INTRODUCTION
The use of herbal origin preparations has increased in the last few years [1] Worldwide herbal medicine practice securing a high demand toward the treatment and management of long-standing disorders [2]. Modernization in lifestyle, negligence toward health, and awareness about the number of side effects upon long-term use of synthetic medicines have pushed the use of herbs and herbal preparations in the recent era of emergence [3]. Medicinal plants are the principle origin offer the new chemical entity, an important benchmark for the pharmaceutical industry to treat, manage, and control a range of illnesses [4]. Phytoconstituents, such as alkaloids, glycosides, terpenoids, polyphenols, and so on, present in medicinal plants have potential therapeutic activity; however, poor bioavailability, solubility, and degradation restrict their effective therapeutic application [5]. A novel drug delivery system combats this limitation by integration with herbal preparation which may aid the bioavailability, sustained release of bioactive compounds, and protection against gastric fluids, Thus, it is essential to incorporate novel drug delivery concept with herbal medicines to achieve their better therapeutic application [6].
A traditional herb saffron (Crocus sativus) is reported for its inherent medicinal properties such as anticancer [7], anti-inflammatory [8], anti-oxidant [9], boosting memory [7,10], neuroprotective [11]), antiobesity [12], hepatoprotective [13], and so on. Saffron stigma contains numerous volatile and promising components inclusive of crocin, crocetin, safranal, and picrocrocin [14]. Crocin is glycoside and hydrolyzed into crocetin which has a remarkable impact on learning and memory. Crocin arrests the amyloid formation and neurotoxicity [15]. The pharmacokinetic study shows crocin is less detected in blood plasma after single or multiple oral administrations of C. sativusextract while its aglycone crocetin found more. The intestine is the principle site where crocin may be hydrolyzed into crocetin and further absorbed into the blood [16]. In vitro studies have shown that after oral administration of C. sativusextract, crocin is readily hydrolyzed by intestinal epithelium enzymes or by the gut microbiota to deglycosylated trans crocetin, which is absorbed through passive diffusion by the intestine [17–19]. It is interesting to note that crocin reaches 75 times higher than crocetin in rat serum after oral administration as compared to only crocetin administration [11]. Oral administration of crocin is more beneficial than crocetin however the major challenges are low bioavailability at the site of action when taken orally and systemically. Many studies have also shown that the extract is not absorbed significantly and is eliminated quickly when taken orally or systemically [20].
Novel drug delivery is intended to cross particular barriers to improving effectiveness and conclusive alternative routes for the delivery of potential phytochemicals at the target site. Phytosomes, liposomes, nanocapsules, nanoparticles, neosomes, solid lipid nanocarriers, and nanoemulsion effectively enhance solubility, the bioavailability of phytoconstituents, as well as protect against physical, chemical, and enzymatic degradation and modification of drug release characteristics [21]. The advantage of such nanoformulation is to overcome the large dose of conventional therapy as well as the effective delivery of bioactive at the target site of action [22]. Nanoemulsion could overcome the associated challenges and form a bridge between conventional therapy and novel drug delivery systems. This is the innovative approach to formulate a stable nanoemulsion consisting of a standardized C. sativusextract rich in bioactive compounds such as crocin, crocetin, safranal, and picrocrocin with the use of low energy method by spontaneous emulsification technique. Carotenoid-enriched C. sativusextract is encapsulated in the novel drug delivery system, i.e., w/o nanoemulsion. Nanoemulsion is a clear transparent biphasic stable isotropic system having a particle size of 20–200 nm [23]. There are three types of emulsion-based systems depending on their compositions—o/w emulsion, w/o emulsion, and multiple emulsions. The techniques for formulating nanoemulsion are high-energy and low-energy methods. High-energy methods are high-pressure homogenizers, micro fluidization, and ultra sonification whereas low-energy methods include phase titration, spontaneous emulsification, and phase inversion methods [24]. The low-energy method is efficient in contrast with the high-energy method as simple stirring is generally required to produce smaller droplet sizes for obtaining kinetically stable nanoemulsion and easy to scale up, less consumption of energy with increased stability of nanoemulsion [25]. A spontaneous emulsification method is adopted for preparing the saffron nanoemulsion where a stepwise addition of water-soluble C. sativusextract is added in a surfactant oil mixture to obtain a small droplet size [26–29].
The present research aimed at highlighting the development of the novel formulation based on standardized extract in which biologically active carotenoid enriched C. sativusextract is encapsulated in w/o nanoemulsion. The optimization of w/o nanoemulsion by Design of Experiment (DoE) is assisting the better quality compliance. The release study performed for formulation revealed the controlled release of bioactive which may be more bioavailable. The quantification of the total content of marker compounds such as total crocin, crocetin, safranal, and picrocrocin present in saffron nanoemulsion was determined and suggested the actual loading of extract in the saffron nanoemulsion.
MATERIALS AND METHODS
Materials
Standardized C. sativusextract and medium chain triglyceride (MCT) were provided by Pharmanza Herbal Pvt. Ltd., Dharmaj, Gujarat. Capmul medium chain monoglyceride (MCM) EP/NF was gifted by Abitec Corporation, USA. Poly glycerol poly ricinoleate (PGPR) was gifted by Palsgaard, Denmark. Transcutol and Span 80 were purchased from Sigma–Aldrich, USA. All chemicals and excipients such as poly ethylene glycol (PEG), Span 20, Span 60, Kolliphore, and Tween 80 were received from Sigma–Aldrich.
Formulation and development
Preformulation studies
Drug-excipient physical incompatibilities
Standardized C. sativusextract and all selected excipients were characterized using Fourier Transform Infra Red Spectroscopy (FTIR) spectroscopy and Differential Scanning Calorimetry (DSC) to check the possibilities of interaction between extract and selected excipients if any.
UV estimation of saffron extract
10 mg of saffron extract was dissolved in 100 ml of water (100 μg/ml) and run into a UV spectrophotometer (UV 1800, Shimadzu, Japan) to obtain absorbance maxima for quantification of marker compounds. From the saffron stock solution, a different concentration, i.e., 10, 20, 30, 40, and 50 μg/ml were prepared and obtained the absorbance at 440, 466.8, 325, and 250 nm wavelength which gives the estimation of crocetin, crocin, safranal, and picrocrocin, respectively. The regression coefficient value was determined [should Not Less Than (NLT) 0.99] from the calibration curve of all constituents for further solubility studies.
Screening of solubility
The solubility of standardized C. sativusextract has a great influence on nanoemulsion formation. The selection of oil, surfactant, and co surfactant for the development of w/o nanoemulsion is a crucial step to obtain the desired particle size. Therefore, it is necessary to determine the solubility of C. sativusextract in different agents including external phase, internal phase, surfactants, co surfactants, and so on, which should be nonirritant, pharmaceutical acceptable, and chemically safe.
Hydrophilic-lipophilic balance (HLB) plays a vital role in the formulation of w/o nanoemulsion. For developing the w/o nanoemulsion, the surfactant with a low HLB range from 3 to 6 is more suitable. The solubility of C. sativusextract was determined by adding an excess known amount of C. sativusextract in known quantity (2 ml) of each oil/surfactant/co surfactant in a closed stopper test tube and placed in digital shaker bath (NOVA Inst. Pvt. Ltd., Ahmedabad, India) for 72 hours. Then, these test tubes were centrifuged at 4,000 rpm for 10 minutes. The supernatant was collected and the absorbance of phyto chemicals in each supernatant was measured by UV spectrophotometrically and calculated the solubility.
The solubility of C. sativusextract was carried out in various oils like MCM, MCT, olive oil, soybean oil, oleic acid, castor oil, and three ratios of MCM and MCT such as 1:1, 3:1, and 2:1. Different surfactant and co surfactants were screened for nanoemulsion were span 80, Tween 80: Span 80 (HLB-5), Span 80: Kolliphore (HLB-5), span 80: PGPR (HLB-4.5), Transcutol, PEG 300, 400, and 200, IPA.
Preliminary optimization of process parameters
The process variables for the preparation of micro-emulsion were batch size, stirring speed, and stirring time. For identifying the optimum process variables, one is kept constant and the other is varied. The different volumes of w/o nanoemulsions such as 10, 20, 30, 40, and 50 ml were formulated by ml keeping other process variables, i.e., speed and time of stirring constant. In a similar manner, the stirring speed was evaluated under the different rpms of the magnetic stirrer, i.e., 500, 1,000, and 1,500. In addition, the optimum time of formulating nanoemulsion was evaluated by preparing the emulsion under variable times such as 30 minutes, 45 minutes, and 1 hour. These all variables were optimized by determination of their particle size and zeta potential.
Construction of pseudo ternary phase diagram for selection of S-mix ratio
The phase behavior study of nanoemulsion was studied by pseudo ternary phase diagram using CHEMIX Software where the determination of the composition of oil, water, and S-mix system that provides stable isotropic nanoemulsion existence zone in which at any point the existence of nanoemulsion can be found. After the selection of oil, surfactant, and co surfactant, a specific ratio of S-mix such as 1:1, 2:1, 3:1, and 1:2 was optimized with the help of pseudo ternary phase diagram in which oil to S-mix mixture is varied as 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 and dropwise water was added using a micropipette with constant stirring and speed until the mixture became clear at a certain point and the number of different phases was recorded to obtain a pseudo ternary phase diagram [30–34].
Optimization of formulation by DoE software- experiment design
Critical process variables were optimized using a 32-factorial design with Design of Expert 13.0.4 software (Stat-Ease, Inc., USA). The concentrations of three different phases were selected as independent variables and their responses on particle size and zeta potential as dependent variables. As it is a mixture component, a simple lattice design with the special cubic model was used for formulation optimization and statistical analysis was done by analysis of variance (ANOVA). The process variables are provided in Table 1.
