INTRODUCTION
Inflammation is the body’s response in combatting pathogens or destructing chemicals (cytokines and histamines). The cascade of inflammatory-related mediators frames the acute inflammatory response, which is activated by recruiting granular white blood cells and frequently resolves the outcome recovery. Understanding how the inflammatory process is triggered might be beneficial for developing the strategies to inhibit the inflammatory responses (Ward and Lentsch, 1999).
Various therapeutics are being used to stop or reduce the inflammation process, such as nonsteroidal anti-inflammatory drugs and corticosteroids. Unfortunately, these drugs have been reported, case by case, for their unfavorable effects, for example, the increase of blood pressure, peptic ulceration, acute kidney dysfunction, and other serious conditions (Attiq et al., 2017).
The plants of Zingiberaceae family, for example, Boesenbergia rotunda (L.) Mansf. (Eng-Chong et al., 2011; Jing et al., 2010; Yusuf et al., 2013), Renealmia alpinia (Nunez et al., 2004), and Zingiber zerumbet (Taha et al., 2010), have been extensively investigated for their potential phytoconstituents and molecular mechanism.
Boesenbergia rotunda contains various phytoconstituents, classified into two major groups – namely, flavonoids and chalcone derivatives (pinocembrin, pinostrombin, alpinetin, panduratin, cardamonin, quercetin, and kaempferol) (Eng-Chong et al., 2012; Rosdianto et al., 2020), which might indicate a great benefit for drug discovery (Jing et al., 2010; Yusuf et al., 2013). Since this plant serves as the wide range of traditional medicine applications, many thorough studies were carried out to assess its pharmacology activities, such as antiulceration (Abdelwahab et al., 2011), hepatoprotective (Mahmood et al., 2010; Salama et al., 2013), Helicobacter pylori inhibitor (Bhamarapravati et al., 2006), anti-inflammatory (Isa et al., 2012), anticancer (Cheah et al., 2011; Isa et al., 2013), antiallergic (Madaka & Tewtrakul, 2011), antibacterial (Udomthanadech et al., 2015; Zainin et al., 2013), antileptospiral (Chander et al., 2016), antioxidant (Chiang et al., 2017), anti-dengue viral (Chee et al., 2010; Kiat et al., 2006), antiherpes viral (Wu et al., 2011), wound-healing (Mahmood et al., 2010), antimutagenic, antibacterial, antifungal, analgesic, antipyretic, antispasmodic, insecticidal, larvicidal, and pupicidal (Ching et al., 2007; Jaiptech et al., 2010; Phukerd et al., 2013) activities.
This review focuses on the bioactive compounds in Boesenbergia sp. and their mechanism as anti-inflammatory agents. Moreover, this paper also provides other utilities of Boesenbergia sp. as well as its future study aspects (Table 1). The required pieces of information were obtained by searching keywords which include Boesenbergia, Zingiberaceae, flavonoids, kaempferol, panduratin, and quercetin, among published articles until March 2020 in authentic scientific databases.
Methods
The literature search was performed on PubMed database using the following keywords: “Boesenbergia sp.” [Medical Subject Headings (MEeSH) terms] or “Zingiberaceae” [all fields] and “B. rotunda” [Subheading] or “Zingiberaceae” [all fields] or “bioactive compounds” [all fields] or “bioactive compounds” [all fields] or “pharmacological activity of B. rotunda” [MeSH terms] or “pharmacological activity of B. rotunda” [all fields] or “anti-inflammatory activity of B. rotunda” [MeSH terms] or “anti-inflammatory activity of Boesenbergia sp.” [all fields]. A search on other scientific databases using the same keywords was done for additional data.
Ethnobotany Facts
Zingiberaceae, a family of lasting herbs, are aromatic, with fleshy tuberous or nontuberous rhizomes. Zingiberaceae plants are widely distributed throughout the tropics, especially in Indonesia, and comprise 150 species of ginger (Habsah et al., 2000; Ibrahim et al., 2010). Ginger plants are extensively utilized to enhance food taste, cure diseases, beverages, perfume, and so forth (Abdelwahab et al., 2010; Taha et al., 2010). Recent molecular studies such as chloroplast DNA, nuclear internal transcribed spacer, random amplified polymorphic DNA, plastid regions, pollen-based classifications, amplified fragment length polymorphism, and single-strand conformation polymorphism have been employed to taxonomically classify Boesenbergia species (Chen and Xia, 2011; Kress et al., 2002; Techaprasan et al., 2006; Techaprasan et al., 2008; Vanijajiva et al., 2005).
