INTRODUCTION
Using natural ingredients in cosmetic production is becoming increasingly popular as many consumers prefer organic and chemical-free products [1,2]. One natural ingredient with potential antimicrobial and antifungal properties is the symbiotic bacteria [3–5]. This bacteria is derived from the fruit of a type of mangrove plant that grows in coastal areas and contains active compounds that have the potential to function as antimicrobials and antifungals. These active compounds fight the growth of microorganisms that cause infections and damage to the skin, such as bacteria and fungi [6].
There is limited information about potential symbiotic bacteria from the sea; one example is symbiotic bacteria that have antimicrobial and antifungal properties, such as the bacterial species Pseudoalteromonas [7] and Bacillus [8]. Pseudoalteromonas is a type of bacteria commonly found in marine environments, especially on the surfaces of marine organisms. The study results on pyocyanin show that biosynthesis activity decreases the pathogenicity of Pseudomonas aeruginosa bacteria. This indicates that pyocyanin is primarily responsible for the initial colonization of P. aeruginosa [9,10].
Symbiotic bacteria of the genus Bacillus are known to be found in several marine hosts, including mangroves, sea cucumbers, mollusks, sediment, and fish. This indicates that Bacillus has a natural affinity for the marine environment and can be categorized as either pathogenic or non-pathogenic. Several non-pathogenic species that have the potential to act as antimicrobial multi-drug resistant (MDR) agents are Bacillus subtilis, Bacillus firmus, and Bacillus oceanissediminis [5]. It means that this type of bacteria has the ability to develop resistance to multiple types of antibiotics. MDR bacteria are able to survive and grow even when exposed to several types of antibiotics commonly used to treat bacterial infections. Treating infections caused by MDR bacteria becomes more difficult and complex. Thus, the bacteria Bacillus is beneficial for health.
Bacillus is a genus of bacteria commonly found in the marine environment that is capable of degrading organic compounds present in seawater. Some Bacillus species can also produce enzymes that are used in food fermentation processes and the production of pharmaceuticals. Due to their adaptive nature and potential to produce valuable compounds, Bacillus bacteria from the sea have become interesting research subjects in biotechnology. Bacillus sp. bacteria are also known as gram-positive bacteria and have the potential to analyze antibacterial activity. These bacteria have the potential to produce important enzymes, some of which have been reported as producers of alginate lyase [11]. Bacillus cereus can be found in seaweed species, such as Eucheuma spinosum, Gracilaria gracilis, and Glacilaria verrucosa [12], while Bacillus oceanisediminis can be found in Holothuria atra [13].
The research results show that the bioactive compounds in the mangrove fruit Xylocarpus sp. are similar to those in its symbiotic bacteria [8]. Using natural ingredients, such as symbiotic bacteria from Xylocarpus sp. fruit, can enhance the safety and sustainability of cosmetic production. Using harmful chemicals in cosmetics can negatively impact human health and the environment. An increasing number of chemical compounds are being added to the formulation of cosmetic products, such as additives, fragrances, preservatives, stabilizers, surfactants, dyes, and shine, to enhance their quality, properties, and shelf life [14]. Harnessing the antimicrobial and antifungal potential of the symbiotic bacteria in Xylocarpus sp. fruit can reduce the presence of toxic chemicals and promote the use of safer and more environmentally friendly natural ingredients. In addition, using symbiotic bacteria from Xylocarpus sp. fruit in the cosmetic bioindustry can add value to the products. Phytochemistry and pharmacology of [15], apart from antioxidants, these natural classes of chemicals have also demonstrated antimicrobial activities against human, animal, and plant pathogens [16]. Consumers are increasingly appreciating cosmetic products that contain natural and beneficial ingredients for their skin. The impact of water exposure and temperature changes on skin barrier function [17], as well as the effects of active ingredients in cosmetics and the possible benefits of these bioactive compounds in rejuvenation and health [2]. By harnessing the potential of the antimicrobial and antifungal properties derived from the symbiotic bacteria of Xylocarpus sp. fruits, research findings can lead to the development of natural cosmetic products that effectively maintain skin cleanliness and health. The antimicrobial and antifungal compounds produced by these bacteria can inhibit the growth of harmful microorganisms in cosmetic products. This will reduce the risk of infections and irritations on the skin caused by the use of cosmetic products. Based on this, the present research aims to determine the potential of symbiotic bacteria from Xylocarpus sp. fruit and a bacterial consortium as ingredients for cosmetic creams for the skin.
