INTRODUCTION
Zebrafish (Danio rerio) has emerged as a widely used animal model in recent years to discover and develop drugs for various disorders and is used as a high-throughput drug screening system [1]. Zebrafish have been demonstrated to have morphological and physiological similarities in the nervous, cardiovascular, and digestive systems as well as genomic phylogeny compared to humans [2]. The zebrafish embryo toxicity test is becoming increasingly popular since it provides a clearly defined developmental period for vertebrate embryos and enables the study of early developmental stages [3]. The zebrafish model can be extremely valuable for intermediate toxicity testing due to its cost-effectiveness, speed, and ease of use. The benefits of the zebrafish model are the reason for the popularity of using this model as an alternative to some vertebrate animal toxicity testing models [4].
Panax notoginseng (PNG) (Burk.) F. H. Chen is a highly valued herb of the Araliaceae family, which has been used in traditional Chinese medicine for more than 400 years [5]. Panax notoginseng saponins (PNS) play a crucial role in the important pharmacological effects of PNG. Numerous studies have demonstrated that PNS possess significant biological activities, including anti-cerebrovascular ischemic properties, anti-arrhythmic effects, vasodilatory effects, improvement of blood rheology and microcirculation, inhibition of platelet aggregation, and anti-thrombotic effects [6].
However, it is still concerned about the toxicity of saponin such as hemolytic toxicity. It is reported that many saponins maintain harmful hemolytic toxicity that causes the lysis of erythrocytes [7]. The harmful effects of saponins might be the reason for the research on the toxicity test of saponins which has been done [8,9]. To reduce the harmful effects of saponins and identify the dose of saponins for animals and humans, the acute toxicity test of PNS using a zebrafish model has been designed and then evaluated for the safety of phytochemical compounds.
The five main saponins in PNG are notoginsenoside R1 (7%–10%), ginsenoside Rb1 (30%–36%), Rg1 (20%–40%), Rd (5%–8.4%), and Re (3.9%–6%). Among them, ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 have been selected as the standard compounds to evaluate the quality of PNG [10]. PNS have high aqueous solubility but low permeability, so PNS belong to class III in the biopharmaceutics classification system [11–13]. Moreover, PNS are unstable in the pH acidic environment [6,14]. At a 1.2 pH, PNS underwent a degradation rate of approximately 50% after 1 hour for notoginsenoside R1 and ginsenoside Rg1, and after 4 hours for ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd [15]. These factors lead to the poor oral bioavailability of PNS.
To enhance the oral bioavailability of PNS, recent studies have been performed such as enteric coating pellet [14,16], enteric microcapsule [17], bio-adhesive pellet [18], micro-porous osmotic pump tablet [19], osmotic pump capsule [20,21], controlled-release tablet [22], and bio-adhesive tablet [23]. Among various solid dosage forms, pellets offer several benefits, including reduced irritation of the gastrointestinal tract, decreased risk of side effects, improved dosage form integrity, and simplified coating [24]. The freeze pelletization technique is a simple approach for preparing matrix pellets. In the dripping method, drugs are dissolved or dispersed in the molten mixture containing carriers such as polymers producing droplets that are rapidly cooled to form spherical pellets [25]. This method offers several advantages including simplicity, ease of preparation, great dissolution rate, high bioavailability, high stability, solvent-free pharmaceutical manufacturing processes, and uniform dosage in each pellet [26].
To quickly release PNS, we prepared core pellets using polyethylene glycols (PEGs) as carriers by the dripping method. Absorption enhancers were added to the pellet formulation to enhance the drug’s permeability. Then, enteric-coated pellets were developed to minimize drug degradation in the stomach.
MATERIALS AND METHODS
Materials
PNS extract containing 6.34% of notoginsenoside R1, 31.29% of ginsenoside Rg1, 3.21% of ginsenoside Re, 31.17% of ginsenoside Rb1, and 8.45% of ginsenoside Rd were extracted from the radix of PNG in the laboratory. PEG 4,000 and 6,000 were purchased from TNJ Chemical Industry Co., Ltd. (China). Sodium lauryl sulfate (SLS) was bought from Emery Oleochemicals Marketing Sdn Bhd (Malaysia). β-cyclodextrin (β-CD) was purchased from Roquette company (France). Hydroxypropyl methylcellulose (HPMC) E6 was provided by Handong Head Co., Ltd. (China). Triethyl citrate (TEC) was purchased from Shanghai Macklin Biochemical Co., Ltd. (China). Eudragit L100 was purchased from Riyuexin Chemical Industrial Co., Ltd. (China). Talc was supplied by Hua Mei Industrial Co., Ltd (China). Titanium dioxide (TiO2) and ethanol were purchased from Xilong Scientific Co., Ltd (China). High performance liquid chromatography (HPLC)-grade acetonitrile and methanol were obtained from Merck (Germany). Purified water was obtained using a GenPure UV-TOC system (Thermo Scientific, Germany). Other substances meet pharmaceutical standards.
Zebrafish
Fish maintenance
Wild-type zebrafish (Danio rerio) were provided by a local vendor specializing in ornamental fish. The care and maintenance of the zebrafish were carried out in accordance with internationally recognized guidelines [27]. The zebrafish were raised in a recirculating tank system with water quality control by changing water, remaining food, and removing feces daily. The temperature and pH of the living environment were maintained at 27°C ± 1°C and 7.0°C ± 0.1°C, respectively. Lighting conditions were regulated through the use of electric lamps set to a constant 14-hour light/10-hour dark cycle.
Breeding conditions and egg production
The zebrafish aged 3 months were selected for natural breeding. A pair of male fish and female fish were placed in a spawning tank and separated by a glass partition in the late afternoon, to be left overnight. The following day, the partition was removed and the fish were allowed to mate, spawn, fertilize, and produce eggs. Embryos were thoroughly washed, collected, and transferred to a petri dish containing 10 ml of a nurturing solution for fish (E3 media) (NaCl, 13.7 mM; KCl, 0.54 mM; MgSO4, 1.0 mM; CaCl2, 1.3 mM; Na2HPO4, 0.025 mM; KH2PO4, 0.044 mM; and NaHCO3, 4.2 mM). The nurturing condition was renewed daily and placed in an incubator at approximately 28.5°C ± 0.5°C until used [28]. The age of the fish eggs was hours post-fertilization (hpf). In addition, unfertilized or abnormally developed embryos or larvae were removed.
