INTRODUCTION
Natural substances offer a promising alternative for medical therapies in managing various health conditions. The bulb of the Dayak onion, Eleutherine bulbosa (E. bulbosa), a notable member of the Iridaceae family found in Southeast Asia, boasts a range of therapeutic benefits. Historically used by the Dayak people to remedy conditions including diabetes, breast cancer, blocked nasal passages, and reproductive health problems [1]. The bulb contains an abundance of chemical components such as phenolic compounds, flavonoids, naphthalene, anthraquinones, and naphthoquinones, all of which enhance its therapeutic capabilities [2]. Synonyms for E. bulbosa include Eleutherine americana, Eleutherine palmifolia, and Eleutherine platifolia [3]. Its documented uses span anticancer [4], antidiabetic [5], antibacterial [6], antifungal [7], antiviral [8], anti-inflammatory [9], dermatological [10], antioxidative [11], and fertility regulatory activities [3]. Despite extensive reports on E. bulbosa’s active components, comparative analysis of these compounds in different solvent extracts from the bulb remains limited. Thus, further exploration of metabolite profiling and its pharmacological relevance in Dayak onions is warranted.
Ultra-performance liquid chromatography-mass spectrometry/mass spectrometry (UPLC-MS/MS) is an advanced and precise analytical technique for profiling metabolites in biological samples [12]but they all have disadvantages either in terms of sensitivity or of selectivity. The number of samples that can be analysed, the low volume of samples available during the experiment and the need to identify different degradates are all obstacles that new techniques are able to overcome. The work presented here summarizes progress in the field of metrology as concerns online solid phase extraction technology coupled with liquid chromatography followed by tandem mass spectrometry detection. Recently developed analytical techniques were validated for both 18 pesticides and their degradates and 17 pharmaceuticals and their degradates. Limits of quantification from 20 to 70 ng L−1 for pharmaceuticals and from 15 to 25 ng L−1 for pesticides and metabolites have been obtained, with linearity range up to 1 μg L−1. The limits of quantification of a few nanograms per litre, the possibility of working on less than 1 mL of sample and the simultaneous quantification of the target products and their transformation products are all advantages that are demonstrated by two environmental applications. The first application concerns the evaluation of ecotoxicological effects of pesticides on aquatic organisms exposed in mesocosms. The second application aims to determine the adsorption constants of pharmaceutical molecules on soils and river sediments. For both applications, the robustness, range of linearity and limit of quantification of the developed analytical methods satisfy the requirements for laboratory experiments conducted under controlled conditions. Specific constraints generated by this type of experiment (adding CaCl2 for the adsorption study and filtration of the water coming from the mesocosms. It is extensively applied to determine the biochemical constituents of plant extracts. The extraction process, pivotal in phytochemical analysis, is influenced by the choice of solvent, which determines the quality and quantity of the metabolites extracted [13]. Ethanol and water extracts are commonly used in ethnopharmacological research due to their varied extraction capabilities.
This research aims to carry out a detailed comparative analysis of the metabolite profiles obtained from the E. bulbosa bulb extracts using ethanol and water as solvents, utilizing UPLC-MS/MS technology. In addition, the research examines the cytotoxic potential of both extracts on T47D human breast cancer cells. Evaluating cytotoxic activity is essential to correlate with the quantity and quality of bioactive compounds obtained using different solvents. The findings will inform the optimal solvent choice for preparing Dayak onion raw material, maximizing its therapeutic efficacy, and contributing to the development of natural anticancer drugs.
MATERIALS AND METHODS
Plant material
E. bulbosa bulbs were gathered from East Kalimantan in Indonesia. The specimens were cataloged at the UPTD Materia Medika in Malang, under the accession number 074/348/102.7/2021. These plants are currently conserved at the Pharmacognosy Laboratory in the Department of Pharmacy at the Maulana Malik Ibrahim State Islamic University of Malang.
Extraction with 96% ethanol
Fifty grams of the powdered plant material were placed into a measuring cup and liquefied with 500 ml of 96% ethanol, which was divided into two clusters (300 and 200 ml). This mixture was then extracted using the ultrasound-assisted extraction (UAE) method with a sonicator for 3 × 10 minutes. Subsequently, the extract was passed through a filter and subsequently dehydrated using a rotary evaporator followed by an oven [14].
Water extraction
One hundred and twenty-two grams of fresh Dayak onion bulbs were extracted using 610 ml of hot water by maceration method. The extract was then filtered and dried using the freeze-drying method.
Thin layer chromatography (TLC) analysis
A standard solution of 1,4-naphthoquinone and the extracts was prepared at a concentration of 10,000 ppm. Two microliters of the standard solution and extracts were then spotted onto a silica Gel 60 F254 plate. The plate underwent elution with a chloroform:methanol (8:2) solvent mixture. Post-elution, it was subjected to UV visualization at 254 and 366 nm wavelengths. Following this, a 10% sulfuric acid (H2SO4) staining solution was applied for derivatization, and the plate was heated to develop visible stain colors, after which it was re-examined under UV light at 366 nm [15].
