INTRODUCTION
Colorectal cancer (CRC) is the third most frequent cancer in both men and women globally. As per the GLOBOCAN 2020 report, it was estimated that the incidence of CRC cases worldwide is about 1,931,590, with the maximum number of cases prevalent in Asia [1]. Environmental, genetic factors, ion-transport mechanism [2], existing disease conditions, and diet [3] are major causes of CRC. Multiple hallmarks responsible for the development of CRC are high cell proliferation, rise in uridine phosphorylase enzyme-1 and β-catenin enzyme, proinflammatory cytokines, oxidative stress, presence of adenomatous polyposis coli (APC), mutation of the growth signal autonomy such as endothelial growth factor receptors, Kirsten rat sarcoma viral oncogene homolog (KRAS), and immune escape [4]. Genetic predisposition is the most critical risk factor in the development of colon cancer in certain populations, along with environmental exposures and abnormal lifestyle [5,6]. Environmental variables, such as sedentary life, overweight, processed foods, liquor, and meat consumption, are causes for the rise in CRC cases [7,8]. Uridine phosphorylase enzyme degrades uridine and aggravates toxicities of 5-fluorouracil (5-FU) in normal tissue. Due to a decrease in uridine level, its cytoprotective effect gets lost [9]. In CRC, the overexpression of β-catenin in the Wnt pathway leads to the upregulation of expression of urokinase plasminogen activator that causes progression of infiltration, metastasis as well as dormancy in human CRC [10]. Tumor necrosis factor-alpha (TNF-α), a proinflammatory cytokine prevalent in the cancer microenvironment, is essential for controlling the body’s immunological and inflammatory reactions. TNF is primarily recognized for its involvement in inducing inflammation, while it is also implicated in a number of physiological and pathological processes. TNF comes in two main varieties. The most well-known type of TNF is TNF-Alpha (TNF-), which is created by a number of immune cells, including macrophages, T cells, and natural killer cells. TNF- is a cytokine that stimulates inflammation and is classified as such. TNF has both inflammatory and apoptotic properties [11–13]. Apart from this, it also increases tumor-associated calcium signal transduction protein-2 expression via the extracellular signal-regulated kinase 1/2 signaling pathway, resulting in colorectal tumor progression [14]. 5-FU is a first-line treatment for CRC, but it has a limitation of cytotoxicity and resistance at advanced stages of CRC. The 1,2-dimethylhydrazine (DMH) is a carcinogen that causes CRC in rats. Preneoplastic abnormalities, such as many plaque lesions, aberrant crypt foci, and well-defined dysplasia, are seen after DMH therapy [15]. Considering the limitation of the 5-FU and multiple markers involved in the progression of CRC, we attempted to treat CRC with natural compounds. In-silico molecular docking was used to target the uridine phosphorylase and β-catenin, which govern cancer growth. Based on a literature review, herbal drugs were chosen for their anti-cancer potential and their capacity to lower other risk factors, such as oxidative stress, cell proliferation markers, and proinflammatory mediators. Herbal extracts include Solanum nigrum, Nigella sativa, Garcinia indica, and Allium sativum, which possess vital phytoconstituents that act on multiple markers of CRC. Solanum nigrum includes phytoconstituents, such as quercetin, Thymol, Naringenin, and others, and it is a traditional treatment with pharmacological properties such as preventing hepatotoxicity and cytotoxicity [16–20]. Thymoquinone, dithymoquinone, anthraquinonequercetin, thymol, and carvacrol are phytoconstituents found in N. sativa that function as anti-inflammatory and immunomodulatory agents [21]. Garcinia indica is beneficial in a number of ways, including as an antioxidant, anti-obesity, antibacterial, hepatoprotective, and cardioprotective substance. Coumaric acid, apigenin, and naringenin are mainly responsible for the action [22]. The phytoconstituents found in A. sativum include Allicin, Naringenin, Anthraquinone, and quercetin. It has been utilized as a medication since ancient times. Allicin is the main physiologically active ingredient in garlic, acting as a possible antioxidant agent that may aid in the treatment of CRC [23]. The mixture of extract of these four plants is hypothesized to treat CRC in the present study.
