INTRODUCTION
Infectious diseases remain one of the top public health threats worldwide. In 2019, infectious diseases caused 13.7 million deaths in the world [1]. The leading causes of death in countries with lower and middle incomes, according to the WHO, include respiratory injuries, tuberculosis, and diarrhea [2]. There are many kinds of infectious diseases and some of them are caused by bacteria, such as tuberculosis, typhoid, pneumonia, cholera, and gonorrhea. With an increase in microbial drug resistance and a lack of novel antibacterial drugs being developed, bacterial infection is on the rise [3].
The search for new antibacterial to overcome infections and resistance problems has been a top priority for the pharmaceutical industry and academia. Plant secondary metabolites are considered as a potential source of new antibacterial agents [4]. It is estimated that 500–800 different secondary metabolites are contained in each plant species. Plant accumulates secondary metabolite with high antibacterial activities, such as alkaloids, coumarins, isoflavonoids, quinones, tannins, and terpenes. It is known that plant secondary metabolites can affect microbial cells through several mechanisms, such as disrupting cell membranes, interrupting bacterial transcription and replication, and inhibiting cell division [5,6]. Based on this knowledge, the search for antibacterial agents from plant sources is worth conducting.
Medicinal plants such as Physalis angulata, Loranthus parasiticus, Plectranthus scutellarioides, Cyperus rotundus, and Terminalia catappa have been used as traditional medicine in Indonesia [7,8]. They possess very diverse ethnopharmacological uses. Physalis angulata is used as a traditional folk remedy and has been empirically used to treat rheumatic diseases, hepatitis, cervical cancer, and mouth and throat inflammatory conditions. Alkaloids, physalins, angulatins, withangulatin A, and withaferin A were known present in P. angulata [9,10]. While L. parasiticus has been used for centuries as a traditional remedy. Bioactive components of this plant mainly belong to triterpenes, biscotoxins, lectins, sesquiterpenes lactones, and flavonoid, which is the most important phenolic compounds [11,12]. Plectranthus scutellariosides are also widely used as a traditional medicine in Indonesia. It is used to treat stomach pain, diarrhea, hemorrhoids, and skin conditions [13,14]. Research on P. scutellarioides has resulted in the separation of flavonoid glycosides, caffeic acid, and abietane or labdane-type diterpenoids. β-sitosterol and stigmasterol, which belong to steroid compounds are also present in this plant [15–19]. Cyperus rotundus, a nut grass that belongs to the family Cyperaceae, is used to treat convulsions, amenorrhea, bronchitis, dysentery, leprosy, diarrhea, and gastric disorders in tropical and sub-tropical countries. This plant contains many secondary metabolites such as terpenoids including sesquiterpenes, alkaloids, fatty acids, steroids, saponins, and phenolic compounds including flavonoids [20]. Terminalia catappa (Combretaceae) is often found and planted in Asia, especially in India. This plant has multipharmacological purposes such as dressing for rheumatic joints and treatment of dermatitis, scabies, and leprosy. Previous reports stated that T. catappa contains flavonoid glycosides, tannin including punicalagin and punicalin, as well as terpenoids and coumarin [21,22].
As part of our ongoing effort to identify natural antimicrobials, we conducted research on the antibacterial properties of these five plants, as well as the isolation of bioactive constituents from a selected plant. The secondary metabolites present in these plants such as alkaloids, terpenes, tannins, saponins, flavonoids, and phenolics have been shown to have antimicrobial activity [23]. The antibacterial activity of the plants, as well as the isolated compounds, was assessed against three pathogenic bacteria, namely Bacillus subtilis, Staphylococcus aureus, and Escherichia coli.
MATERIALS AND METHODS
Materials
Five species of medicinal plants, namely P. angulata fruits, the aerial parts of L. parasiticus, P. scutellarioides leaves, C. rotundus rhizome, and T. catappa leaves were obtained from Yogyakarta, Indonesia. The authentication of all plants was determined by D. Santosa (Faculty of Pharmacy, Gadjah Mada University, Indonesia).
