INTRODUCTION
Boesenbergia rotunda (L.) Mansf, also known as lesser galangal or fingerroot, is a plant native to Southeast Asia that has been traditionally used for medicinal purposes in Thai traditional medicine, including the treatment of oral diseases. It belongs to the family Zingiberaceae and is commonly found in Thailand, Indonesia, and Laos. In addition to treating oral diseases, B. rotunda has been used to treat various ailments such as inflammation, gastrointestinal disorders, and infections. The rhizomes of B. rotunda are often used as decoctions or infusions in traditional medicines [1].
Oral diseases, such as dental caries and periodontitis, represent significant global health challenges, arising from a complex interplay of bacterial and fungal elements within the oral biofilm. While Streptococcus mutans and Candida albicans are key contributors, the ecological plaque hypothesis suggests that these diseases result from broader microbial dysbiosis rather than the actions of a single pathogen. Streptococcus mutans, known for its acidogenic capacity, plays a central role in tooth decay by producing acids and glucans, which not only lead to enamel demineralization but also facilitate an environment that favors cariogenic species over noncariogenic commensal microbes. In addition, S. mutans interacts synergistically with C. albicans, a fungus commonly present in the oral cavity, contributing to the complexity of cariogenic biofilms [2].
Panduratin A and its derivatives are prenylated flavonoids from B. pandurata that exhibit a broad range of biological activities, including strong antibacterial, anti-inflammatory, and anti-cancer activities [3]. Among fingerroot’s primary ingredients, panduratin A exhibits antiviral activity against HIV-1 and dengue virus [4]. The essential oil from B. rotunda, as demonstrated in recent research, exhibits potent antibacterial activity against key oral pathogens, a discovery that significantly enhances its profile as an effective agent in oral healthcare [5]. However, there have been limited scientific investigations into the pharmacological properties of B. rotunda, particularly concerning its potential use in the treatment of oral and respiratory tract infectious diseases.
Although the human coronavirus primarily causes respiratory infections, it has been detected in droplets emitted from the oral cavity of infected individuals, from where it can be potentially transmitted [6]. Therefore, investigating the potential anti-viral and antimicrobial activity of B. rotunda against common oral pathogens is crucial to reducing the risk of transmission of SARS-CoV2, which may coexist in the oral cavity.
This study aimed to evaluate the antimicrobial and antiviral activities of the ethanolic extract of B. rotunda rhizomes and its active component, panduratin A, against oral pathogens such as S. mutans, Streptococcus pyogenes, Streptococcus sobrinus, Candida albicans, and human coronavirus 229E. In addition, this study aimed to assess the cytotoxicity of the extract and determine its antioxidant activity, as well as to identify and quantify its chemical composition to provide a comprehensive evaluation of the therapeutic potential of B. rotunda in treating oral and respiratory tract infections.
MATERIAL AND METHODS
Preparation of plant extracts
Boesenbergia rotunda rhizomes were sourced from suppliers in Chiangmai, Thailand (specimen voucher no. MJU 2102-005), and verified by the Maejo University Herbarium before extraction.
Rhizomes were washed thoroughly with tap water, chopped into small pieces, and dried in an oven at 50°C for 3 days. The plant material was macerated in 95% ethanol at room temperature with agitation at 100 rpm for 72 hours. The extract was filtered through Whatman No. 1 filter paper under vacuum and then concentrated by removing the solvent using a rotary evaporator and freeze drying [7]. The obtained extracts were stored at 4°C until use.
Column chromatography and thin-layer chromatography (TLC)-bioautography
Column chromatography and TLC-bioautography were used to identify the active compounds in the B. rotunda extract that exhibited antibacterial activity as previously described [8]. The plant extract (25 g) was fractionated using column chromatography. Each elution (700 ml) was performed using the following nine mobile phase steps: 1) hexane, 2 to 6) hexane: ethyl acetate (9:1, 4:1, 3:2, 2:3, and 1:4, respectively), 7) ethyl acetate, 8) ethyl acetate: methanol (1:1), and 9) methanol. The fractionated extracts were collected, concentrated under reduced pressure, and freeze dried to obtain dry extracts. The yields of the crude and fractionated extracts were determined by weighing the dried extracts and determining their chemical composition patterns using TLC.