MATERIALS AND METHODS
Bacterial purification
The purification of bacterial colonies, namely Alcaligenes aquatilis (Isolate X1.63), Sinomicrobium oceani (Isolate X2.52), S. oceani (Isolate X1.54), Pseudomonas khazarica (Isolate X1.64), S. oceani (Isolate X1.53), and Proteus mirabilis (Isolate X1.65), stored on Zobell 2216 E agar medium was conducted. The purification was carried out using the streak plate method until pure colonies were obtained [7].
Antibacterial and antifungal testing
Antibacterial and antifungal testing was conducted using the pour-plate method. Six isolates were tested for antibacterial activity using paper discs against the pathogenic bacteria Micrococcus luteus, P. aeruginosa, and Staphylococcus epidermidis. Antifungal testing was conducted on the pathogenic fungi Malassezia furfur and Candida albicans.
Laboratory cream making
A bacteria culture was conducted using Marine Zobell Broth. The cultured bacteria were then extracted using 96% ethanol. The evaporation process was carried out using a rotary evaporator to obtain a concentrated extract. Cream preparation involved the use of the oil-in-water (O/W) emulsion method. Stearic acid was placed in a porcelain dish and melted using a water bath. Then, the aqueous phase and the selected bacterial extract were added to a beaker and heated on a hot plate. The oil phase was poured into the aqueous phase, which was contained in a mortar. The mixture was homogenized using an aluminum spatula until the desired cream was formed. The cream was made with the following ingredients: bacteria extract (various), stearic acid (14.2 g), triethanolamine (1 g), glycerol (10 g), nipagin (0.12 g), and distilled water (100 ml).
Next, the organoleptic test [18], cream pH test [19], cream adhesive test [18], cream spreading test, and cream stability tests were conducted on the obtained cream [20].
In vivo test on male mice (Mus musculus)
In vivo tests were conducted on male mice (M. musculus). Before the experiment, the mice were acclimatized for 7 days to adjust to their new environment. Then, the test animals were administered ketamine-xylazine (KX) medication at a dosage of 0.02/20gr kgBW, which was injected to induce anesthesia and ensure sterilization. After sterilization, a 1 cm long and 1 mm deep parallel incision was made on the dorsal skin. The purpose of performing the wound healing test on the M. musculus (mouse) model is to study and understand the cellular and molecular mechanisms involved in wound healing. This model allows researchers to investigate different aspects of wound healing, such as inflammation, tissue regeneration, and scar formation. The result can identify various factors and pathways that influence wound healing by inducing controlled wounds in mice and subsequently analyzing the healing process. This information can be valuable in developing new therapeutics and interventions to enhance human wound healing. A total of 22 Balb/c mice were divided into 11 groups: positive control (povidone-iodine), negative control (cream without additives), PA1 (0.5% extract of bacteria A), PA2 (5% extract of bacteria A), PA3 (20% extract of bacteria A), PB1 (0.5% extract of bacteria B), PB2 (5% extract of bacteria B), PB3 (20% extract of bacteria B), PC1 (0.5% extract of bacteria C), PC2 (5% extract of bacteria C), and PC3 (20% extract of bacteria C). The application and observation process was performed over 14 days [21]. The selected bacterial extract was derived from two isolated samples, chosen for their excellent antifungal and antibacterial properties against a range of test pathogens for a consortium of bacterial isolates. The concentrations of each extract used were 0.5%, 5%, and 20%.
Histology slide preparation
Next, the test animal was euthanized using ether and cervical dislocation methods. Mus musculus was preserved using 10% formalin. Excision was done on the skin tissue extracted from the tested area. The collected epidermis sample was prepared for histology according to the standard histology procedure. The test animal’s epidermal tissue was observed under a microscope at various magnifications.
RESULTS AND DISCUSSION
Antibacterial test
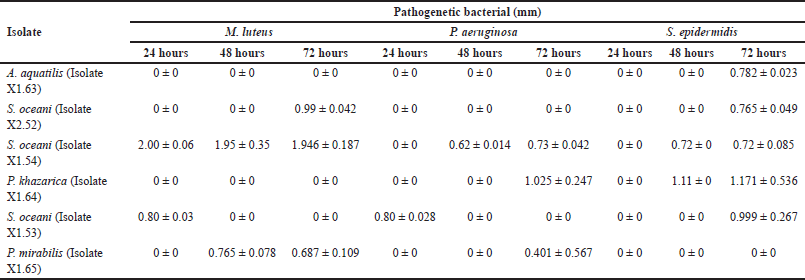
The antibacterial test of symbiotic bacteria showed that S. oceani (Isolate X1.54) had the largest clear zone against all three tested pathogens, measuring 1.946 ± 0.187 mm after 72 hours of observation. This isolate also exhibited activity against all tested pathogens, as shown in Table 1.