The analysis of UPLC-QToF-MS
Using UPLC-MS instruments with a QToF detector and positive electrospray ionization for ion generation, the analysis through UPLC-QToF-MS was carried out on an Acquity C18 column with dimensions of 1.8 μm; 2.1 × 150 mm. A mixture of water (of HPLC grade) and formic acid (sourced from Merck, Darmstadt, Germany) was used in a 99.9 to 0.1 [v/v] ratio. The eluent employed was acetonitrile (provided by Merck, Darmstadt, Germany), used in a 99.9 to 0.1 [v/v] ratio, within a gradient elution setup. The desolvation temperature was 350°C, while the source temperature was 100°C. Following the solution of a 10 mg extract in an absolute methanol-filled 10 ml volumetric flask, 5 μl volumes were introduced into the UPLC-MS apparatus. Area was expressed as a percentage based on chromatogram data. The study parameters were established using the positive ion mode, and spectra were acquired across a mass range from m/z 120 to 1,000. Masslynx software version 4.1 (by Waters, Massachusetts, USA) was utilized to process the chromatograms. Component identification was performed by comparing the measured m/z ratios in Masslynx with those listed in PubChem (https://pubchem.ncbi.nlm.nih.gov/). Confirmation of a compound’s identity was determined through MS/MS fragment comparison and a deviation of less than 5 ppm to ensure accuracy [16].
Cell culture
The T47D breast cancer cell line was procured from Dr. Masashi Kawaichi at the Nara Institute of Science and Technology in Japan. These cells were maintained in a monolayer using high-glucose Dulbecco’s Modified Eagle Medium from Gibco, USA, enriched with 10% (v/v) fetal bovine serum from Sigma, USA, along with 150 IU/ml of penicillin and 150 μg/ml of streptomycin from Gibco, USA, plus 1.25 μg/ml of amphotericin B also from Gibco, USA. They were kept at 37°C in a humidified environment with 5% CO2 and 100% atmosphere. The T47D cells were utilized for the experiments when they reached 80%–90% confluency [17].
Cell viability assay
The growth rate of T47D cells was assessed via the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Initially, T47D cells (2 × 10³ cells/well) were distributed into a 96-well plate and left to attach throughout the night. Post-attachment, the cells were treated for 24 hours with varying agents: 96% ethanol extract of E. bulbosa, water extract of E. bulbosa, 1,4-Naphthoquinone (from Sigma-Aldrich, USA) at concentrations ranging from 50–500 μM, and doxorubicin (DOX) (from Sigma-Aldrich, USA) at 0.01–10 μM. Cells without treatment served as the negative control. Following the exposure to treatments, each well received 100 μl of MTT solution (0.5 mg/ml, supplied by Biovision) and was incubated for an additional 4 hours at 37°C in an atmosphere of 5% CO2. The MTT formazan product was then dissolved using an sodium dodecyl sulfate halt agent blended with 0.01 N HCl and left in the dark overnight. The solubilized purple formazan’s absorbance was measured at 595 nm using an ELISA plate reader (Corona SH-1000). These treatments were replicated three times to determine the IC50 value—the necessary concentration to inhibit cell growth by 50% relative to the control group [18].
Cell cycle analysis
Flow cytometry was used to conduct cell cycle analysis, employing propidium iodide (PI) for staining. T47D cells were grown to a density of 2 × 105 cells per well in six-well plates. Following treatments with extracts of E. bulbosa, 1,4-Naphthoquinone (50–500 μM), and DOX (0.01–10 μM) from Sigma-Aldrich, USA, the culture medium was discarded, and the cells were detached using trypsin, then centrifuged at 2,000 rpm for 3 minutes. The cell harvest was then fixed in ethanol at 4°C for half an hour. Subsequently, the cells were rinsed with cold phosphate-buffered saline (PBS) and centrifuged again under the same conditions. The resulting cell sediment was then reconstituted in a PI mixture (50 μg/ml in PBS with 1% Triton X-100 from Merck) and RNase A devoid of DNase (20 μg/ml), and incubated at 37°C for 30 minutes. The final cell analysis was conducted using a flow cytometer (FACS Calibur, BD Biosciences, USA), where cell debris was electronically excluded, and the red fluorescence intensity was measured using the FL1 channel in logarithmic mode [14].
Apoptosis assay
The apoptosis assay was conducted utilizing the Annexin V-fluorescein isothiocyanate (FITC)/PI staining protocol for flow cytometry after treatments with E. bulbosa extract, DOX, and 1,4-naphthoquinone. In summary, after collection, cells were stained for 10 minutes in darkness at ambient temperature using the Annexin-V-FLUOS kit by Roche, which includes 100 μl of binding buffer, 2 μl of Annexin V, and 2 μl of PI. Following staining, the cells were subjected to analysis with a flow cytometer (FACS Calibur, BD Biosciences, USA). FITC was detected by measuring the fluorescence intensity with the FL-1H channel. The proportion of apoptotic cells was then calculated using the Cell Quest software provided by BD Bioscience [19].
RESULTS AND DISCUSSION
Extract yield analysis
In this study, the yield of the 96% ethanol extract was found to be higher (yield percentage: 4.465% ± 0.21%) compared to the yield of the water extract (yield percentage: 4.394% ± 0.52%). The extraction results using 96% ethanol solvent had a higher average than those using water as the solvent. This is presumed to be due to the use of the UAE method which can extract E. bulbosa more optimally by using ultrasonic waves compared to the maceration extraction method, which is just a soaking process without the aid of additional forces. Moreover, the drying method used is also believed to affect the results obtained. The freeze-drying method can produce extracts with a very lightweight because all water content in the extract has been optimally dried during the drying process [20]. If the weight of the extract is very light, then the yield value will also be low. Therefore, the extract obtained from water solvent extraction using the freeze-drying method has a lower yield value than the extract obtained from 96% ethanol solvent extraction.
Identification with TLC
The results of the identification using the TLC approach indicated that the standard for 1,4-naphthoquinone was at an retention factor (used in chromatography) (Rf) of 0.90. Stains at the same Rf were also detected in both ethanol and water extracts. The 96% ethanol extract identified stains of 1,4-naphthoquinone, alkaloids, flavonoids, terpenoids, steroids, and tannins. Whereas the water extract showed stains of compounds such as 1,4-naphthoquinone, alkaloids, flavonoids, and steroids.