Acute toxicity of PNS using zebrafish model
10 embryos were distributed into each Petri dish. PNS was dissolved in E3 media to obtain corresponding concentrations of 0.01, 0.05, 0.1, 0.5, 1, 5, 10, and 50 μg/ml. Then, the embryos were exposed to each 10 ml of PNS sample on a petri dish and the control group was replaced with E3 media. The experimental samples were investigated under a light stereo microscope (LEIKA, Germany). Survival embryonic rate was recorded at 24, 48, 72, 96, and 120 hpf [29]. In addition, the morphological abnormalities and the heartbeat of zebrafish were observed at 120 hpf. The 50% lethal concentration (LC50) value of PNS was calculated using GraphPad Prism 9 software. The experiment was repeated three times.
HPLC analysis
The concentration of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd in the samples was determined by HPLC method according to the Chinese Pharmacopoeia 2015. HPLC Agilent 1,260 Infinity system was connected with DAD Detector G1315D. An Agilent C18 column (4.6 × 250 mm; 5 μm) was used. The mobile phase consisted of a mixture of acetonitrile (A) and purified water (B) in various proportions, with a gradient elution program: 0–20 minutes (20% A), 20–45 minutes (25%–46% A), 45–55 minutes (46%–55% A), and 55–60 minutes (55% A). A UV detector was set up at 203 nm. The column temperature was maintained at 25°C ± 0.1°C. The flow rate was set at 1.5 ml/minute and the injection volume was 20 μl.
Preparation of enteric-coated pellets of PNS
Core pellets
PEGs are commonly employed in industrial manufacturing processes due to hydrophilicity and low melting point [30,31]. Therefore, PEGs were chosen as carriers. PEG 4000 and PEG 6000 with various ratios were selected as carriers in the pellet formulations. SLS and β-CD were used as absorption enhancers. The composition of formulations P1, P2, P3, P4, P5, P6, P7, and P8 was provided in Table 1. Core pellets were prepared by the dropping method. First, β-CD and SLS were finely ground in a porcelain mortar and sieved through a 125 μm mesh sieve. PEGs were melted in a beaker at 80°C using a hot water bath, and then, PNS, β-CD, and SLS (if used) were dispersed in the melt. Finally, the mixture was stirred with a glass rod for 5 minutes until it became homogeneous. Using a self-made device (Fig. 1), the liquid mixture was poured into a metal tube connected to a needle with a 1 mm diameter. The molten matrix was maintained at a stable temperature by being immersed in a hot water bath with a thermostat and dropped into cold liquid paraffin to form pellets. The dripping and cooling temperatures were checked every 5 minutes. The prepared pellets were dried with soft paper and stored in a desiccator at room temperature.
Barrier layer
The barrier layer plays a role in protecting the active ingredients from direct contact with the enteric layer [32]. The formulation of the barrier layer is shown in Table 2. HPMC E6 and PEG 6000 were dissolved in approximately 150 ml of purified water in a beaker equipped with a magnetic stirrer to obtain a polymeric solution. Talc and TiO2 were finely ground and passed through a 125 μm mesh sieve, then mixed well to form a powder mixture and dispersed in the polymeric solution. Purified water was added to an adequate volume of 200 ml. The technological parameters of the barrier coating process were presented in Table 3 using a mini coater drier (MCD-2, Caleva, England). The core pellets were coated to achieve a weight gain of 5% w/w.
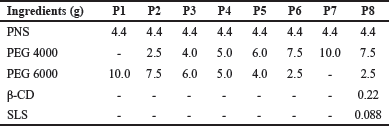 | Table 1. The composition of pellets using various carriers. [Click here to view] |
Enteric-coated layer
The formulation of the enteric-coated layer is shown in Table 2. Initially, Eudragit L100 was dissolved in approximately 150 ml of 70% ethanol in a beaker. Then, TEC was added and stirred to obtain a polymeric solution. After that, micronized Talc and TiO2 were dispersed into this polymeric solution to form a homogeneous suspension. Finally, 70% ethanol was added to make a total volume of 200 ml. The technological parameters used for the enteric coating process are shown in Table 3 using a mini coater drier (MCD-2, Caleva, England). The enteric-coated pellets were coated to achieve a weight gain of 25% w/w compared to the barrier-coated pellets.
Evaluation of pellets
Core pellets
Roundness and average diameter
Ten pellets were randomly selected and their diameters were measured using a micrometer caliper with a minimum division of 0.01 mm. The average diameter and relative standard deviation (RSD) were then calculated. In addition, the ratio of the longest diameter and the shortest diameter of the pellets was also determined.
Drug content
Approximately 300 mg of ground uncoated pellet sample was accurately weighed in a 50 ml volumetric flask. After adding 40 ml of 70% methanol, the mixture was sonicated for 30 minutes. The volume was then adjusted to 50 ml with 70% methanol and the obtained solution was filtered through a 0.2 μm filter membrane. The content of notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rd, and ginsenoside Re was determined using the HPLC method. Then, the content of the PNS was calculated.
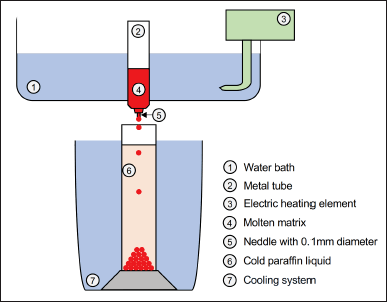 | Figure 1. The design of using a self-made device. [Click here to view] |
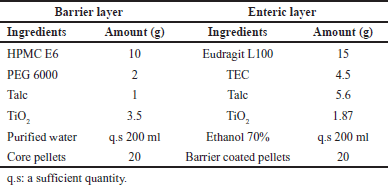 | Table 2. Formulations of the barrier layer and enteric-coated layer. [Click here to view] |
 | Table 3. Process parameters of barrier coating pellets and enteric-coating pellets. [Click here to view] |
Weight variation
Twenty core pellets were accurately weighed, and then the average weight was determined.
Friability
Approximately 3 g of pellets were accurately weighed and placed into a TAR 120 Erweka (Germany) friability test apparatus. The process was carried out for 100 cycles at 25 ± 1 rpm. The pellets were then withdrawn and re-weighed. The friability was determined as the percentage of weight loss compared to the initial weight of the pellet [33].
In vitro dissolution testing
In vitro dissolution studies were conducted using Dissolution Apparatus 708 DS (Agilent) with a USP 44 type-II paddle apparatus. Pellets equivalent to 60 mg of PNS were placed in 900 ml of pH 6.8 phosphate buffer solution and stirred at 100 rpm. The temperature of the dissolution medium was maintained at 37°C ± 0.5°C. After 5, 10, 15, 30, and 45 minutes, the samples were withdrawn and filtered through filter paper, and the same volume of the medium was added. 10 ml samples of the dissolved solution were transferred to a glass test tube and evaporated to dryness at 60°C under vacuum. The dried sample was dissolved in 1 ml of 70% methanol, shaken for 2 minutes to ensure complete dissolution, filtered through a 0.2 μm filter membrane, and subjected to the HPLC system. The drug release was measured using the following formula:
Drug release rate (%) = (weight of dissolved PNS/weight of theoretical PNS) × 100.