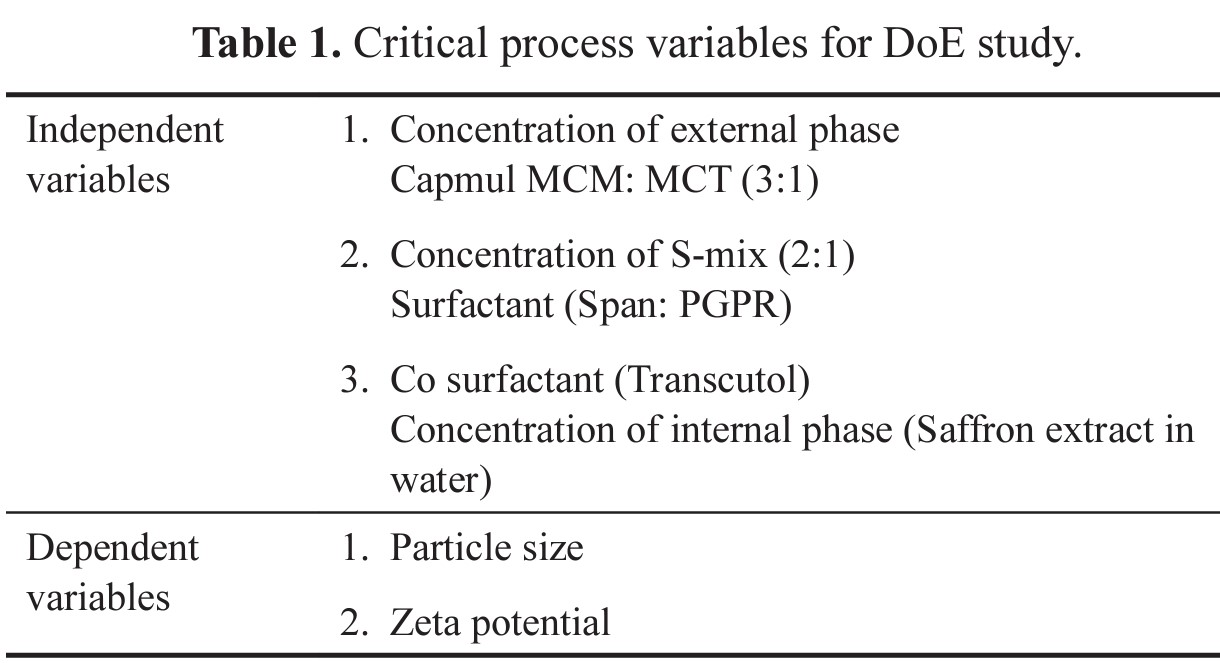 | Table 1. Critical process variables for DoE study. [Click here to view] |
According to the adopted design total of 17 different formulations were prepared and their response for the particle size and zeta potential were obtained. The data were placed to design expert software. The polynomial equation was generated and optimized by using ANOVA in the software. The models were assessed with respect to statistically significant coefficient and R2 values. The coded and actual values of independent variables are described in below Table 2.
The relationship between variables and the responses was studied by 3-D surface plots and contour plots. Depending on the smallest particle size and possible values of zeta potential, optimum formulation were selected. The checkpoint batch was again prepared and evaluated for response. The resulting responses were compared with the predicted responses.
Preparation of saffron-loaded nanoemulsion
Carotenoid enriched C. sativusextract loaded w/o nanoemulsion also termed as saffron nanoemulsion was prepared by low energy method with spontaneous emulsification [35–38].
Crocus sativus extract at the required quantity of 75 mg was dissolved in 1 ml of purified water filtered through 0.45 μm termed as a saffron solution.
A required quantity of selected oil was taken in a previously clean and dried beaker to that surfactant- co-surfactant S-mix ratio was added with constant stirring using a magnetic bead on the magnetic stirrer (DBK mag. stirrer) at 1,500 rpm. All the formulation parameters and process parameters were optimized.
Saffron solution was drop wise added into the above oil and S-mix mixture till the formation of a homogeneous and transparent emulsion under constant agitation.
Pharmaceutical characterization
The batch size of 50 ml w/o nanoemulsion was prepared by using an optimized formula and subjected to characterization using the following tests.
Inverted microscopic study
The size and shape of particles in the formulated nanoemulsion were checked by microscopic evaluation to determine the homogenous distribution of the internal phase using an Inverted Microscope-Nikon TS 100 at a magnification of 40 × 10.
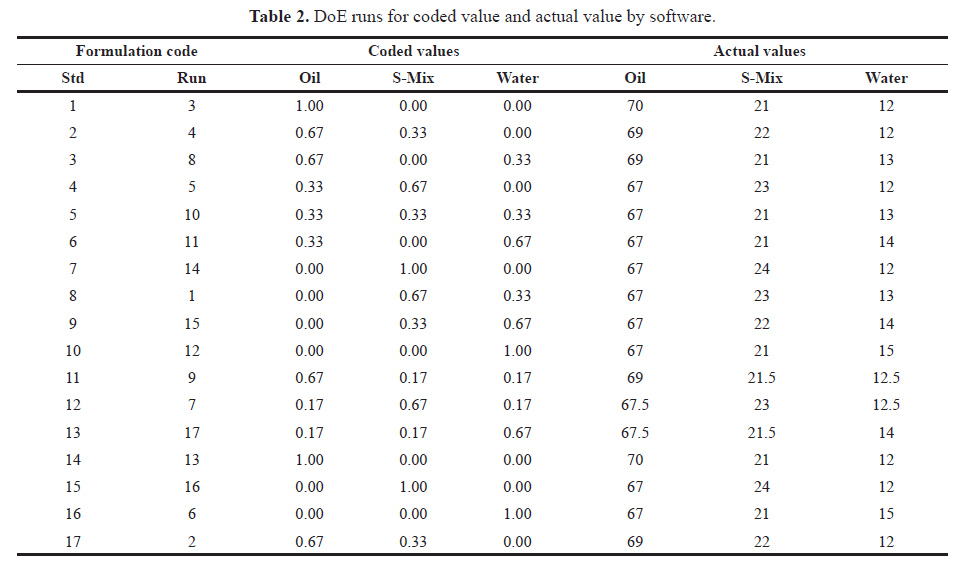 | Table 2. DoE runs for coded value and actual value by software. [Click here to view] |
Determination of droplet size and zeta potential
The droplet size of the internal phase and zeta potential of saffron-loaded nanoemulsion were analyzed using a dynamic light scattering method (Zeta Sizer-Malvern Instrument). All the measurements were done after overnight storage of nanoemulsion for reliable and accurate results by performing the experiment triplicate.
Surface morphology by transmission electron microscopy
The surface morphology as well as the structure of the optimized nanoemulsion was observed using Transmission Electron Microscopy (TEM). The TEM analysis was performed in SICART Lab, Vallabh-Vidyanagar, Anand.
Shear viscosity
Optimized nanoemulsion was assayed by measuring the viscosity digitally by viscometer (LABMAN Scientific Instrument). The spindle is rotated at 100 rpm for 10 minutes.
Content uniformity
The optimized saffron nanoemulsion was assayed using the robust, precise, accurate, sensitive, specific, and linear High Performance Liquid Chromatography- Photo Diode Array (HPLC-PDA) method (Pharmanza Herbal Pvt. Ltd.) for the total content of crocin, crocetin, picrocrocin, and safranal. The simultaneous quantification of marker compounds was achieved by Phenomenex Luna, 5 μ, 150 × 4.6 mm column, flow rate 1.0 ml/minute, injection volume of 10 μl, run time of 25 minutes with a gradient program of 0.1% formic acid in water and acetonitrile. The detection wavelength for crocin and crocetin are 440 nm, picrocrocin at 250 nm, and safranal at 320 nm [39].
In vitro release studies
A dissolution study was performed to evaluate the extent of drug release from the formulation. In vitro drug release of saffron nanoemulsion was carried out by placing the saffron nanoemulsion equivalent to 10 mg in a dialysis bag consisting of cellulose membrane (Sigma–Aldrich) that was introduced in 300 ml of dissolution media consisting of pH 7.4 phosphate buffer as release media using USP type II apparatus at 50 rpm and 37°C ± 2°C to predict the dissolution pattern in general in-vivo environment. A sample was withdrawn at periodic time intervals of 1, 2, 4, 5, 7, 9, 12, and 24 hours and at each time point was replaced by fresh media to maintain sink condition. The samples were analyzed spectrophotometrically by performing the experiment in triplicate. By using the calibration curve the total content from saffron nanoemulsion was determined and % cumulative release was calculated [40–43].
Stability study
The stability of the optimized saffron loaded w/o nanoemulsion was determined by keeping at accelerated conditions of 40°C ± 2°C and 75% ± 5% RH for 90 days as per International Council for Harmonisation (ICH) guidelines. Samples were analyzed for appearance, phase separation, particle size, and zeta potential and content of marker compound by HPLC at the end of 0, 30, 60, and 90 days [44].
RESULTS AND DISCUSSION
In the 21st century, the way toward herbal preparation is drastically increased. Although great advancements of new chemical moiety or synthetic drugs, traditional medicinal plants, and herbal preparation attract more and more research to improve quality of life [45]. Unfortunately, due to a lack of scientific evidence of pharmacology, pharmacokinetics, pharmacovigilance, poor bioavailability, shelf life, and so on, herbal preparations require more research in terms of fundamental principles and quality evaluation for their global acceptance [46]. In recent years, the use of phyto-chemicals extended their attentiveness to improving the quality of life, and among them, carotenoids are playing an important role in maintaining health due to potential therapeutic application. Crocin (digentiobiosyl crocetin) is a distinctive carotenoid present in the traditional herb C. sativusand is highly water soluble. It makes a difference than other carotenoids which can be attributed to sugar moiety binding to the carboxylic acid group. The present study focused on carotenoid-enriched C. sativusextract which was incorporated in a novel drug delivery system.