Metabolite profiling
Metabolite profiling is a method used to analyze compounds with the aim of profiling the metabolite compounds contained in a plant [21]. This instrument was chosen because it has advantages over other analytical tools such as higher resolution which results in more efficient compound separation, smaller column particles leading to higher sensitivity, shorter analysis time due to higher flow rates, the ability to separate smaller compounds because of higher pressure, and requires less quantity [22].
The choice of solvent in the extraction process is a critical factor due to the differing solubility of compounds. Solvents such as ethanol are often used in herbal extractions because of their ability to extract a wide range of chemical compounds, including both hydrophobic and hydrophilic substances. Meanwhile, water is a more selective solvent and typically only extracts highly polar compounds [23].
This research employed the advanced analytical method of UPLC-QToF-MS/MS for profiling metabolites[12]but they all have disadvantages either in terms of sensitivity or of selectivity. The number of samples that can be analysed, the low volume of samples available during the experiment and the need to identify different degradates are all obstacles that new techniques are able to overcome. The work presented here summarizes progress in the field of metrology as concerns online solid phase extraction technology coupled with liquid chromatography followed by tandem mass spectrometry detection. Recently developed analytical techniques were validated for both 18 pesticides and their degradates and 17 pharmaceuticals and their degradates. Limits of quantification from 20 to 70 ng L−1 for pharmaceuticals and from 15 to 25 ng L−1 for pesticides and metabolites have been obtained, with linearity range up to 1 μg L−1. The limits of quantification of a few nanograms per litre, the possibility of working on less than 1 mL of sample and the simultaneous quantification of the target products and their transformation products are all advantages that are demonstrated by two environmental applications. The first application concerns the evaluation of ecotoxicological effects of pesticides on aquatic organisms exposed in mesocosms. The second application aims to determine the adsorption constants of pharmaceutical molecules on soils and river sediments. For both applications, the robustness, range of linearity and limit of quantification of the developed analytical methods satisfy the requirements for laboratory experiments conducted under controlled conditions. Specific constraints generated by this type of experiment (adding CaCl2 for the adsorption study and filtration of the water coming from the mesocosms. This method enables the detection and measurement of substances with great precision and clarity. The information garnered from the UPLC-QToF-MS/MS apparatus is displayed in the form of chromatograms. The chromatograms produced are shown in Figures 1 and 2.
Based on the metabolite profiling analysis results, it is known that the ethanol extract of E. bulbosa contains 32 identified compounds (Table 1). In contrast, the water extract has 27 compounds that have been successfully identified (Table 2). This difference may be attributed to ethanol’s efficiency in extracting a greater number of compounds, including nonpolar ones.
Tables 1 and 2 display the compounds detected via UPLC-QToF-MS/MS in both the ethanol extract and the water extract. One factor that may influence the differences in the metabolite profiles is the variance in solvents used. Ethanol and water solvents possess different polarities where ethanol is a semi-polar solvent and water is a polar solvent. Ethanol is considered a universal solvent, thereby capable of extracting the majority of compounds that are both polar and nonpolar in nature from the plant material [24,25]. The percentage levels of transresveratrol, oxyresveratrol, and 1,4-naphthoquinone compounds in the ethanol extract of E. bulbosa have been identified in Table 1, at peak numbers 10, 17, and 21, respectively, with percentages of 1.035%, 1.453%, and 1.753% in order. Although the concentrations of these compounds in the extract are not very high, they seem to play a role in anticancer activity.
The analysis of the metabolite profiles revealed a total of 32 compounds from the ethanol extract and 27 from the aqueous extract, with 3 compounds common to both extracts, namely 1,4-naphthoquinone (C10H6O2), L-lysine sulfate (C6H14N2O4S), and Epirizole (C11H14N4O2). The presence of these compounds in both extracts indicates that they are sufficiently polar to be soluble in water, yet they also possess adequate solubility in ethanol. This data was obtained using a Venn diagram (Fig. 3).
Cytotoxicity test results on breast cancer cell line
In this study, after the identification of phytochemical in the ethanol and water extract of E. bulbosa, we proceeded to conduct in vitro verification through cytotoxicity testing on the T47D cells. The purpose of this test is to validate the anticancer activity of both extracts.
The cytotoxic test (Fig. 4 and Table 3) indicates that the ethanol extract has low anticancer potential with an IC50 value of 202.37 μg/ml. This IC50 value refers to the concentration at which the extract can inhibit the growth of 50% of the tested cancer cell population. Meanwhile, the water extract demonstrated no anticanceractivity, with an IC50 value of 1,020 μg/ml. According to the National Cancer Institute, an extract is considered active when it exhibits cytotoxic activity with an IC50 value <30 μg/ml. Moderate activity is defined when the IC value is ≥30 μg/ml and the IC50 is <100 μg/ml. The extract is deemed to have low activity or is inactive when the IC50 value is >100 μg/ml [26,27].
Induction of apoptosis and cell cycle arrest
As further evidence from the cytotoxicity assay results showing that compounds in the ethanol extract of E. bulbosa have a low inhibitory effect on T47D cells. In this study, further evaluation of the effects of ethanole extract through apoptosis and cell cycle trials was conducted. In addition, the apoptotic activity and the influence of cell cycles were also compared with the chemotherapeutic drugs DOX and the 1,4-naftokuinon compound. On the contrary, the quantitative assessment of cell death, which included the early and late stages of apoptosis, indicated that the use of ethanol extracts of E. bulbosa differed significantly in effect when compared to the nontreated control group, as well as with the groups treated with Doxoroubicine and 1,4-naphtokuinon (p < 0.001).