Antibacterial evaluation of plant extracts
Five species of Indonesian medicinal plants were extracted with methanol and chloroform (1:5) using the ultrasonication technique. These extracts were then tested to determine the antibacterial activities using standard microdilution-3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test against B. subtilis NBRC 13719 and S. aureus NBRC 100910, which are Gram-positive bacteria, as well as E. coli NBRC 102203, a Gram-negative bacteria [24]. Yeast Polypeptone (YP) was used as a medium for bacterial culture which is comprised of 0.2% yeast extract (BD Difco™, USA), 1% polypeptone (Nihon Pharmaceutical Co., Ltd., Japan), agar (2%), and 0.1% hydrous magnesium sulfate (Nacalai Tesque Inc., Japan). Initially, bacteria were inoculated on YP agar plates under 37°C overnight incubation. Bacterial strains were then transferred to grow in liquid YP medium (without agar) and incubated at 37°C under shaking conditions for 12 hours. A stock sample solution (medicinal plant extracts) was prepared in dimethyl sulfoxide (DMSO) with a concentration of 10 mg/ml. Liquid YP medium and bacteria were put in 96-well plates and a sample solution was applied. Medium-contained microbial strains and samples were then further diluted into varying concentrations (200 and 100 μg/ml). The positive controls, ampicillin, and kanamycin (Nacalai Tesque) were also treated as the extract and finally, the plate was incubated at a temperature of 37°C overnight. 50 μl of MTT (Sigma-Aldrich, USA) solution (5 mg/ml in isopropanol-Cl) were added into each well and then incubated for 1 hour. The experiment was conducted in triplicates. The extract that showed inhibition of bacterial colony growth which could be observed visually (clear yellow color) both at concentrations of 200 and 100 mg/ml was selected for further isolation.
Antibacterial evaluation of isolated compounds
The antibacterial activity of compounds isolated from a selected plant, P. scutellarioides, was performed using the same method as the plant extracts. The sample stock solution in DMSO (5 mM) was first prepared and then diluted to various concentrations (200, 100, 80, 60, 50, 25, and 12.5 μM) with YP medium in 96-well plates containing bacterial culture. After incubation at 37°C overnight, the minimum inhibitory concentration (MIC) values were visually observed by the addition of 50 μl of MTT solution into each well, followed by 1 hour incubation at 37°C. The experiment was performed in triplicates. After the incubation, the result was observed by the unaided eye. The MIC of isolated compounds is defined as the lowest concentration of the compounds that completely inhibits the growth of the bacteria (clear yellow color).
Extraction and isolation of compounds from P. scutellarioides
Extraction of P. scutellarioides was performed using powdered leaves (300 g) with CHCl3 as a solvent. The sample was macerated under sonication for 1.5 hours at room temperature using 2 l of solvents and then remacerated two times. The filtrate was collected and evaporated by a vacuum rotary evaporator to yield CHCl3 extract (25.2 g). The extract was fractionated using normal phase medium pressure liquid chromatography (Büchi Labortechnik, Switzerland) with silica gel as stationary phase (100 × 460 mm sample column; 40–50 μm and 1.85 kg silica; flow rate = 30 ml/minute), and mixtures of n-hexane–ethyl acetate (EtOAc) from 1:0 to 0:1 as eluents. A total of 18 fractions were collected from the process. Fraction 11 (2,470 mg) was separated using reversed-phase column chromatography (Cosmosil 75C18-OPN, Nacalai Tesque Inc., Japan) and H2O–methanol (MeOH) (1:1) as eluent resulting in four subfractions (F11-1: 180 mg; F11-2: 83 mg; F11-3: 450 mg; F11-4: 812 mg). Purification of sub-fraction F11-1 by column chromatography with n-hexane:EtOAc (2:1) as solvent system followed by reversed-phase preparative Thin Layer Chromatography (TLC) (RP-18F254 plates, Merck, Germany, eluents CH3CN:H2O 7:3) afforded known compound 1 (4.8 mg). Further purification of sub-fraction F11-2 using normal phase open column chromatography and solvent system of n-hexane–EtOAc (2:1) yielded a known compound 2 (2.1 mg). Subsequently, Fr. 13 was separated by reversed-phase column chromatography to afford several sub-fractions. Purification of sub-fraction F13-3 by semipreparative High Performance Liquid Chromatography (HPLC) (Agilent 1260 Infinity series G1311B) with CH3CN–H2O (45:55) furnished known compound 3 (2.2 mg; flow rate = 2 ml/minute, Rt 35 minutes). The structure of all isolated compounds was analyzed and determined by 1D/2D NMR (Varian UNITY 600 spectrometer; 1H NMR for 600 MHz, 13C NMR for 150 MHz, and JEOL JNM-ECA500II; 1H NMR for 500 MHz, 13C NMR for 125 MHz) and MS spectra (JEOL MStation JMS-700 High-Resolution Electron Impact Mass Spectrometer and Waters SYNAPT G2-Si HDMS High-Resolution Electron Spray Ionization Mass Spectrometer), then cross-referenced with previously published data.