The weight of PNS was calculated based on the total weight of five saponins including notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd.
Powder X-ray diffraction
The X-ray diffraction patterns of pure PNS, excipient samples, physical mixture, and core pellet were examined using a high-resolution X-ray diffractometer (D8 Advance, Bruker, Germany). The range of diffraction angles (2θ) scanned for the sample was from 2° to 60°.
Differential scanning calorimetry (DSC) thermal analysis
The thermal properties of PNS, excipients, physical mixture, and core pellet were assessed using a PT1000 LINSEIS DSC apparatus (Germany). Samples weighing approximately 5–10 mg were accurately weighed onto an aluminum cup and subjected to heating from 40°C to 250°C at a rate of 10°C/minute under argon gas flow at 40 ml/minute.
Fourier-transform infrared spectroscopy (FTIR) spectrum
An Agilent Cary 630 FT-IR spectrophotometer (USA) was employed to perform FTIR spectroscopy on PNS, excipients, physical mixture, and core pellet samples. A quantity of 10–20 mg of the sample was deposited directly onto a diamond surface. The spectrum was obtained using an FTIR infrared spectrophotometer with a resolution of 0.4 cm−1 and a wavelength range of 4,000–600 cm−1.
Histological evaluation
Pigs were slaughtered at a local abattoir. Tissue samples of jejunum were excised immediately after slaughter, then cleaned with ice-cold KBR (120 mM NaCl, 5.5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 20 mM NaHCO3, and 11 mM glucose; pH 7.4). 3 × 3 cm pieces were collected and exposed to the PNS solution and the dissolved pellet solution in a pH 7.4 phosphate buffer with a corresponding PNS concentration of 100 μg/ml for 2 hours. These samples were subjected to dehydration using a 10% buffered formaldehyde solution. The dehydrated specimens were then embedded in paraffin wax and stained with hematoxylin and eosin. Slides were imaged under a light microscope (MT5300L Meiji, Japan). Microphotographs were acquired using a digital camera equipped with software (Meiji Techno HD1500MET Color HD).
Enteric-coated pellets
SEM analysis
The morphology of coated pellets was observed using the FE-SEM S-4800 scanning electron microscope (Hitachi, Japan) at an acceleration voltage of 10 kV. The samples were affixed onto an aluminum stub using double-sided adhesive tape and subjected to gold coating under vacuum conditions.
Bulk density and tapped density
An accurate amount of pellets (M0) was placed in a measuring cylinder and the bulk volume (V0) was recorded. The cylinder was then tapped for 5 minutes to obtain the tapped volume (Vt). The bulk density (D0) and tapped density (Dt) were calculated using the following formulas: D0 = M0/V0, Dt = M0/Vt. Carr’s index (CI) was determined as below: CI = 100 × (Dt-D0)/Dt (%).
Flowability
The flowability of pellets was measured using a GTL Erweka (Germany) flowability tester with a funnel diameter of 10 mm. The flow rate was calculated using the following formula: V = m/t, where V is the flow rate of the pellets (g/s), m is the weight of the pellets (g), and t is the flow time (s).
Drug content
An appropriate amount of enteric-coating pellets was pulverized and dissolved in 70% methanol. The samples were filtered through a 0.2 μm filter membrane. The resulting supernatant was subjected to HPLC analysis under the described conditions.
In vitro dissolution testing
In vitro drug release studies were performed according to the dissolution criteria of delayed-released dosage forms (Method A) in USP 44. Pellet samples equivalent to 60 mg of PNS were placed in 750 ml of 0.1 N hydrochloric acid medium. The dissolution medium was maintained at a temperature of 37°C ± 0.5°C with a stirring rate of 100 rpm. After 15 and 120 minutes, the amount of drug released in the acid medium was calculated. Then, 250 ml of 0.2 mol/l trisodium phosphate buffer solution was added to the mixture and dissolution was continued for an additional 45 minutes. After 5, 10, 15, 30, and 45 minutes, the samples were treated and quantified by the HPLC method as described in the previous section.
RESULTS AND DISCUSSION
Acute toxicity of PNS in zebrafish
Survival and abnormal rates of embryos
As shown in Figure 2A, the survival embryonic rate and abnormal embryonic rate of zebrafish larvae depended on the concentration of PNS with increasing toxicity observed at high concentrations. After 48 hpf of exposure, the survival embryos significantly decreased compared to 24 hpf. At 96 hpf, the percentages of survival eggs at PNS concentrations of 5, 10, and 50 μg/ml were less than 50% compared to the control sample. The survival rates of zebrafish embryos treated with 10 and 50 μg/ml of PNS declined to zero at 120 hpf (Fig. 2B). Based on the survival rate, the LC50 value of PNS was calculated to be 0.9671 μg/ml. Moreover, at this time, the percentage of embryonic abnormality treated with 10 and 50 μg/ml of PNS also reached a peak of 100%, which increased significantly compared to the control group (p < 0.001) (Fig. 2C). Morphological defects of embryos including pericardial edema and curved tail could be seen in Figure 3.
Heartbeat of zebrafish embryos
Figure 2D showed the heartbeat of zebrafish at 120 hpf with various levels. The normal heartbeat range for zebrafish embryos is between 120 and 180 beats per minute (bpm) [34]. There was no statistically significant difference in heart rate between the treatment groups receiving PNS concentrations ranging from 0.01 to 1 μg/ml and the control group. However, it could be observed that embryos treated with 10 μg/ml of PNS died at 120 hpf due to no heartbeat.
In summary, the results showed that exposure of zebrafish embryos to oral administration of PNS at concentrations higher than 1 μg/ml may induce acute toxicity with morphological changes such as pericardial edema and curved tail. This finding is consistent with a previous study. Wang et al. [35] indicated that following 21 days of daily oral administration, decocted extract of PNG at levels of 0.5, 1.5, and 5.0 μg/ml was found to decrease the body weight, body length, and vertebral count of zebrafish embryos, indicating the presence of developmental toxicity. The toxicity mechanism of high-dose PNS could be associated with the dysregulation of lipid metabolism, amino acid metabolism, and energy metabolism [36]. However, Xiong et al. [37] revealed that there was no observed toxicity on the survival of zebrafish larvae in the treated groups with raw PNG powder at 50 μg/ml; raw PNG extract at 50 μg/ml; steamed PNG powder at 50 μg/ml; and steamed PNG extract at 50 μg/ml. In this research, the investigation of the toxicity of PNS was necessary. Based on the result of the developmental toxicity, it can be identified the dose of PNS for the next research using the zebrafish as a study model. In this research, we also prepared the pellets containing PNS to enhance the absorption and permeability of PNS. Thus, we can use the safe dose to determine the activity of the phytochemical compound and compare it to new formulations. In addition, these findings revealed that it could be careful when administering different types of dosage forms containing PNS for children [35].