In addition to triggering cell mortality, the amplification of anticancer activities may also be achieved by altering the cell cycle. The analysis of cell cycle phases post-treatment was conducted using flow cytometry. T47D cells underwent a 24-hour treatment with 10 nM DOX, the half-maximal inhibitory concentration (IC50) of E. bulbosa ethanol extract, and the IC50 of 1,4-naphthoquinone. Results in Figure 6A and B indicate that there was a substantial impact on the distribution of cell cycle phases of cells treated with E. bulbosa ethanol extract (p < 0.0001). Administration of this extract resulted in an increase in the sub G1 and S phases, as well as a significant decrease in accumulation in the G2-M and G1 phases. Conversely, the administration of DOX and 1,4-naphthoquinone had no effect on the cell cycle. In Figure 6A and B, it is noted that the cell cycles of DOX and 1,4-naphthoquinone do not differ from the untreated group (p > 0.0001).
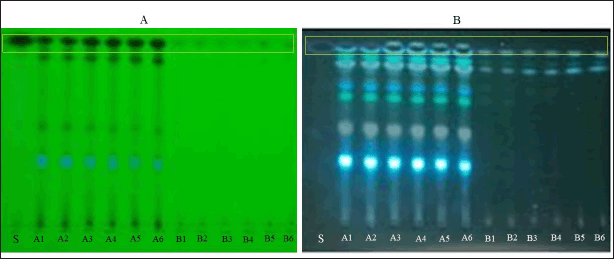 | Figure 1. TLC results at UV 254 nm; S = standard 1,4-naphthoquinone; A1–A6 = Eleutherine bulbosa ethanol extract; B1–B6 = Eleutherine bulbosa water extract (A). UV 366 nm = standard 1,4-naphthoquinone; A1-A6 = Eleutherine bulbosa ethanol extract; B1–B6 = Eleutherine bulbosa water extract (B). [Click here to view] |
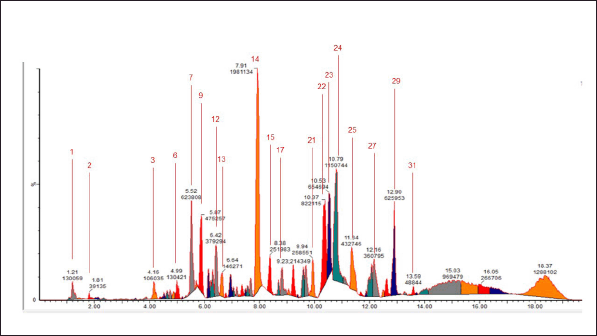 | Figure 2. Chromatogram of the 96% ethanol extract of E. bulbosa. [Click here to view] |
This study utilized LC-MS/MS techniques to identify chemical components in the ethanolic extract of E. bulbosa. According to the results displayed in Table 1, it was determined that the extract contains the compound oxyresveratrol, known for its significant anticancer potential [28].
Previous research has recognized oxyresveratrol as a compound that can affect the expression of genes related to apoptosis (programmed cell death) and cell cycle control, as well as DNA repair in Michigan cancer foundation-7 (a breast cancer cell line) (MCF-7) cells. This effect is crucial in cancer therapy as it can halt the growth of cancer cells and induce death in damaged cells [29].
In addition, studies suggest that oxyresveratrol has the ability to suppress the proliferation of human lung squamous carcinoma cells by inducing arrest in the S-phase of the cell cycle and promoting cell death through apoptosis. The arrest in the S phase prevents cancer cells from continuing DNA replication, an essential step in cell division. Apoptosis induction compels the cell to activate its own death mechanisms [30,31].
In addition to that in Table 1 it has been identified that the ethanolic extract of E. bulbosa contains trans resveratrol, in previous studies it had been that trans-resveratrole is capable of inducing apoptosis in breast cancer cells MCF-7. The process involves activating the path Mitogen-activated protein kinases, which include c-Jun N-terminal kinase and p38 mitogen-activated protein kinase[32].