The procedures of TLC-bioautography were performed as described previously with modifications [9]. Two silica gel plates of Silica Gel 60 F254 (MERCK, Germany) were prepared and subjected to identical conditions. One μl of the B. rotunda extract was applied to the TLC plates. AR-grade hexane (Fisher Scientific, UK), ethyl acetate (RCI Labscan Ltd., Thailand), and dichloromethane (RCI Labscan Ltd.) were used for TLC separation. The extract was separated using hexane: ethyl acetate: dichloromethane (11:8:1) mobile phase. Separation was performed in a saturated chamber. The TLC plates were dried in a fume hood for 15 minutes to remove the solvent. The compounds separated on the first TLC plate (I) were UV-visualised (Upland, USA, 254 nm), spots were pencil-marked, and retention values (Rf) were measured. A second TLC plate (II) was overlaid with molten brain heart infusion agar, seeded with bacteria (0.5 McFarland), and incubated in a moist chamber at 37°C for 24 hours and then sprayed with 1% thiazolyl blue tetrazolium bromide (AppliChem, Spain), before incubation at room temperature for 30 minutes. The clear spots appearing on the purple background suggested the presence of an antibacterial agent. Fractions with the same TLC bioautographic patterns were pooled.
High-performance liquid chromatography (HPLC) for panduratin Analysis
The presence of panduratin A was determined by the Thailand Institute of Scientific and Technology Research using HPLC following the method described previously [10].
Antimicrobial activity
The oral pathogens used for testing were S. mutans DMST 18777, S. pyogenes DMST 30563, S. sobrinus DMST 35719, and C. albicans DMST 5815 (obtained from the Department of Medical Sciences, Ministry of Public Health, Thailand). The broth dilution method was used to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), as described previously [11]. Briefly, the extract was prepared at concentrations from 0.06 to 128 mg/ml in 10% dimethyl sulfoxide (DMSO). The plates were incubated at 37°C for 24 hours in an anaerobic jar for bacteria and under aerobic conditions for C. albicans.
Virus inhibition assay
The antiviral activity of an extract was determined by the Tropical Medicine Diagnostic Reference Laboratory, Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Thailand, using a virus inhibition assay in Vero cells with a 50% tissue culture infectious dose (TCID50) assay, in accordance with ASTM E1052-20 [12]. Briefly, the human coronavirus 229E (ATCC® VR-740TM) was multiplied by propagating Vero cells and stored at −80°C. The extract was prepared at a concentration of 1,600 μg/ml and was incubated with 1 × 105 TCID50/ml of human coronavirus at 37°C for 30 minutes.
Cytotoxicity assay
The cytotoxicity of the extracts was determined by the School of Science, King Mongkut’s Institute of Technology Ladkrabang, Thailand, using the MTT assay on Vero cells, following the method as previously described [13]. Briefly, the Vero cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin-streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Cells were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 hours. The cells were then treated with the extract at concentrations of 125, 250, 500, 1,000, and 2,000 μg/ml for 24 hours. After treatment, the cells were incubated with MTT (5 mg/ml) for 4 hours at 37°C. The formazan crystals were dissolved in DMSO and the absorbance at 570 nm was measured using a microplate reader. The 50% cytotoxic concentration (CC50) causing visible morphological changes in 50% of the Vero cells was determined.
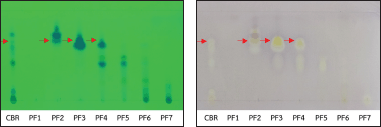 | Figure 1. TLC of CBR and PF1-PF7 (A) and TLC-bioautography against S. mutans (B). [Click here to view] |
Antioxidant assay
Boesenbergia rotunda crude extract (CBR) and fractionated B. rotunda (FBR) were determined for antioxidant activity using a 2,2-Diphenyl-1-picrylhydrazyl radical scavenging assay, as previously described [14]. The total phenolic and flavonoid contents were determined using a previously described method [15].
RESULTS
Column chromatography and TLC-bioautography
Figure 1A shows the TLC pattern of CBR and pooled fractions PF1-PF7 under UV 254 nm. Figure 1B shows the TLC-bioautography of CBR and PF1-PF7 against S. mutans. CBR presented several inhibition zones, whereas PF2-PF4 presented a common inhibition zone at Rf = 0.66, as indicated by the arrows. PF3 exhibited the strongest inhibition zone at Rf = 0.66, followed by PF2 and PF4. Similar results were obtained for S. pyogenes and S. sobrinus (data not shown).