MATERIAL AND METHODS
Experimental animals
Fifty-four male Sprague-Dawley rats with body weights around 200–250 gm and ages 8–10 weeks were used. The animal experiment protocol (RPCP/IAEC/2021-22/R8) was approved by the Institute Animal Ethics Committee. The rats were kept in polypropylene cages with a hygienic corn cob bed with a 12-hour dark/12-hour light cycle. Temperature and relative humidity were maintained at 25°C ± 2°C and 50% ± 10%, respectively. The animal study was modified and prepared in alignment with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (The ARRIVE Essential 10 and The Recommended Set) as well as CPCSEA regulation, INDIA. All animals were given free access to a pellet diet and regular drinking water [24].
Chemical procurement
DMH was purchased from Sigma Aldrich, while herbal extracts were purchased from Nutan Ayurvedic Research Centre, Gujarat. 5-FU manufactured by Celon Lab. Standard compounds such as thymoquinone, quercetin, and ellagic acid were purchased from Yucca Enterprise. TNF-α (RTA1021) and β-catenin (K11-0879) ELISA kits were purchased from Krishgen Biosystem, and the Uridine phosphorylase (MBS2605124) ELISA kit was purchased from My BioSource.
Experimental design
All the animals were divided into seven groups. DMH, a carcinogen, was administered to all groups except the normal control (NC) group. DMH was dissolved in normal saline containing 1.5% potassium EDTA as a vehicle. Late final pH was adjusted to 6.5–7 with 1 N sodium hydroxide solution and administered subcutaneously in each animal [25,26]. After 10 weeks, group 3 animals received 5-FU alone, whereas groups 4 and 5 received standard and test drug treatment at high and low doses. The remaining groups 6 and 7 were given test drug therapy alone at low and high doses respectively.
Group 1: NC; six animals; saline 10 ml/kg/day p.o.
Group 2: Disease control (DC); eight animals; DMH (35 mg/kg s.c.)—Once a week for 10 weeks [week 1 to 10].
Group 3: Standard control (STD); eight animals; DMH (35 mg/kg s.c.) [week 1 to 10] followed by 5-FU (10 mg/kg once a week for 5 weeks i.p.) [week 11 to 15].
Group 4: Standard + high test dose (STD + THP); eight animals; DMH (35 mg/kg s.c.) [week 1 to 10] followed by 5-FU (10 mg/kg i.p.) [week 11 to 15] + Polyherbal mixture (Low dose) (SN: 35 mg/kg; NS: 100 mg/kg; GI:75 mg/kg; AS: 30 mg/kg daily for 5 weeks p.o.) [week 11 to 15].
Group 5: Standard + low test dose (STD + TLP); eight animals; DMH (35 mg/kg s.c.) [week 1 to 10] followed by 5-FU (10 mg/kg i.p.) [week 11 to 15] + Polyherbal mixture (High dose) (SN: 140 mg/kg; NS: 400 mg/kg; GI:300 mg/kg; AS: 120 mg/kg daily for 5 weeks p.o.) [week 11 to 15].
Group 6: Test drug (TLP); eight animals; DMH (35 mg/kg s.c.) [week 1 to 10] followed by Polyherbal mixture (Low dose) (SN: 35 mg/kg; NS: 100 mg/kg; GI:75 mg/kg; AS: 30 mg/kg daily for 5 weeks p.o.) [week 11 to 15].
Group 7: Test drug (THP); eight animals; DMH (35 mg/kg s.c.) [week 1 to 10] followed by Polyherbal mixture (High dose) (SN: 140 mg/kg; NS: 400 mg/kg; GI:300 mg/kg; AS: 120 mg/kg daily for 5 weeks p.o.) [week 11 to 15].
The dose of each test drug (Solanum nigrum-SN, Nigella sativa-NS, Garcinia indica-GI, Allium sativum-AS) was selected based on acute oral toxicity (OECD guideline 423) and published literature [27–32].
