INTRODUCTION
Dyslipidaemia or hyperlipidemia is characterized by abnormal lipid panels that include increased levels of total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), and/or decreased levels of low high-density lipoprotein cholesterol (HDL-C) levels, which contribute to morbidity and mortality due to cardiovascular and cerebrovascular disease [1].
Statins are the gold standard drug for the treatment of dyslipidemia. However, several studies have reported that statins can cause serious side effects such as hepatotoxicity, skeletal muscle-related toxicity, renal toxicity, neurocognitive and neurological effects, and other conditions [2,3]. Therefore, medication or novel agents for dyslipidemia treatment without undesirable side effects remain a challenging question for medical researchers.
The enhancement of oxidative stress phenomena is associated with the dysregulation of lipid metabolism leading to hyperlipidemia [4,5]. The oxidative modulation of the lipid profile especially LDL-C with increased formation of free radicals and lipid peroxidation products such as malondialdehyde (MDA) leads to biomolecular damage and cellular dysfunction [6]. These complications occurring in the context of hyperlipidemia are due in part to reduced activities of the endogenous enzymatic antioxidant defense system such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which induce oxidative damage [7].
Herbal tea drinking has earned a lot of attention in recent times because of the increase in awareness of food nutritive value and healthcare. Many studies have reported that herbal teas have a wide variety of active phytochemicals with biological properties in the prevention of metabolic disorders such as obesity, diabetes type 2, and hypercholesterolemia [8,9].
Makiang (MK) or Cleistocalyx nervosum var. paniala belongs to the Myrtaceae family, which is widely grown in southeast Asia including northern Thailand [10]. Moreover, it is also classified as a preservation plant under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn project on the MK Heredity Conservation in Thailand [11]. Various parts of MK including leaves, flowers, buds, fruits, and seeds have long been used in traditional medicine [12] because of their valuable pharmacological and biological actions such as antimutagenic, antiaging, anticarcinogenic, antimicrobial, hepatoprotection, neuroprotection, immune enhancement activities especially antioxidant properties [13–15]. Previous studies by Manosroi et al. [16] reported that the highest total phenolic contents (TPCs) [1,032.49 ± 18.11 μg gallic acid equivalents (GAEs)/mg] and flavonoid contents (TFCs) (262.96 ± 2.98 μg quercetin equivalents/mg) were found in methanol extracts of young MK leaves. This extract also exhibited the highest free radical scavenging abilities (SC50 values of 0.02 ± 0.004 mg/ml), lipid peroxidation inhibition (IPC50 values of 0.02 ± 0.004 mg/ml), and tyrosinase inhibition capacities [50% inhibitory concentration (IC50) values of 0.02 ± 0.006 mg/ml]. In addition, a concentration of 0.1 mg/ml in methanol of MK leaf extract presented the highest matrix metalloproteinases-2 inhibition of 91.14% ± 1.67%. Despite being a rich source of polyphenolic compounds and having great antioxidant activities, there are no clinical studies demonstrating anti-hyperlipidemic or antioxidant properties of MK tea. Therefore, the present study was undertaken to determine the effect of MK tea on the lipid profile and serum biomarkers of oxidative stress in subjects with hyperlipidemia.
MATERIALS AND METHODS
MK tea preparation
Fresh young MK leaves were obtained from a farm at Maejo University, Phrae Campus, Phrae, Thailand, at the end of August. The steps of nonfermented MK tea processing: picking, screening, rolling, roasting, and baking were done by Assist. Prof. Dr. Rattaphong Pokkaew, Department of Food Science and Technology, Maejo University, Phrae Campus, Thailand. Each nylon teabag contained 3 g of loose MK tea leaves.
Determination of total polyphenol and TFC of MK tea
The Folin Ciocalteu method [17] and a colorimetric assay [18] were used for the analysis of the TPC and TFCs of MK tea, respectively. All measurements were carried out in triplicate. The TPC was calculated from a calibration curve using gallic acid as standard and expressed as mg GAE/g of MK tea leaves. The TFC was calculated through the calibration curve of catechin and expressed as mg CE/g of MK tea leaves. Measurements were conducted in triplicate.