Antifungal testing
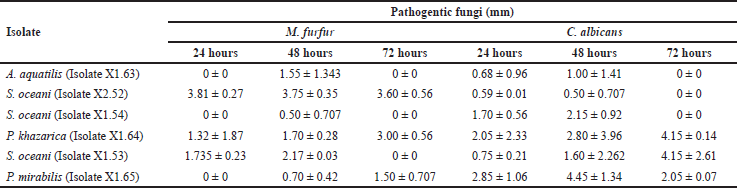
The results of antifungal testing showed that S. oceani (Isolate X2.52) has the highest efficacy against the fungal pathogen M. furfur. The bacterial isolate that produces the largest clear zone against C. albicans is P. mirabilis (Isolate X1.65), with a diameter of 2.85 ± 1.060 mm after 24 hours of observation, and 4.45 ± 1.344 mm after 48 hours of observation, as shown in Table 2.
Antagonistic test
Based on the antagonistic test conducted on each isolate, no bacterial isolate was found to be dominant over the others. This is evidenced by the absence of clear zones in the trial, indicating that all six isolates synergize to create a bacterial consortium.
Selection of isolates for cream formulation
Based on their excellent antifungal and antibacterial abilities against various test pathogens, the bacterial extracts selected for this research were isolated X1.64 (P. khazarica), X1.54 (S. oceani), and a consortium of bacterial isolates.
Organoleptic test
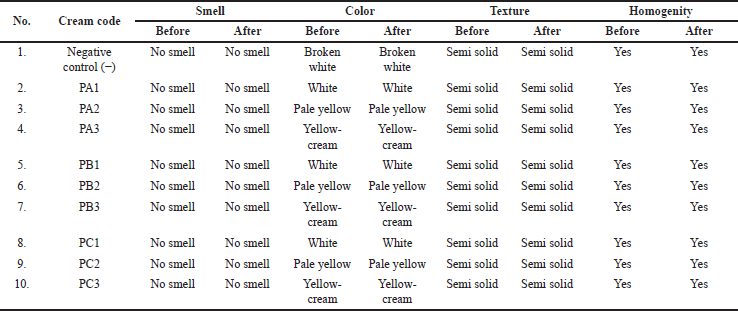
Based on the organoleptic test, the cream product did not have a smell and had a semi-solid texture. The cream color varied from white to cream with a higher concentration of added bacteria extract. As stated in Table 3 and Figure 1, the cream color became more concentrated in yellow.
Stability test
Stability testing was used to compare the test creams before and after a cycle test. The results showed that PB2 cream has a stable pH. The creams with the most stable spreadability were PC1 and PC3, while the creams with the most stable adhesiveness were PB3, as shown in Table 4.
The results of in vivo testing of cream on M. musculus mice showed that the fastest wound healing formula was the PC isolate sample, which had the highest concentration of bacterial extract (20% bacterial extract C, bacterial consortium) compared to the PA (bacterial extract A = X1.64, P. khazarica) and PB (bacterial extract B = X1.54, S. oceani) isolates (Table 5). On the first day of testing, the mouse’s skin appeared wounded and bleeding (day 0). However, over time (day 7), the condition of the mouse’s skin improved, and it became dry by day 12, as depicted in Figure 2. However, in the PA and PB isolate samples, it can be seen that the wounded mouse skin had not completely dried by day 12. The results showed that all of the in vivo tests were conducted on day 12, and the PC cream had the lowest standard deviation compared to others. This result showed that the test has closer similarities between each repetition.
 | Table 1. Antibacterial test of Xylocarpus sp. symbiont bacteria isolates. [Click here to view] |
 | Table 2. Antifungal test of Xylocarpus sp. symbiont bacteria isolates. [Click here to view] |
 | Table 3. Organoleptic test on cream. [Click here to view] |
Histology of the mouse epidermis
The histological analysis revealed that the structure of the epidermis in the positive control group (K+) was not fully formed, with only about 90% intact out of the total five layers of the epidermis. In the negative control group (K−), the epidermis structure developed well but appeared thin, and hair follicles were present. Furthermore, in treatment PA3, the epidermis had a complete layer compared to treatments PA1 and PA2, but the hair follicles were structurally incomplete (Fig. 3).