MOLECULAR DOCKING
The in-silico approach employs molecular docking software, which anticipates the interaction of specific enzymes, proteins, or genes with ligands. Some software names for molecular docking are AutoDock, FlexX, Autodock vina, and so on [33–35]. The interaction of uridine phosphorylase, β-catenin, and phytoconstituents was studied using the in vitro molecular docking tool “Autodock Vina.” Targets were identified using the protein data bank (PDB), and the structure of phytoconstituents for interaction was designed using ChemDraw. The standard drug for molecular docking against both enzymes is 5-FU. From UniProtKB Data Base (https://www.uniprot.org/), the sequence, structure, and functional information of β-Catenin and Uridine phosphorylase were retrieved with UniProt ID: P35222 (CTNB1_HUMAN), Q16831 (UPP1_HUMAN), respectively. β-Catenin and Uridine phosphorylase 3-D structures were downloaded from Research Collaboratory for Structural Bioinformatic PDB (https://www.rcsb.org/) with PDB IDs: 1JDH, 3NBQ, respectively, with resolutions of 1.90 Å and 2.30 Å. BIOVIA Discovery Studio 21.1 Visualizer was used to remove the co-crystallized ligands. Thymol, carvacrol, anthraquinone, naringenin, quercetin, thymoquinone, dithymoquinone, and allicin had their 3-D structures retrieved from the PubChem database [https://pubchem.ncbi.nlm.nih.gov/]. A structural and active site investigation of all three proteins was carried out using the computed atlas of surface topography of proteins server (http://sts.bioe.uic.edu/castp). Molecular docking was performed using Autodock vina V.1.2.0.
EVALUATION PARAMETERS
Qualitative analysis-thin layer chromatography (TLC)
TLC was performed using toluene: ethyl acetate: glacial acetic acid as a mobile phase (4.5: 4: 0.5), and ALUGRAM® Xtra SIL G/UV254 precoated TLC sheets were used as a stationary phase.
Change in body weight
As an important marker of cachexia and decrease in food intake during CRC progression, change in body weight was measured by determining the difference between final body weight (at the end of 15th weeks) and initial body weight (before carcinogen induction) [36,37].
Modified Bowen’s score scale
Based on the consistency of the stool, different scores were assigned to determine colitis and colon dysbiosis. 0 for regular stool, 1 for moist and soft stool (mild diarrhea), 2 for moist, and unformed stool (moderate diarrhea), 3 for watery stool (severe diarrhea), and 4 for occult blood stool [38–40].
Colon length-to-weight ratio
After sacrificing each animal, the colon was isolated. The length and weight of the colon were measured. The following formula was used to determine the ratio [41–43]:
Colon length to weight ratio = Colon length/Animal weight.
Liver index
Following the sacrifice of the animals, the liver was removed. Later, the weight of the liver was measured. The final ratio was calculated using the following formula [41–43]:
Liver index=Weight of liver/weight of the animal.
Spleen index
As the spleen can be considered the graveyard of blood cells, an increase in the spleen’s weight can be considered as high cellular mortality. Following the sacrifice of the animals, the spleen was removed. The weight of each spleen was measured. The final ratio was calculated using the following formula [41–43]:
Spleen index = Weight of spleen/weight of animal.
Complete blood count (CBC)
Considering cellular turnover changes during carcinogenesis, CBC was estimated from each rat’s blood (200 μl) using a Mindray BC-5130 analyzer. The percentage of lymphocyte, neutrophil, monocyte, and red blood cells (RBCs), and platelets were measured [44].
ELISA of TNF-α, uridine phosphorylase, and β-catenin
Proinflammatory cytokine, cell proliferation, and detoxifying protein levels are the main players in judging the success of the oncotherapy. A 100 μl plasma was used to estimate TNF-α and uridine phosphorylase, while 40 μl colon homogenate was used to estimate β-catenin by ELISA. At the end of the test, absorbances were measured at 450 nm using a microplate reader, and the results were interpreted.
Histopathological analysis
Based on palpation and morphological changes, the suspected colon part and liver were isolated and cleaned with normal saline before being placed in formalin for cell fixation. Tissues of the colon and liver were sent to a laboratory for histology in 10% formalin solution. Light microscopy was used to examine paraffin-embedded samples stained with hematoxylin and eosin (H and E). Slide images were captured using an inverted trinocular microscope (Carl Zeiss, Axio vert ALFL) [45].