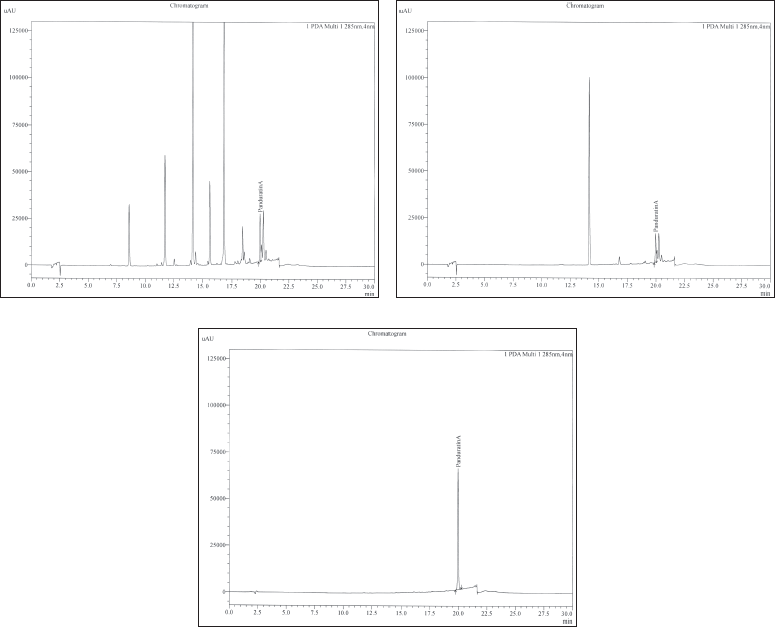 | Figure 2. HPLC chromatogram of panduratin A in CBR (A), FBR (B), and the standard panduratin A (C). [Click here to view] |
Characterization of panduratin A by HPLC
The chemical composition of PF3 was analyzed by HPLC because it showed the strongest antibacterial activity against the tested bacteria. From this point onward, PF3 is referred to as FBR. Our results showed the presence of panduratin A in CBR and FBR at the same retention time as that of the standard panduratin A (19.9 minutes) (Fig. 2). The identification of panduratin A in both the CBR and FBR samples suggested that it remained stable after column chromatography.
The quantitation of panduratin A in CBR and FBR, as shown in Table 1, revealed concentrations of 3.75 and 13.76 g/100 g, respectively. This indicates that FBR contains 4.3 times more panduratin A than CBR. The high concentration of panduratin A in FBR may be attributed to the fractionation process, which allowed the isolation of the compound from other components in the crude extract.
 | Table 1. Quantitation of panduratin A in crude extract and FBR by HPLC. [Click here to view] |
Antimicrobial activity
In the present study, the antimicrobial activities of CBR and FBR against oral pathogens were evaluated using a microdilution assay. The MIC and MBC values of both extracts against S. mutans, S. pyogenes, S. sobrinus, and C. albicans are listed in Table 2. The MIC/MBC ratios for CBR against the tested bacteria ranged from 0.01/0.06 to 0.03/0.12 mg/ml, whereas the MIC/MBC ratios for FBR against the tested bacteria ranged from 0.002/0.008 to 0.008/0.03 mg/ml. The MIC/MBC ratios of CBR and FBR against C. albicans were 0.25/0.25 and 0.12/0.25 mg/ml, respectively. CBR and FBR demonstrated stronger antimicrobial activity against the tested bacteria than against the tested yeast.
Antiviral activity and cytotoxicity
Exposure of human coronavirus 229E to CBR and FBR at a concentration of 1,600 μg/ml for 30 minutes resulted in more than 99.99% efficacy, with more than 4 log reduction against the virus. The 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay showed that CBR and FBR exhibited low toxicity toward the Vero cells, with IC50 values exceeding 2,000 μg/ml. These findings suggest that the concentration used to eliminate the virus was unlikely to be toxic to the cells.