Preparation of pellets by the dripping method
The fabrication of pellets containing PNS could be influenced by various factors: the ratio of excipients, melting point temperature, cold fluid temperature, the distance of dripping, and the height of cold fluid. The roundness (the ratio of the largest diameter and the smallest diameter of the pellets) was used as an indicator to evaluate pellet preparation.
The effect of ingredients on the preparation of pellets
The ratio of PEGs
The effect of various amounts of PEGs on the roundness of pellets was shown in Figure 4A. The result showed that pellet shape in all formulations except for P4 could be considered round with roundness values ranging from 1.1 to 1.2 and RSD ranging from 1.2 to 3.0. P6 formulation (PEG 4000/PEG 6000: 3/1, w/w) was chosen for further studies.
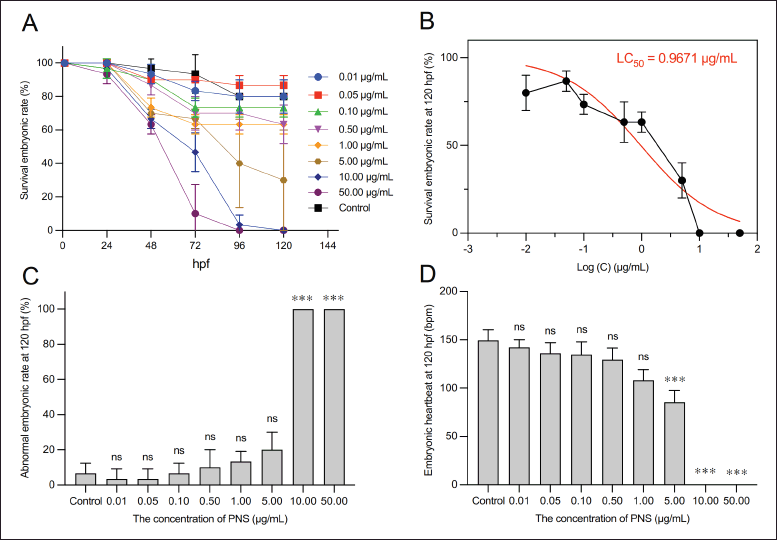 | Figure 2. The effect of PNS various levels on (A–B) survival embryonic rate, (C) Abnormal embryonic rate, and (D) Embryonic heart rate. The data were shown as means ± SD (n = 3); ns: not significant, *** p < 0.001 compared with the control group, one-way ANOVA, and Tukey post-hoc analysis. [Click here to view] |
Absorption enhancers
Cyclodextrins are effective in enhancing the bioavailability of numerous drugs [38], including ginsenosides extracted from Panax ginseng [39,40]. SLS is an anionic surfactant that enhances drug permeation across the membrane by solubilizing the intestinal mucosa, thereby releasing the proteins and phospholipids of the mucosal layer [41]. Therefore, from our preliminary experimental research (data not shown), β-CD and SLS were used in a corresponding ratio of 5% and 2% (w/w) relative to PNS to improve the permeability of PNS. The addition of absorption enhancers was found to not affect the shape of the pellets. Therefore, the best formulation of pellets was selected as follows: PNS, 4.4 g; PEG 4000, 7.5 g; PEG 6000, 2.5 g; β-CD, 0.22; and SLS, 0.088 g.
The effect of parameters on the preparation of pellets
Melting point temperature
Figure 4B displayed the influence of melting liquid temperature on the formation of pellets. The results indicated that a higher temperature may lead to a rounder shape with a roundness value closer to one. However, to stabilize the drug, the optimal temperature was determined to be 80°C.
Cold fluid temperature
As shown in Figure 4C, at temperatures below 0°C, the pellets were spherical and uniform. However, at temperatures above 0°C, elongated droplets were formed, which resulted in non-spherical pellets. In particular, as the temperature increased, the pellets tended to elongate droplets at the bottom of the container. The appropriate condensate temperature of paraffin liquid was from −5°C to 0°C.
The distance of dripping
The effect of the distance of dripping on forming pellets was presented in Figure 4D. When the distance was too short, the droplets were prone to floating and condensing on the surface of the paraffin due to the surface tension, which deformed the pellets. When the distance was too long, owing to the gravity of the droplets, a part of the droplets was broken into small particles. They contacted the surface of the paraffin liquid, affecting the mass and efficiency of pellet formation. A dripping distance of 13 cm was selected for further research studies.
The height of cold fluid
Figure 4E showed that at a height of 20 cm in the cold column, the pellet particles are deformed. However, at heights above 20 cm from 25 to 40 cm, the pellet particles have a spherical shape, even surface and smooth texture. In fact, as the height of the paraffin column increased, the pellet particles became more uniformly spherical. In addition, the longer the moving time of the pellets inside the column, the more perfectly round their shape becomes. A height of 35 cm for the cooled paraffin column was suitable for this study.
In conclusion, the optimal parameters for preparing pellets were chosen as follows: melting point temperature: 80°C; cold fluid temperature: −5°C → 0°C; the distance of dripping: 13 cm; and the height of cold fluid: 35 cm.
Characterization of pellets
Dripping pellets
Average diameter, drug content, weight variation, friability, and in vitro dissolution study
The prepared pellets displayed a mean weight of 32.1 ± 0.6 mg with low weight variation and a mean particle diameter of 3.60 ± 0.05 mm (Table 4, Fig. 5A). The drug content in the dripping pellets was 29.86% ± 0.58% (w/w) equivalent to 9.59 ± 0.19 mg of PNS in each dripping pellet, the HPLC chromatograms of PNS was shown in Figure 5B. Friability is a crucial criterion for pellets that are used for coating. The friability of pellets was less than 1%, which indicated that pellets may be considered acceptable for the coating process according to USP 44. It could be seen that the drug was released rapidly due to the presence of PEGs in the formulation [42,43]. More than 90% of the drug was dissolved after 15 minutes of dissolution testing (Fig. 6D), which revealed that pellets could be released quickly in a pH 6.8 buffer solution.