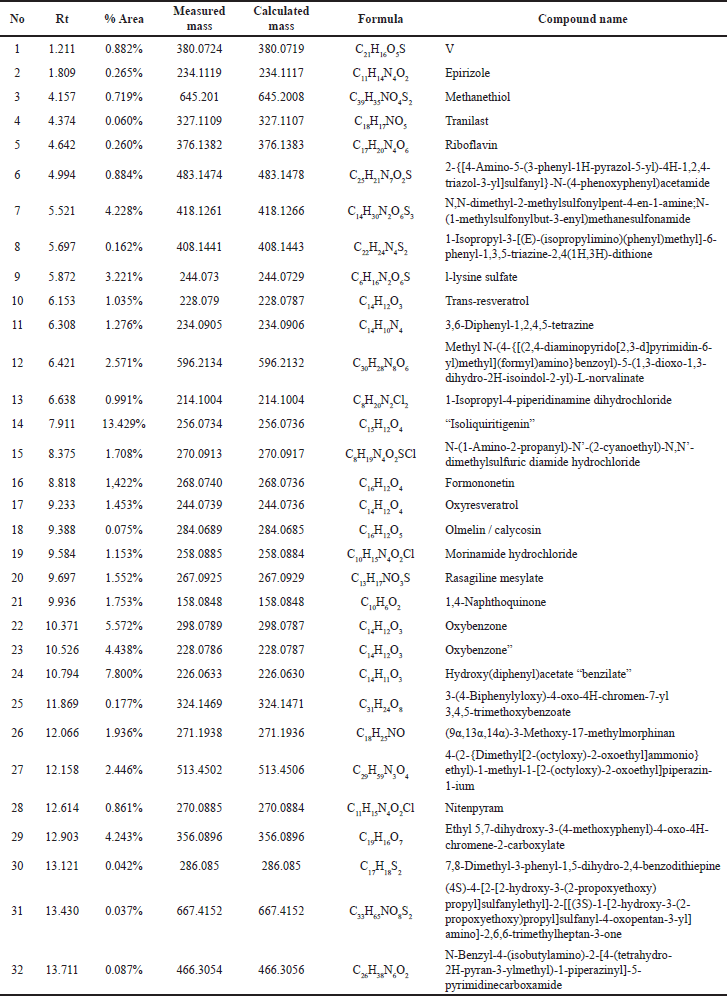 | Table 1. Chromatogram analysis results of LCMS/MS from the 96% ethanol extract of E. bulbosa. [Click here to view] |
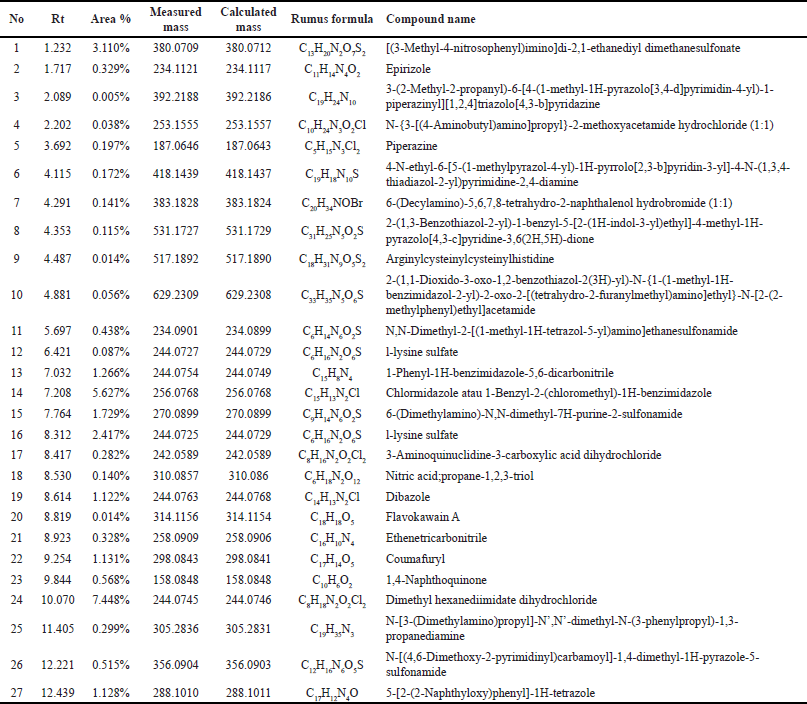 | Table 2. Outcomes of the Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LCMS/MS) chromatogram analysis conducted on the water extract of E. bulbosa. [Click here to view] |
Therefore, these findings provide additional evidence to support the role of oxyresveratrol and trans resveratrol as active components in E. bulbosa extract that may contribute to the observed anticancer activity.
In vitro validation results regarding the anticancer activity of E. bulbosa ethanolic extract show significant induction of apoptosis, and strong potential in upregulating the cell cycle through an increase in the sub-G1 and S phases, along with a significant decrease in accumulation in the G2-M and G1 phases [33]cyclins D1 and E and the cyclin-dependent kinase inhibitors p21 (Waf1/Cip1.
These findings concur with earlier studies which found that the ethanol extract of E. bulbosa hindered the growth of retinoblastoma cancer cells, demonstrating a half-maximal inhibitory concentration (IC50) of 15.7 μg/ml. Cells showed initial indications of apoptosis and increased regulation of the cell cycle [34].
Genetically, the data shows a heightened expression of genes that advance apoptosis (like Bax, p53, and Caspases) and a reduced expression of genes that deter apoptosis (such as Bcl-2 and Nrf-2) [34]. The p53 gene is an important marker in controlling DNA quality and triggers apoptosis in the event of DNA damage [35]. Caspases are a family of proteins that function in the execution of apoptosis [36]. On the other hand, the Bcl-2 and Nrf-2 genes are involved in the cell’s defense mechanisms to prevent apoptosis and oxidative stress [37].
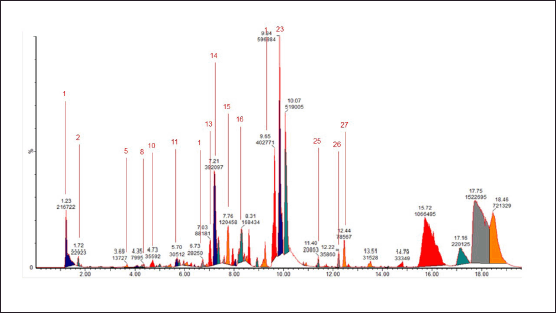 | Figure 3. Chromatogram of the water extract of E. bulbosa. [Click here to view] |
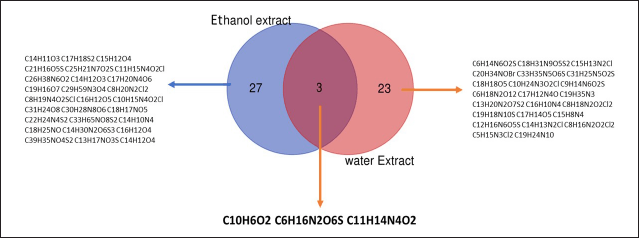 | Figure 4. Venn diagram illustrating the compound profiles for ethanol and water extracts of E. bulbosa. [Click here to view] |
 | Table 3. IC50 value (50% inhibitory concentration) of E. bulbosa extract against cell line T47D. [Click here to view] |
In summary, the findings of this research verify that the ethanolic extract of E. bulbosa, which includes oxyresveratrol, is noted to exert a considerable effect on the proliferation of cancer cells [28–31]. Using LC-MS/MS techniques, this study successfully identified this compound and linked it to anticancer activity in vitro. In addition, there is evidence that oxyresveratrol contributes to the halting of the cell cycle and the induction of apoptosis, including changes in the expression of genes associated with the apoptosis process.