Determination of DPPH radical scavenging activity of MK tea
The antioxidant capacity of MK tea leaves was measured by the diphenyl-picrylhydrazyl (DPPH) method with some modifications. First, 1 ml of DPPH solution was added to 0.2 ml of the MK tea leaves diluted with ethanol. After 30 minutes, the absorbance was measured at 517 nm using a spectrophotometer. The free radical scavenging activity of MK tea on the DPPH assay was expressed as the IC50 value. The assay was performed in triplicate. In this case, Trolox served as the reference standard.
Study design, sample sizes, and participants
This single-blinded, randomized, and controlled clinical trial was conducted in accordance with the principles of the Declaration of Helsinki on human subjects and approved by the Ethics Committee for Human Research of the University of Phayao (No. UP-HEC 1.3/047/65).
The current study was designed for 64 patients, including 35 women and 29 men (aged 30–65 years). The sample size of the number of subjects per group was calculated using Stata software, considering α = 0.05, 90% power, and based on Gheflati et al. [19]. All subjects were recruited from the outpatient department of Phayao Hospital, Phayao Province, Thailand, between November 2022 and February 2023. A flow chart of the clinical study enrolment is shown in Figure 1.
Hyperlipidemia diagnosis was made by an endocrinologist based on lipid profile abnormalities (TG between 150 and 300 mg/dl; TC between 200 and 250 mg/dl; and LDL-C between 130 and 190 mg/dl). Subjects who were taking cholesterol-lowering medications, antioxidant agents, multivitamin supplementation or who suffered from severe dyslipidemia (TC more than 250 mg/dl; TG more than 300 mg/dl), hepatic disease, diabetes mellitus, heart failure, severe hypertension, renal failure, body mass index (BMI) more than 30 kg/m2, malignancy, pregnancy, metabolic bone disorders, infectious diseases, alcohol use, cigarette use, psychiatric and neurologic disorders, and allergic reactions to herbal or plant supplements were excluded from this study. Signed informed consent was provided by each participant, fulfilling all of the above-mentioned criteria, before any study procedures.
Trial intervention
Each subject was randomly allocated to the control group (n = 32) or the intervention group (n = 32) using simple random sampling. Neither the researchers nor the subjects themselves knew the group they belonged to. The control group drank one cup of the placebo bag infused in warm water (250 ml) once a day after breakfast during the experimental period, and the intervention group drank one cup of MK tea (MK tea 3 g/bag infused in 250 ml hot water for 10 minutes) once a day after a meal in the morning. MK teabags are tightly packed and sealed, the leaves of dried MK tea did not have enough room to expand and release flavor as they were steeped. All subjects were asked to continue consuming their usual daily food and regular lifestyle during the experiment. To ensure the participants in the intervention group consumed the MK tea, they were asked to bring the waste MK teabags to the next follow-up appointment. In addition, all participants were instructed to record 24-hour dietary recall, the dietary record [20] and were advised to avoid consuming other nutraceutical products or antioxidant supplements, to maintain their normal eating habits and to avoid any confounding error in the analysis of data during the study. The average energy intake of all participants was calculated using Nutritionist IV software (First Databank Inc., Hearst Corp., San Bruno, CA).