Phytoconstituents
The active phytoconstituents of B. rotunda are (1) flavonoids including alpinetin, boesenbergin, cardamonin, pinostrobin, pinocembrin, geraniol, panduratin, and silybin (Fig. 1) (Ching et al., 2007; Morikawa et al., 2008; Yusuf et al., 2013); (2) essential oils including camphor, cineole, fenchene, hemanthidine, and limonene (Fig. 2) (Baharudin et al., 2015); and (3) polyphenols including caffeic acid, coumaric acid, chlorogenic acid, hesperidin, kaempferol, naringin, and quercetin (Fig. 3) (Jing et al., 2010; Rosdianto et al., 2020).
Anti-Inflammatory Mechanism of Boesenbergia sp.
Table 1 shows all the pharmacological activities of B. rotunda; however, this review study will only focus on the anti-inflammatory mechanism of this plant.
In the Asia region, particularly in Indonesia, B. rotunda has been empirically utilized to treat various types of inflammation. Its flavonoids (panduratin A, 4-hydroxypanduratin A, cardamonin, 2′,4′,6′-trihydroxychalcone, uvangoletin, panduratin C, boesenbergin A, 2′,6′-dihydroxy-4′-methoxychalcone, hydroxypanduratin A, (−)-isopanduratin A, (+)-krachaizin B, (−)-krachaizin B, quercetin, and kaempferol) extracted from the tuberous root of B. pandurata had been studied for their anti-inflammatory activity (Chahyadi et al., 2014; Isa et al., 2012; Rho et al., 2011; Tewtrakul et al., 2009; Tuchinda et al., 2002; Yun et al., 2003).
Panduratin A and Hydroxypanduratin A inhibit TNF-α and the production of nitric oxide
Nitric oxide (NO) plays a key role in maintaining vascular function. The overproduction of NO could damage the tissue and is related to acute and chronic inflammation. An anti-inflammatory study in Thailand reported that phytoconstituents isolated from the extract of B. rotunda strongly inhibit NO production, for example, panduratin A, hydroxypanduratin A, and cardamonin. Moreover, a medium strength of inhibitory activity on tumor necrosis factor-alpha (TNF-α) was observed for both panduratin A and hydroxypanduratin A (Tewtrakul et al., 2009). The NO inhibitors are favorable because NO regulates cerebral blood flow and nociception in migraine-induced animal models (Wong and Lerner, 2015).
Panduratin A and Hydroxypanduratin A inhibit PGE2 production
Prostaglandin synthase catalyzes two separate reactions: (1) the addition of O2 to oxygenate the arachidonic acid molecule until an unstable prostaglandin G2 (PGG2) is produced and (2) PGG2 then migrates to the peroxidase site where it reacts with the hemin group to generate prostaglandin H2 (PGH2) (Levita et al., 2009). PGH2 is subsequently converted into the active PGE2, PGI2, PGD2, PGF2α, and thromboxane A2 (Nørregaard et al., 2015). Both panduratin A and hydroxypanduratin A strongly inhibit PGE2 production (Tewtrakul et al., 2009). The inhibition of PGE2 production could lessen inflammatory symptoms and pain (Sugita et al., 2016).
Boesenbergia rotunda inhibits the infiltration of inflammatory cells in the hepatic bile ducts
The extract of B. rotunda reduces the inflammation caused by Opisthorchis viverrini and induced by N-nitrosodimethylamine administration in rats. This study proved that there was a decrease in the number of inflammatory cells infiltrated into the hepatic bile ducts as well as the serum alanine transaminase and direct bilirubin level (Boonjaraspinyo et al., 2010).
Boesenbergia rotunda accelerates wound healing in rats
A wound recovery is a dynamic process of repairing cellular structures in damaged tissue. Wound abridgment occurs throughout the recovery process commencing in the fibroblastic stage followed by the proliferative stage (Midwood et al., 2004). Flavonoids have been proven to promote the wound-healing process due to their antimicrobial activities, which is responsible for wound contraction and increased the rate of epithelialization. Flavonoids could inhibit lipid peroxidation by preventing the onset of cell necrosis and improving vascularity. Therefore, any compound that reduces lipid peroxidation is predicted, which might be able to enhance the viability of collagen fibers, increase blood circulation, halt the cell damage, and stimulate the DNA synthesis (Getie et al., 2002).