Statistical analysis
All the values were expressed as mean ± SEM of six animals. Parameters were statistically analyzed with one-way ANOVA followed by Tukey’s multiple comparison test and Kruskal Wallis test (for scoring) using graph pad prism software. p < 0.05 is considered a significant difference. Statistical analysis was done with GraphPad Prism 8.4.3 software [46].
RESULTS
Molecular docking
The molecular docking was carried out between phytoconstituents and individually between the enzymes β-catenin and uridine phosphorylase enzyme-1, respectively (Table 1).
According to the molecular docking score, all of the phytoconstituents were closer to or had a high affinity for the β-catenin enzyme. Naringenin and quercetin obtained a higher docking score than other phytoconstituents and the standard medication. This indicates that both Naringenin as well as quercetin exhibited a greater affinity for the β-catenin enzyme. Dithymoquinone and anthracene obtained higher docking scores than 5-FU. Thus, they have a stronger affinity for the β-catenin enzyme than 5-FU.
According to the molecular docking score, all of the phytoconstituents were closer to or had a high affinity for the uridine phosphorylase enzyme-1. Naringenin obtained a higher docking score than all other phytoconstituents and the standard medication. This indicates that Naringenin exhibited a greater affinity for the uridine phosphorylase enzyme-1. Following Naringenin, quercetin also displayed a higher docking score, indicating good binding affinity toward the uridine phosphorylase enzyme-1. Anthracene and dithymoquinone obtained higher docking scores than 5-FU. That is, they have a stronger affinity for the uridine phosphorylase enzyme-1 than 5-FU.
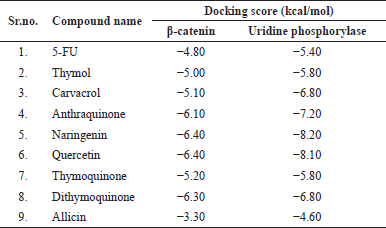 | Table 1. Molecular docking score of phytoconstituents of herbal test drugs. [Click here to view] |
Overall, Naringenin and quercetin obtained a higher docking score than other phytoconstituents and the traditional medicines for both the enzymes uridine phosphorylase enzyme-1 and β-catenin (Fig. 1).
Qualitative analysis-TLC
The standard marker was placed on the first track, while the test herbal extract was placed on the second track. The presence of a specific phytoconstituent was confirmed by comparing it to a standard marker. After reaching the maximum height of the mobile phase, “Retention factor” (Rf value) was determined for test and standard. Rf sof the quercetin and ellagic acid were found to be 0.49 and 0.45, respectively.
Modified Bowen’s score
NC group has shown semi-solid brown color stool and thus assigned a score “0” which indicates the absence of diarrhea. The DC group was assigned a score “4” because it displayed occult blood. The STD group was assigned a score “3” as it displayed moderate to severe diarrhea. The STD + TLP and STD + THP were assigned a score “1” because they showed mild diarrhea.
Change in body weight
The body weight of the DC group was significantly low (p < 0.05) due to cachexia and a decrease in food intake as compared to the NC and STD groups. The body weight of the THP and STD + THP groups was found to be comparable with the STD group. There is a significant decrease in body weight of TLP and STD + TLP as compared to the NC group and STD group, respectively (Fig. 2a).
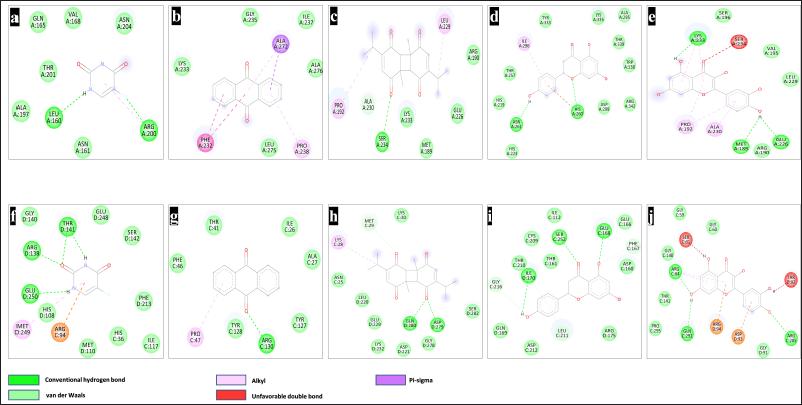 | Figure 1. 2-D interaction of phytoconstituents with β-catenin (1JDH) and UPP-1 (3NBQ). Interaction of β-catenin with 5-FU (a), Anthracene (b), Dithymoquinone (c), Naringenin (d), quercetin (e), Interaction of UPP-1 with 5-FU (f), Anthracene (g), Dithymoquinone (h), Naringenin (i), and quercetin (j). [Click here to view] |
Colon length/weight ratio
The colon length-to-weight ratio of the DC group was significantly lower as compared to all other groups. Amongst all test groups, STD + THP gave improved results when compared with the STD group. All other test groups gave comparable results with the STD group (Fig. 2b).