X-ray powder diffraction (XRPD) analysis
The XRPD patterns of PNS, excipients, physical mixture, and dripping pellets were presented in Figure 6A. The XRPD data of PNS showed no shape peak, suggesting that PNS may exist as an amorphous form [44,45]. The XRPD pattern of SLS had characteristic diffraction peaks intensely at 2.2°, 4.3°, and 6.6° [46]. PEGs had diffraction peaks at 19.1° and 23.2°, which was consistent with previous studies [47,48]. Meanwhile, the X-ray diffraction (XRD) spectra of the physical mixture and dripping pellet had diffraction peaks that were similar to those of PEGs due to the high proportion of PEGs in the pellet formulation (nearly 70%) and no strange diffraction peak was observed. This may demonstrate that the drug still existed in an amorphous state after the pellet manufacturing stages.
 | Table 4. The characteristics of dripping pellets: average diameter, drug content, weight variation and friability (n = 3). [Click here to view] |
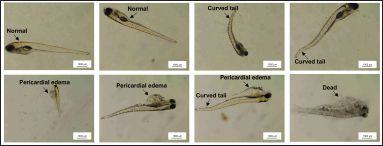 | Figure 3. Morphological defects of embryos at 120 hpf. [Click here to view] |
DSC analysis
The DSC thermogram of PNS exhibited an endothermic peak at 210°C (Fig. 6B), which might be the melting point of the PNS [49]. PEG 4000 and PEG 6000 showed sharp respective endothermic peaks at 64°C and 65°C, which corresponded to their respective melting points [50,51]. The melting peak of β-CD was 102°C [52]. The dripping pellet and the physical mixture showed similar endothermic peaks at 64°C, which corresponded to the presence of PEGs in the formulation. In addition, the peak area of the pellet samples (corresponding to a heat of fusion value of 161.79 J/g) was found to be similar to that of the physical mixture (corresponding to a heat of fusion value of 163.11 J/g), which suggested that the drug still remained in an amorphous form and dispersed uniformly in the carrier mixture.
FTIR spectroscopy
FTIR spectroscopy of samples was shown in Figure 6C. The FTIR spectrum of PNS is characterized by bands at 3,228 cm−1 (O-H bonds), 2,929 cm−1 (C-H bonds), and 1,252 cm−1, 1,088 cm−1, and 1,047 cm-−1 (C-O bonds) [15]. The FT-IR spectra of PEGs are characterized by strong vibration bands at 2,881 cm−1, 1,465 cm−1, 1,341 cm−1, 1,278 cm−1, 1,238 cm−1, 958 cm−1, and 842 cm−1 (C-H bond) and 1,100 cm−1 (C-O-C bond) [47,53]. These peaks were found on the spectra of the physical mixture and dripping pellet. In addition, no peak shift was found in the pellet formulation. From the results of X-ray analysis, DSC analysis and FTIR spectroscopy, it could be concluded that the drug remained in an amorphous state and there was no interaction between the drug and excipients in the preparation of pellets.
Histology
As shown in Figure 7A, the epithelial tissue cells in the control group revealed normal morphological features with no observed instances of exfoliation or loss of membrane continuity. The jejunum after exposure to PNS maintained a homogeneous distribution of villi cells (Fig. 7B). This finding could demonstrate that the oral administration of PNS is safe owing to no mucosal irritation. The use of permeation enhancers in the pellet formulation could result in mild disruption of the mucosal layer (Fig. 7C). However, the submucosal layer remained intact and the pouches of mucus-secreting cells were distributed along the villi. These results may also be attributed to SLS, which altered the surface of the small intestine mucosa, thereby improving the permeability of PNS. These findings are consistent with a prior study by Twarog et al. [54] in which the authors demonstrated that the use of permeation enhancers including sodium salcaprozate and sodium caprate, resulted in minor damage to the colonic tissue. However, this disruption and damage may be fully restored. Berg et al. [55] observed erosion of villus epithelial cells after a 10–60 minute exposure to 300 mM sodium caprate. After 120 minutes, the intestinal cell layer exhibited recovery with tissue covering the villi under the mucosa. In this study, the use of permeation enhancers had a minor impact on the surface of the small intestine mucosa.
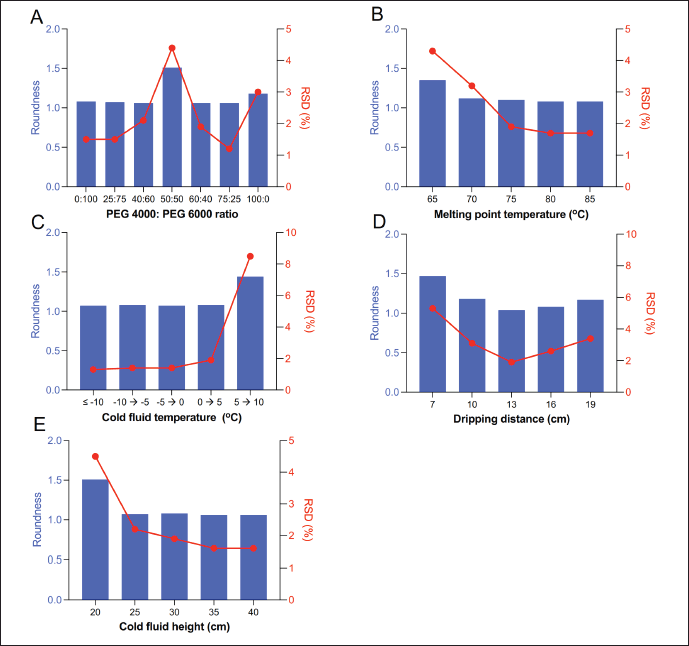 | Figure 4. The optimized process is based on the roundness and RSD of prepared pellets. (A) Effects of the ratio of PEGs, (B) Effects of melting point temperatures, (C) Effects of cold fluid temperatures, (D) Effects of dripping distances, and (E) Effects of cold fluid heights. [Click here to view] |
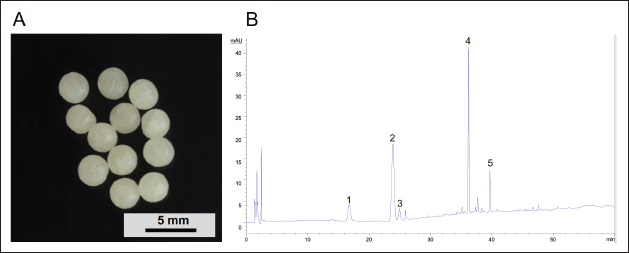 | Figure 5. (A) The appearance of dripping pellets and (B) HPLC chromatograms of PNS: (1) notoginsenoside R1, (2) ginsenoside Rg1, (3) ginsenoside Re, (4) ginsenoside Rb1, and (5) ginsenoside Rd. [Click here to view] |
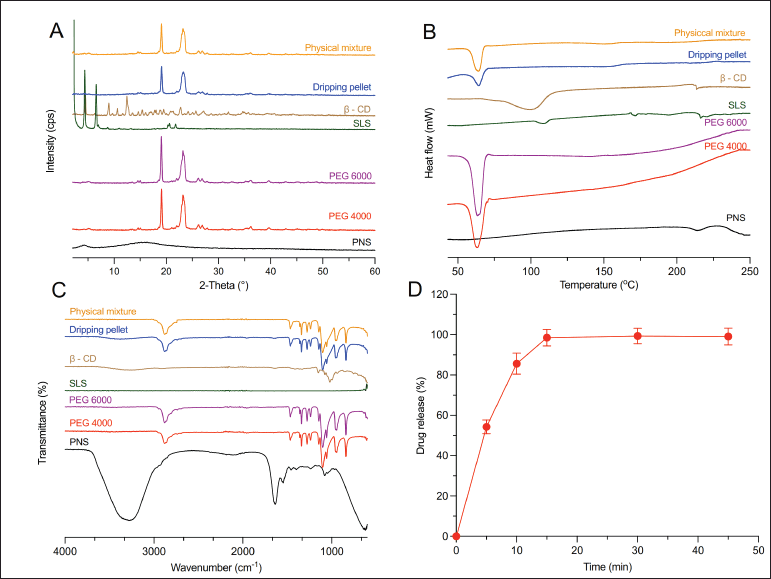 | Figure 6. (A) XRPD diffractograms, (B) DSC analysis, and (C) FTIR spectroscopy of samples. (D) The dissolution profile of dripping pellets. [Click here to view] |
 | Figure 7. Histopathological observation of jejunum of pigs. (A) Control group, (B) Exposure to PNS, and (C) Exposure to dripping pellet. [Click here to view] |
Enteric-coated pellets
Appearance
The appearance of enteric-coated pellets is shown in Figure 8C. Prepared pellets were spherical and uniform.