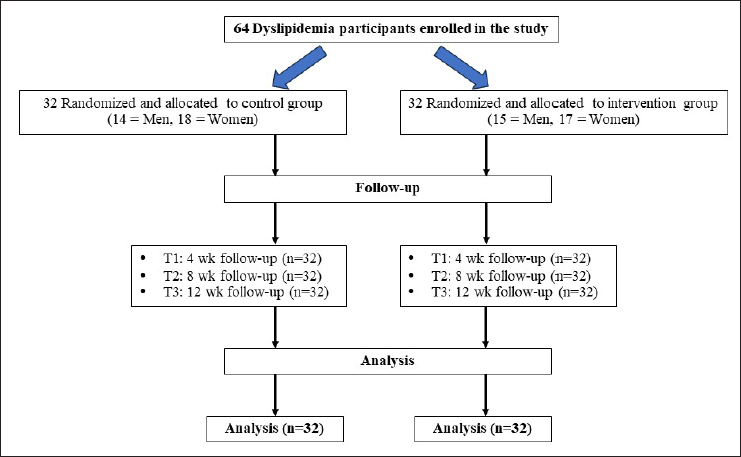 | Figure 1. Consort flow diagram describing the clinical trial study design. MK tea, Makiang tea. [Click here to view] |
The duration of the experiment was 12 weeks, all subjects were followed up every 4 weeks to ensure ongoing safety, compliance of trial participants, and counting of returned MK teabags. Each weekday, each subject’s height and weight were determined, and the BMI was calculated according to the formula: Weight (kg) / height (m)2. The lipid profile levels, including TC, TG, HDL-C, and LDL-C, and serum oxidative markers such as SOD, CAT, GPx, and MDA, were measured at baseline and after 12 weeks of the experimental period.
Participant blood samples
The concentrations of all lipid profiles were determined through fasting blood samples (10 ml) at the beginning and the end of the experimental trial. Venous whole blood samples were collected in blood collection tubes and isolated serum for determination of biochemical and oxidative markers as mentioned earlier.
Lipid marker measurements
Serum TC concentration was estimated using the colorimetric method with cholesterol esterase and oxidase, and the TG and HDL-C concentrations were determined by an enzyme-colorimetric method using commercial kits (Biocon Diagnostik GmbH, Germany) following the manufacturer’s instructions. The LDL-C concentration was calculated using Friedewald’s formula: LDL = TC − (HDL + 0.2 TG).
Serum oxidative marker measurements
The total antioxidant enzyme activities were determined using commercial kits: Sigma-Aldrich No. 19160 for SOD activity, Sigma-Aldrich No. 219265 for CAT activity, and Sigma-Aldrich, CS1020 for GPx activity. MDA concentration, a marker of lipid peroxidation and oxidative stress, was estimated using the assay kit Sigma-Aldrich No. 437639. All serum oxidative markers were measured using a UV spectrophotometer at 450, 540, and 500 nm, respectively.
Statistical analyses
The demographic data of the subjects were analyzed using descriptive statistics. The Kolmogorov–Smirnov test was used for checking the normal distribution of the data set. All analyses were performed using GraphPad Prism, version 8 software (GraphPad Software Inc., San Diego, CA). Normal continuous variables were presented as means ± SD (standard deviation). To compare the differences within each group before and after the intervention, an independent Student’s t-test for normally distributed data and Wilcoxon signed ranks test for nonnormally distributed data were performed. A comparison of pre and postintervention data between the control and the intervention groups was calculated by the independent t-test for normally distributed data and the Mann–Whitney U test for nonnormally distributed data, respectively. A p-value < 0.05 was considered to be of statistical significance.
RESULTS
The first part of this study involved measuring the TPC, TFC, and antioxidant capacity of MK tea leaves. The results are shown in Table 1. The MK tea leaves presented the highest TPC (775.92 ± 5.37 mg GAE/g of MK tea), TFC (273.58 ± 6.04 mg CE/g of MK tea), and the highest free radical scavenging activity by the DPPH method (88.17% ± 0.11%), with IC50 values of 5.04 ± 2.68 μg/ml.
In the clinical trial, a total of 64 dyslipidemia patients completed this study and were included in the final testing. The demographic information of the subjects is shown in Table 2. There were no significant differences between the control and the intervention groups that received MK tea in regard to gender, age, weight, height, BMI, and duration of dyslipidemia. This indicates that they were homogeneous in the proportion of participant characteristics in both groups.