RESULTS AND DISCUSSION
Antibacterial activity of five Indonesian medicinal plants
Antibacterial activity screening of five species of Indonesian medicinal plants was performed by using microdilution-MTT assay against B. subtilis, S. aureus, and E. coli (Table 1). According to the screening result, the chloroform extract of P. angulata’s fruit exerted antibacterial activity against B. subtilis (MIC 200 μg/ml). The methanol extract of T. catappa’s leaves showed antibacterial activity against S. aureus (MIC 200 μg/ml). The chloroform extract of P. scutellarioides’ leaves showed antibacterial activities against B. subtilis (MIC 200 μg/ml) and S. aureus (MIC 100 μg/ml). Hence, P. scutellarioides leaf extract was chosen to be further investigated.
Plectranthus scutellarioides (synonym: Coleus scutellarioides, Coleus blumei, Coleus atropurpureus) is an ornamental plant belonging to the Lamiaceae family. This plant is widely distributed in Indonesia, the Philippines, India, China, and Australia [25]. Previous research on the biological properties of the isolated compounds from P. scutellarioides showed the potential for antibacterial, anti-inflammatory, and antiproliferative properties as well as antioxidant properties [15,26–28]. The result of this study corresponds to the study by Bismelah et al. [29] which reported that P. scutellarioides extract showed antibacterial activity against some bacteria strains, including S. aureus and B. subtilis [29]. The extract of P. scutellarioides can disrupt the bacteria’s cell wall, leading to cell death.
Isolation of compounds from P. scutellarioides
The CHCl3 extract of P. scutellarioides was subjected to fractionation and purification to obtain known compounds 1, 2, and 3 (Fig. 1).