Then, histological results of treatments PB1 and PB2 showed a thin overall epidermis layer [structurally similar to K(−)], with hair follicles present. Treatment PB3 had a similar epidermal layer to PB1, but it was slightly better, although not significantly. Hair follicles were also present. Treatment PC1 indicated an intact epidermal structure with five layers: a thin stratum corneum, a thin stratum lucidum, a normal stratum granulosum, a normal stratum spinosum, and a thicker than average stratum basal. Treatment PC3 showed better hair follicles compared to PC1, with an intact epidermal structure consisting of five layers. This included a thicker stratum corneum, stratum lucidum, stratum granulosum, and stratum basal compared to PC1, as well as a normal stratum spinosum.
 | Figure 1. Examples of cream samples (a) F7, (b) F8, and (c) F9. [Click here to view] |
The results of the histological analysis for each treatment showed that all tested creams can promote hair follicle growth (Fig. 3). Cream formula PC3 has the best ability to restore the intact structure of the four layers of the stratum corneum in the epidermis.
The development of each stratum in the epidermis appears to be thicker than the epidermis treated with other formulas. The testing cream containing S. oceani extract (Isolate X1.54, cream code PB) and consortium extract (cream code PC) shows a linear relationship, wherein the higher the concentration of the extract added to the cream, the greater its ability to regenerate the epidermal layers of the test animals and promote hair follicle growth. This indicates that formulating a cream by adding bacterial extracts can help the skin regenerate and hair follicles grow (Fig. 4).
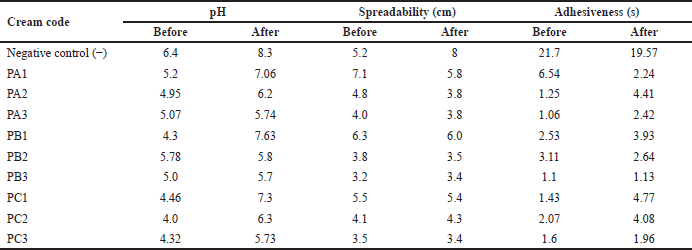 | Table 4. Cream stability test. [Click here to view] |
Each cream formulation can stimulate the growth of different layers of the epidermis. The PA1 (Fig. 3a), PA2 (Fig. 3b), and PA3 (Fig. 3c) cream formulation have a complete epidermal layer, but the hair follicles are structurally incomplete. The epidermal structure formed by the PB3 cream formulation has a thin layer in each stratum (Fig. 3d). PC3 formulation (Fig. 3e) is the most effective formulation, as indicated by the increased thickness of the stratum corneum, stratum lucidum, and stratum granulosum, as well as the stratum spinosum and stratum basal (Fig. 3e.2) compared to the other formulations. Hair follicles can be seen in Figure 3b.1 and 3c.1. K(−) cream formulation forms a perfect epidermal structure, albeit thin (Fig. 3f). The overall epidermal structure formed by the K(+) cream formulation in the test animal is not yet fully developed, with approximately 90% of the total five epidermal layers (Fig. 3g). On the 12th day, all wounds had healed. However, the most complete healing was observed in the treatment with PC (20% bacterial extract C, bacterial consortium). Moreover, on the seventh day, the appearance of the mouse skin in each treatment was different. This means that the wound healing results were better with the PC treatment. In general, skin wounds will heal on their own around day 14 [21]. Similarly, this research result revealed that on day 12, which is close to day 14, the injured mice’s skin was healed. The mice’s skin treated with PC showed more complete healing and a smoother skin surface.
Previous research has identified six symbiotic bacterial isolates from mangrove fruit, namely A. aquatilis (Isolate X1.63), S. oceani (Isolate X2.52), S. oceani (Isolate X1.54), P. khazarica (Isolate X1.64), S. oceani (Isolate X1.53), and P. mirabilis (Isolate X1.65). All of these bacterial isolates have the potential to form bacterial consortia. The research results showed that all isolates can act as antibacterial agents against the pathogenic bacteria M. luteus, P. aeruginosa, and S. epidermidis. The bacterial isolates also have the potential to act as antifungal agents against the pathogenic fungi M. furfur and C. albicans, as shown in Tables 1 and 2. This means that all bacterial isolates have the potential to act as antibacterial and antifungal agents.
The research results showed that the extract of S. oceani bacteria (Isolate X1.53) inhibits the growth of M. luteus and P. aeruginosa within 24 hours (Table 1), but not within 48 or 72 hours. Similarly, in the antifungal test, the extracts of all tested isolates inhibited the growth of M. furfur and C. albicans after 48 hours. However, no inhibition zones were observed after 72 hours (Table 2). Many factors contribute to the condition. Some types of bacteria have specific periods of activity in producing substances or compounds that inhibit the growth of pathogenic bacteria. After these periods, bacteria no longer produce these substances, so the inhibition zone does not form. Another factor that can cause this is the rapid reproduction of pathogenic bacteria. In such cases, the compounds produced by inhibitory bacteria may not be effective in restraining the growth of the pathogenic bacterial population.