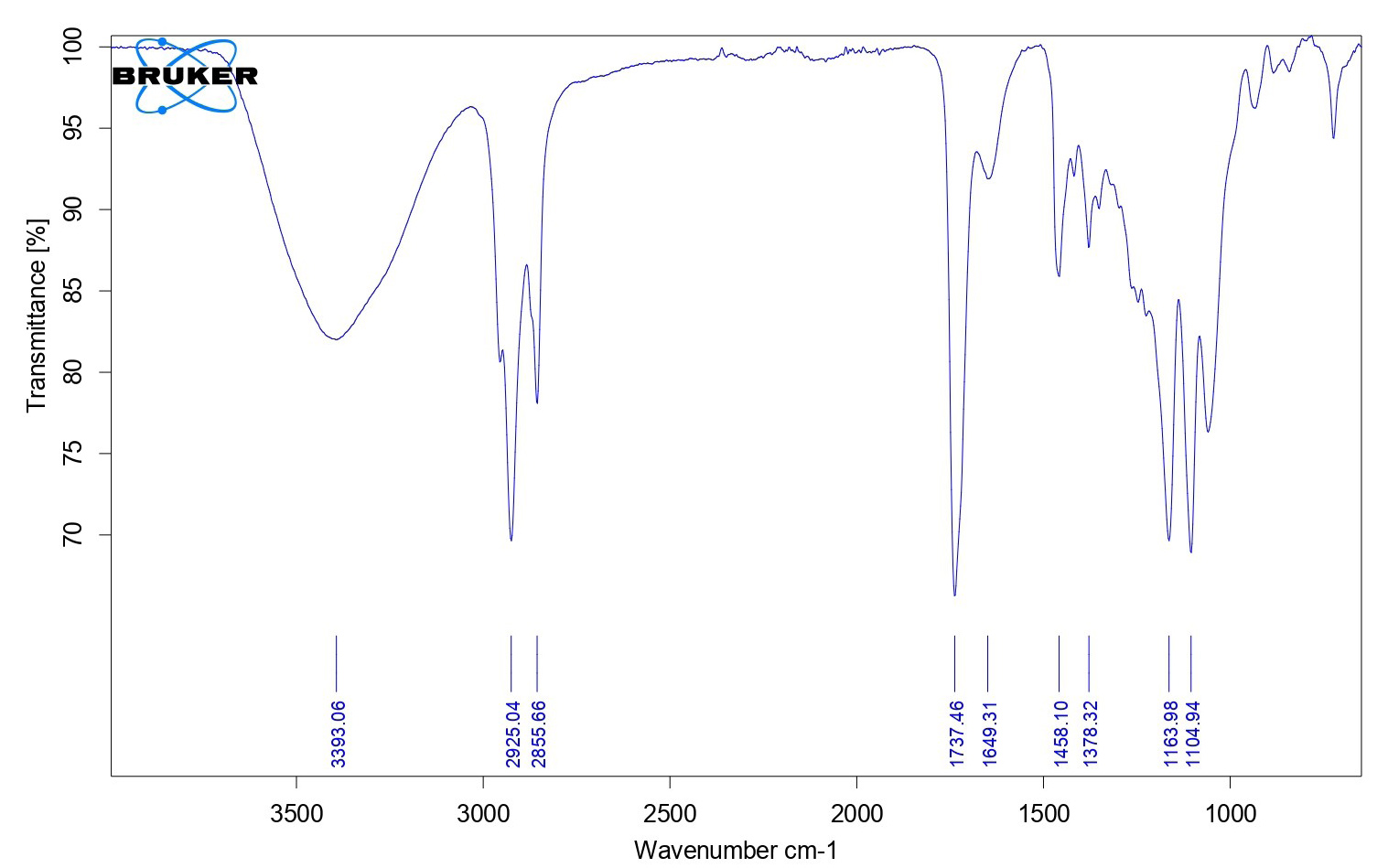
 | Figure 1. FTIR spectra of C. sativusextract. [Click here to view] |
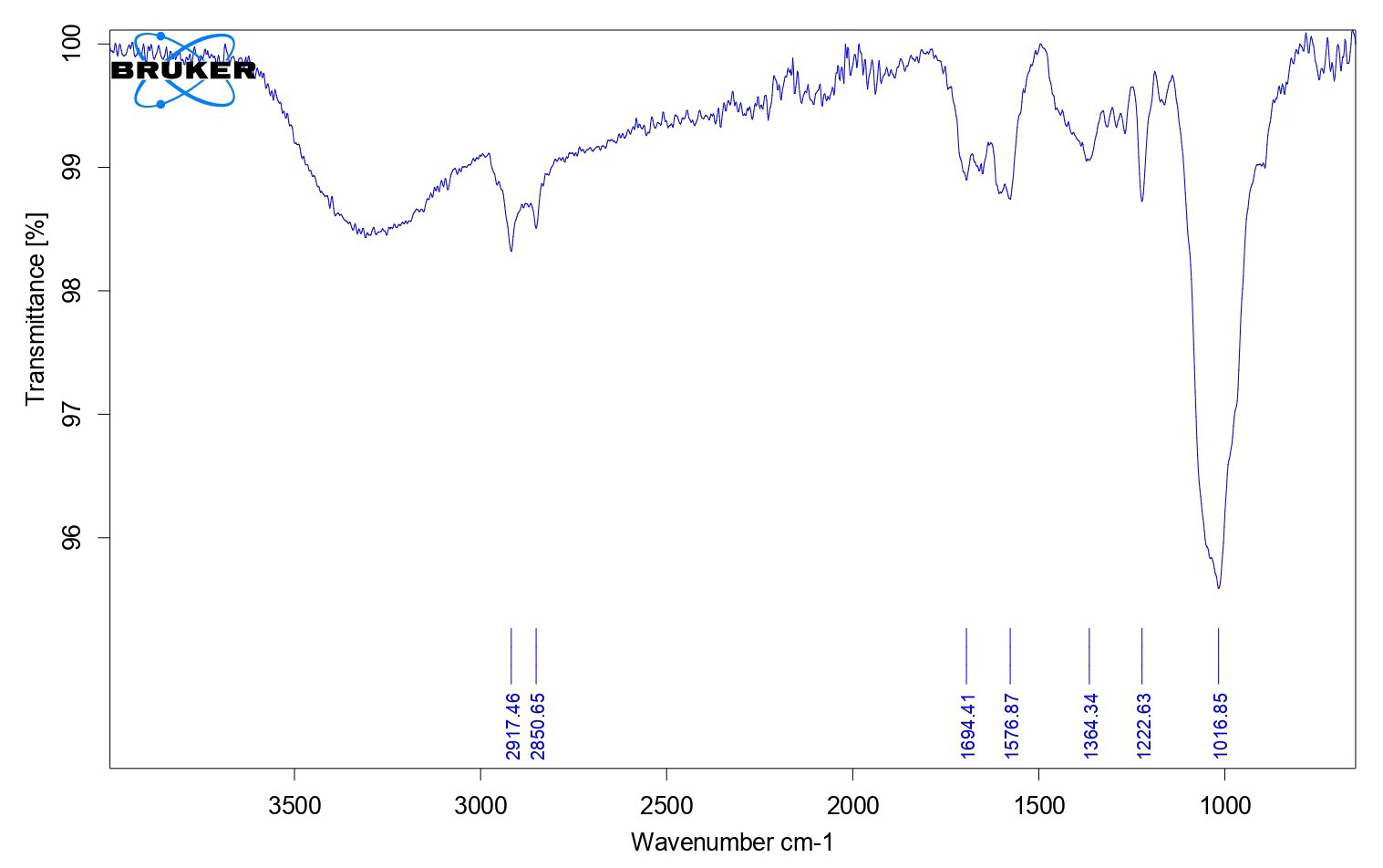
| Figure 2. FTIR spectra of the saffron formulation. [Click here to view] |
Formulation and development
Preformulation studies
Drug-excipients physical incompatibilities
The FTIR spectra and DSC graphs of C. sativusand the developed formulation were obtained to find out the possibilities of drug-excipient interaction if any. The FTIR spectra of saffron loaded w/o nanoemulsion were very similar to that of pure C. sativusextract and showed many of the characteristic peaks of pure extract. This observation was similar when DSC graphs were obtained from developed formulation and pure extract that suggested that no more possible interactions were found. The FTIR spectra of C. sativusextract and formulation are provided in Figures 1 and 2. The developed w/o nanoemulsion and pure C. sativuswere analyzed through DSC for possible interaction with excipients if any. The DSC graphs of C. sativusextract and formulation are provided in Figures 3 and 4. This indicated that the selected components required to formulate the nanoemulsion were found compatible and observed no drug-excipient interaction.