Antioxidant activity
The antioxidant potential of CBR and FBR extracts revealed a more pronounced activity in CBR. Both extracts exhibited significant phenolic and flavonoid contents, essential for their antioxidant properties, with a marked concentration of phenolics in FBR as shown in Table 3. Relative to standard antioxidants, the extracts demonstrated considerable antioxidant effectiveness, suggesting their utility in pharmaceutical applications as natural antioxidant sources.
 | Table 2. MIC and MBC of CBR and FBR against oral pathogens by broth dilution assay. [Click here to view] |
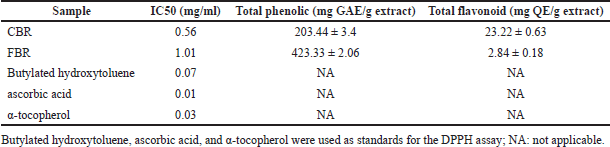 | Table 3. Antioxidant activity (Inhibitory concentration 50%, IC50), total phenolic, and total flavonoid contents of CBR and FBR. [Click here to view] |
DISCUSSION
Our study corroborates and extends the previous findings [16] by demonstrating that both CBR and FBR exhibit significant antimicrobial activity against oral pathogens, with FBR showing greater potency. These findings align with other researchers [17], who also reported the antimicrobial efficacy of fingerroot extracts. Furthermore, our results are in line with the previous study [18] about the antibacterial activities of B. pandurata extracts and suggested a mechanism involving cell wall and membrane disruption.
The direct inhibitory effect of CBR and FBR against human coronavirus 229E adds a new dimension to the antiviral potential of B. rotunda. This is consistent with recent studies indicating the pre-and post-infection inhibition of SARS-CoV-2 [16] and the inhibition of the main protease of SARS-CoV-2 by panduratin A [19]. The molecular docking studies previously [20] further underscore the potential of panduratin A in targeting 2′-O-methyltransferase, a crucial enzyme in viral replication.
In addition to these antimicrobial and antiviral properties, our investigation into the antioxidant activities of CBR and FBR aligns with previous findings on the antioxidant potential of B. rotunda. Consistent with the previous observation [7], our results highlight the antioxidant properties inherent in B. rotunda extracts. In addition, previous studies [16,21] further reinforce the relevance of panduratin A in antioxidant activity and its protective role against oxidative stress in biological systems. These findings collectively emphasize the multifaceted therapeutic potential of B. rotunda, especially in combating oxidative stress-related cellular damage.
The safety profile of these extracts, indicated by their low toxicity to Vero cells, suggests their suitability for therapeutic use. However, the translation from in vitro efficacy to clinical application necessitates a cautious approach. The mechanisms through which panduratin A exerts its antimicrobial and antiviral effects require further elucidation. In vivo studies and clinical trials are essential to confirm these findings and to understand the pharmacokinetics and pharmacodynamics of panduratin A.
CONCLUSION
This study has demonstrated the significant potential of both CBR and FBR of B. rotunda against oral pathogens and human coronavirus 229E. The direct inhibitory effect on human coronavirus 229E notably contributes to its antiviral potential, aligning with recent advancements in antiviral research and the pre- and post-infection inhibition of SARS-CoV-2. This highlights B. rotunda’s capacity to address current global health challenges, particularly in the context of emerging viral threats. Furthermore, the substantial antimicrobial activity against Streptococcus species and C. albicans, as indicated by the MIC and MBC ratios, emphasizes its role in combating oral infections. The antioxidant properties of these extracts, revealed through their phenolic and flavonoid contents, further underscore their therapeutic potential, offering a holistic approach to oral healthcare. In conclusion, B. rotunda, particularly through its bioactive components such as Panduratin A, presents itself as a promising natural resource for pharmaceutical development. This study suggests promising pharmaceutical applications of B. rotunda in the treatment of oral infections and viral diseases, positioning it as a valuable herbal medicine for natural and effective treatments in oral and antiviral healthcare.
AUTHOR CONTRIBUTION
NW and DS conceived and designed the project. KB and RW performed the experiments. NW, DS, IF, and PS analyzed the results. NW drafted the manuscript. NW, DS, IF, and PS revised and finalized the manuscript, which was reviewed and approved by all authors.