Table 3 presents the results of the statistical analysis of the lipid profile before and after 12 weeks of treatment from the control and MK tea groups. No statistically significant difference (p > 0.05 all) was observed in the baseline values of the serum lipid panel, including TC, TG, HDL-C, and LDL-C in both groups. At the endpoint of this study, there was a statistically significant reduction (p < 0.05 all) in the mean TC, TG, and LDL-C of the MK tea-treated group compared with the control group and baseline. However, MK tea drinking did not show any significant alteration in HDL-C concentration compared with the control group and baseline.
Scientific evidence suggests that hyperlipidemia and hypercholesterolemia are associated with enhanced oxidative stress. Therefore, the final part of this study aimed to determine the effect of MK tea consumption on systemic indices of oxidative stress. At the beginning of the trial, there was no significant difference in the mean of three major antioxidant enzyme families, namely SOD, CAT, and GPx activities in both groups. Interestingly, the mean of these antioxidant enzyme activities was increased significantly (p < 0.05 all) in the MK tea-treated group compared with the control group after 12 weeks of treatment. Furthermore, the activities of all antioxidant enzymes as mentioned earlier, were significantly increased in the MK tea-treated group compared to the baseline. Moreover, the MDA level was significantly (p < 0.05) reduced in the MK tea-treated group compared to the baseline and the endpoint of the trial. The results are summarized in Table 4.
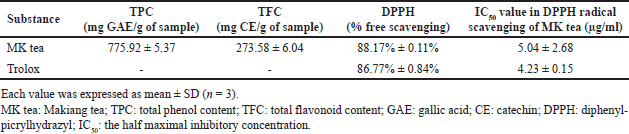 | Table 1. Total polyphenols, TFC contents, and antioxidant activities of the MK tea. [Click here to view] |
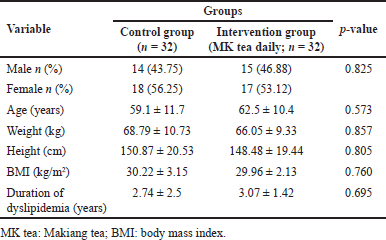 | Table 2. Baseline demographic data of participants between two groups before initiation of the trial. [Click here to view] |
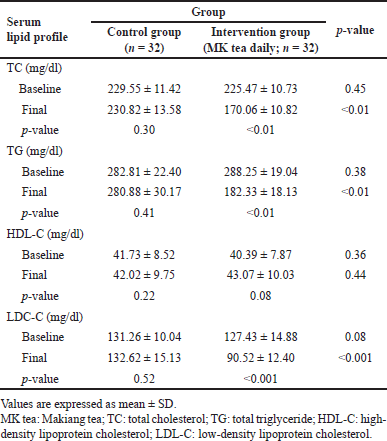 | Table 3. Comparison of lipid profile between two groups before and after the intervention. [Click here to view] |
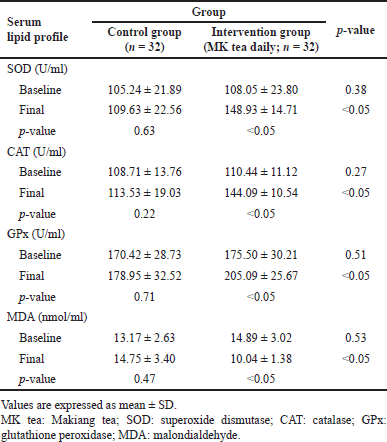 | Table 4. Comparison of serum oxidative stress biomarkers between two groups before and after the intervention. [Click here to view] |
DISCUSSION
There is no information in the literature about clinical trials to study the effect of herbal tea made from the dried leaves of MK for the management of dyslipidemia. In this trial study, we demonstrated that MK tea supplementation (3 g/day for 12 weeks), a source of polyphenols, leads to a significant alteration in the serum lipid profile comprising TC, TG, and LDL-C, but no alterations were observed in HDL-C levels. This phenomenon seems to be related to the antioxidant activity by decreasing the MDA concentration and increasing the SOD, CAT, and GPx activities in the serum of participants with dyslipidemia. In addition, no adverse reactions or side effects were reported by subjects drinking MK tea during the study.