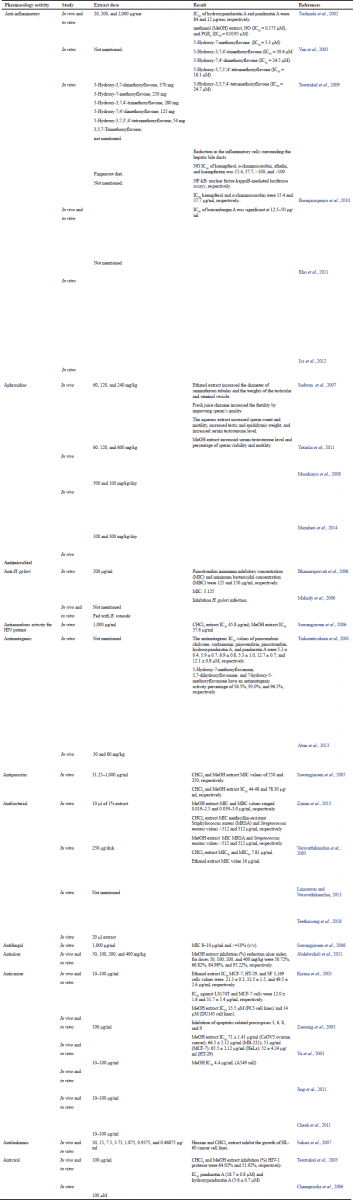 | Table 1. Pharmacology activity of B. rotunda. [Click here to view] |
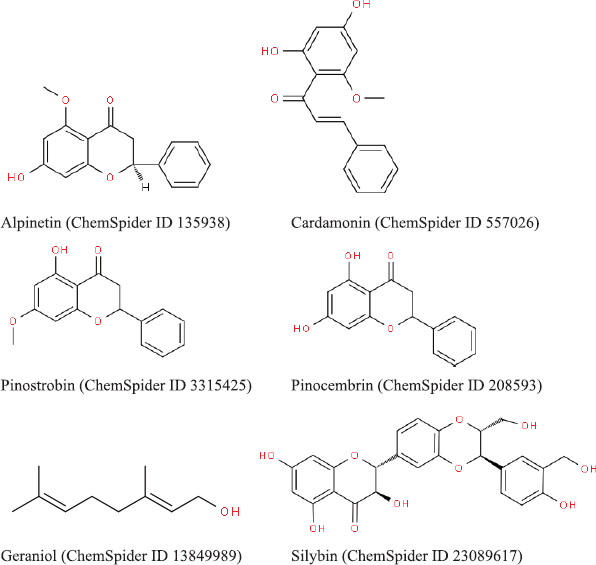 | Figure 1. 2D structure of flavonoids in B. rotunda (downloaded from http://www.chemspider.com/). [Click here to view] |
The ethanolic extract of B. rotunda rhizome could accelerate wound healing in rats (Mahmood et al., 2010). This plant extract, which contains various types of free radical scavenging molecules – for example, flavonoids and polyphenols, has exhibited antioxidant activity (Shindo et al., 2006). Antioxidants significantly play an important role in the wound-healing process and block the oxidative damage (Martin, 1996).
Boesenbergia rotunda and pinostrobin reduce ulcer inflammation
Boesenbergia rotunda has been utilized empirically to cure ulcers by the people in Thailand and Indonesia. The antiulcer activity of the methanol extract of B. rotunda and its phytoconstituent pinostrobin has been studied by Abdelwahab et al. It was reported that B. rotunda extract and pinostrobin revealed the cytoprotective effects on ulcer-induced rats. This plant extract also significantly decreased submucosal edema and leukocyte infiltration (Abdelwahab et al., 2011).
Boesenbergia rotunda and panduratin A as anticancer
Kirana et al. (2003) assayed through eleven species of Zingiberaceae and discovered that B. rotunda and Zingiber aromaticum indicated the highest inhibition toward the growth of MCF-7 breast cancer and human HT-29 colon cancer cells (Kirana et al., 2003). An additional study of panduratin A on the same cell lines has also proven similar potent inhibitory properties and a nontoxic result to the rats (Kirana et al., 2007).
B. rotunda volatile oils revealed cytotoxic activities against MCF-7 (IC50 31.7 ± 5.4 μg/ml) and LS174T cell lines (Zaeoung et al., 2005). In a separate study, Jing et al. (2011) demonstrated that B. rotunda possessed a moderate inhibitory activity against CaOV3 ovarian cancer, breast cancer malone dialdehyde-MB-231, MCF-7, HeLa cervical cancer, and HT-29 colon cancer cell growth as compared to three other Boesenbergia species: B. pulchella var. attenuate and B. armeniaca (Jing et al., 2011).
In 2006, Yun et al. demonstrated that panduratin A could prevent the growth of prostate cancer cell lines (PC3 and DU145) in a time- and dose-dependent manner. An immunofluorescence assay revealed that panduratin A activated the induction of apoptosis in both cell lines by inhibiting apoptotic-related procaspases 3, 6, 8, and 9 (Yun et al., 2006). Panduratin A also exhibited inhibitory activities against the growth of A549 human non-small cell lung cancer cells (Cheah et al., 2011).