The research results used for the skin cream include the isolate P. khazarica (Isolate X1.64), which is referred to as cream PA; the isolate S. oceani (Isolate X1.54), which is referred to as cream PB; and the bacterial consortium isolated from these six bacterial species, which is referred to as cream PC. These bacterial isolates are known to have potential as antibacterial and antifungal agents. Furthermore, the bacterial isolates contain a highly dominant compound called 9-octadecenoic acid. This compound is present in two bacterial isolates, P. khazarica and S. oceani, as well as in the bacterial consortium isolate (Table 3 and Fig. 2). The results of creating a bacterial extract cream showed that the cream had optimal pH, spreadability, and adhesion for the test.
The compound 9-octadecenoic acid, also known as oleic acid, is found in herbal hair oil that is widely available in Indonesia. The main ingredients of this oil are olive oil, candlenut oil, and castor oil. It is assumed that this compound offers several benefits for the skin, such as moisturizing, preventing dehydration, healing dry and scaly skin, reducing inflammation, and promoting hair growth, as stated on the product’s commercial brands. Oleic acid compounds are beneficial for hair and have been used in commercial products in Indonesia, such as candlenut oil. Candlenut oil helps promote hair growth; its main ingredient is the candlenut fruit. It is known that candlenut fruit contains oleic acid [22]. From this information, it can be inferred that oleic acid promotes hair growth, similar to what occurs in symbiotic bacteria in mangroves that contain oleic acid.
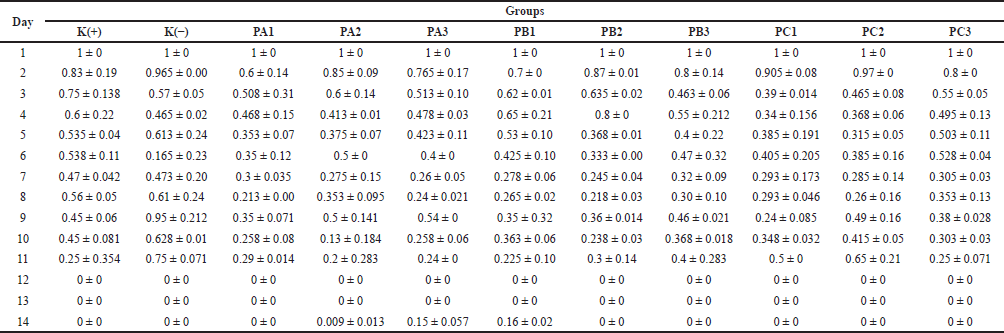 | Table 5. The results of histology analysis of the bacteria simbiont Xylocarpus sp. in mice. [Click here to view] |
 | Figure 2. Wound healing process on M. musculus. [Click here to view] |
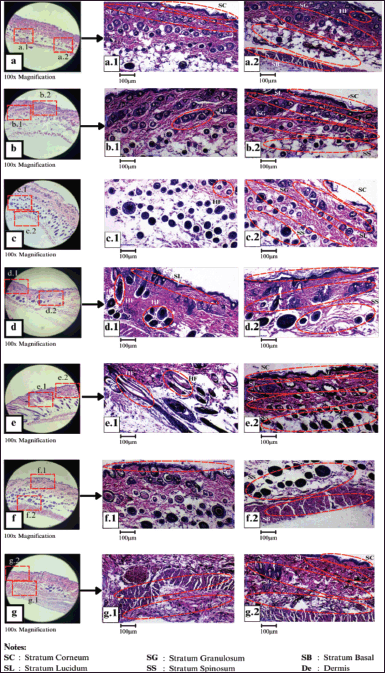 | Figure 3. Histological results of the epidermis of test animals M. musculus formula (a) PA1, (b) PA2, (c) PA3, (d) PB3, (e) PC3, (f) K(−), and (g) K(+). [Click here to view] |
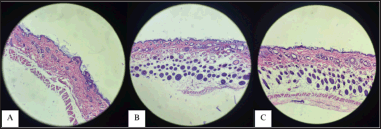 | Figure 4. Histology of the growth of epidermal layers (A) PC1, (B) PC2, and (C) PC3. [Click here to view] |
Research results show that the oleic acid found in the symbiotic bacteria of Xylocarpus sp., a mangrove fruit, has the potential to promote hair growth on the skin. This discovery can be utilized in the cosmetic industry to create hair growth products (Table 5 and Fig. 2). Such compounds are highly sought after by bald individuals. The similarity in compound composition between the symbiotic bacteria and their host makes this research particularly promising for the future. The findings strongly support conservation efforts because it is possible to obtain the desired compounds from the symbiotic bacteria without causing harm to the mangrove plants.