SEM analysis
The cross-sectional view and surface of the pellets are shown in Figure 8A and B, respectively. Enteric-coated pellets were spherical with a smooth surface. The structure of the dripping pellet exhibited a high degree of compactness. The homogeneously enteric coating layer was applied uniformly onto the core pellet.
Bulk density and tapped density
The bulk density, tapped density, and CI of prepared pellets were 0.64 g.cm−1, 0.70 g.cm−1, and 8.57%, respectively. The prepared pellets had good flowability due to a low CI of less than 10% [56].
Flowability
The flowability characterization of pellets was good at a flow rate of 31.1 g.s−1. Pellets were used as an intermediate product, subsequently compressed into tablets or formulated into capsules. The results of flow rate and CI indicated that pellets exhibited excellent flowability, which facilitates the filling of pellets into hard capsules or their compression into tablets [14].
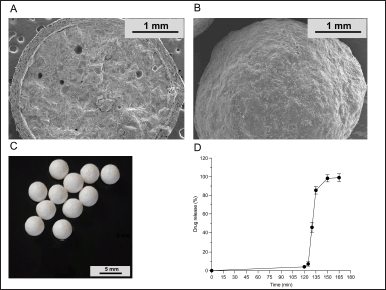 | Figure 8. The SEM images of (A) the cross section and (B) the surface, (C) The appearance and (D) The drug release of the enteric-coated pellets. [Click here to view] |
Drug content
The content of PNS in prepared pellets was 22.71% ± 0.76% (w/w) equivalent to 9.57 ± 0.21 mg of PNS in each enteric-coated pellet.
In vitro dissolution testing
As shown in Figure 8D, less than 10% of the drug (4.1% ± 0.9%) was released within 2 hours in the 0.1 N hydrochloric acid medium, suggesting that the drug could not be leaked in the acid stage. Meanwhile, more than 80% of the drug (92.1% ± 0.5%) was released within 45 minutes in the medium of phosphate buffer pH 6.8. These results revealed that prepared pellets meet the USP 44 dissolution test for delayed-release dosage forms. Eudragit L100 is a frequently employed polymer in the coating layer of modified-release drug formulations to impede drug release in the stomach [57]. The weight gain of enteric polymers ranged from 15% to 30% [58]. In this study, 25% of the polymer weight gain was needed to control drug release in a 0.1 N hydrochloric acid medium after a 2-hour period. Furthermore, the drug also was released quickly in a pH 6.0 phosphate buffer. These findings are consistent with previous studies [59,60].
CONCLUSION
The obtained results showed that the acute toxicity of PNS in zebrafish larvae indicated a potential risk associated with the clinical use of these substances in children. The dripping pellets had the characteristics of easy preparation, good uniformity, great friability, high dissolution rate, and high stability. Absorption enhancers were loaded successfully into the core pellets to enhance the permeability of PNS and caused a small impact on the surface of the jejunal mucosa via histological observation. In addition, the enteric-coated pellets exhibited a smooth surface, good sphericity, flowability, content uniformity, stability in a pH 1.2 acidic medium, and quick drug release in a pH 6.8 buffer solution.
ACKNOWLEDGMENT
Nguyen Van Khanh was funded by the PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), VINIF.2022.TS057.
AUTHOR CONTRIBUTIONS
Khanh Van Nguyen: investigation, analysis, writing, review, and editing. Nhung Thi Hong Nguyen: investigation and analysis. Hoa Thi Dinh: investigation and analysis. Hanh Thi Hong Vu: writing, review, and editing. Giang Thi Thu Vu: writing, review, and editing.
CONFLICTS OF INTEREST
All of the authors have declared that there is no conflict of interest.
ETHICAL APPROVALS
All animal experiments were conducted in strict adherence to a protocol approved by the Ethics Committee in Biomedical Research of the Hanoi University of Pharmacy, following the guidelines outlined in Consent Form No. 2023.06/ PCT-HDDD issued on 20 April 2023.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wang X, Robertson AL, Li J, Chai RJ, Haishan W, Sadiku P, et al. Inhibitors of neutrophil recruitment identified using transgenic zebrafish to screen a natural product library. Dis Model Mech. 2014;7(1):163–9. CrossRef
2. Han HS, Jang GH, Jun I, Seo H, Park J, Glyn-Jones S, et al. Transgenic zebrafish model for quantification and visualization of tissue toxicity caused by alloying elements in newly developed biodegradable metal. Sci Rep. 2018;8(1):13818. CrossRef
3. Hermsen SAB, van den Brandhof EJ, van der Ven LTM, Piersma AH. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol In Vitro. 2011;25(3):745–53. CrossRef
4. Chahardehi AM, Arsad H, Lim V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants (Basel). 2020;9(10):1345. CrossRef
5. Xia PG, Zhang SC, Liang ZS, Qi ZH. Research history and overview of chemical constituents of Panax notoginseng. Chin Tradit Herb Drugs. 2014;45:2564–70.