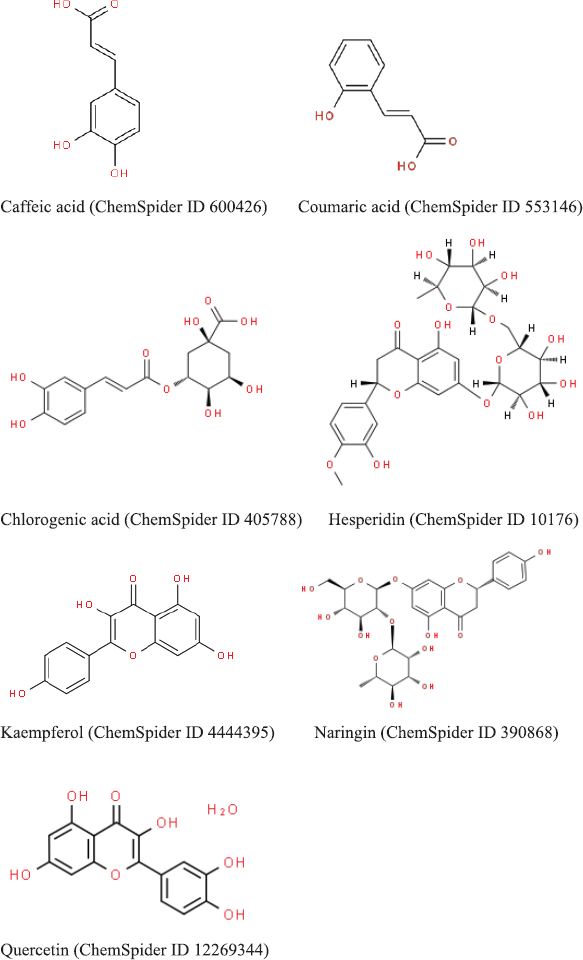 | Figure 2. 2D structure of essential oils in B. rotunda (downloaded from http://www.chemspider.com/). [Click here to view] |
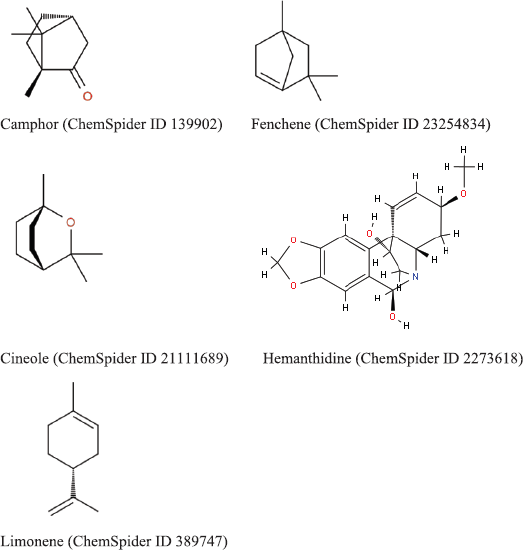 | Figure 3. 2D 2D structure of polyphenols in B. rotunda (downloaded from http://www.chemspider.com/). [Click here to view] |
The antileukemia activity of B. rotunda rhizome extracts has been investigated and revealed that the chloroform extract and boesenbergin A could inhibit the growth of HL-60 cell line (Sukari et al., 2007).
Panduratin A inhibits NF-kappaB translocation to the nucleus
Panduratin A could inhibit the translocation of NF-kappaB from the cytoplasm to nuclei (Cheah et al., 2011).
Toxicity Study
The toxicity of the B. rotunda extract was studied in normal healthy rats by exposing the animals to high doses of the rhizome extract (2 and 5 g/kg of BW) (Mahmood et al., 2010; Manosroi et al., 2017; Salama et al., 2012). An in vivo study indicated that the ethanol extract of B. rotunda was not toxic as there were no significant changes in the body weight of the rats. Moreover, all hematological and histopathological parameters did not show any adverse changes (Lim, 2016; Saraithong et al., 2010). Meanwhile, pinostrobin and pinocembrin revealed no mutagenic effect or toxicity toward Wistar rats, which confirmed the safety of these compounds (Charoensin et al., 2010).
CONCLUSION
The traditional utilities of B. rotunda rationalize that this plant could be upgraded to the next level of drug discovery study. Nonetheless, the molecular mechanism of panduratin A and 4-hydroxypanduratin A of B. rotunda has described their activity in inhibiting the production of nitric oxide and PGE2 as well as on TNF-α. Panduratin A also inhibits the translocation of NF-kappaB to the nucleus, which might contribute to this plant’s anti-inflammatory activity. Furthermore, the ethanolic extract of B. rotunda was considered not toxic as it did not alter the body weight and hematological parameters of rats.
ACKNOWLEDGMENT
The publication fee is funded by Doctoral Dissertation Grant 2019 of the Ministry of Research and Technology and Higher Education, the Republic of Indonesia (10/E1/KP.PTNBH/2019).
CONFLICTS OF INTEREST
There are no conflicts of interest related to the publication of this paper.
FUNDING
None.
