. CrossRef
90. Enerstvedt KH, Jordheim M, Andersen ØM. Isolation and identification of flavonoids found in Zostera marina collected in Norwegian Coastal waters. Am J Plant Sci. 2016 May 10;7(7):1163–72. CrossRef
91. Takagi M, Funahashi S, Ohta K, Nakabayashi T. Phyllospadine, a new flavonoidal alkaloid from the sea-grass Phyllosphadix iwatensis. Agric Biol Chem. 1980;44(12):3019–20. CrossRef
92. Lee SW, Kim JH, Song H, Seok JK, Hong SS, Boo YC. Luteolin 7-sulfate attenuates melanin synthesis through inhibition of CREB- and MITF-mediated tyrosinase expression. Antioxidants. 2019 Apr;8(4):87. CrossRef
93. Chen BJ, Shi MJ, Cui S, Hao SX, Hider RC, Zhou T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from Sargassum fusiforme by degradation. Int J Biol Macromol. 2016 Nov 1;92:715–22. CrossRef
94. Okeke ES, Nweze EJ, Chibuogwu CC, Anaduaka EG, Chukwudozie KI, Ezeorba TPC. Aquatic phlorotannins and human health: bioavailability, toxicity, and future prospects. Nat Prod Commun. 2021 Dec 1;16(12):1934578X211056144. CrossRef