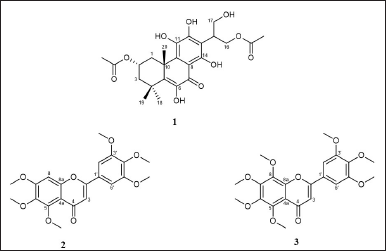 | Figure 1. Structure of compounds isolated from P. scutellarioides. [Click here to view] |
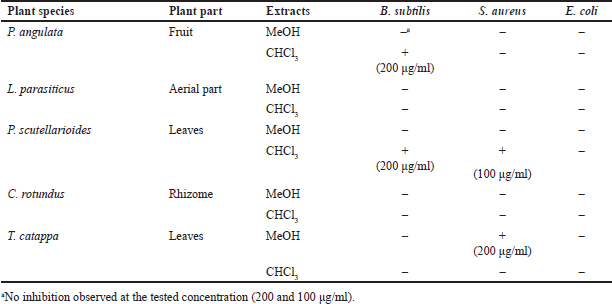 | Table 1. Result of antibacterial activity screening of Indonesian medicinal plants. [Click here to view] |
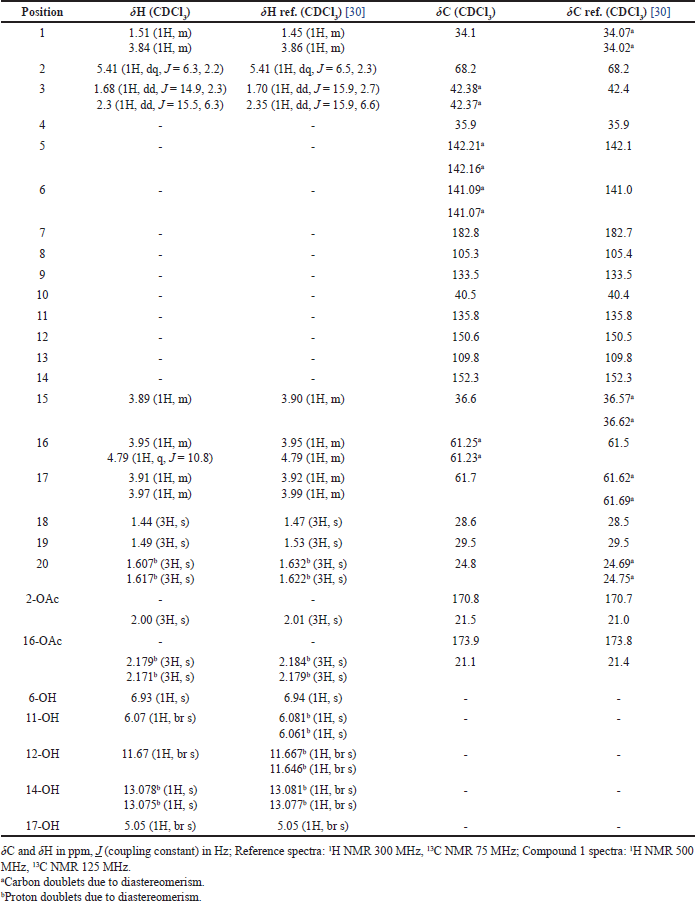 | Table 2. Comparison of NMR spectra of compound 1 and reference. [Click here to view] |
Compound 1 was isolated as yellow amorphous, and from the ESIMS analysis (m/z 478 [M]+), the molecular formula of compound 1 was determined to be C24H30O10 in accordance with NMR data. The 1H NMR analysis (500 MHz, CDCl3) revealed the signals for two aliphatic methylene groups [δH 3.84 (1H, m, H-1a), 1.51 (1H, m, H-1b), 2.31 (1H, dd, J = 15.5, 6.3 Hz, H-3a), and 1.68 (1H, dd, J = 14.9, 2.3 Hz, H-3b)]; one oxygenated methine proton [δH 5.41 (1H, dq, J = 6.3, 2.2 Hz, H-2)], two oxygenated methylene groups [δH 4.79 (1H, q, J = 10.8 Hz, H-16a), 3.95 (1H, m, H-16b), 3.97 (1H, m, H-17a), and 3.91 (1H, m, H-17b)]; five hydroxyl groups [δH 6.93 (1H, s, 6-OH), 6.07 (1H, br s, 11-OH), 11.67 (1H, br s, 12-OH), δH 13.07 (1H, s, 14-OH), and 5.05 (1H, br s, 17-OH)]; methine proton [δH 3.89 (1H, m, H-15)], and five singlet methyl protons [δH 2.00 (3H, s, 2-OCOCH3), 2.17 (3H, s, 16-OCOCH3), 1.44 (3H, s, H-18), 1.49 (3H, s, H-19), and 1.61 (3H, s, H-20)]. Several proton signals at H-20, 14-OH, and 16-OCOCH3 appeared as “doublets” with the difference of chemical shift not more than 0.01 ppm, but in fact, they were overlapping singlets. This data suggested the possibility that the compounds were a mixture of diastereomers.