REFERENCES
Abdelwahab SI, Abdul AB, Devi N, EhassanTaha MM, AlZubairi AS, Mohan S, Marion AA. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: involvement of mitochondria-regulated apoptosis. Experiment Toxicol Pathol, 2010; 62:461–9. CrossRef
Abdelwahab SI, Mohan S, Abdulla MA, Sukari MA, Abdul AB, Taha MM, Syam S, Ahmad S, Lee KH. The methanolic extract of Boesenbergia rotunda (L.) Mansf. and its major compound pinostrobin induces anti-ulcerogenic property in vivo: possible involvement of indirect antioxidant action. J Ethnopharmacol, 2011; 137(2):963–70. CrossRef
Attiq A, Jalil J, Husain K, Ahmad W. Raging the war against inflammation with natural products. Front Pharmacol, 2018; 9:976. CrossRef
Baharudin M, Hamid S, Susanti D. Chemical composition and antibacterial activity of essential oils from three aromatic plants of the Zingiberaceae family in Malaysia. J Phys Sci, 2015; 26:71–81. Available via http://web.usm.my/jps/26-1-15/26-1-7.pdf
Bhamarapravati S, Juthapruth S, Mahachai W, Mahady G. Antibacterial activity of Boesenbergia rotunda (L.). Mansf. and Myristica fragrans Houtt. against Helicobacter pylori. Songklanakarin J Sci Technol. 2006; 28(Suppl.1):157–63. Available via http://rdo.psu.ac.th/sjst/journal/28-Suppl-1/20_Antibacterial_activity.pdf
Boonjaraspinyo S, Boonmars T, Aromdee C, Kaewsamut B. Effect of fingerroot on reducing inflammatory cells in hamster infected with Opisthorchis viverrini and N-nitrosodimethylamine administration. Parasitol Res. 2010; 106(6):1485–9. CrossRef
Chahyadi A, Hartati R, Wirautisna KR, Elfahmi. Boesenbergia pandurata Roxb., An indonesian medicinal plant: phytochemistry, biological activity, plant biotechnol. Procedia Chem. 2014; 13(2014):13–37. CrossRef
Chander MP, Vinod Kumar K, Lall C, Vimal Raj R, Vijayachari P. GC/MS profiling, in vitro anti-leptospiral and haemolytic activities of Boesenbergia rotunda (L.) Mansf. used as a medicinal plant by Nicobarese of Andaman and Nicobar Islands. Nat Prod Res, 2016; 30(10):1190–2. CrossRef
Charoensin S, Punvittayagul C, Pompimon W, Mevatee U, Wongpoomchai R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J Toxicol, 2010; 25(1):29–40. Available via https://www.semanticscholar.org/ paper/Toxicological-and-clastogenic- evaluation-of-and-in-Charoensin -Punvittayagul/3dd5b3fc8fdf541e5e16e1a659fa10e0134704ad
Cheah SC, Appleton DR, Lee ST, Lam ML, Hadi AH, Mustafa MR. Panduratin A inhibits the growth of A549 cells through induction of apoptosis and inhibition of NF-kappaB translocation. Molecules, 2011; 16(3):2583–98. CrossRef
Chee CF, Abdullah I, Buckle MJC, Rahman NA. An efficient synthesis of (±)-panduratin A and (±)-isopanduratin A, inhibitors of dengue-2 viral activity. Tetrahedr Lett, 2010; 51(3):495–8. CrossRef
Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg Med Chem, 2006; 14(6):1710–14. CrossRef
Chen J, Xia NH. Pollen morphology of Chinese Curcuma L. and Boesenbergia Kuntz (Zingiberaceae): taxonomic implications. Flora, 2011; 206(5):458–67. CrossRef
Chiang M, Kurmoo Y, Khoo TJ. Chemical-and cell-based antioxidant capacity of methanolic extracts of three commonly edible plants from Zingiberaceae family. Free Radic Antioxid, 2017; 7:57–62. CrossRef
Ching A, Wah T, Sukari M, Lian G, Rahmani M, Khalid K. Characterization of flavonoid derivatives from Boesenbergia rotunda (L.) Malays. J Anal Sci, 2007; 11:154–9. Available via https://inis.iaea.org/search/searchsinglerecord.aspx? recordsFor=SingleRecord&RN=42092333
Eng-Chong T, Teck FG, Ming WS, Rahman NA, Khalid N, Karsani SA, Othman S, Yusof R. Optimization of two-dimensional gel electrophoresis protocol for Boesenbergia rotunda in vitro suspension culture. J Med Plants Res, 2011; 5:3777–80.