The wound shown did not get smaller with time during treatment (Table 5). The wound sizes on day 11 were larger than those on day 10 when treated with PB3, PC1, PC2, and the negative control. The variation in wound sizes in the negative control was abnormal. The results showed that the wound size for K(−) on day 4 was 0.465 mm. On day 5, the size increased to 0.613 mm; on day 6, it was 0.165 mm; and on day 7, it was 0.473 mm (Table 5). The results were not as expected after treating the injured mice with bacterial cream or the control treatment. The environment surrounding the wound can also affect the healing process. Cleanliness, moisture, and exposure to irritating substances or infections can slow down the recovery process and hinder the reduction of damage.
Furthermore, the study’s results showed that PC cream applied to injured skin appeared to promote faster healing than other creams. On the other hand, there was an increase in the number of hair follicles on the skin. PC cream is a cream that contains a consortium of bacteria consisting of six isolates. The results of the study showed that these six bacteria, each with their respective contents, are as follows: S. oceani bacteria containing hexadecanoic acid, methyl ester compound (CAS) (27.52%); S. pectinilyticum containing 9-octadecenoic acid (Z)-, 35.61% methyl ester (CAS); A. aquatilis containing 9-octadecenoic acid, methyl ester, (E)- (CAS) 44.02%; P. khazaric containing octadecenoic acid, methyl ester, (E)- (35.64% area); P. mirabilis containing 9-octadecenoic acid, methyl ester, (E)- 33.28%; and S. oceani containing the highest content of 9-octadecenoic acid, methyl ester, (E)- (46.58%). In addition, all bacteria have the potential to be antibacterial and antifungal agents [8]. The consortium of bacterial isolates contains dominant compounds such as 9-octadecenoic acid, oleic acid, hexadecanoic acid, and palmitic acid.
Hexadecanoic acid, also known as palmitic acid, is one of the essential components in forming the skin barrier. The skin barrier protects the skin from environmental influences such as bacteria, germs, pollution, and moisture. Hexadecanoic acid forms a protective layer on the skin surface, which helps to maintain skin moisture and prevent water loss. In addition, palmitic acid also has anti-inflammatory and antimicrobial properties [23,24]. Therefore, this compound is used in various skincare products, such as moisturizers, creams, and acne treatments.
The research results on the symbiotic bacteria Xylocarpus sp. found in mangrove fruit, which is known for its potential as an antibacterial and antifungal agent, as well as its ability to promote hair growth on bald skin, show promising potential for the cosmetic bioindustry. The desired cosmetic products can hydrate the skin, prevent dehydration, heal dry skin, have anti-inflammatory properties, and function as anti-aging agents and free radical fighters [25,26]. Hydrating cosmetic products help maintain skin moisture, keeping the skin soft, smooth, and hydrated. Preventing skin dehydration helps protect the skin from losing moisture due to low humidity, sunlight, and other environmental exposures.
Moreover, it has the potential to heal dry and scaly skin, indicating its intensive moisturizing properties that can aid in improving issues related to dry and scaly skin [27,28]. It can also soften and smooth the skin, as well as reduce inflammation or irritation that may occur. In addition, it has anti-inflammatory properties, which means it can help reduce any inflammation in the skin, including acne and eczema [29,30].
On the other hand, products that have the potential to act as anti-aging agents, meaning compounds that can help reduce skin aging, such as fine lines, wrinkles, and loss of elasticity, can be associated with oleic acid. Oleic acid is the most abundant type of monounsaturated fatty acid in food and belongs to the group of beneficial fatty acids. Oleic acid can stimulate the production of collagen and elastin in the skin, which is necessary to maintain its elasticity and strength [31]. Furthermore, products that can combat free radicals are sought-after in the cosmetics industry. The mangrove fruit, Xylocarpus sp., contains antioxidants [32]. Antioxidant compounds possess strong antioxidant properties that aid in combating skin damage caused by free radicals [33]. This helps maintain healthy skin and inhibit premature aging. The bacterial consortium of the Xylocarpus sp. mangrove fruit is suspected to have the potential to fight free radicals due to the presence of similar bioactive compounds.