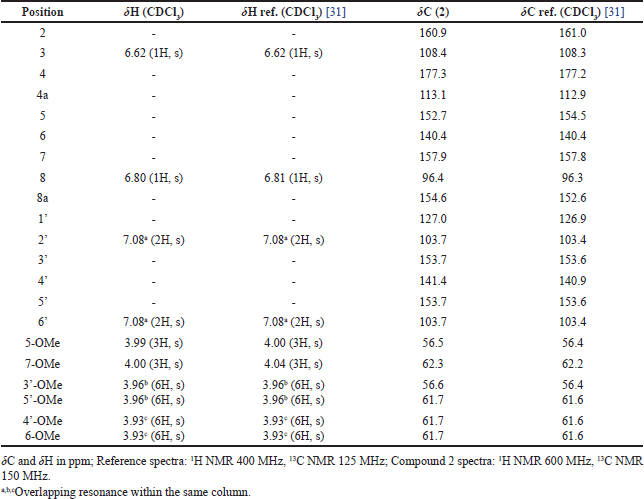 | Table 3. Comparison of NMR spectra of compound 2 and reference. [Click here to view] |
The 13C NMR (125 MHz, CDCl3) indicated 24 signals including 2 methylenes [δC 34.1 (C-1), 42.3 (C-3)], 2 oxygenated methylenes [δC 61.2 (C-16), 61.7 (C-17)], methine carbon [δC 36.6 (C-15)], oxygenated methine carbon [δC 68.2 (C-2)], 2 quaternary carbons [δC 35.9 (C-4), 40.5 (C-10)], 5 methyl carbons [δC 29.5 (C-19), 28.6 (C-18), 24.8 (C-20), 21.5 (2-OCOCH3), 21.1 (16-OCOCH3)], 8 olefinic carbons [δC 142.2 (C-5), 141.1 (C-6), 105.3 (C-8), 133.5 (C-9), 135.8 (C-11), 150.6 (C-12), 109.8 (C-13), 152.3 (C-14)], ketone carbonyl [δC 182.8 (C-7)], and 2 ester carbonyls [δC 173.9 (16-OCOCH3), 170.8 (2-OCOCH3)]. Similar to the proton signals, the carbon signals of those at C-2, C-5, C-6, and C-16 appeared as “doublets” with the difference of chemical shift not more than 0.02 ppm, rather than singlets. The 1H and 13C NMR confirmed the possibility of the diastereomeric mixture presence of compound 1. From NMR and ESIMS analysis, it is concluded that compound 1 is an abietane-type diterpene, identical to those of 2,16-diacetoxy-6,11,12,14,17-pentahydroxy-abieta-5,8,11,13-tetraene-7-one, published in previous literature (Table 2) [30]. The previous finding by Ragasa et al. [30] led to the conclusion that this compound was a 1:1 mixture of diastereomers and it was proposed to be diastereomeric at C-15.
Compound 2 was obtained as a pale-yellow amorphous solid. Compound 2 was analyzed with ESIMS and showed m/z 403 [M+H]+, consistent with the molecular formula C21H22O8. The 1H and 13C NMR data of 2 were nearly similar to those of reference (Table 3) [31]. Thus, compound 2 was determined to be 5,6,7,3′,4′,5′-hexamethoxy flavone.
Compound 3 was isolated as a yellow amorphous solid. The molecular formula was established as C22H24O9 based on its EIMS peak at m/z 432 [M]+. The 1H NMR and 13C NMR data of 3 showed similarity to compound 2 with the addition of one methoxy group. Moreover, the 1H NMR data of 3 are similar to those of Rumbero et al. [32] (Table 4). Thus, compound 3 was determined to be 5,6,7,8,3′,4′,5′-heptamethoxy flavone (5′ -methoxynobiletin). Compounds 2 and 3 are polymethoxyflavones, and as far as our knowledge they were first isolated from P. scutellarioides.
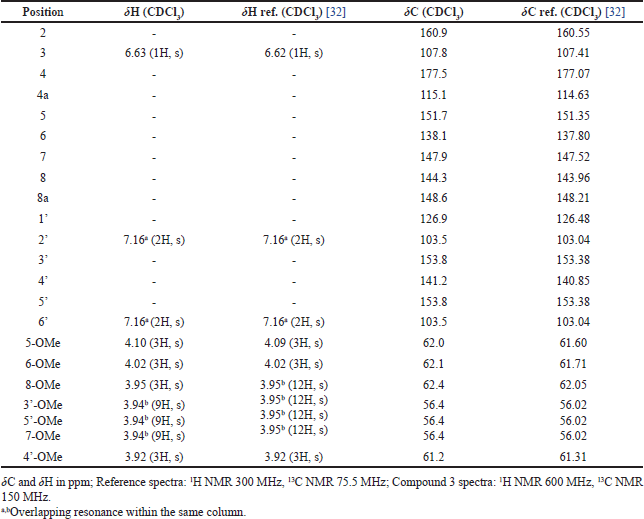 | Table 4. Comparison of NMR spectra of compound 3 and reference. [Click here to view] |
Antibacterial activity of extracts and isolated compounds
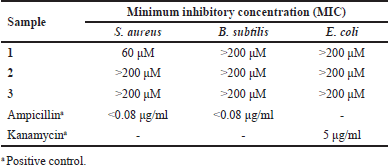
The isolated compounds from P. scutellarioides were tested for their antibacterial activities against Gram-positive bacteria S. aureus and B. subtilis, as well as Gram-negative bacteria, E. coli (Table 5).