FINANCIAL SUPPORT
This study was supported by Naresuan University and Maejo University, Thailand.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ongwisespaiboon O, Jiraungkoorskul W. Fingerroot, Boesenbergia rotunda, and its aphrodisiac activity. Pharmacogn Rev. 2017;11(21):27–30. CrossRef
2. Metwalli KH, Khan SA, Krom BP, Jabra-Rizk MA. Streptococcus mutans, Candida albicans, and the human mouth: a sticky situation. PLoS Pathog. 2013;9(10):e1003616. CrossRef
3. Chahyadi A, Hartati R, Wirasutisna KR, Elfahmi. Boesenbergia pandurata Roxb., an Indonesian medicinal plant: phytochemistry, biological activity, plant biotechnology. Procedia Chem. 2014;13:13–37. CrossRef
4. Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg Med Chem. 2006;14(5):1710–4. CrossRef
5. Sanguansermsri D, Sanguansermsri P, Buaban K, Kawaree R, Wongkattiya N. Antimicrobial activity and time-kill kinetics of Boesenbergia rotunda essential oil and geraniol alcohol against oral bacterial pathogens. J Appl Pharm Sci. 2024;12(2). (In Press).
6. Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19(8):528–45. CrossRef
7. Atun S, Handayani S, Rakhmawati A. Potential bioactive compounds isolated from Boesenbergia rotunda as antioxidant and antimicrobial agents. Pharmacogn J. 2018;10(5):513–8. CrossRef
8. Wongkattiya N, Sanguansermsri P, Assawatheptawee K, Niumsup PN, Fraser I, Sanguansermsri D. Antibacterial activity of ajwain oil on multidrug-resistant Enterobacteriaceae isolated from human faeces. Songklanakarin J Sci Technol. 2022;44(3):785–93. CrossRef
9. Lomarat P, Phanthong P, Wongsariya K, Chomnawang MT, Bunyapraphatsara N. Bioautography-guided isolation of antibacterial compounds of essential oils from Thai spices against histamine-producing bacteria. Pak J Pharm Sci. 2013;26(3):473–7.
10. Yusuf NA, Suffian M, Annuar M, Khalid N. Existence of bioactive flavonoids in rhizomes and plant cell cultures of Boesenbergia rotunda (L.) Mansf. Kulturpfl. Aust J Crop Sci. 2013;7(5):730–4.
11. Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed. CLSI standard M07. Wayne, PA: CLSI; 2018.
12. ASTM International. ASTM E1052-20, Standard practice to assess the activity of microbicides against viruses in suspension. [cited 2022 Oct 26]. Available from: https://www.astm.org/Standards/E1052.htm
13. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. CrossRef
14. Fukumoto L, Mazza G. Assessing antioxidant and pro-oxidant activities of phenolic compounds. J Agric Food Chem. 2000;48(7):3597–604. CrossRef
15. Derakhshan Z, Ferrante M, Tadi M, Ansari F, Heydari A, Hosseini MS, et al. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice, and seeds. Food Chem Toxicol. 2018;114:108–11. CrossRef
16. Kanjanasirirat P, Saksatu A, Manopwisedjaroen S, Munyoo B, Tuchinda P, Jearawuttanakul K, et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component. Sci Rep. 2020;10:19963. CrossRef
17. Mazlan RNAR, Zakaria MPM, Rukayadi Y. Antimicrobial activity of fingerroot [Boesenbergia rotunda (L.) Mansf. A.] extract against Streptococcus mutans and Streptococcus sobrinus. J Pure Appl Microbiol. 2016;10(4):2965–72.
18. Limsuwan S, Voravuthikunchai SP. Bactericidal, bacteriolytic, and antibacterial virulence activities of Boesenbergia pandurata (Roxb) Schltr extract against Streptococcus pyogenes. Trop J Pharm Res. 2013;12(6):1023–8. CrossRef
19. Vergoten G, Bailly C. Interaction of panduratin A and derivatives with the SARS-CoV-2 main protease (mpro): a molecular docking study. J Biomol Struct Dyn. 2022;17(1):1–11. CrossRef
20. Boonserm P, Khunrae P, Sutthibutpong T. A computational study on the molecular mechanisms of panduratin A as a potential inhibitor on SARS-CoV-2 protein targets. Heliyon. 2023;9:e12780. CrossRef
21. Salama SM, AlRashdi AS, Abdulla MA, Hassandarvish P, Bilgen M. Protective activity of panduratin A against Thioacetamide-induced oxidative damage: demonstration with in vitro experiments using WRL-68 liver cell line. BMC Comp Alt Med. 2013;13:279. CrossRef