CONCLUSION
All creams that were tested can promote hair follicle growth. The cream with the consortium bacteria extract formula can stimulate hair follicle growth in test animals. Furthermore, the tested cream with S. oceani extract (Isolate X1.54, PB cream code) and consortium extract (PC cream code) have linear abilities. This means that the higher the concentration of extract added to the cream, the greater its ability to regenerate the epidermal layer of the test animal and stimulate hair follicle growth. The tested cream with P. khazarica extract (Isolate X1.64, PA cream code) does not have the same linear ability as PB and PC creams. This study suggests that the concentration of P. khazarica bacteria extract has the maximum potential to enhance skin regeneration and stimulate hair follicle growth. Based on the obtained results, the cream formulation can promote the formation of hair follicles on the skin. This is achieved through a combination of bacteria extracts, which aid in regeneration and growth. Testing a cream formulation containing bacteria extract can be an alternative treatment for alopecia (baldness).
AUTHOR CONTRIBUTIONS
DP, WAS, and AI designed and investigated. DP, WAS, and DA contributed to the original draft preparation, review writing, and editing. In addition, DP and DA contributed to the literature review and the paper submission process. All authors have made an equal contribution and have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
This research received funding from Research and Community Services “LPPM” Universitas Diponegoro for funding “Riset Publikasi Internasional” under No: 569-61/ UN7. D2/PP/IV/2023 and Rector:182/UN7.A/HK//II/2023 and 42/UN7.A/HK//IV/2023.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The study protocol was approved by the Institutional Ethics Committee (No :65/EC-H/KEPK/FK-UNDIP/VI/2023).
DATA AVAILABILITY
All data generated and analyzed are included within this article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliations.
REFERENCES
1. Gubitosa J, Rizzi V, Fini P, Cosma P. Inonotus obliquus extract as an inhibitor of α-MSH-induced melanogenesis in B16F10 mouse melanoma cells. Cosmetics. 2019;6(1):1–16.
2. Liu JK. Natural products in cosmetics. Nat Prod Bioprospect. 2022;12(40):1–43. CrossRef
3. Cui J, Deng Z, Li J, Fu H, Proksch P, Lin W. Phragmalin-type limonoids from the mangrove plant Xylocarpus granatum. Phytochemistry. 2005;66(19):2334–9. CrossRef
4. Dai YG, Wu J, Padmakumar KP, Shen L. Sundarbanxylogranins A–E, five new limonoids from the Sundarban Mangrove, Xylocarpus granatum. Fitoterapia. 2017;122:85–9. CrossRef
5. Pringgenies D, Setyati WA, Djunaedi A, Pramesti R, Rudiyanti S, Ariyanto D. Exploration of antimicrobial potency of mangrove symbiont against multi-drug resistant bacteria. J Ilm Perikan dan Kelaut. 2021;13(2):102–12. CrossRef
6. Pringgenies D, Wilis AS, Feliatra F, Ariyanto D. The antibacterial and antifungal potential of marine natural ingredients from the symbiont bacteria of mangrove. Glob J Environ Sci Manag. 2023;9(4):819–32. CrossRef
7. Pringgenies D, Setyati WA. Antifungal strains and gene mapping of secondary metabolites in mangrove sediments from Semarang city and Karimunjawa islands, Indonesia. AIMS Microbiol. 2021;7(4):499–512. CrossRef
8. Pringgenies D, Setyati WA. Metabolites of mangrove sediment bacteria from Semarang and Karimunjawa as anti-fungal and antibacterial. Trends Sci. 2023;20(5):6774. CrossRef
9. El-Fouly MZ, Sharaf AM, Shahin AAM, El-Bialy HA, Omara AMA. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J Radiat Res Appl Sci. 2015;8:36–48. CrossRef
10. Sultan M, Arya R, Kim KK. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int J Mol Sci. 2021;22:12152. CrossRef
11. Zilda DS, Yulianti Y, Sholihah RF, Subaryono S, Fawzya YN, Irianto HE. A novel Bcillus sp. isolated from rotten seaweed: identification and characterization alginate lyase its produced. Biodiversitas. 2019;20(4):1166–72. CrossRef
12. Pringgenies D, Retnowati EI, Ariyanto D, Dewi K, Viharyo MAS, Susilowati R. Symbiotic microbes from various seaweeds with antimicrobial and fermentative properties. AACL Bioflux. 2020;13(4):2211–7.
13. Santosa GW, Djunaedi A, Susanto AB, Pringgenies D, Ariyanto D. Characteristics of bioactive compounds of Holothuria atra (Jaeger, 1833) associated bacteria. AACL Bioflux. 2020;13(4):2161–9.