Liver index
The liver weight was increased in the DC group due to dysplasia, and the animal weight decreased due to cachexia, from which it was concluded that this group has a high liver index as compared to all other groups. Only the THP group showed a significant decrease (p < 0.05) in liver weight, which is comparable with the NC group. All other test groups gave comparable results to that of the STD group (Fig. 2c).
Spleen index
There was an increase in spleen weight due to high blood cell turnover, whereas there was a decrease in the body weight of animals in the DC group, from which it was concluded that the spleen index of the DC group was higher as compared to all other groups. The spleen index of the THP group was significantly (p < 0.05) lower as compared to the NC, DC, and STD groups. The spleen index of STD + THP and STD + TLP were also comparable with the NC group (Fig. 2d).
Complete blood count
Percentage of lymphocytes
DC group displayed a significant decrease (p < 0.05) in the lymphocyte count as compared to all other groups, which can be associated with failure of antitumor immunity. The TLP and THP groups displayed an increase in the number of lymphocytes as compared with the STD group. The STD + TLP and STD + THP groups had similar results when compared with the STD group, which can be an indication of STD causing a decrease in overall number of lymphocytes (Fig. 3a).
Percentage of neutrophils
The DC group showed a significantly higher (p < 0.05) neutrophil count as compared to all groups, which is an indication of poor prognosis. The THP, STD + THP, and STD + TLP groups displayed a similar number of neutrophils when compared with the STD group. The TLP group showed a significant decrease in the percentage of neutrophils as comparable to NC, DC, and STD groups (Fig. 3b).
Percentage of monocytes
The monocyte count was higher in the DC group as compared to all other groups, indicating poor prognosis. The results of all test groups were found to have comparable results with the NC group and STD group (Fig. 3c).
RBC
Only the THP group was found to have comparable results with the STD group. The STD + THP, TLP, and STD + TLP groups were found to have decreased RBC count as compared to the STD group, possibly due to lower values found in a few animals (Fig. 3d). When CRC reaches the advanced stage, there are chances of the development of anemia that were not found in our study.
Enzyme-linked immunosorbent assay
TNF-α
The TNF- α levels were found to be low in the NC group, whereas they were highest during diseased conditions. The results of THP and TLP were comparable with STD. The TNF- α levels of STD + THP and STD + TLP were significantly lower (p < 0.05) as compared to STD, which can be an indication of the synergistic effect of STD and polyherbal formulation (Fig. 4b).
β-Catenin
The amount of β-catenin in the NC group was found to be low but too high in the DC group. The STD + THP group displayed a low amount of β-catenin as compared to STD and all other test groups; it gave comparable results with the NC group (helps in the reduction of tumor cell proliferation). The THP, TLP, and STD + TLP groups have comparable results with the STD group (Fig. 4c).
Uridine phosphorylase 1
Inhibition of uridine phosphorylase-1 is responsible for cytoprotection and lowering chemo-induced toxicities in vital organs. The uridine phosphorylase-1 enzyme concentration was lowest in the NC group and highest in the DC group as compared to all other groups, respectively. The uridine phosphorylase-1 levels were significantly lower (p < 0.05) in the THP group, STD + THP group, and TLP group as compared to the STD group. The uridine phosphorylase-1 levels in the STD + TLP group were comparable with the STD group (Fig. 4a).