1,4-naphthoquinone exhibits a higher level of cytotoxic activity compared to the ethanol extract (Table 3), but it does not have an impact on apoptosis and the cell cycle (Fig. 5). This suggests that 1,4-naphthoquinone may have potential as an anticancer agent, but not through the pathways of apoptosis and the cell cycle. In previous studies, it has been reported that 1,4-naphthoquinone exhibits highly potent anticancer activity in several cell lines, including HepG2, HuCCA-1, A549, and MOLT-3. In this study, it was found that 1,4-naphthoquinone has a high-potential IC50; however, in apoptosis and cell cycle tests, it did not show any effects. This may be due to the compound working as an anticancer agent, not through the induction of apoptosis and the cell cycle, but possibly through other mechanisms. Previous research has reported that quinone-based drugs function as anticancer agents through the production of reactive oxygen species. In addition, the cytotoxic effects of clinically used quinone-based anticancer drugs are closely related to the inhibition of the DNA topoisomerase II enzyme [38]. DNA topoisomerase II is a crucial enzyme required for DNA replication, chromosome condensation, and chromosome segregation [39]. The DNA opening process is a crucial step where the double-stranded DNA helix needs to be unwound to allow the continuation of the DNA replication process [40].
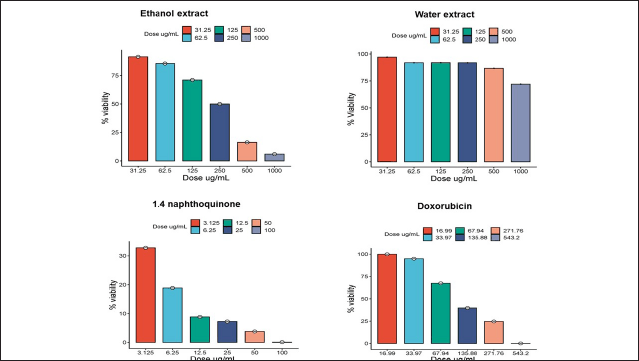 | Figure 5. T47D cell viability after treatment with ethanol extract of E. bulbosa, water extract, DOX, and 1,4-naphthoquinone. Over a 24-hour period, test samples at different concentrations were administered to T47D cells, and the MTT assay was used to determine cell viability. The profile of cell survival is reported as the average ± SD from three separate trials. [Click here to view] |
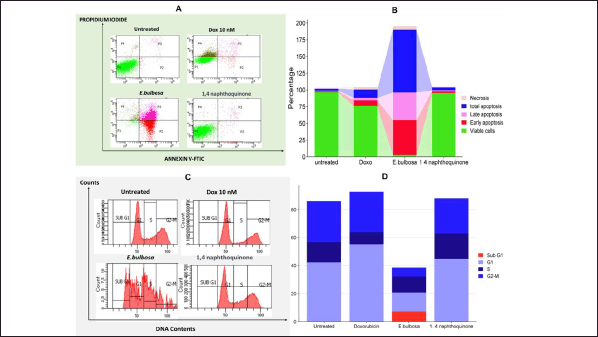 | Figure 6. Triggering cell death and altering cell cycle progression in T47D cells of breast cancer by treatment with E. bulbosa ethanol extract, DOX, and 1,4-naphthoquinone. Cells were treated with E. bulbosa ethanol extract, DOX, and 1,4-naphthoquinone for 24 hours. Further examination assessed the proportion of cell mortality (Fig. A and B) and the allocation of cells in various stages of the cell cycle (Fig. C and D), using flow cytometry after labeling with Annexin V-FITC/PI and PI, correspondingly. The lines indicate the average ± SD from three separate studies. A p-value less than 0.001 (calculated through a post-hoc LSD test among the groups) was deemed to reflect statistical significance. [Click here to view] |
However, this study is still limited to the use of one type of cancer cell and has not yet deeply explored the specific molecular mechanisms of oxyresveratrol. In addition, in vitro results may not fully reflect the same effects within living organisms due to the complexity of interactions within biological systems. Therefore, future research should lead to in vivo studies to evaluate the effectiveness and safety of the E. bulbosa ethanolic extract and oxyresveratrol in animal models. Further research should also consider testing against various types of cancer cells to expand understanding of the anticancer activity spectrum of this compound. In addition, research that delves deeper into the molecular mechanisms and signaling pathways involved in the anticancer effects of oxyresveratrol will provide valuable insights for the development of more effective cancer therapies.
CONCLUSION
The metabolite profile obtained from the ethanol extract of E. bulbosa revealed the presence of 32 compounds, while its water extract disclosed the presence of 27 compounds. Venn diagram analysis identified three compounds present in both extracts: 1,4-Naphthoquinone (C10H6O2), l-lysine sulfate (C6H16N2O6S), and Epirizole (C11H14N4O2). In cytotoxic tests, the ethanol extract of E. bulbosa demonstrated low anticancer ability with an inhibitory concentration of 50% (IC50) of 202.37 μg/ml. On the other hand, the water extract did not exhibit anticancer effects with an IC50 of 1,020 μg/ml. Despite its low activity, the ethanol extract of E. bulbosa is effective in inducing apoptosis and potentially influencing the life cycle of T47D cancer cells. Therefore, this finding suggests that ethanol is a more suitable solvent for extracting bioactive components from E. bulbosa that are beneficial in the development of anticancer therapy.