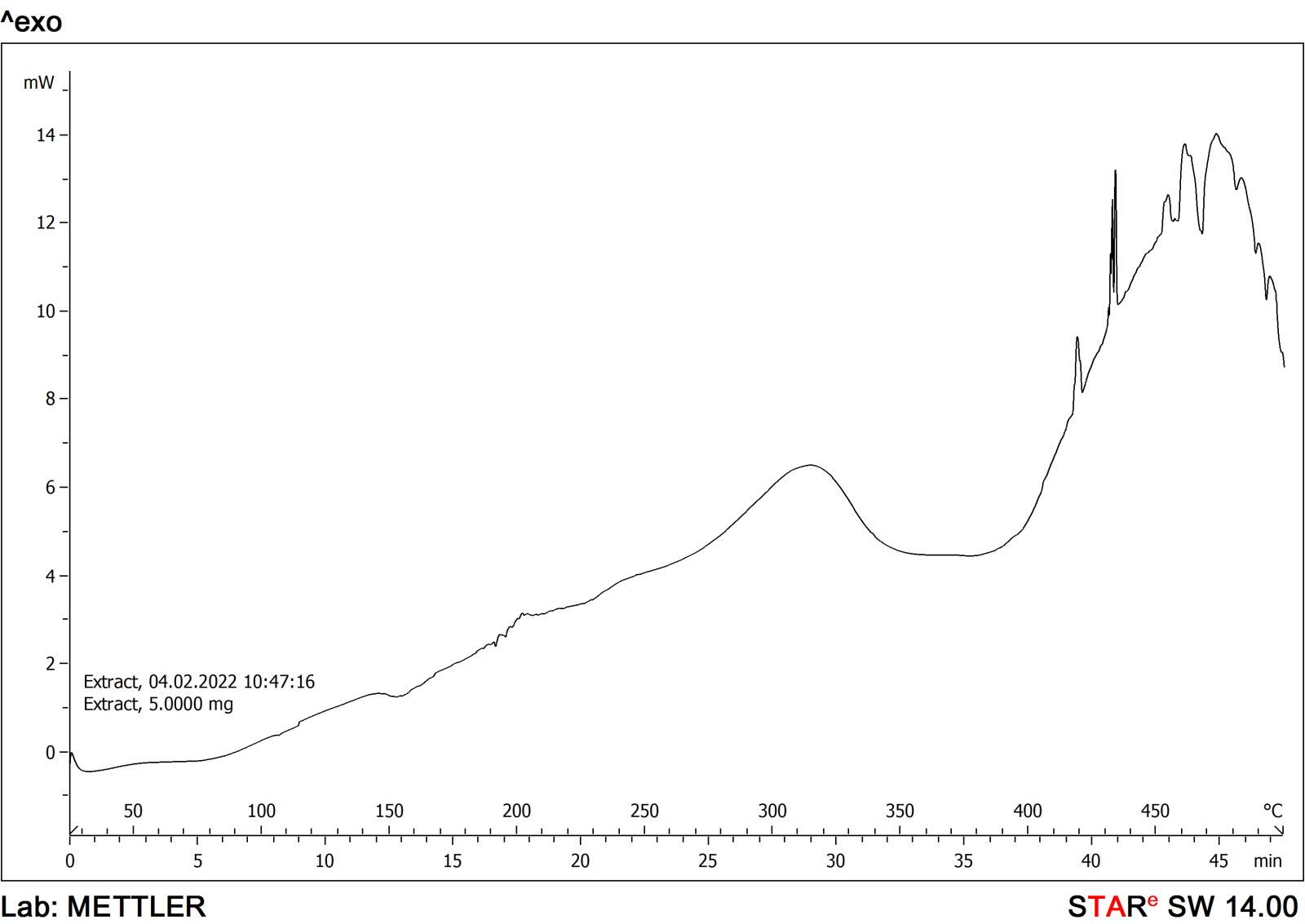 | Figure 3. DSC graph of C. sativusextract. [Click here to view] |
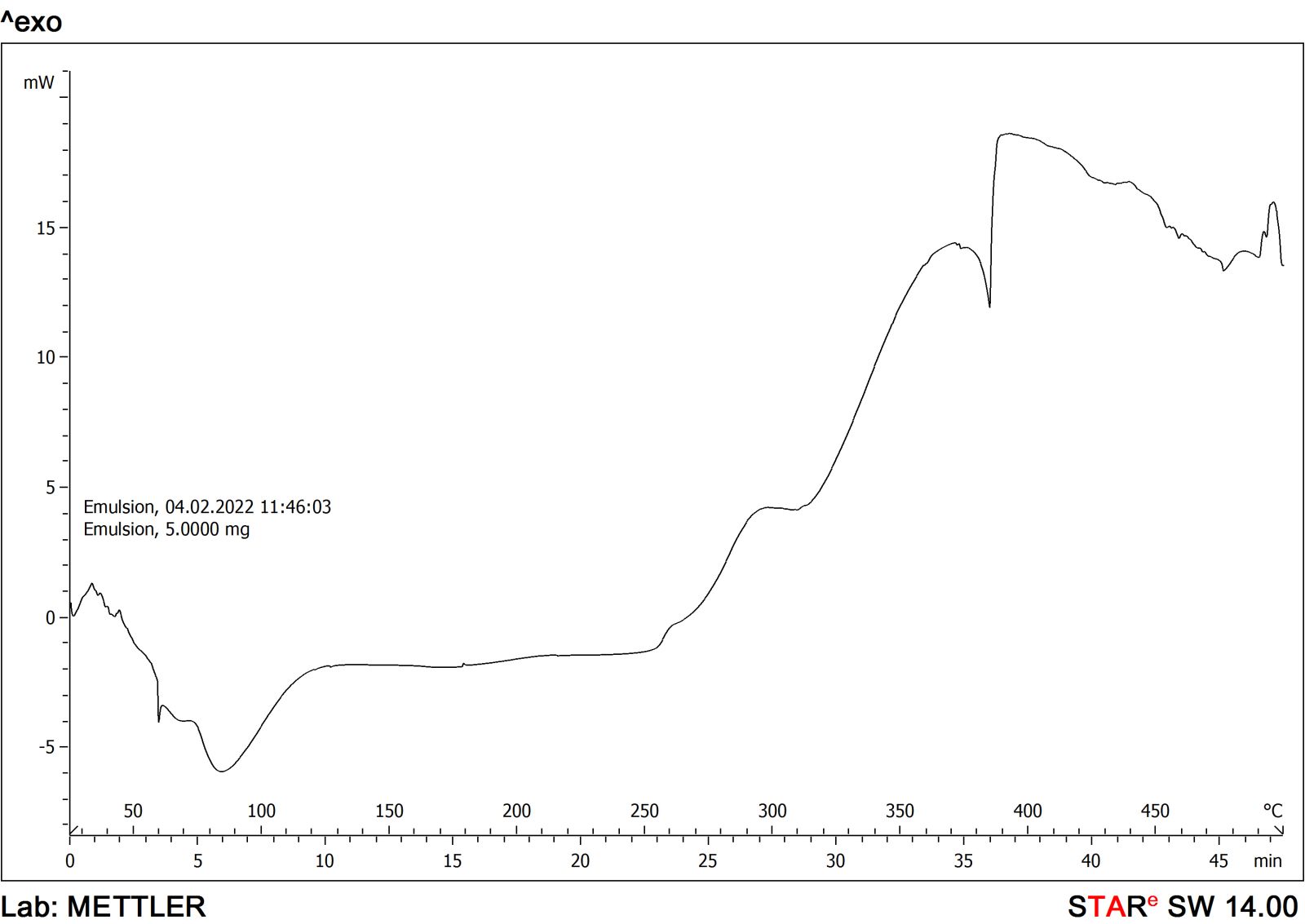 | Figure 4. DSC graph of saffron nanoemulsion. [Click here to view] |
UV estimation of saffron extract
The different concentrations, i.e., 10, 20, 30, 40, and 50 μg/ml of C. sativuswere prepared and scanned from 800 to 200 nm under UV absorbance spectroscopy and absorbance of crocin, crocetin, safranal, and picrocrocin were observed at 466.8, 440, 325, and 250 nm wavelength respectively were noted and provided in Table 3 and correlation coefficient of each phytoconstituent was determined Not More Than (NMT) 0.99 which is shown in Figure 6. The scanning of C. sativusextract in a UV spectrophotometer at different concentrations is provided in Figure 5.
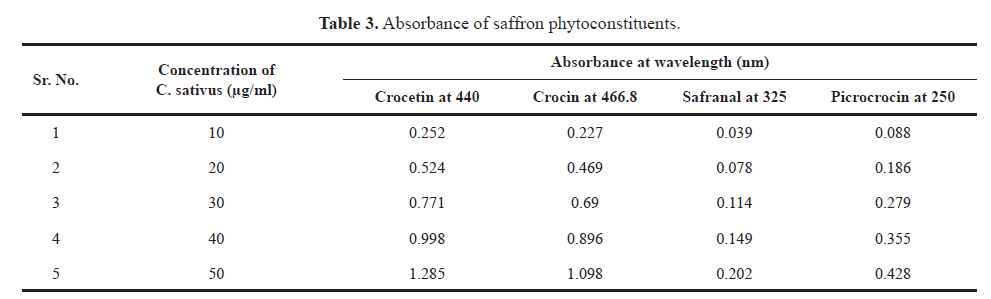 | Table 3. Absorbance of saffron phytoconstituents. [Click here to view] |
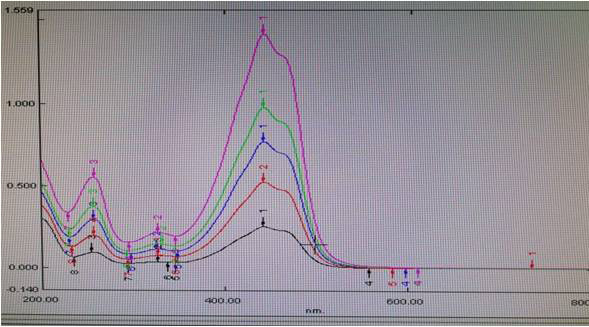 | Figure 5. Overlay of scanning of different concentrations of C. sativus. [Click here to view] |
Screening of solubility study
Based on the above results, a solubility study of C. sativusin various oils, surfactants, and co-surfactants was found. All the observations are in triplicate.
Selection of oils. Capmul MCM, MCT, and their different ratio were screened by determining the solubility of C. sativusin it and their results are shown in Table 4 and Figure 7. From the results obtained, it can be seen that the ratio 3:1 was considered as further optimization of formulation as lesser solubility as compared to others with minimum solubility, i.e., 1 ± 0.312 mg/ml of C. sativusextract.