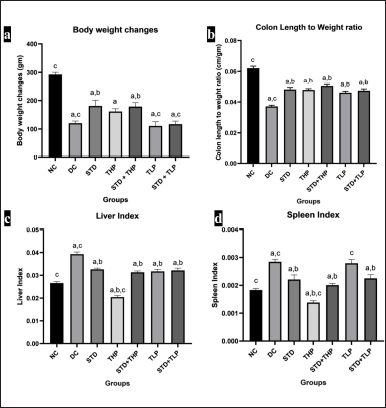 | Figure 2. Morphological changes after CRC treatment. Body weight changes (a), colon length to weight ratio (b), liver index (c), and spleen index (d), respectively. a, b, c significantly different from NC, DC, and Standard (Std), respectively, at p < 0.05 and n = 6. [Click here to view] |
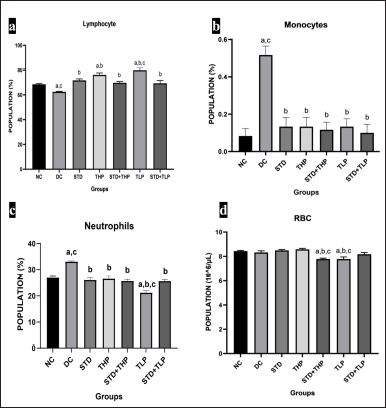 | Figure 3. Hematological changes after CRC treatment for lymphocyte population (%) in different groups (a), Monocytes population (%) in different groups (b), Neutrophils population (%) in different groups (c), RBC population (106/μl) in different groups. a, b, and c are significantly different from NC, DC, and Standard (Std), respectively, at p < 0.05 and n = 6. RBC = Red blood cells. [Click here to view] |
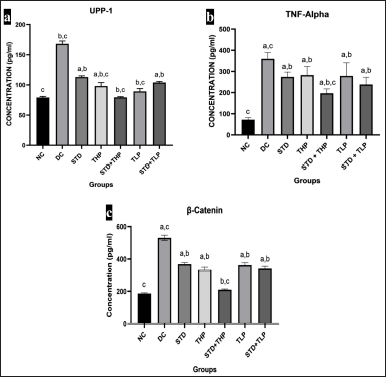 | Figure 4. Cytokine and driver proteins change after CRC treatment. ELISA for UPP-1 concentration (pg/ml) in different groups (a), TNF-α concentration (pg/ml) in different groups (b), and β-catenin concentration (pg/ml) in different groups (c). a, b, and c are significantly different from NC, DC, and Standard (Std), respectively, at p < 0.05 and n = 6. CRC = Colorectal cancer; ELISA = Enzyme link immunosorbent assay; TNF-α = Tumour necrosis factor–α; UPP-1 = Uridine phosphorylase-1. [Click here to view] |
Histopathological study
NC group animals have all normal layers of mucosa, submucosa, muscularis, and serosa with normal cell proliferation with a proper nucleocytoplasmic ratio in the colon and absence of liver metastasis. The DC animals showed moderately differentiated adenocarcinoma infiltrated to lamina propria in colon and liver dysplasia. The STD and STD + THP groups showed mild dysplasia in the colon with absence of liver metastasis. The TLP group, STD + TLP, and THP groups displayed adenocarcinoma in the colon. Mild liver dysplasia was observed in the TLP and STD + TLP groups, whereas no liver metastasis was observed in the case of the THP group (Fig. 5).