Eng-Chong T, Yean-Kee L, Chin-Fei C, Choon-Han H, Sher-Ming W, Li-Ping CT, Gen-Teck F, Khalid N, Rahman NA, Karsani SA, Othman S, Othman R, Yusof R. Boesenbergia rotunda: from ethnomedicine to drug discovery. Evid Based Complement Alternat Med, 2012; 2012:473637. CrossRef
Getie M, Gebre MT, Reitz R, Neubert, RH. Evaluation of the release profiles of flavonoids from topical formulations of the crude extract of the leaves of Dodonea viscosa (Sapindaceae). Pharmazie, 2002; 57:320–2. Available via https://www.ncbi.nlm.nih.gov/pubmed/12061256
Habsah M, Amran M, Mackeen MM, Lajis NH, Kikuzaki H, Nakatani N, Rahman AA, Ghafar A, Ali M. Screening of Zingiberaceae extracts for antimicrobial and antioxidant activities. J Ethnopharmacol, 2000; 72:403–10.CrossRef
Ibrahim MY, Abdul ABH, Ibrahim TAT, AbdelWahab SI, Elhassan MM, Mohan S. Attenuation of cisplatin-induced nephrotoxicity in rats using zerumbone. Afr J Biotechnol, 2010; 9:4434–41. Available via https://doi.org/10.5897/AJB10.1075
Isa N, Abdelwahab S, Mohan S, Abdul A, Sukari M, Taha M, Syam S, Narrima P, Cheah SC, Ahmad S, Mustafa MR. In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.). (fingerroot). Braz J Med Biol Res, 2012; 45:524–30. CrossRef
Jaipetch T, Reutrakul V, Tuntiwachwuttikul P, Santisuk T. Flavonoids in the black rhizomes of Boesenbergia pandurate. Phytochemistry, 1983; 22(2):625–6. CrossRef
Jing L, Mohamed M, Rahmat A, Abu BM. Phytochemicals, antioxidant properties and anticancer investigations of the different parts of several gingers species (Boesenbergia rotunda, Boesenbergia pulchella var attenuata and Boesenbergia armeniaca). J Med Plants Res, 2010; 4:27–32. CrossRef
Jing LJ, Abu Bakar MF, Mohamed M, Rahmat A. Effects of selected Boesenbergia species on the proliferation of several cancer cell lines. J Pharmacol Toxicol, 2011; 6(3):272–82. Available via http://dx.doi.org/10.3923/jpt.2011.272.282
Kiat TS, Pippen R, Yusof R, Ibrahim H, Khalid N, Rahman NA. Inhibitory activity of cyclohexenyl chalcone derivatives and flavonoids of fingerroot, Boesenbergia rotunda (L.). towards dengue-2 virus NS3 protease. Bioorg Med Chem Lett, 2006; 16:3337–40. CrossRef
Kirana C, Jones GP, Record IR, McIntosh GH. Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J Nat Med, 2007; 61(2):131–7. CrossRef
Kirana C, Record IR, McIntosh GH, Jones GP. Screening for antitumor activity of 11 species of Indonesian zingiberaceae using human MCF-7 and HT-29 cancer cells. Pharm Biol, 2003; 41(4):271–76. CrossRef
Kress WJ, Prince LM, Williams KJ. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. Am J Bot, 2002; 89(10):1682–96. CrossRef
Levita J, Istyastono EP, Nawawi A, Mutalib A, de Esch IJP, Ibrahim S. Analyzing the interaction of andrographolide and neoandrographolide, diterpenoid compounds from andrographis paniculata (Burm.F) nees, to cyclooxygenase-2 enzyme by docking simulation. ITB J Sci, 2009; 41A(2):110–9. CrossRef
Lim TK. 2016. Edible medicinal and non-medicinal plants: Vol 12, modified stems, roots, bulbs. New York, NY: Springer Internat Pub, pp. 227–8. CrossRef
Limsuwan S, Voravuthikunchai SP. Bactericidal bacteriolytic, and antibacterial virulence activitities of Boesenbergia pandurata (Roxb) schltr extract against Streptococcus pyogenes. Trop J Pharm Res, 2013;12(6):1023–8. CrossRef
Madaka F, Tewtrakul S. Anti-allergic activity of some selected plants in the genus Boesenbergia and Kaempferia. Songklanakarin J Sci Technol, 2011; 33:301–4. Available via http://rdo.psu.ac.th/sjstweb/journal/33-3/0125-3395-33-3-301-304.pdf
Mahmood AA, Mariod AA, Abdelwahab SI, Ismail S, Al-Bayaty F. Potential activity of ethanolic extract of Boesenbergia rotunda (L.) rhizomes extract in accelerating wound healing in rats. J Med Plants Res, 2010; 4:1570–6. CrossRef