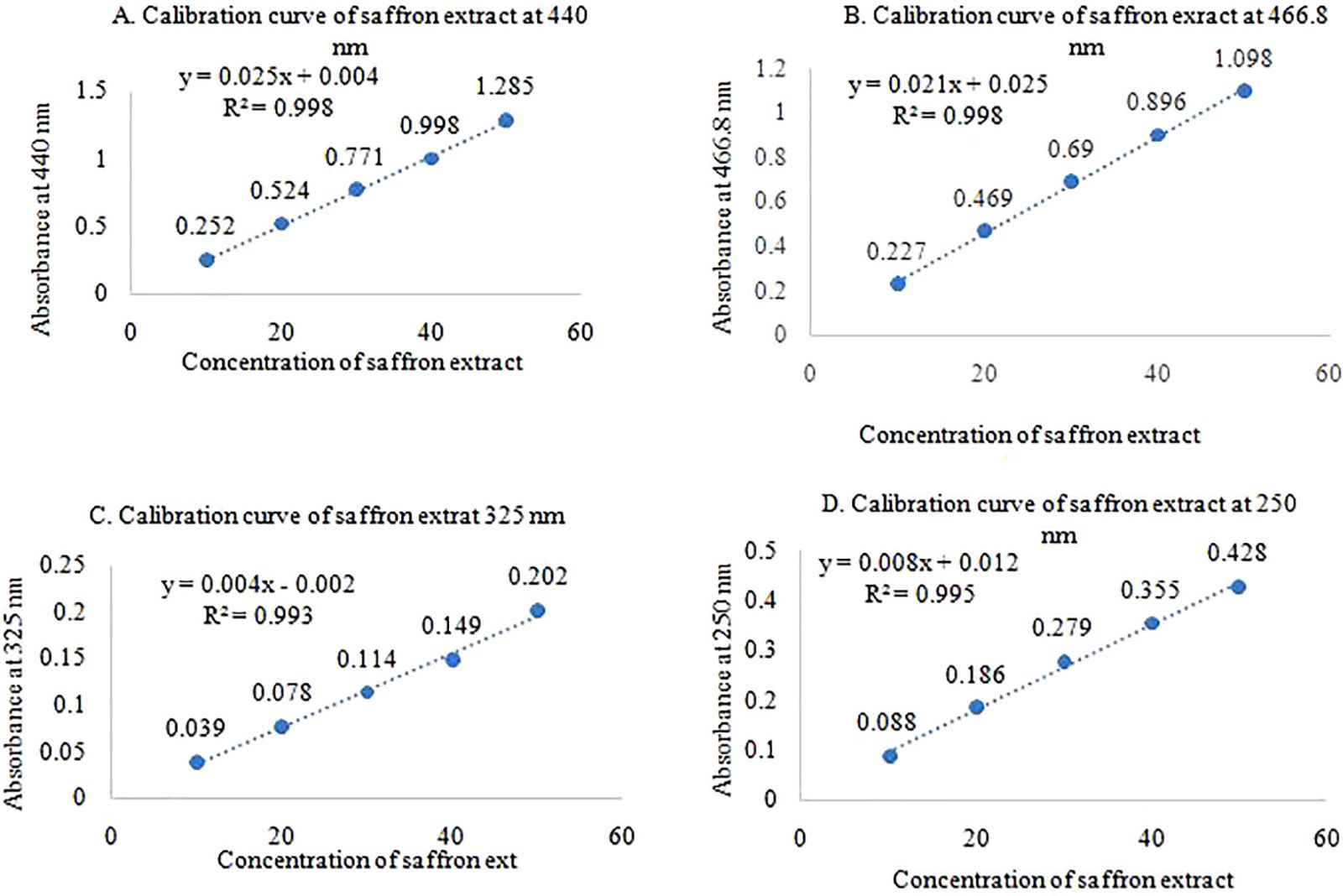 | Figure 6. Calibration curve of saffron phytoconstituents. [Click here to view] |
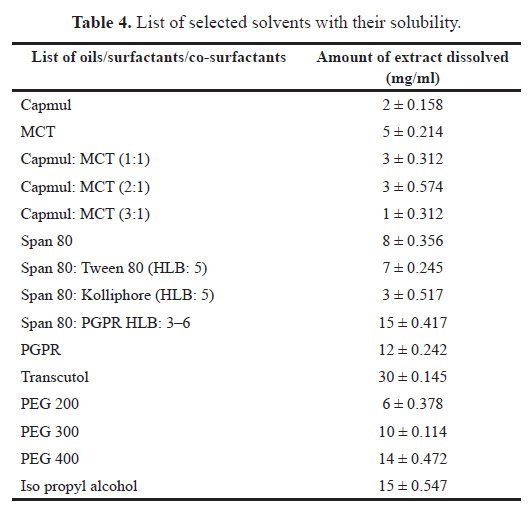 | Table 4. List of selected solvents with their solubility. [Click here to view] |
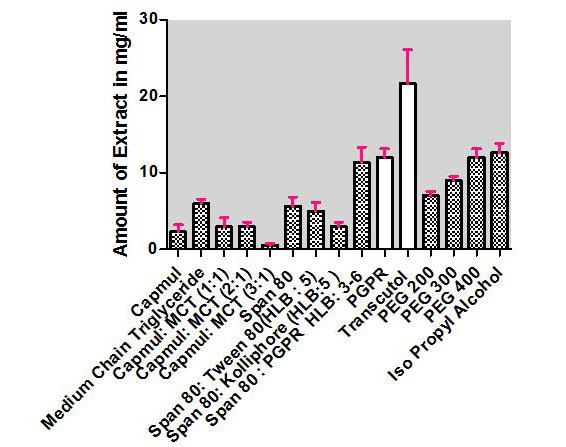 | Figure 7. Solubility data of C. sativusextract in different solvents. [Click here to view] |
Selection of surfactant and co-surfactant. For the preparation of w/o nanoemulsion, different surfactants, and co-surfactants having lower HLB values between 3 and 6 were screened, and from the results the Span 80: PGPR and transcutol were employed for the development of nanoemulsion which has the highest solubility as compared to other surfactants.
Preliminary optimization of process parameters
Table 5 shows the preliminary process parameters by considering the one that is changing with respect to other parameters that were kept constant.
Construction of pseudo ternary phase diagram for selection of S-mix ratio
The three axes of pseudo pseudo-ternary phase diagram are three axes were oil phase, the water phase, and the fixed ratio of the S-mix phase. Different ratios of surfactants and co surfactants, i.e., 1:1, 2:1, 3:1, and 1:2 were screened and their co-relation was shown in Figure 8. The nanoemulsion zone and turbid zone were clearly separated. Among the different ratios of S-mix, the 2:1 ratio of surfactant and co-surfactant showed the best area of nanoemulsion existence, and hence, the 2:1 ratio was used for further optimization of nanoemulsion.
Optimization of formulation by DoE software
The resulting observed responses were kept in DoE software to compare with the predicted responses. Their response along with actual value was provided in Table 6.
Details of response 1-particle size and zeta potential
A linear versus special cubic model was suggested by software for particle size and zeta potential. The model was satisfactory in terms of nonsignificant lack of fit F-value obtained was 5.23. A nonsignificant lack of fit is good. Further p-values less than 0.0500 indicate model terms were significant and again the Model F-value of 5.87 implies the model was significant. The adjusted R2 of 0.6464 and predicted R2 of 0.4628 values were closed, i.e., the difference of both the agreements was less than 0.2 which was considered an acceptable design model.