ACKNOWLEDGMENT
The researchers would like to thank the National Research and Innovation Agency for the funding provided for this research, allowing it to proceed smoothly.
AUTHOR CONTRIBUTIONS
All authors have made an equal contribution to the writing of this article.
FINANCIAL SUPPORT
This research was funded by the National Research and Innovation Agency (BRIN) based on the decision of the Deputy for Research and Innovation Facilitation of the National Research and Innovation Agency number 37/II.7/HK/2023 in the Research and Innovation Program for Advanced Indonesia wave 4.
CONFLICTS OF INTEREST
The authors affirm that there are no conflicts of interest.
ETHICAL APPROVALS
This research was approved by the Research Ethics Committee on Health of the Faculty of Medicine and Health Science at UIN Maulana Malik Ibrahim Malang with the number: No.139/EC/KEPK-FKIK/2022.
DATA AVAILABILITY
All data generated and analyzed that are included in this research article are available upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ieyama T, Gunawan-Puteri MDPT, Kawabata J. α-Glucosidase inhibitors from the bulb of Eleutherine americana. Food Chem. 2011;128(2):308–11. CrossRef
2. Insanu M, Kusmardiyani S, Hartati R. Recent studies on phytochemicals and pharmacological effects of Eleutherine americana Merr. Procedia Chem. 2014;13:221–8. CrossRef
3. Kamarudin AA, Sayuti NH, Saad N, Razak NAA, Esa NM. Eleutherine bulbosa (Mill.) Urb. Bulb: review of the pharmacological activities and its prospects for application. Int J Mol Sci. 2021;22(13):6747. CrossRef
4. Mutiah R, Sari RA, Firsyaradha WY, Listiyana A, Indrawijaya YY, Wafi A, et al. Activity and toxicity of Eleutherine palmifolia (L.) Merr. Extract on BALB/c mice colitis-associated colon cancer model. Asian Pac J Cancer Prev APJCP. 2020;21(12):3579–86. CrossRef
5. Hairani MAS, Abdul Majid FA, Zakaria NH, Hudiyanti D, Fadhlina A, Sheikh HI. Anti-diabetic properties of traditional herbal concoction containing Eleutherine palmifolia (L.) Merr., Momordica charantia L., and Syzygium polyanthum (Wight.): a bibliometric analysis. Food Prod Process Nutr. 2023;5(1):60. CrossRef
6. Harlita TD, Oedjijono, Asnani A. The antibacterial activity of Dayak onion (Eleutherine palmifolia (L.) Merr) towards pathogenic bacteria. Trop Life Sci Res. 2018;29(2):39–52. CrossRef
7. Masfria M, Tampubolon MSA. The antifungal activity of n-hexane extract of Eleutherine palmifolia (L). Merr bulbs against Candida albicans and trichophyton mentagrophytes. Open Access Maced J Med Sci. 2019;7(22):3777–80. CrossRef
8. Nafisa BB, Santoso S, Rahmah Z, Muti’ah R. Potential antiviral activity of eleutherine, isoeleutherine, eleuthinone and elecanacine compounds in Eleutherine palmifolia (L.) Merr against NSP3 SARS-COV-2: in silico study. GSC Biol Pharm Sci. 2023 [cited 2023 Nov 05];22(2):049–57. Available from: https://gsconlinepress.com/journals/gscbps/content/potential-antiviral-activity-eleutherine-isoeleutherine-eleuthinone-and-elecanacine
9. Hanh PT, Thao DT, Nga NT, Phuong NT, Hung LN, Thien DT, et al. Toxicity and anti-inflammatory activities of an extract of the Eleutherine bulbosa rhizome on collagen antibody-induced arthritis in a mouse model. Nat Prod Commun. 2018;13(7):1934578X1801300. CrossRef
10. Biworo A, Atanta LW, Arianto IS, Hamidah S, Suhartono E. Ameliorative effect of tuber extract from bawang dayak (Eleutherine palmifolia (L.) merr) against acute UV-induced skin oxidative damage in Rattus norvegicus. AIP Conf Proc. 2019;2108(1):020010. CrossRef
11. Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci Nutr. 2019;7(1):148–54. CrossRef
12. Togola A, Baran N, Coureau C. Advantages of online SPE coupled with UPLC/MS/MS for determining the fate of pesticides and pharmaceutical compounds. Anal Bioanal Chem. 2014;406(4):1181–91. CrossRef
13. Khaw KY, Parat MO, Shaw PN, Falconer JR. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules. 2017;22(7):1186.
14. Mutiah R, Listiyana A, Suryadinata A, Annisa R, Hakim A, Anggraini W, et al. Activity of inhibit the cell cycle and induct apoptosis in HeLa cancer cell with combination of Sabrang onion (Eleutherine palmifolia (L.) Merr) and Starfruit Mistletoe (Macrosolen cochinchinensis (Lour.) Tiegh). J Appl Pharm Sci. 2018;8(10):122–8.
15. Mutiah R, Hadya CM, Bhagawan WS, Annisa R, Indrawijaya YY, Huwaida FI, et al. Metabolite profiling of Eleutherine palmifolia (L.) Merr. By HPTLC-densitometry and its correlation with anticancer activities and in vitro toxicity. Indones J Pharm. 2019;30(3):157.
16. Rachmawati E, Suharti S, Sargowo D, Kinasih LS, Octaviano YH, Mutiah R, et al. Metabolite profiling, hypolipidemic, and anti-atherosclerosis activity of mixed vegetable fermentation extract. Saudi Pharm J. 2023;31(5):639–54.