6. Li Y, Zhang Y, Zhu CY. Pharmacokinetics and correlation between in vitro release and in vivo absorption of bio-adhesive pellets of Panax notoginseng saponins. Chin J Nat Med. 2017;15(2):142–51. CrossRef
7. Zheng S, Wang Y, Liu H, Chang W, Xu Y, Lin F. Prediction of hemolytic toxicity for saponins by machine-learning methods. Chem Res Toxicol. 2019;32(6):1014–26. CrossRef
8. Lin B, Qi X, Fang L, Zhao L, Zhang R, Jing J, et al. In vivo acute toxicity and mutagenic analysis of crude saponins from Chenopodium quinoa Willd husks. RSC Adv. 2021;11(8):4829–41. CrossRef
9. Wisløff H, Uhlig S, Scheie E, Loader J, Wilkins A, Flåøyen A. Toxicity testing of saponin-containing Yucca schidigera Roetzl. juice in relation to hepato-and nephrotoxicity of Narthecium ossifragum (L.) Huds. Toxicon. 2008;51(1):140–50. CrossRef
10. Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui F, et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. 2016;188:234–58. CrossRef
11. Xiong J, Guo J, Huang L, Meng B, Ping Q. Self-micelle formation and the incorporation of lipid in the formulation affect the intestinal absorption of Panax notoginseng. Int J Pharm. 2008;360(1):191–6. CrossRef
12. Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84(2–3):187–92. CrossRef
13. Tian Z, Pang H, Zhang Q, Du S, Lu Y, Zhang L, et al. Effect of aspirin on the pharmacokinetics and absorption of Panax notoginseng saponins. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1074–1075:25–33. CrossRef
14. Zhao YL, Zhang SQ, Lu WX, Shen SZ, Wei L. Preparation of Panax notoginseng flower saponins enteric-coated sustained-release pellets and its pharmacokinetics and in vitro-in vivo correlation. J Drug Deliv Sci Technol. 2021;62(11):1–21. CrossRef
15. Nguyen KV, Dang TK, Pham HT, Nguyen BT, Thu Vu G, Nguyen HT, et al. Development of Panax notoginseng saponins-loaded orodispersible films: a potential approach to enhance delivery efficacy in older adults. J Appl Pharm Sci. 2022;12(04):044–53. CrossRef
16. Lai L, Wen L. Correlation between in vivo absorption and in vitro release of Panax notoginseng saponins enteric pellets. Chin J Exper Tradit Med Form. 2011;17(24):97–100.
17. Lai L, Lu SH. Pharmacokinetics and correlation between in vivo absorption and in vitro release of Panax notoginseng saponins enteric microcapsule. Chin J New Drug. 2012;21(6):693–6.
18. Chen XN, Li DQ, Zhao MD, Yu GY, Du SY, Lu Y, et al. Pharmacokinetics of Panax notoginseng saponins in adhesive and normal preparation of Fufang Danshen. Eur J Drug Metab Pharmacokinet. 2018;43(2):215–25. CrossRef
19. Chen QY, Wang WP. Drug release in vitro mathematical model of Panax notoginoside micro-porous osmotic pump tablet. Chin Tradit Patent Med. 2009;31(4):538–40.
20. Nie X, Wang B, Hu R, Lu W, Chen J, Liu S, et al. Development and evaluation of controlled and simultaneous release of compound Danshen based on a novel colon-specific osmotic pump capsule. AAPS PharmSciTech. 2020;21(2):1–12. CrossRef
21. Shao L, Sun C, Lu W, Chen J, Su D, Gao S, et al. Effects of borneol on the release of compound Danshen colon-specific osmotic pump capsule in vitro and pharmacokinetics study in beagle dogs. AAPS PharmSciTech. 2020;21(8):1–12. CrossRef
22. Wu Y, Lin N. Study on preparation and release characteristics of Panax notoginseng saponins controlled-release preparations. J Hubei Univ Chin Med. 2013;15(2):33–6.
23. Feng H, Chen W, Zhu C. Pharmacokinetics study of bio-adhesive tablet of Panax notoginseng saponins. Int Arch Med. 2011;4(1):1–8. CrossRef
24. Jain SP, Singh PP, Amin PD. Alternative extrusion-spheronization aids. Drug Dev Ind Pharm. 2010;36(11):1364–76. CrossRef
25. Chang CW, Wong CY, Wu YT, Hsu MC. Development of a solid dispersion system for improving the oral bioavailability of resveratrol in rats. Eur J Drug Metab Pharmacokinet. 2017;42(2):239–49. CrossRef
26. Chang CW, Wang CY, Wu YT, Hsu MC. Enhanced solubility, dissolution, and absorption of lycopene by a solid dispersion technique: the dripping pill delivery system. Powder Technol. 2016;301:641–8. CrossRef
27. Busquet F, Halder BT, Gourmelon LA, Kleensang A, Belanger SE, Carr GJ, et al. OECD guidelines for the testing of chemicals 236-fish embryo acute toxicity (FET) test. OECD; Organisation for Economic Co-operation and Development: Paris, France. 2013.
28. Riesco MF, Martínez-Pastor F, Chereguini O, Robles V. Evaluation of zebrafish (Danio rerio) PGCs viability and DNA damage using different cryopreservation protocols. Theriogenology. 2012;77(1):122–30. CrossRef
29. Mektrirat R, Yano T, Okonogi S, Katip W, Pikulkaew S. Phytochemical and safety evaluations of volatile terpenoids from Zingiber cassumunar Roxb. on mature carp peripheral blood mononuclear cells and embryonic zebrafish. Molecules. 2020;25(3):613. CrossRef
30. Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50(1):47–60. CrossRef
31. Paberit R, Rilby E, Göhl J, Swenson J, Refaa Z, Johansson P, et al. Cycling stability of poly(ethylene glycol) of six molecular weights: influence of thermal conditions for energy applications. ACS Appl Energy Mater. 2020;3(11):10578–89. CrossRef
32. Kuang C, Sun Y, Li B, Fan R, Zhang J, Yao Y, et al. Preparation and evaluation of duloxetine hydrochloride enteric-coated pellets with different enteric polymers. Asian J Pharm Sci. 2017;12(3):216–26. CrossRef
33. Hu L, Shi Y, Li JH, Gao N, Ji J, Niu F, et al. Enhancement of oral bioavailability of curcumin by a novel solid dispersion system. AAPS PharmSciTech. 2015;16(6):1327–34. CrossRef
34. De Luca E, Zaccaria GM, Hadhoud M, Rizzo G, Ponzini R, Morbiducci U, et al. ZebraBeat: a flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci Rep. 2014;4(1):4898. CrossRef
35. Wang RR, Li T, Zhang L, Hu ZY, Zhou L, Shan LT, et al. Acute developmental toxicity of Panax notoginseng in zebrafish larvae. Chin J Integr Med. 2023;29(4):333–40. CrossRef
36. Fei QQ, Wei YJ, Wang J, Huang YP, Chen Y, Chen B. Acute toxicity mechanism of Panax notoginseng saponins in larvae zebrafish based on metabonomics. Zhongguo Zhong Yao Za Zhi. 2019;44(17):3798–805.