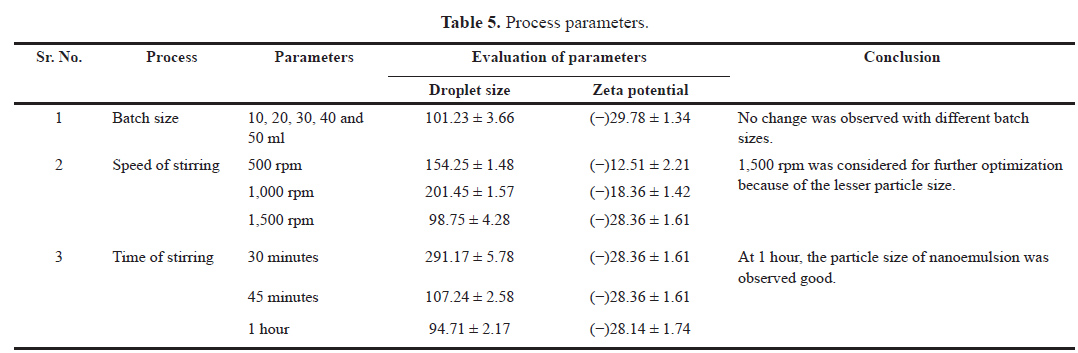 | Table 5. Process parameters. [Click here to view] |
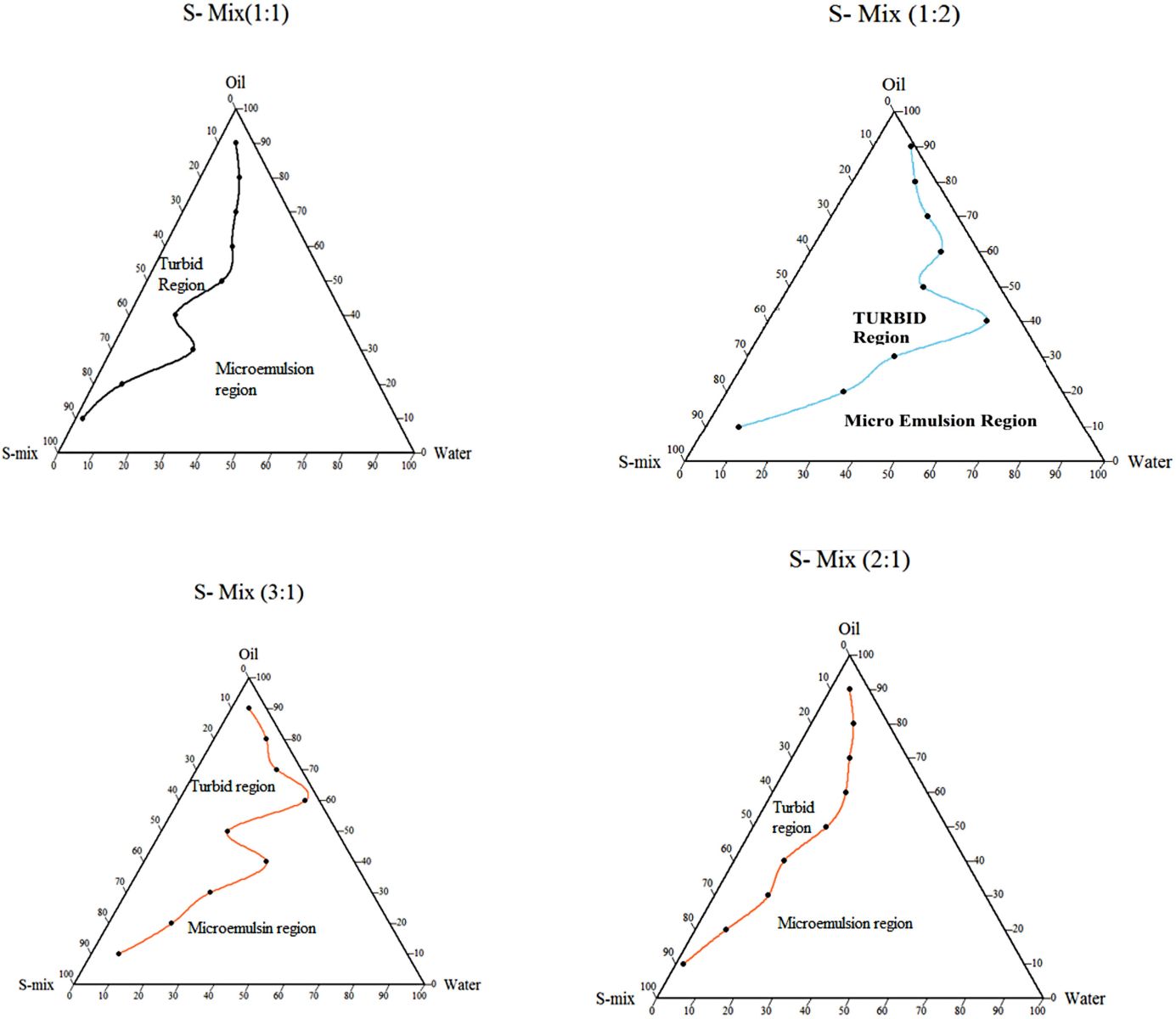 | Figure 8. Pseudo ternary phase diagram of different S-mix ratios. [Click here to view] |
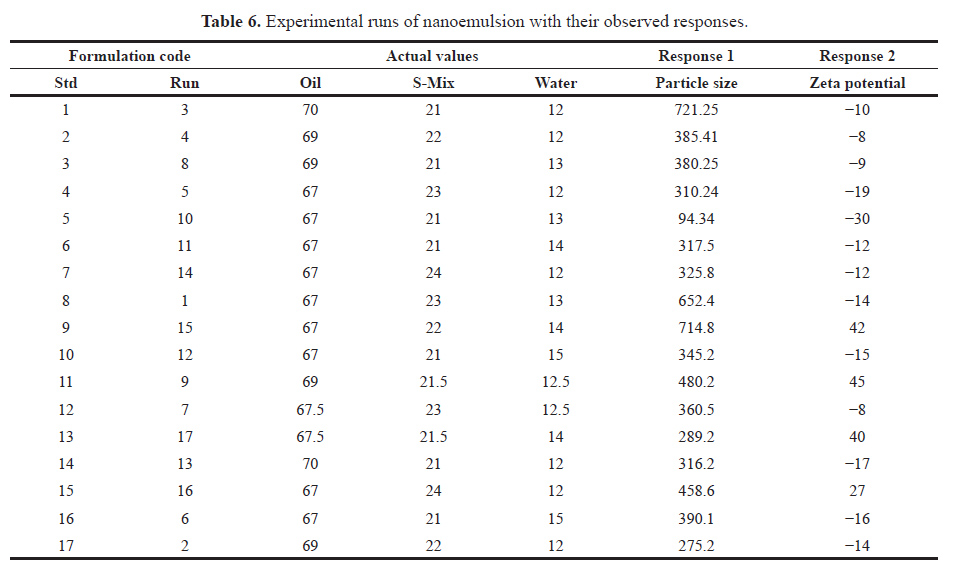 | Table 6. Experimental runs of nanoemulsion with their observed responses. [Click here to view] |
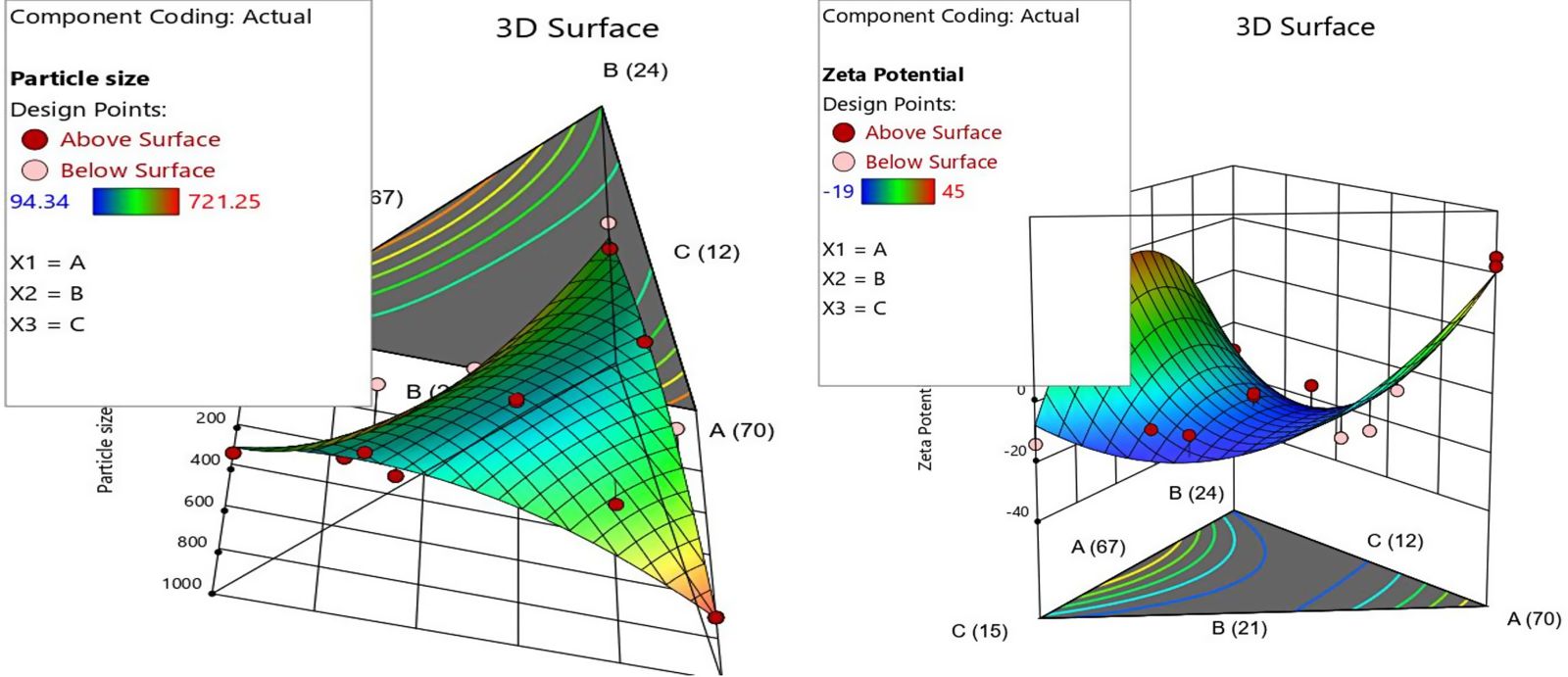 | Figure 9. DoE plots for dependent variables with response. [Click here to view] |
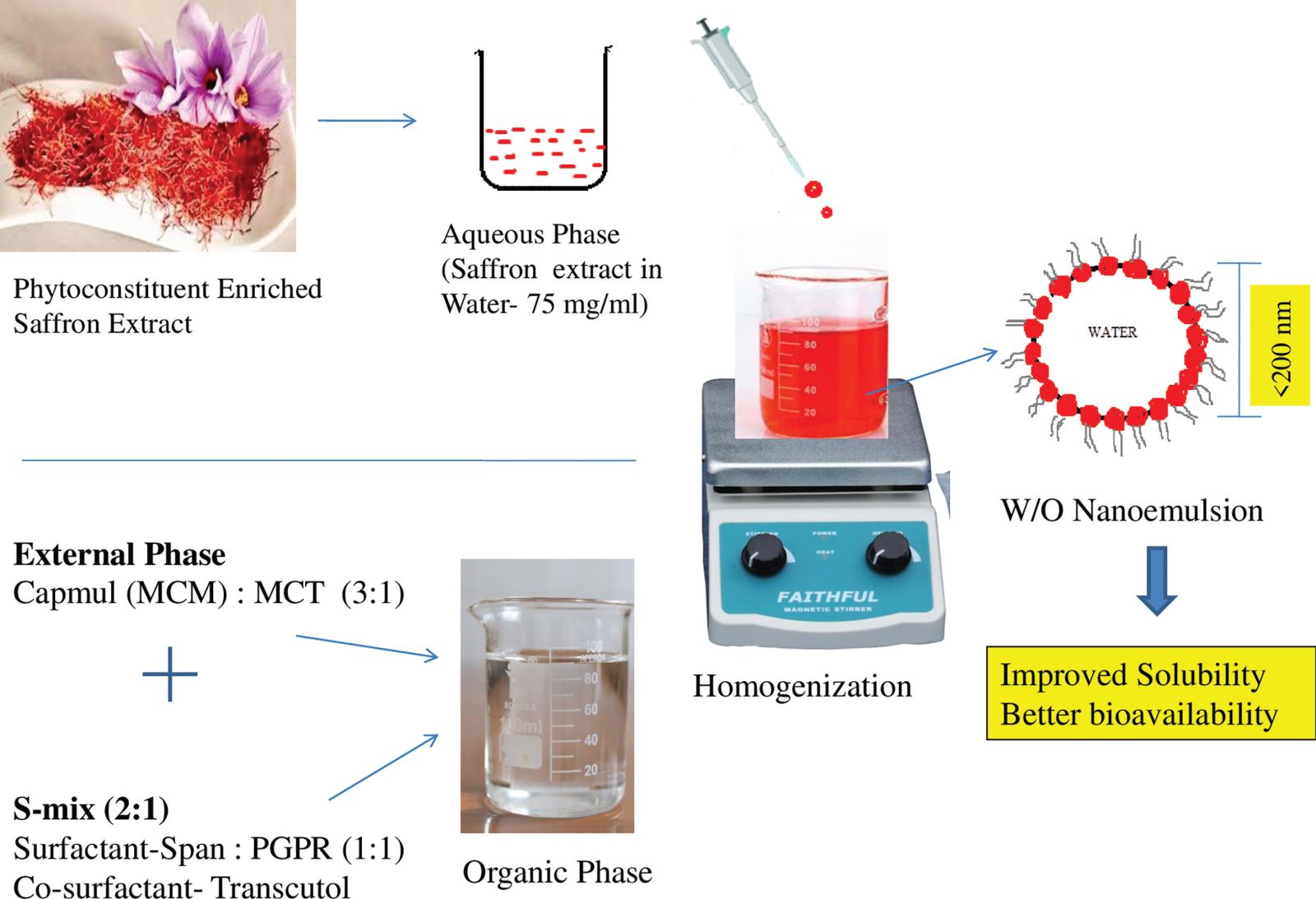 | Figure 10. Experimental design of saffron nanoemulsion. [Click here to view] |
The contour plots for responses are provided in Figure 9. Numerical optimization following the desirability approach was preferred to predict the optimum levels of the studied variables. The optimized formulation that yielded minimized size was generated as follows: 67% v/v oil concentration, 21% surfactant-co surfactant, and 12% internal phase concentration with desirability of 0.997. The C. sativusextract concentration in water was 75 mg/ml. Thus, 10 ml of optimized nano-emulsion formulation with 1.2 ml of water contains 90 mg of C. sativus. The predicted globule size of 94.89 nm was in accordance with the observed one (94.34 nm) indicating the successfulness of the optimization process.
Preparation of saffron-loaded nanoemulsion
Saffron loaded w/o nanoemulsion was prepared by low energy method with spontaneous emulsification. At one end, C. sativusextract accurately weighed 75 mg was dissolved in 1 ml of water. The other end required a quantity of MCM, MCT was added in a clean and dried beaker and allowed to disperse by keeping on the magnetic stirrer with optimum stirring speed and the S-mix mixture was added till obtained a clear liquid. In this resultant mixture, the aqueous phase containing saffron solution was added drop-wise which forms turbulent movement between the aqueous phase and oil phase with fast migration of water miscible C. sativusextract leading to larger water-oil interfacial area obtained w/o nanoemulsion loaded with C. sativusextract. The entire experiment design is provided in Figure 10.