17. Amalina ND, Salsabila IA, Zulfin UM, Jenie RI, Meiyanto E. In vitro synergistic effect of hesperidin and doxorubicin downregulates epithelial-mesenchymal transition in highly metastatic breast cancer cells. J Egypt Natl Cancer Inst. 2023;35(1):6. CrossRef
18. Jenie R, Utomo R, Susidarti R, Novitasari D, Kirihata M, Meiyanto E. The evaluation of cytotoxic properties from CCB-2 sugar complexes against TNBC and non-TNBC cells. Asian Pac J Cancer Prev. 2021;22(1):151–5. CrossRef
19. Mutiah R, Listiyana A, Indradmojo C, Griana TP, Dwi HH, Atmaja RRD. Induction of apoptosis and phase-cell cycle inhibition of G0-G1, S, G2-M of T47D breast cancer cells on treatment with ethyl acetate fraction of Jackfruit parasite leaves (Macrosolen cochinensis). J Appl Pharm Sci. 2017;07(10):138–43.
20. Bui LT, Coad RA, Stanley RA. Properties of rehydrated freeze dried rice as a function of processing treatments. LWT. 2018;91:143–50.
21. Krastanov A. Metabolomics—the state of art. Biotechnol Biotechnol Equip. 2010;24(1):1537–43. CrossRef
22. Khan MR, Wabaidur SM, Alothman ZA, Busquets R, Naushad M. Method for the fast determination of bromate, nitrate and nitrite by ultra performance liquid chromatography–mass spectrometry and their monitoring in Saudi Arabian drinking water with chemometric data treatment. Talanta. 2016;152:513–20.
23. Carr AG, Mammucari R, Foster NR. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem Eng J. 2011;172(1):1–17. CrossRef
24. Cvetanovic A. Extractions without organic solvents: advantages and disadvantages. Chem Afr. 2019;2(3):343–9. CrossRef
25. Khiari Z, Makris DP, Kefalas P. An investigation on the recovery of antioxidant phenolics from onion solid wastes employing water/ethanol-based solvent systems. Food Bioprocess Technol. 2009;2(4):337–43. CrossRef
26. Thornburg CC, Britt JR, Evans JR, Akee RK, Whitt JA, Trinh SK, et al. NCI program for natural product discovery: a publicly-accessible library of natural product fractions for high-throughput screening. ACS Chem Biol. 2018;13(9):2484–97. CrossRef
27. Haryanti S, Zulfin UM, Salsabila IA, Wulandari F, Meiyanto E. The cytotoxic and anti-migratory properties of Caesalpinia sappan and Ficus septica, in combination with doxorubicin on 4T1 TNBC cells with nephroprotective potential. Asian Pac J Cancer Prev. 2022;23(2):743–52. CrossRef
28. Rahman MdA, Bishayee K, Sadra A, Huh SO. Oxyresveratrol activates parallel apoptotic and autophagic cell death pathways in neuroblastoma cells. Biochim Biophys Acta BBA—Gen Subj. 2017;1861(2):23–36. CrossRef
29. Radapong S, Chan K, Sarker SD, Ritchie KJ. Oxyresveratrol modulates gene expression of cell cycle control, apoptosis, autophagy and DNA repair in MCF-7 cells. Front Pharmacol. 2021;12:694562.
30. Chuang CH, Tan KT, Tung YT, Lin CC. Oxyresveratrol inhibits the growth of human lung squamous cell carcinoma cells by triggering S-phase arrest and apoptosis. J Food Bioact. 2019;6:131–9.
31. Mutiah R, Hariz MF, Indrawijaya YYA, Ma’arif B. In silico prediction of isoliquiritigenin and oxyresveratrol compounds to BCL-2 dan VEGF-2 receptors. Indones J Cancer Chemoprev. 2019;10(2):51–9. CrossRef
32. Filomeni G, Graziani I, Rotilio G, Ciriolo MR. Trans-resveratrol induces apoptosis in human breast cancer cells MCF-7 by the activation of MAP kinases pathways. Genes Nutr. 2007;2(3):295–305. CrossRef
33. Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem. 2006;97(2):261–74. CrossRef
34. Kamarudin AA, Sayuti NH, Saad N, Razak NAA, Esa NM. Induction of apoptosis by Eleutherine bulbosa (Mill.) Urb. bulb extracted under optimised extraction condition on human retinoblastoma cancer cells (WERI-Rb-1). J Ethnopharmacol. 2022;284:114770. CrossRef
35. Issaeva N. p53 signaling in cancers. Cancers. 2019;11(3):332. CrossRef
36. Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19(2):448. CrossRef
37. Radha G, Raghavan SC. BCL2: a promising cancer therapeutic target. Biochim Biophys Acta BBA—Rev Cancer. 2017;1868(1):309–14. CrossRef
38. Abdelaziz AA, Nawaz M, Izzeldin I, Abubshait HA, Alsadig A, Gomaa MS, et al. Molecular docking and anticancer activity of some synthesized 1,4- naphthoquinone derivatives against human cancer cell line. J Mol Struct. 2023;1275:134702. CrossRef
39. Mohamady S, Gibriel AA, Ahmed MS, Hendy MS, Naguib BH. Design and novel synthetic approach supported with molecular docking and biological evidence for naphthoquinone-hydrazinotriazolothiadiazine analogs as potential anticancer inhibiting topoisomerase-IIB. Bioorg Chem. 2020;96:103641.
40. Prachayasittikul V, Pingaew R, Worachartcheewan A, Nantasenamat C, Prachayasittikul S, Ruchirawat S, et al. Synthesis, anticancer activity and QSAR study of 1, 4-naphthoquinone derivatives. Eur J Med Chem. 2014;84:247–63.