Several scientific reports and ethnobotanical studies have demonstrated that there are many medicinal plants used for making tea and herbal tea [21,22]. Herbal tea contains several ingredients such as flavonoids, minerals, alkaloids, terpenoids, volatile oils, theanine, organic acids, and polysaccharides especially polyphenolic compounds [23,24]. Our study revealed that the strong antioxidant ability of herbal tea made from the dried leaves of MK might be associated with the total polyphenol and flavonoid concentrations. These results are consistent with prior studies reporting that there is a strong correlation between the antioxidant properties and the amount of polyphenol contents in herbal teas [25,26]. In addition, our results are compatible with previous work by Pokkaew et al. [27] who demonstrated that the methanol extract from MK leaves contains a large number of flavonoid and phenolic compounds with strong antioxidant abilities. Concerning the DPPH assay IC50, the herbal tea made from leaves of MK showed good antioxidant capacity. However, IC50 values for DPPH radicals of MK tea were lower than Trolox (the standard antioxidant), suggesting that Trolox solution is a single compound serving as a free radical scavenger, while the MK dried leaf tea is a pool of polyphenolic compounds, which consists of the active and inactive forms leading to a masking effect on the DPPH radical scavenging activity.
Multiple lines of evidence show that hypercholesterolemia and hypertriglyceridemia are associated with LDL oxidation leading to alteration of lipid metabolism and an elevation of lipid peroxidation, which could lead to enhancement of oxidative stress in dyslipidemia patients [28,29]. Previous studies found that the consumption of dietary polyphenols including tea could directly reduce the LDL-C level and hyperlipidemia, both effects which were associated with its bioactive compounds such as polyphenols, acting as protective agents against oxidative damage [30,31]. Correspondingly, our results showed that the biomarker of oxidative stress (MDA) was increased, and the levels of antioxidant scavenging enzymes (SOD, CAT, and GPx) were decreased in the control group. However, drinking herbal tea made from the dried leaves of MK can appreciably decrease TC, TG, and LDL-C and mitigate the MDA level but increase antioxidant enzyme activities in hyperlipidemia subjects. Our results are in conformity with the previous observational studies which reported that drinking tea rich in polyphenols can improve lipid profiles and reduce oxidative stress in dyslipidemia [32–36] and are consistent with several prior animal studies reporting the lipid-lowering effects of tea [37–39].
All our data confirm the hyperlipidemia effects and the antioxidant activity of herbal tea produced from the leaves of the MK plant. Unfortunately, there was no significant difference in the mean levels of HDL-C between the group drinking MK tea and the control group, indicating that MK tea has no effect on boosting HDL-C levels. In addition, body weights and BMI in both groups did not change significantly from the prestudy level until the end of the experiment.
It is also noteworthy that in our study, all subjects were instructed to maintain their former diet during the experiment and data revealed that the population diet did not change significantly during the interventional studies. Therefore, dietary factors could not be considered as confounding factors in the biological interpretation of biochemical variables.
From a review of the literature, we found that the possible mechanism for the hypolipidemic effects of polyphenolic compounds contained in tea or herbal tea decreased cholesterol levels by reducing cholesterol absorption in the intestine [40], increasing fecal lipid excretion [41], regulating fat metabolism [42] and preventing LDL oxidation [43].