Hydrophilic compounds are more challenging to reach at the target site of action for their therapeutic application and to overcome this limitation standardized C. sativusextract enriched with carotenoids is encapsulated in a nanoemulsion system which is thermodynamically stable and clear having particle sizes between 20 and 200 nm [47]. The nanoemulsion incorporating the standardized C. sativusextract was formulated with the help of a low-energy method using a spontaneous emulsification process [48]. The low-energy method is principally important in terms of thermal-sensitive bioactive compounds [49]. The w/o nanoemulsion was optimized by the Quality by Design (QbD) approach with DoE software may increase the industrial application.
Pharmaceutical characterization
Inverted microscopic study
Preliminary w/o nanoemulsion was observed under an inverted microscope at 40 × 10 magnification for distribution of internal phase and images were captured shown in Figure 11.
Droplet size and zeta potential measurement
The mean globule size of saffron w/o nanoemulsion was found to be 94.85 ± 2.25 nm and zeta potential was obtained—28.25 ± 1.26 mv. The high content of medium chain mono glyceride and MCT have contributed toward negative zeta potential value. Figure 12 shows droplet size distribution in saffron nanoemulsion.
Surface morphology by transmission electron microscopy
The TEM image provided in Figure 13 of saffron nanoemulsion shows uniform surface morphology. TEM images of saffron nanoemulsion indicated the actual morphology of the system where the measurement of formed globule size observed NMT 200 nm with uniform distribution in the w/o nanoemulsion system. The formulation has a droplet size in the nano range which is well distinct from low values of polydispersity. It is defined as the standard deviation of the mean droplet size of the internal phase in the nanoemulsion system. This was perfectly evident in the uniformity of droplet size within the nanoemulsion system.
Shear viscosity
The viscosity of optimized w/o nanoemulsion was found to be 136.25 ± 1.78 using a digital viscometer. All observation was recorded in triplicate. The recording is shown in Figure 14.
Content uniformity
The standardized C. sativuswas incorporated as an aqueous phase in the optimized nanoemulsion therefore % total content present in formulation is required to determine the loading of C. sativusin nanoemulsion in terms of overall total content. The optimized formulation of w/o nanoemulsion comprising 9 mg/ml of C. sativusextract with 12% internal phase, 21% S-mix, and 67% oil phase was assayed by developed and validated HPLC method. The loading of total content present in nanoemulsion was determined 83% which showed good loading capacity of C. sativusconsidered one of the important requirements of nanoemulsion. The HPLC chromatogram is shown in Table 7.
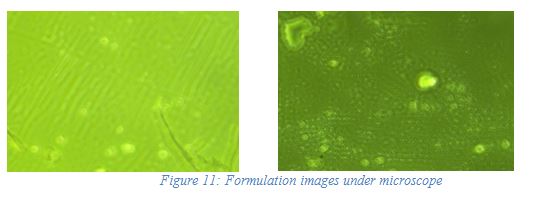 | Figure 11. Formulation images under a microscope. [Click here to view] |
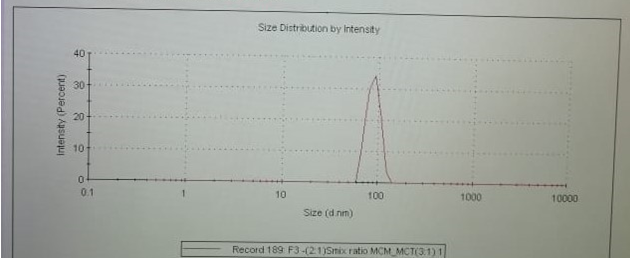 | Figure 12. Particle size determination by zeta sizer. [Click here to view] |
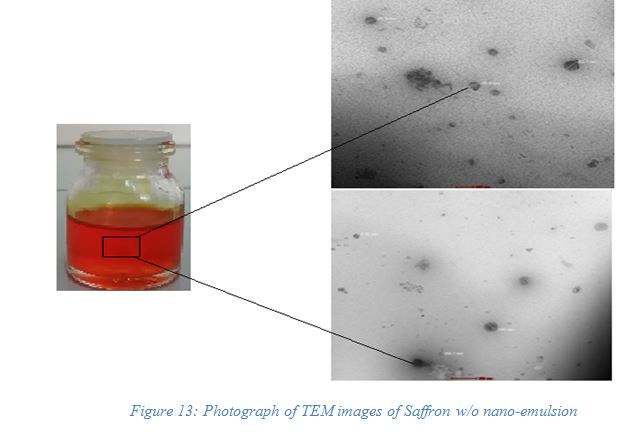 | Figure 13. Photograph of TEM images of saffron w/o nanoemulsion. [Click here to view] |
 | Figure 14. Measurement of viscosity of saffron nanoemulsion under digital viscometer. [Click here to view] |
In vitro release studies
At the desired time points such as 1, 2, 4, 5, 7, 9, 12, and 24 hours, the aliquots were withdrawn and analyzed through UV spectroscopy and measure the absorbance. By using the calibration curve the total content was determined and % cumulative release from formulation was calculated. The below graph in Figure 15 shows the release pattern. The drug release showed an initial burst release of 60% from the nanoemulsion at 1 hour, and a slow release of the drug after 1 hour. which allows more absorption in Gastro Intestinal Tract (GIT). The initial burst release was obtained due to the smaller particle size of the nanoemulsion. The smaller globule size and large surface area due to structural or physical properties of nanoemulsion could accelerate the initial burst release of phytoconstituents and further slow release indicated the controlled release.
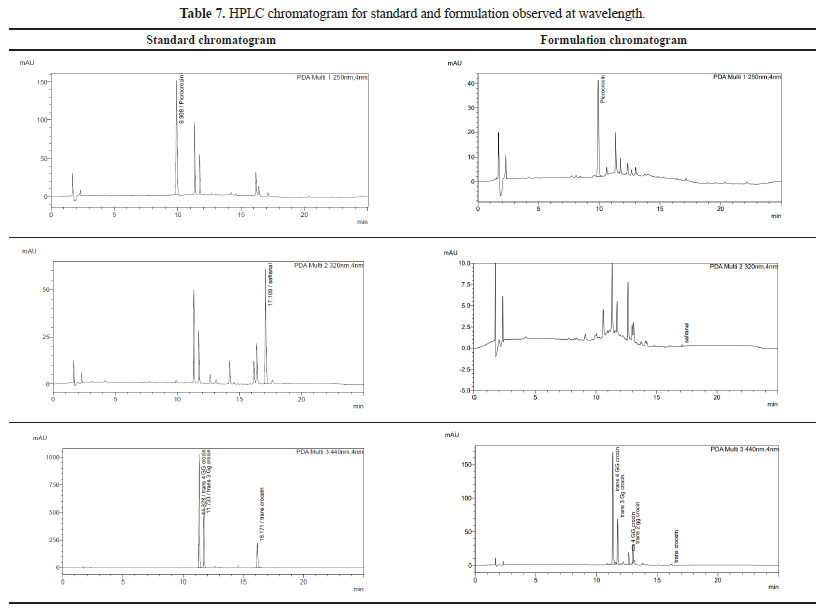 | Table 7. HPLC chromatogram for standard and formulation observed at wavelength. [Click here to view] |
Stability study
The evaluation of nanoemulsion kept under accelerated stability conditions, i.e., 40°C and 75% RH with respect to appearance, phase separation, globule size, zeta potential, and % total content was shown in Table 8. The % total content was determined as the average of three samples with a standard deviation NMT 2.0%. There was no significant difference in results obtained even after storage of nanoemulsion at a particular time period. Based on the stability results it was observed that the saffron nanoemulsion was found to be stable for up to 90 days without any signs of phase separation and the reduction of total content was found to be very less.
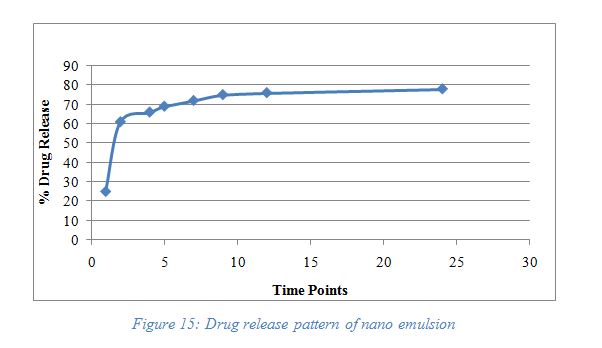 | Figure 15. Drug release pattern of nanoemulsion. [Click here to view] |
Highly hydrophilic bioactive principles present in herbs have poor absorption via lipid membranes that arrests their biological efficacy and pharmacokinetics. This novel drug delivery system has the ability to potentiate the efficacy of bioactive molecules by improving solubility and permeability, reducing side effects, lowering the dose, improving absorption profile, and so on [50]. The nanoemulsion can route the herbal phytoconstituents at the targeted site by increasing the drug-plasma concentration over a longer period of time which may enhance the bioavailability [51].
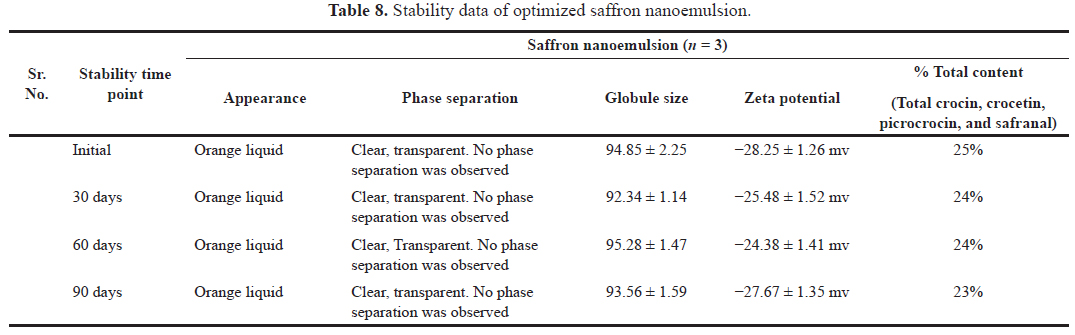 | Table 8. Stability data of optimized saffron nanoemulsion. [Click here to view] |
CONCLUSION
In this experiment, we attempted the development and optimization of w/o nanoemulsion of standardized C. sativusextract using the spontaneous emulsification method with low energy methods for pharmaceutical applications. The pharmaceutical characterization and quality by design studied for optimized w/o nanoemulsion of C. sativusextract facilitates its industrial application. The novel approach presented here may address the limitation of traditional herbal medicines by increased bioavailability, and controlled release of bioactive compounds with improved therapeutic response. The in vitro release from the formulation indicated that the formulation would be more advantageous with controlled drug release. In addition to this, the focused research area will widen the further research in preclinical and clinical investigation in the field of herbal medicines.