14. Bilal M, Mehmood S, Iqbal HMN. The beast of beauty: environmental and health concerns of toxic components in cosmetics. Cosmetics. 2020;7(1):1–18. CrossRef
15. Dey D, Quispe C, Hossain R, Jain D, Ahmed Khan R, Janmeda P, et al. Ethnomedicinal use, phytochemistry, and pharmacology of Xylocarpus granatum J. Koenig. Evid Based Complement Altern Med. 2021;2021:1–16. CrossRef
16. Mitra S, Naskar N, Chaudhuri P. A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomed Plus. 2021;1(4):100107. CrossRef
17. Herrero-Fernandez M, Montero-Vilchez T, Diaz-Calvillo P, Romera-Vilchez M, Buendia-Eisman A, Arias-Santiago S. Impact of water exposure and temperature changes on skin barrier function. J Clin Med. 2022;11(2):298. CrossRef
18. Rohmani S, Miararani N, Yugatama A, Ermawati DE, Prihapsara F. Formulation and the release of eugenol from cream using glycerin base. IOP Conf Ser Mater Sci Eng. 2019;578(1):1–6. CrossRef
19. Luu NDH, Dang LH, Vo Le TV, Ngoc Do TD, Thi Nguyen TT, Thi Nguyen TT, et al. Topical cream based on nanoformulation of Chromolaena odorata extract for accelerating burn wound healing. J Drug Deliv Sci Technol [Internet]. 2023;82:104360. CrossRef
20. Whangsomnuek N, Mungmai L, Amornlerdpison D, Mengamphan K. Efficiency of skin whitening cream containing etlingera elatior flower and leaf extracts in volunteers. Cosmetics. 2019;6(3):1–10. CrossRef
21. Sagástegui-Guarniz WA, Silva-Correa CR, Villarreal-La Torre VE, González-Blas MV, Sagástegui-Guarniz WO, Calderón-Peña AA, et al. Wound healing by topical application of Momordica charantia L. formulations on mice. Vet World. 2021;14(10):2699–704. CrossRef
22. Erawati TM, Rosita N, Rachmania I. The activity of candlenut oil in the nanostructured lipid carrier system on hair growth in rats. J Public Health Africa. 2023;14:118–22.
23. Huang CB, George B, Ebersole JL. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch Oral Biol. 2011;55(8):555–60. CrossRef
24. Charlet R, Le Danvic C, Sendid B, Nagnan-Le Meillour P, Jawhara S. Oleic acid and palmitic acid from Bacteroides thetaiotaomicron and Lactobacillus johnsonii exhibit anti-inflammatory and antifungal properties. Microorganisms. 2022;10(9):1–20. CrossRef
25. Sharma RR, Deep A, Abdullah ST. Herbal products as skincare therapeutic agents against ultraviolet radiation-induced skin disorders. J Ayurveda Integr Med. 2022;13(1):100500. CrossRef
26. Kashyap N, Kumari A, Raina N, Zakir F, Gupta M. Prospects of essential oil loaded nanosystems for skincare. Phytomed Plus. 2022;2(1):100198. CrossRef
27. Ratz-Lyko A, Arct J, Pytkowska K. Moisturizing and antiinflammatory properties of cosmetic formulations containing Centella asiatica extract. Indian J Pharm Sci. 2016;78(1):27–33. CrossRef
28. Aguirre-Cruz G, León-López A, Cruz-Gómez V, Jiménez-Alvarado R, Aguirre-Álvarez G. Collagen hydrolysates for skin protection: oral administration and topical formulation. Antioxidants. 2020;9(2):181. CrossRef
29. Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2018;19(70):1–21. CrossRef
30. Martins AM, Gomes AL, Boas IV, Marto J, Ribeiro HM. Cannabis-based products for the treatment of skin inflammatory diseases: a timely review. Pharmaceuticals. 2022;15(210):1–25. CrossRef
31. Cardoso CR, Favoreto S, Oliveira LL, Vancim JO, Barban GB, Ferraz DB, et al. Oleic acid modulation of the immune response in wound healing: a new approach for skin repair. Immunobiology. 2011;216(3):409–15. CrossRef 10.1016/j.imbio.2010.06.007
32. Analuddin K, Septiana A, Nasaruddin, Sabilu Y, Sharma S. Mangrove fruit bioprospecting: nutritional and antioxidant potential as a food source for coastal communities in the Rawa Aopa Watumohai National Park, Southeast Sulawesi, Indonesia. Int J Fruit Sci. 2019;19(4):423–36. CrossRef
33. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4(8):118–26. CrossRef