The present study represents the first step in creating a tea product made from the dried leaves of the MK plant that possesses anti-hyperlipidemia effects. The possible mechanisms underlying the anti-hyperlipidemia effects of MK tea may occur via its antioxidant and free radical scavenging properties and by decreasing LDL oxidation. Regarding the limitations of this study, first, the exact mechanisms of MK tea phytochemicals possibly capable of cholesterol-lowering effects are complex and remain unclear. For this reason, more research is needed to identify the precise mechanisms and pharmacokinetics of MK tea which are responsible for their cholesterol-lowering effect. Second, a crossover study is a better design than a parallel study to compare the effectiveness of novel treatments. Therefore, future research is required to elucidate this issue.
CONCLUSION
This study provides evidence that drinking 3 g of tea made from the leaves of the MK plant per day is an effective supplement in lowering levels of LDL-C, TG, and TC. Possible underlying mechanisms are associated with its antioxidant effect to scavenge oxidative stress and enhance the activities of the three main antioxidant enzyme families, namely SOD, CAT, and GPX, and decrease lipid peroxidation. All results seem to suggest that MK tea may be a candidate in lipid-lowering nutraceuticals for the management of dyslipidemia disorders.
ACKNOWLEDGMENTS
This research was supported by (i) the University of Phayao, (ii) Thailand Science Research and Innovation (TSRI), and (iii) the National Science, Research and Innovation Fund (NSRF) (Grant No.FF66-RIM059).
LIST OF ABBREVIATIONS
MK, Makiang; TC, total cholesterol; LDL-C, low-density lipoproteins; TG, triglyceride; HDL-C, high-density lipoproteins; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde; TPC, total phenolics; TFC, total flavonoids contents; CE, catechin equivalents; GAE, gallic acid equivalent; DPPH, 2,2 diphenylpicrylhydrazyl; IC50, 50% inhibitory concentration.
AUTHOR CONTRIBUTIONS
W. Phachonpai: Designer and project manager, performed the experiment, doing statistical analysis and manuscript writing. K. Wuthiyan: Harvesting MK tea leaves. A. Junkaew: Harvesting MK tea leaves. R. Pokkaew: MK tea making and manufacturing process management. K. Suttikullabud: Health assessment testing of subjects in this study. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
All authors declare that we have no conflicts of interest.
ETHICAL APPROVALS
This clinical trial was conducted in accordance with the principles of the Declaration of Helsinki on human subjects and approved by the Ethics Committee for Human Research of the University of Phayao (No. UP-HEC 1.3/047/65).
DATA AVAILABILITY
All data generated and analyzed are included in this article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hadaegh F, Harati H, Ghanbarian A, Azizi F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran lipid and glucose study. Eur J Cardiovasc Prev Rehabil. 2006;13(4):571–7. CrossRef
2. Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016 Nov;32(6):631–9.
3. Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328–50. CrossRef
4. Vaziri ND. Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin Exp Nephrol. 2014;18(2):265–8. CrossRef
5. Kim MH, Choi WS. The association between subclinical inflammation and abnormal glucose and lipid metabolisms in normal-weight Korean individuals. Nutr Metab Cardiovasc Dis. 2018;28(11):1106–13. CrossRef
6. Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008;43(3):154–8. CrossRef
7. Guevara-Cruz M, Medina-Vera I, Cu-Cañetas TE, Cordero-Chan Y, Torres N, Tovar AR, et al. Chaya leaf decreased triglycerides and improved oxidative stress in subjects with dyslipidemia. Front Nutr. 2021;8:666243. CrossRef
8. Chandrasekara A, Shahidi F. Herbal beverages: bioactive compounds and their role in disease risk reduction—a review. J Tradit Complement Med. 2018;8(4):451–8. CrossRef
9. Sá CM, Ramos AA, Azevedo MF, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int J Mol Sci. 2009;10(9):3937–50. CrossRef
10. Thongma, S. Botanical description of Makiang. Ma-kiang. Bangkok, Thailand: Lampang Agricultural Research and TrainingCenter; 2002. pp 66–96.