ACKNOWLEDGMENT
The author acknowledges Parul University for providing the research facility and Pharmanza Herbal Pvt. Ltd., for providing the C. sativusextract and analytical support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an authors as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors have no conflicts of interest regarding this investigation.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Fowler MW. Plants, medicines and man. J Sci Food Agric. 2006;86(12):1797–804. CrossRef
2. Rivera J, Loya A, Ceballos R. Use of herbal medicines and implications for conventional drug therapy medical sciences. Altern Integ Med. 2013;2(6):1–6. CrossRef
3. Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta. 2013;1830(6):3670–95. CrossRef
4. Boligon AA, Athayde ML. Importance of HPLC in analysis of plants extracts. Austin Chromatogr. 2014;1(3):2.
5. Kumar S, Saini R, Suthar P, Kumar V, Sharma R. Plant secondary metabolites: their food and therapeutic importance. In: Sharma, A.K., Sharma A, (eds) Plant Secondary Metabolites. Springer, Singapore.; 2022. pp 371–413. CrossRef
6. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MdP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):1–33. Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-018-0392-8
7. Cerdá-Bernad D, Valero-Cases E, Pastor J-J, Frutos MJ. Saffron bioactives crocin, crocetin and safranal: effect on oxidative stress and mechanisms of action. Crit Rev Food Sci Nutr. 2022;62(12):3232–49. CrossRef
8. Premkumar K, Thirunavukkarasu C, Abraham S, Santhiya S, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25(2):79–84. CrossRef
9. Rathore B, Jaggi K, Thakur SK, Mathur A, Mahdi F. Anti-inflammatory activity of Crocus sativus extract in experimental arthritis. Int J Pharm Sci Res. 2015;6(4):1473. CrossRef
10. Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents, crocin and safranal. Pharmacogn Mag. 2009;5(Suppl 2):419–24.
11. Finley JW, Gao S. A perspective on Crocus sativus L. (saffron) constituent crocin: a potent water-soluble antioxidant and potential therapy for Alzheimer’s disease. J Agric Food Chem. 2017;65(5):1005–20. CrossRef
12. Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol. 2013;52:163–70. CrossRef
13. Razavi BM, Hosseinzadeh H. Saffron: a promising natural medicine in the treatment of metabolic syndrome. J Sci Food Agric. 2017;97(6):1679–85. CrossRef
14. Omidi A, Riahinia N, Torbati MBM, Behdani MA. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J Phytomed. 2014;4(5):330. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4224710/
15. Butnariu M, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Singh L, Aborehab NM, et al. The pharmacological activities of Crocus sativus L.: a review based on the mechanisms and therapeutic opportunities of its phytoconstituents. Oxid Med Cell Longev. 2022;2022. CrossRef
16. Xi L, Qian Z, Du P, Fu J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine. 2007;14(9):633–6. CrossRef
17. Zhang Y, Fei F, Zhen L, Zhu X, Wang J, Li S, et al. Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;15:1–7. CrossRef
18. Christodoulou E, Grafakou M-E, Skaltsa E, Kadoglou N, Kostomitsopoulos N, Valsami G. Preparation, chemical characterization and determination of crocetin’s pharmacokinetics after oral and intravenous administration of saffron (Crocus sativus L.) aqueous extract to C57/BL6J mice. J Pharm Pharmacol. 2019;71(5):753–64. CrossRef
19. Almodóvar P, Briskey D, Rao A, Prodanov M, Inarejos-García AM. Bioaccessibility and pharmacokinetics of a commercial saffron (Crocus sativus L.) extract. Evid Based Complement Alternat Med. 2020;2020 Jan 30:2020:1575730 2020. CrossRef
20. Girme A, Pawar S, Ghule C, Shengule S, Saste G, Balasubramaniam AK, et al. Bioanalytical method development and validation study of neuroprotective extract of Kashmiri saffron using ultra-fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS): in vivopharmacokinetics of apocarotenoids and carotenoids. Molecules. 2021;26(6):1815. CrossRef
21. Garavand F, Rahaee S, Vahedikia N, Jafari SM. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci Technol. 2019;89:26–44. CrossRef
22. Rahaiee S, Shojaosadati SA, Hashemi M, Moini S, Razavi SH. Improvement of crocin stability by biodegradeble nanoparticles of chitosan-alginate. Int J Biol Macromol. 2015;79:423–32. CrossRef
23. El-Aasser MS, Sudol ED. Miniemulsions: overview of research and applications. JCT Res. 2004;1(1):21–31.
24. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–41. CrossRef
25. Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci. 2012;17(5):246–54. CrossRef
26. Solans C, Morales D, Homs M. Spontaneous emulsification. Curr Opin Colloid Interface Sci. 2016;22:88–93. CrossRef
27. Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1–2):241–51. CrossRef
28. López-Montilla JC, Herrera-Morales PE, Pandey S, Shah DO. Spontaneous emulsification: mechanisms, physicochemical aspects, modeling, and applications. J Dispers Sci Technol. 2002;23(1–3):219–68. CrossRef
29. Saberi AH, Fang Y, McClements DJ. Fabrication of vitamin E-enriched nanoemulsions: factors affecting particle size using spontaneous emulsification. J Colloid Interface Sci. 2013;391:95–102. CrossRef
30. Esakkiraja N, Paul A. A novel concept of pseudo ternary diffusion couple for the estimation of diffusion coefficients in multicomponent systems. Scripta Materialia. 2018;147:79–82. CrossRef
31. Ghosh S, Mali SN, Bhowmick D, Pratap AP. Neem oil as natural pesticide: pseudo ternary diagram and computational study. J Indian Chem Soc. 2021;98(7):100088. CrossRef
32. Anwer MK, Jamil S, Ibnouf EO, Shakeel F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J Oleo Sci. 2014;63(4):347–54. CrossRef
33. Harika K, Debnath S. Formulation and evaluation of nanoemulsion of amphotericin B. Int J Novel Trends Pharm Sci. 2015;5(4):114–22. Available from: https://scienztech.org/index.php/ijntps/article/view/146
34. Wang Z, Pal R. Enlargement of nanoemulsion region in pseudo-ternary mixing diagrams for a drug delivery system. J Surfactants Deterg. 2014;17(1):49–58. Available from: https://link.springer.com/article/10.1007/s11743-013-1497-6
35. Mehrnia M-A, Jafari S-M, Makhmal-Zadeh BS, Maghsoudlou Y. Rheological and release properties of double nano-emulsions containing crocin prepared with Angum gum, Arabic gum and whey protein. Food Hydrocolloids. 2017;66:259–67. CrossRef
36. Uson N, Garcia MJ, Solans C. Formation of water-in-oil (W/O) nano-emulsions in a water/mixed non-ionic surfactant/oil systems prepared by a low-energy emulsification method. Colloids Surf Physicochem Eng Asp. 2004;250(1–3):415–21. CrossRef
37. Laxmi M, Bhardwaj A, Mehta S, Mehta A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells Nanomed Biotechnol. 2015;43(5):334–44. CrossRef
38. Peng L-C, Liu C-H, Kwan C-C, Huang K-F. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf Physicochem Eng Asp. 2010;370(1–3):136–42. CrossRef
39. Girme A, Saste G, Pawar S, Ghule C, Mirgal A, Patel S, et al. Quantitative determination and characterization of a Kashmir saffron (Crocus sativus L.)-based botanical supplement using single-laboratory validation study by HPLC-PDA with LC–MS/MS and HPTLC investigations. ACS Omega. 2021;6(36):23460–74. CrossRef
40. Joshi SA, Chavhan SS, Sawant KK. Rivastigmine-loaded PLGA and PBCA nanoparticles: preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur J Pharm Biopharm. 2010;76(2):189–99. CrossRef
41. Gurpreet K, Singh S. Review of nanoemulsion formulation and characterization techniques. Indian J Pharm Sci. 2018;80(5):781–9.
42. Gulati N, Kumar Chellappan D, Tambuwala MM, Aljabali AAA, Prasher P, Kumar Singh S, et al. Oral nanoemulsion of fenofibrate: formulation, characterization, and in vitro drug release studies. ASSAY Drug Dev Technol. 2021;19(4):246–61. CrossRef
43. Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J. Anti HIV nanoemulsion formulation: optimization and in vitro–in vivoevaluation. Int J Pharm. 2014;462(1–2):129–34. CrossRef
44. Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. J Appl Pharm Sci. 2012;2:129–38. CrossRef
45. Sharma R, Kuca K, Nepovimova E, Kabra A, Rao M, Prajapati P. Traditional Ayurvedic and herbal remedies for Alzheimer’s disease: from bench to bedside. Expert Rev Neurother. 2019;19(5):359–74. CrossRef
46. Chauhan A, Semwal DK, Mishra SP, Semwal RB. Ayurvedic research and methodology: present status and future strategies. Ayu. 2015;36(4):364–369. CrossRef
47. Dutta L, Mukherjee B, Chakraborty T, Das MK, Mondal L, Bhattacharya S, et al. Lipid-based nanocarrier efficiently delivers highly water soluble drug across the blood–brain barrier into brain. Drug Deliv. 2018;25(1):504–16. CrossRef
48. Kotta S, Khan AW, Ansari S, Sharma R, Ali J. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015;22(4):455–66. CrossRef
49. Safaya M, Rotliwala Y. Nanoemulsions: a review on low energy formulation methods, characterization, applications and optimization technique. Mater Today Proc. 2020;27:454–9. CrossRef
50. Harwansh RK, Deshmukh R, Rahman MA. Nanoemulsion: promising nanocarrier system for delivery of herbal bioactives. J Drug Deliv Sci Technol. 2019;51:224–33. CrossRef
51. Abdul Wahab R, Al-obaidi NG, Yahya NA, Che Marzuki NH, Mohd Bohari SP. Formulation of a stable water-in-oil nanoemulsion rich in anti-diabetic components of the roselle extract for controlled release. Chem Pap. 2022;76(4):2341–56. Available from: https://link.springer.com/article/10.1007/s11696-021-02030-x