The results of the antibacterial investigation suggested that compound 1, which belongs to acylhydroquinone abietanoids, was found to be selectively active against S. aureus with a MIC value of 60 μM. This result corresponds to the previous studies on Coleon U, an acylhydroquinone isolated from P. grandidentatus and P. forsteri that displays potent antibacterial activities against S. aureus and other bacteria strains [33,34]. It has been proposed that the potent activities of the acylhydroquinone abietanoids are due to the presence of oxygenated functions in the chromophoric system on the B and C rings. A number of studies suggested that these specific characteristics were linked to the compound’s capacity to cross or degrade bacterial cell membranes [35,36]. In contrast, compound 1 was inactive against B. subtilis and E. coli.
Compounds 2 and 3 were inactive against all tested bacteria, S. aureus, B. subtilis, and E. coli (MIC > 200 μM). Previous research on the antibacterial activity of polymethoxy flavones mainly discussed the activity of those compounds on Gram-negative bacteria. Yao et al. [37] stated that polymethoxy flavones such as nobiletin and tangeretin were weak/inactive against Pseudomonas fluorescens and Pseudomonas aeruginosa. Other research showed that polymethoxylated flavones such as tangeritin and nobiletin are less active than flavanones in inhibiting the growth of Helicobactor pylori [38]. According to Shamsudin et al. [39], methoxylation at C3’ and C5 on flavonoid structure could decrease its antibacterial action [39]. This is due to the lipophilicity of polymethoxylated flavones that cause minimal or no antibacterial activities [40].
 | Table 5. Antibacterial activities of isolated compounds from P. scutellarioides. [Click here to view] |
CONCLUSION
This research unfolds the antibacterial properties of P. scutellarioides extract and the isolated compounds. Among five Indonesian medicinal plants that are tested for antibacterial activity, P. scutellarioides extract exerted the most effective antibacterial activity against Gram-positive bacteria, B. subtilis (MIC 200 μg/ml), and S. aureus (MIC 100 μg/ml). An abietane diterpene was isolated, as well as two polymethoxy flavones which were recognized to be first isolated from this plant. The abietane diterpene compound possessed potential antibacterial activity against S. aureus (MIC 60 μM) but was inactive against B. subtilis and E. coli. Whereas two polymethoxy flavones were inactive against tested bacteria (S. aureus, B. subtilis, and E. coli) due to their lipophilicity. Further research regarding the antibacterial activity mechanisms of the abietane diterpene compound was needed to provide an alternative therapy for antibacterial infection.
ACKNOWLEDGMENTS
The authors thank D. Santosa (Faculty of Pharmacy, Gadjah Mada University, Indonesia) for authentication of the plant material. The authors also thank Y. Okamoto (Tokushima Bunri University, Tokushima, Japan) for NMR spectra recording and Mass Spectroscopy measurement.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (22H02777 to H.M.).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ikuta KS, Swetschinski LR, Robles Aguilar G, Sharara F, Mestrovic T, Gray AP, et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022 Dec;400(10369):2221–48.