Manosroi A, Tangjai T, Chankhampan C, Manosroi W, Najarut Y, Kitdamrongtham W, Manosroi J. Potent phosphodiesterase inhibition and nitric oxide release stimulation of anti-impotence thai medicinal plants from "MANOSROI III" database. Evid Based Complement Alternat Med, 2017; 2017:9806976. CrossRef
Martin A. The use of antioxidants in wound healing. Dermatol Surg, 1996; 22:156–60. CrossRef
Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol, 2004; 36(6):1031–7. CrossRef
Morakinyo A, Adeniyi O, Arikawe A. Effects of Zingiber officinale on reproductive functions in the male rat. Afr J Biomed Res, 2008; 11:329–34. CrossRef
Morikawa T, Funakoshi K, Ninomiya K, Yasuda D, Miyagawa K, Matsuda H, Yoshikawa M. Medicinal foodstuffs. XXXIV. Structures of new prenylchalcones and prenylflavanones with TNF-α and aminopeptidase n inhibitory activities from Boesenbergia rotunda. Chem Pharm Bull, 2008; 56:956–62. CrossRef
Nørregaard R, Tae-Hwan Kwon, TH, Frøkiær J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res Clin Pract, 2015; 34(4):194–200. CrossRef
Núñez V, Otero R, Barona J, Saldarriaga M, Osorio RG, Fonnegra R, Jiménez SL, Díaz A, Quintana JC. Neutralization of the edema-forming, defibrinating and coagulant effects of Bothrops asper venom by extracts of plants used by healers in Colombia. Braz J Med Biol Res, 2004; 37:969–77. CrossRef
Phukerd U, Soonwera M. Insecticidal effect of essential oil from Boesenbergia rotunda (L.) Mansf. and Curcuma zedoaria Rosc. againts dengue vector mosquito, Aedes aegypti (Linn.). J Agric Technol. 2013; 9(6):1573–83. Available via https://www.semanticscholar.org/ paper/Insecticidal-effect-of-essential-oils -from-rotunda-Phukerd-Soonwera/d6a814d873a636d8a457e6d255a2cd1a4898c928
Rho HS, Ghimeray AK, Yoo DS, Ahn SM, Kwon SS, Lee KH, Cho DH, Cho JY. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules, 2011; 16(4):3338–44. CrossRef
Rosdianto AM, Puspitasari IM, Lesmana R, Levita J. Determination of Quercetin and Flavonol Synthase in Boesenbergia rotunda Rhizome. Pak J Biol Sci, 2020; 23(3):264–70. CrossRef
Salama SM, Abdulla MA, Alrashdi AS, Hadi AH. Mechanism of Hepatoprotective Effect of Boesenbergia rotunda in Thioacetamide-Induced Liver Damage in Rats. Evid Based Complement Alternat Med, 2013; 2013:157456. CrossRef
Salama SM, Bilgen M, Al Rashdi AS, Abdulla MA. Efficacy of Boesenbergia rotunda treatment against thioacetamide-induced liver cirrhosis in a rat model. Evid Based Complement Alternat Med, 2012; 2012:137083. CrossRef
Saraithong P, Saenphet S, Saenphet K. Safety evaluation of ethanol extracts from Boesenbergia rotunda (L.) Mansf. in male rats. Trends Res Sci Technol, 2010; 2(1):19–22. Available via https://www.researchgate.net/publication/216040189_ Safety_Evaluation_of_Ethanol_Extracts_from_Bosenbergia_rotunda_L_Mansf_in_Male_Rats
Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, Srisuwan N, Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitol Res, 2006; 98(6):588–92. CrossRef
Shindo K, Kato M, Kinoshita A, Kobayashi A, Koike Y. Analysis of antioxidant activities contained in the Boesenbergia rotunda Schult. Rhizome. Biosci Biotechnol Biochem, 2006; 70:2281–4. Available via https://doi.org/10.1271/bbb.60086 CrossRef
Sudwan P, Saenphet K, Aritajat S, Sitasuwan N. Effects of Boesenbergia rotunda (L.) Mansf. on sexual behaviour of male rats. Asian J Androl, 2007; 9(6):849–55. CrossRef
Sugita R, Kuwabara H, Sugimoto K, Kubota K, Imamura Y, Kiho T, Tengeiji A, Kawakami K, Shimada K. A novel selective prostaglandin E2 synthesis inhibitor relieves pyrexia and chronic inflammation in rats. Inflammation, 2016; 39(2):907–15. CrossRef
Sukari MA, Ching AYL, Lian GEC, Rahmani M, Khalid K. Cytotoxic constituents from Boesenbergia pandurata (Roxb.) Schltr. Nat Prod Sci. 2007; 13(2):110–13. Available via http://kpubs.org/article/articleMain.kpubs?articleANo=E1HSBY_2007_v13n2_110
Taha MME, Abdul AB, Abdullah R, Tengku Ibrahim TA, Abdelwahab SI, Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet). Chemico-Biol Interact, 2010; 186:295–305. CrossRef