37. Xiong Y, Halima M, Che X, Zhang Y, Schaaf MJM, Li M, et al. Steamed Panax notoginseng and its saponins inhibit the migration and induce the apoptosis of neutrophils in a zebrafish tail-fin amputation model. Front Pharmacol. 2022;13:946900. CrossRef
38. Loftsson T, Moya-Ortega MD, Alvarez-Lorenzo C, Concheiro A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J Pharm Pharmacol. 2016;68(5):544–55. CrossRef
39. Li H, Zhang G, Wang W, Chen C, Jiao L, Wu W. Preparation, characterization, and bioavailability of host-guest inclusion complex of ginsenoside Re with gamma-cyclodextrin. Molecules. 2021;26(23):7227. CrossRef
40. Sharma A, Lee HJ. Ginsenoside compound K: insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules. 2020;10(7):1028. CrossRef
41. Junginger HE. Excipients as absorption enhancers. In: Krishna R, Yu L, editors. Biopharmaceutics applications in drug development. Boston, MA: Springer US; 2008. p 139–74. CrossRef
42. Biswal S, Sahoo J, Murthy PN, Giradkar RP, Avari JG. Enhancement of dissolution rate of gliclazide using solid dispersions with polyethylene glycol 6000. AAPS PharmSciTech. 2008;9(2):563–70. CrossRef
43. Xu XL, Zhu Q, Bian TZ, Liu KH. Preparation of vitamin C dripping pill and its quality evaluation. Adv Mater Res. 2013;602–604:1215–8. CrossRef
44. Zhou B, Zhang W, Wu Y, Yang Y, Wang N, Li J, et al. Improved efficacy of Panax notoginseng saponin loaded into BSP/alginate microspheres for the treatment of alcoholic gastric ulcers. Int J Pharm. 2021;596:120218. CrossRef
45. Zhang Y, Zhao X, Li W, Zu Y, Li Y, Wang K. Preparation, characterization, and dissolution rate in vitro evaluation of total Panax notoginsenoside nanoparticles, typical multicomponent extracts from traditional chinese medicine, using supercritical antisolvent process. J Nanomat. 2015;2015:439540. CrossRef
46. Cao X, Wang J, Liu M, Chen Y, Cao Y, Yu X. Chitosan-collagen/organomontmorillonite scaffold for bone tissue engineering. Front Mater Sci. 2015;9(4):405–12. CrossRef
47. Fu X, Weibo K, Zhang Y, Jiang L, Wang J, Lei J. Novel solid-solid phase change materials with biodegradable trihydroxy surfactant for thermal energy storage. RSC Adv. 2015;5:68881–9. CrossRef
48. Martínez-Ohárriz MC, Martín C, Goñi MM, Rodríguez-Espinosa C, Tros-Ilarduya MC, Zornoza A. Influence of polyethylene glycol 4000 on the polymorphic forms of diflunisal. Eur J Pharm Sci. 1999;8(2):127–32. CrossRef
49. Zhou Z, Sun X, Cheng J, Ban Q, Guo M. Physicochemical, digestive, and sensory properties of Panax notoginseng saponins encapsulated by polymerized whey protein. Foods (Basel, Switzerland). 2021;10(12):2942. CrossRef
50. Jayaramudu T, Raghavendra GM, Varaprasad K, Subba Reddy G, Reddy A, Sudhakar K, et al. Preparation and characterization of poly (ethylene glycol) stabilized nano silver particles by a mechanochemical assisted ball mill process. J Appl Polym Sci. 2016;133:43027. CrossRef
51. Barghi L, Asgari D, Barar J, Valizadeh H. Synthesis of PCEC copolymers with controlled molecular weight using full factorial methodology. Adv Pharm Bull. 2015;5:51–6.
52. David I, Orboi MD, Simandi MD, Chiril? CA, Megyesi CI, R?dulescu L, et al. Fatty acid profile of Romanian’s common bean (Phaseolus vulgaris L.) lipid fractions and their complexation ability by β-cyclodextrin. PLoS One. 2019;14(11):e0225474. CrossRef
53. Altamimi MA, Elzayat EM, Qamar W, Alshehri SM, Sherif AY, Haq N, et al. Evaluation of the bioavailability of hydrocortisone when prepared as solid dispersion. Saudi Pharm J. 2019;27(5):629–36. CrossRef
54. Twarog C, McCartney F, Harrison SM, Illel B, Fattal E, Brayden DJ. Comparison of the effects of the intestinal permeation enhancers, SNAC and sodium caprate (C10): isolated rat intestinal mucosae and sacs. Eur J Pharm Sci. 2021;158:105685. CrossRef
55. Berg S, Kärrberg L, Suljovic D, Seeliger F, Söderberg M, Perez-Alcazar M, et al. Impact of intestinal concentration and colloidal structure on the permeation-enhancing efficiency of sodium caprate in the rat. Mol Pharm. 2022;19(1):200–12.
56. Goyal A, Sharma V, Sihag MK, Tomar SK, Arora S, Sabikhi L, et al. Development and physico-chemical characterization of microencapsulated flaxseed oil powder: a functional ingredient for omega-3 fortification. Powder Technol. 2015;286:527–37. CrossRef
57. Widjaja M, Gan J, Talpaneni JSR, Tjandrawinata RR. Determination of Eudragit® L100 in an enteric-coated tablet formulation using size-exclusion chromatography with charged-aerosol detection. Sci Pharm. 2018;86(3):38. CrossRef
58. Duki?-Ott A, De Beer T, Remon JP, Baeyens W, Foreman P, Vervaet C. In-vitro and in-vivo evaluation of enteric-coated starch-based pellets prepared via extrusion/spheronisation. Eur J Pharm Biopharm. 2008;70(1):302–12. CrossRef
59. Shravani D, Lakshmi PK, Balasubramaniam J. Preparation and optimization of various parameters of enteric coated pellets using the Taguchi L9 orthogonal array design and their characterization. Acta Pharm Sin B. 2011;1(1):56–63. CrossRef
60. Kovacevic J, Mladenovic A, Djuris J, Ibric S. Evaluation of powder, solution and suspension layering for the preparation of enteric coated pellets. Eur J Pharm Sci. 2016;85:84–93. CrossRef