2. WHO. Noncommunicable diseases progress monitor 2020. Geneva, Switzerland: WHO; 2020.
3. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(07): 309–18. CrossRef
4. Gorlenko CL, Kiselev HY, Budanova EV, Zamyatnin AA, Ikryannikova LN. Plant secondary metabolites in the battle of drugs and drug-resistant bacteria: new heroes or worse clones of antibiotics? Antibiotics. 2020 Apr 10;9(4):170. CrossRef
5. Gupta PD, Birdi TJ. Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med. 2017 Oct–Dec;8(4):266–75. CrossRef
6. Radulovi NS, Blagojevi PD, Stojanovi-Radi ZZ, Stojanovi NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem. 2013;20:932–52. CrossRef
7. Silalahi M, Nisyawati, Pandiangan D. Medicinal plants used by the Batak Toba Tribe in Peadundung Village, North Sumatra, Indonesia. Biodiversitas. 2019;20(2):510–25. CrossRef
8. Fathir A, Haikal M, Wahyudi D. Ethnobotanical study of medicinal plants used for maintaining stamina in Madura Ethnic, East Java, Indonesia. Biodiversitas. 2021;22(1):386–92. CrossRef
9. Sharma V, Sharma N, Bano A, Dhaliwal HS. A pharmacological comprehensive review on a pharmacological comprehensive review on “Rassbhary” Physalis angulata (L.) Int J Pharm Pharm Sci. 2015;7(8):30–4.
10. Vieceli PS, Juiz PJL, Lauria PSS, Couto RD, Tomassini TCB, Ribeiro IM, et al. Physalis angulata reduces the progression of chronic experimental periodontitis by immunomodulatory mechanisms. J Ethnopharmacol. 2021 Jun;273:113986. CrossRef
11. Park MJ, Park JE, Han JS. Inhibitory effects of Loranthus parasiticus extract on carbohydrate digestive enzymes and postprandial hyperglycemia. J Life Sci. 2020;30(1):18–25.
12. Moghadamtousi SZ, Kamarudin MNA, Chan CK, Goh BH, Kadir HA. Phytochemistry and biology of Loranthus parasiticus Merr, a commonly used herbal medicine. Am J Chin Med. 2014;42(1):23–35. CrossRef
13. Quattrocchi U. CRC world dictionary of medicinal and poisonous plants. Boca Raton, FL: CRC Press; 2016. CrossRef
14. Gharge S, Hiremath SI, Kagawad P, Jivaje K, Palled MS, Suryawanshi SS. Curcuma zedoaria Rosc (Zingiberaceae): a review on its chemical, pharmacological and biological activities. Futur J Pharm Sci. 2021 Aug 23;7(1):166. CrossRef
15. Cretton S, Saraux N, Monteillier A, Righi D, Marcourt L, Genta-Jouve G, et al. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry. 2018 Oct;154:39–46. CrossRef
16. Astuti AD, Yasir B, Rahim A, Natzir R, Subehan, Nakagawa-Goto K, et al. Isolation and characterization of stigmasterol and β-sitosterol from Plectranthus scutellarioides var. color blaze dark star and cytotoxicity of its fraction. Egypt J Chem. 2022 Mar 1;65(3):255–60.
17. Ito T, Rakainsa SK, Nisa K, Morita H. Three new abietane-type diterpenoids from the leaves of Indonesian Plectranthus scutellarioides. Fitoterapia. 2018 Jun 1;127:146–50. CrossRef
18. Kubínová R, Gazdová M, Hanáková Z, Jurkaninová S, Dall’Acqua S, Cva?ka J, et al. New diterpenoid glucoside and flavonoids from Plectranthus scutellarioides (L.) R. Br. S Afr J Bot. 2019 Jan 1;120:286–90. CrossRef
19. Lukhoba CW, Simmonds MSJ, Paton AJ. Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol. 2006 Jan;103(1):1–24. CrossRef
20. Pirzada AM, Ali HH, Naeem M, Latif M, Bukhari AH, Tanveer A. Cyperus rotundus L.: traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol. 2015;174:540–60. CrossRef
21. Anand AV, Divya N, Kotti PP. An updated review of Terminalia catappa. Pharmacogn Rev. 2015;9:93–8. CrossRef
22. Venkatalakshmi P, Vadivel V, Brindha P. Phytopharmacological significance of Terminalia catappa L.: an updated review. Int J Res Ayurveda Pharm. 2016 May 5;7(2):130–7. CrossRef
23. Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in middle Eastern Plants. Front Microbiol. 2019;10:911. CrossRef
24. Malekinejad H, Bazargani-Gilani B, Tukmechi A, Ebrahimi H. A cytotoxicity and comparative antibacterial study on the effect of Zataria multiflora Boiss, Trachyspermum copticum essential oils, and enrofloxacin on Aeromonas hydrophila. Avicenna J Phytomed. 2012;2(4):188–95.