11. Janpaijit S, Lertpatipanpong P, Sillapachaiyaporn C, Baek SJ, Charoenkiatkul S, Tencomnao T, et al. Anti-neuroinflammatory effects of Cleistocalyx nervosum var. paniala berry-seed extract in BV-2 microglial cells via inhibition of MAPKs/NF-κB signaling pathway. Heliyon. 2022;8:e11869. CrossRef
12. Prasanth MI, Sivamaruthi BS, Sukprasansap M, Chucha-wankul S, Tencomnao T, Chaiyasut C. Functional properties and bioactivities of Cleistocalyx nervosum var. paniala berry plant: a review. Food Sci Technol. 2020;40:369–73. CrossRef
13. Taya S, Punvittayagul C, Inboot W, Fukushima S, Wongpoomchai R. Cleistocalyx nervosum extract ameliorates chemical-induced oxidative stress in early stages of rat hepatocarcinogenesis. Asian Pac J Cancer Prevent. 2014;15:2825–30. CrossRef
14. Phachonpai W, Wattanathorn J. Neuroprotective effect of Cleistocalyx nervosum var. paniala extract in a rat model of ischemic stroke. Naresuan Phayao J. 2015;8(3):137–41.
15. Chariyakornkul A, Inboot N, Taya S, Wongpoomchai R. Low-polar extract from seed of Cleistocalyx nervosum var. paniala modulates initiation and promotion stages of chemically- induced carcinogenesis in rats. Biomed Pharm. 2021;133:110963. CrossRef
16. Manosroi J, Chankhampan C, Kumguan K, Manosroi W, Manosroi A. In vitro anti- aging activities of extracts from leaves of Ma Kiang (Cleistocalyx nervosum var. paniala). Pharm Biol. 2015;53:862–9. CrossRef
17. Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V. A reproducible, rapid and inexpensive Folin-Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem J. 2009;91:107–10. CrossRef
18. Kivits GAA, van der Sman FJP, Tijburg LBM. Analysis of catechins from green and black tea in humans: a specific and sensitive colorimetric assay of total catechins in biological fluids. Int J Food Sci Nutr. 1997;48(6):387–92. CrossRef
19. Gheflati A, Adelnia E, Nadjarzadeh A. The clinical effects of purslane (Portulaca oleracea) seeds on metabolic profiles in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. Phytother Res. 2019;33(5):1501–9. CrossRef
20. Leech RM, Worsley A, Timperio A, McNaughton SA. Understanding meal patterns: definitions, methodology and impact on nutrient intake and diet quality. Nutr Res Rev. 2015;28:1–21. CrossRef
21. Liu YJ, Ahmed S, Long CL. Ethnobotanical survey of cooling herbal drinks from southern China. J Ethnobiol Ethnomed. 2013;9:82. CrossRef
22. Jin B, Liu Y, Xie J, Luo B, Long C. Ethnobotanical survey of plant species for herbal tea in a Yao autonomous county (Jianghua, China): results of a 2-year study of traditional medicinal markets on the Dragon Boat Festival. J Ethnobiol Ethnomed. 2018;14(1):58. CrossRef
23. Ye L, Wang H, Duncan SE, Eigel WN, O’Keefe SF. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015;172:416–22. CrossRef
24. Yan Z, Zhong Y, Duan Y, Chen Q, Li F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr. 2020;6:115–23. CrossRef
25. Fu L, Xu BT, Gan RY, Zhang Y, Xu XR, Xia EQ, et al. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int J Mol Sci. 2011;12:2112–24. CrossRef
26. Kumari A, Kumar D. Evaluation of antioxidant and cytotoxic activity of herbal teas from Western Himalayan region: a comparison with green tea (Camellia sinensis) and black tea. Chem Biol Technol Agric. 2022;9:33. CrossRef
27. Pokkaew R, Wuthiyan K, Chaitawatwithi T, Junkaew A. Tea fromyoung leaves of Ma-Kiang. In: Chareonsap PP, editor. Ma-kiang: the conservation plant of plant genetic conservation project under the Royal initiative of Her Royal Highness Princess Maha Chakri Sirindhorn. Chiangmai, Thailand: Vanida Printing; 2015. pp 131–9.