Techaprasan J, Klinbunga S, Jenjittikul T. Genetic relationships and species authentication of Boesenbergia (Zingiberaceae) in Thailand based on AFLP and SSCP analyses. Biochem Syst Ecol, 2008; 36(5–6):408–16. CrossRef
Techaprasan J, Ngamriabsakul C, Klinbunga S, Chusacultanachai S, Jenjittikul T. Genetic variation and species identification of Thai Boesenbergia (Zingiberaceae) analyzed by chloroplast DNA polymorphism. J Biochem Mol Biol, 2006; 39(4):361–70. CrossRef
Teethaisong Y, Pimchan T, Srisawat R, Hobbs G, Eumkeb G. Boesenbergia rotunda (L.) Mansf. extract potentiates the antibacterial activity of some β-lactams against β-lactam-resistant staphylococci. J Glob Antimicrob Resist, 2018; 12:207–13. CrossRef
Tewtrakul S, Subhadhirasakul S, Kummee S. HIV-1 protease inhibitory effects of medicinal plants used as self medication by AIDS patients. Songklanakarin J Sci Technol, 2003; 25(2):239–43. CrossRef
Tewtrakul S, Subhadhirasakul S, Karalai C, Ponglimanont C, Cheenpracha S. Anti-inflammatory effects of compounds from Kaempferia parviflora and Boesenbergia pandurata. Food Chem, 2009; 115(2):534–538. Available via http://dx.doi.org/10.1016/j.foodchem.2008.12.057
Trakoontivakorn G, Nakahara K, Shinmoto H, Takenaka M, Onishi-Kameyama M, Ono H, Yoshida M, Nagata T, Tsushida T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J Agric Food Chem, 2001; 49(6):3046–50. CrossRef
Tuchinda P, Reutrakul V, Claeson P, Pongprayoon U, Sematong T, Santisuk T, Taylor WC. Anti-inflammatory cyclohexenyl chalcone derivatives in Boesenbergia pandurata. Phytochem, 2002; 59(2):169–73. CrossRef
Udomthanadech K, Vajrodaya S, Paisooksantivatana Y. Antibacterial properties of the extracts from some Zingibereous species in Thailand against bacteria causing diarrhea and food poisoning in human. Int Trans J Eng Manage Appl Sci Technol, 2015; 6:203–13.
Vanijajiva O, Sirirugsa P, Suvachittanont W. Confirmation of relationships among Boesenbergia (Zingiberaceae) and related genera by RAPD. Biochem Syst Ecol. 2005; 33(2):159–70. CrossRef
Voravuthikunchai SP, Phongpaichit S, Subhadirasakul S. Evaluation of antibacterial activity of medicinal plants widely used among AIDS patients in Thailand. Pharm Biol, 2005; 43:701–6. CrossRef
Ward PA, Lentsch AB. The acute inflammatory response and its regulation. Arch Surg, 1999; 134(6):666–9. CrossRef
Wong V, Lerner E. Nitric oxide inhibition strategies. Future Sci OA, 2015; 1(1):FSO35. CrossRef
Wu N, Kong Y, Zu Y, Fu Y, Liu Z, Meng R, Liu X, Efferth T. Activity investigation of pinostrobin towards herpes simplex virus-1 as determined by atomic force microscopy. Phytomedicine, 2011; 18:110–8. CrossRef
Yu JM, Kwon H, Hwang JK. In vitro anti-inflammatory activity of panduratin A isolated from Kaempferia pandurata in RAW264.7 cells. Planta Med, 2003; 69:1102–8. CrossRef
Yun JM, Kwaeon MH, Kwon H, Hwang JK, Mukhtar H. Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis, 2006; 27(7):1455–64. CrossRef
Yusuf N, Annuar M, Khalid N. Existence of bioactive flavonoids in rhizomes and plant cell cultures of Boesenbergia rotunda (L.). Mansf. Kulturpfl. Aust J Crop Sci, 2013; 7:730–4. Available via https://www.researchgate.net/publication/288138724_ Existence_of_bioactive_ flavonoids_in_rhizomes_and_plant_cell_cultures_ of_boesenbergia_rotunda_L_Mansf_Kulturpfl
Zaeoung S, Plubrukarn A, Keawpradub N. Cytotoxic and free radical scavenging activities of Zingiberaceous rhizomes. Songklanakarin J Sci Technol, 2005; 27(4):799–812. Available via http://rdo.psu.ac.th/sjstweb/journal/27-4/11-Zingiberaceous-rhizomes.pdf
Zainin N, Lau K, Zakaria M, Son R, Abdull RA, Rukayadi Y. Antibacterial activity of Boesenbergia rotunda (L.). Mansf. A. extract against Escherichia coli. Internat Food Res J, 2013; 20:3319–23. Available via http://www.ifrj.upm.edu.my/ 20%20(06)%202013/50%20I FRJ%2020%20(06)%202013%20Rohman%20Yaya%20422.pdf