25. Hanelt P, Buttner R, Mansfeld R. Mansfeld’s encyclopedia of agricultural and horticultural crops (except ornamentals). New York, NY: Springer; 2001. CrossRef
26. Levita J, Sumiwi A, Pratiwi TI, Ilham E, Sidiq SP, Moektiwardoyo M. Pharmacological activities of Plectranthus scutellarioides (L.) R.Br. leaves extract on cyclooxygenase and xanthine oxidase enzymes. J Med Plants Res. 2016;10(20):261–9.
27. Astuti AD, Yasir B, Subehan, Alam G. Comparison of two varieties of Plectranthus scutellarioides based on extraction method, phytochemical compound, and cytotoxicity. J Phys Conf Ser. 2019;1341:072012. CrossRef
28. Wardojo MM, Sumiwi A, Iskandar Y, Novinda D, Mustarichie R. Antioxidant aactivity and phytochemical screening of Plectranthus scutellarioides L. leaves ethanol and water extracts by DPPH method. Res J Pharm Biol Chem Sci. 2018;9(1):955–61.
29. Bismelah NA, Ahmad R, Mohamed Kassim ZH, Ismail NH. Coleus blumei extract as a potential antibacterial oral rinse. IOP Conf Ser Earth Environ Sci. 2019;269:012015. CrossRef
30. Ragasa CY, Templora VF, Rideout JA. Diastereomeric diterpenes from Coleus blumei. Chem Pharm Bull. 2001;49(7):927—9. CrossRef
31. Passador EAP, Da Silva FDGF, Fo ER, Fernandes JB, Vieira PC, Pirani JR. A pyrano chalcone and a flavanone from Neoraputia magnifica. Phytochemistry. 1997;45(7):1533–7. CrossRef
32. Rumbero A, Arriaga-Giner FJ, Wollenweber E. A new oxyprenyl coumarin and highly methylated flavones from the exudate of Ozothamnus lycopodioides (Asteraceae). Z Naturforsch C J Biosci. 2000 Jan–Feb;55(1–2):1–4. CrossRef
33. Gaspar-Marques C, Rijo P, Simões MF, Duarte MA, Rodriguez B. Abietanes from Plectranthus grandidentatus and P. hereroensis against methicillin- and vancomycin-resistant bacteria. Phytomedicine. 2006 Mar 13;13(4):267–71. CrossRef
34. Wellsow J, Grayer RJ, Veitch NC, Kokubun T, Lelli R, Kite GC, et al. Insect-antifeedant and antibacterial activity of diterpenoids from species of Plectranthus. Phytochemistry. 2006 Aug;67(16):1818–25. CrossRef
35. Urzúa A, Rezende MC, Mascayano C, Vásquez L. A structure-activity study of antibacterial diterpenoids. Molecules. 2008;13:882–91. CrossRef
36. Rijo P, Faustino C, Fátima Simões M. Antimicrobial natural products from Plectranthus plants. In: Mendez-Vilas A, ed. Microbial pathogens and strategies for combating them: science, technology and education. Badajoz, Spain: Formatex Research Center; 2013. vol. 2, pp 922–31.
37. Yao X, Zhu X, Pan S, Fang Y, Jiang F, Phillips GO, et al. Antimicrobial activity of nobiletin and tangeretin against Pseudomonas. Food Chem. 2012 Jun 15;132(4):1883–90. CrossRef
38. Yi ZB, Yu Y, Liang YZ, Zeng B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium Citri reticulatae of a new citrus cultivar and its main flavonoids. LWT. 2008;41(4):597–603. CrossRef
39. Shamsudin NF, Ahmed QU, Mahmood S, Shah SAA, Khatib A, Mukhtar S, et al. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules. 2022;27:1149. CrossRef
40. Omosa LK, Midiwo JO, Mbaveng AT, Tankeo SB, Seukep JA, Voukeng IK, et al. Antibacterial activities and structure–activity relationships of a panel of 48 compounds from Kenyan plants against multidrug resistant phenotypes. Springerplus. 2016 Dec 1;5(1):1–15. CrossRef