28. Annuzzi G, Bozzetto L, Costabile G, Giacco R, Mangione A, Anniballi G, et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial. Am J Clin Nutr. 2014;99(3):463–71. CrossRef
29. Sun P, Zhao L, Zhang N, Zhou J, Zhang L, Wu W, et al. Bioactivity of dietary polyphenols: the role in LDL-C lowering. Foods. 2021;10(11):2666. CrossRef
30. Yi D, Tan X, Zhao Z, Cai Y, Li Y, Lin, X, et al. Reduced risk of dyslipidaemia with oolong tea consumption: a population-based study in southern China. BJN. 2014;111(8):1421–9. CrossRef
31. Fang X, Azain M, Crowe-White K, Mumaw J, Grimes JA, Schmiedt C. Effect of acute ingestion of green tea extract and lemon juice on oxidative stress and lipid profile in pigs fed a high-fat diet. Antioxidants. 2019;8:195. CrossRef
32. Huang S, Li J, Wu Y, Ranjbar S, Xing A, Zhao H, et al. Tea consumption and longitudinal change in high-density lipoprotein cholesterol concentration in Chinese adults. J Am Heart Assoc. 2018;7(13):e008814. CrossRef
33. Stensvold I, Tverdal A, Solvoll K, Foss OP. Tea consumption. relationship to cholesterol, blood pressure, and coronary and total mortality. Prev Med. 1992;21(4):546–53. CrossRef
34. Kono S, Shinchi K, Ikeda N, Yanai F, Imanishi K. Green tea consumption and serum lipid profiles: a cross-sectional study in northern Kyushu, Japan. Prev Med. 1992;21(4):526–31. CrossRef
35. Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ. 1995;310(6981):693–6. CrossRef
36. Khongrum J, Yingthongchai P, Boonyapranai K, Wongtanasarasin W, Donrung N, Sukketsiri W, et al. Antidyslipidemic, antioxidant, and anti-inflammatory effects of jelly drink containing polyphenol-rich roselle calyces extract and passion fruit juice with pulp in adults with dyslipidemia: a randomized, double-blind, placebo- controlled trial. Oxid Med Cell Longev. 2022;2022:4631983. CrossRef
37. Choi UK, Lee OH, Yim JH, Cho CW, Rhee YK, Lim SI, et al. Hypolipidemic and antioxidant effects of dandelion (Taraxacum officinale) root and leaf on cholesterol-fed rabbits. Int J Mol Sci. 2010;11(1):67–78. CrossRef
38. Yang C, Yifan L, Dan L, Qian Y, Ming-yan J. Bamboo leaf flavones and tea polyphenols show a lipid-lowering effect in a rat model of hyperlipidemia. Drug Res (Stuttg). 2015;65(12):668–71. CrossRef
39. Chan PT, Fong WP, Cheung YL, Huang Y, Ho WK, Chen ZY. Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet. J Nutr. 1999;129(6):1094–101. CrossRef
40. Davies MJ, Judd JT, Baer DJ, Clevidence BA, Paul DR, Edwards AJ, et al. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J Nutr. 2003;133(10):3298S–302S. CrossRef
41. Hsu TF, Kusumoto A, Abe K, Hosoda K, Kiso Y, Wang MF, et al. Polyphenol-enriched oolong tea increases fecal lipid excretion. Eur J Clin Nutr. 2006;60;1330–6. CrossRef
42. Liu C, Guo, Sun L, Lai X, Li Q, Zhang W, et al. Six types of tea reduce high-fat-diet induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019;10(4):2061–74. CrossRef
43. Yang TT, Koo MW. Inhibitory effect of Chinese green tea on endothelial cell-induced LDL oxidation. Atherosclerosis. 2000;148(1):67–73. CrossRef