DISCUSSION
Uridine phosphorylase degrades uridine to uracil by the pyrimidine salvage pathway and increases 5-FU toxicities in normal tissues. It gets overexpressed in many gastric cancer cells. Uridine is a biochemical modulator that lowers 5-FU host toxicity and maintains the drug’s antitumor efficacy [47]. The β-catenin protein regulates cell division. By boosting transcriptional factors, intranuclear beta-catenin promotes cell proliferation and malignancy. Wnt/catenin signaling is necessary for intestinal homeostasis and APC gene mutations. APC works as a tumor suppressor gene, preventing cells from dividing and developing too swiftly or uncontrolled. TNF-α accelerated cell proliferation and metastasis as well as induced chemotherapy resistance [48]. DMH metabolized in the liver, releasing metabolic intermediates such as azoxymethane and methyl azoxy methanol that are subsequently transformed into active electrophilic methyl diazonium glucuronide in the liver and get discharged into the intestinal lumen by organotropism. In mucosal cells, bacterial glucuronidases hydrolyze glucuronides to form active carbonium ions, which methylate nucleic acids and proteins, producing oxidative stress and cancer. In addition to colon selectivity, DMH alkylates hepatocellular DNA and acts as a hepatic necrogenic agent [49]. Adenocarcinoma, on the other hand, is a cancer that originates from the epithelial cells of glands or glandular-like structures [50]. Successful treatment necessitates anti-cancer activity on tumor cells and cytoprotection to normal cells. Single herbal medicine does not have all the necessary properties to work at multiple hallmarks of the CRC, so the best four herbal extracts were chosen based on molecular docking and available literature. The multiple therapeutic effects were reported by polyherbal mixture like activation of the caspase 2/3/9 for apoptosis, [51] anti-inflammatory, [51,52] and antioxidant effect [51–53]. Diarrhea is an indication of colitis, loss of aquaporin channels from the tissue, or side effects of the test drug due to gastrointestinal damage [54]. Polyherbal mixture lowered the modified Bowen’s score compared to the DC and STD groups. Compared to the STD alone, the THP with 5-FU normalizes the colon length-to-weight ratio. TNF-α was reduced in both the standard and polyherbal test mixtures at high and lower doses compared to the STD alone. Compared to the STD, the β-catenin level in the STD + THP group was lower, indicating that the polyherbal combination reduced the β-catenin level. Polyherbal mixture raises the uridine level and accelerates the cytoprotection against 5-FU by decreasing UPP-1 in STD + THP compared to the STD, implying that polyherbal combination inhibits UPP-1 enzyme and increases the uridine level. STD + THP group animals showed dysplasia and a normal liver in histopathology. As per the result, the polyherbal mixture showed a synergistic effect with the 5-FU when given at a higher dose. The polyherbal combination improved the 5-FU antitumor efficacy while lowering its toxicity. Histopathological analysis of the colon from DMH administered to a different group of rats has shown mild dysplasia, tumor cell infiltration up to the lamina propria, moderately differentiated adenocarcinoma, and liver metastasis. Mild dysplasia was observed in the STD and STD + THP groups. Therefore, it can be deduced that STD + THP can prevent cancerous tumor growth in the tissue. In addition, the high dose of polyherbal mixture prevents the spread of cancer cells to the liver, thereby avoiding invasive adenocarcinoma.
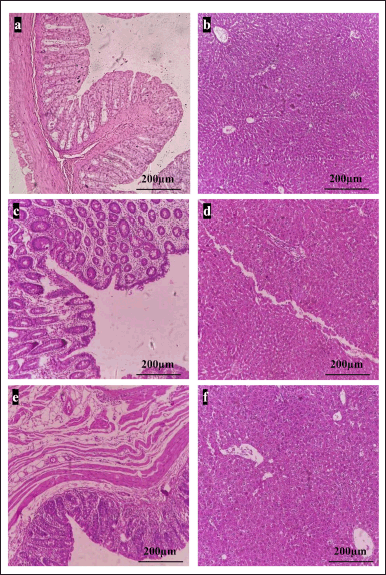 | Figure 5. Polyherbal mixture ameliorated DMH-induced CRC. H and E staining of rat colon and liver. A NC group of colon (a) and liver (b) shows normal histoarchitecture; DC group of colon (c) shows infiltration up to the lamina and moderately to poorly differentiated adenocarcinoma, while liver (d) shows mild to moderate dysplasia, colon of STD + THP (e) shows mild dysplasia but no malignancy, whereas in liver (f) metastasis was not observed (Scale = 200 μm). CRC = Colorectal cancer, DMH = 1,2-dimethylhydrazine. [Click here to view] |
CONCLUSION
The uridine phosphorylase-1 enzyme is responsible for reducing uridine levels in host tissue. The rise in uridine level reduces 5-FU toxicity in normal cells. By inhibiting β-catenin, tumorigenesis can be halted at the dysplasia level. TNF-α inhibition by polyherbal mixture reduces oxidative stress and inflammatory changes. The positive modulation by a combination of herbal medicine with 5-FU is supported by improvement in prognosis measured by changes in body weight, blood cell counts, and diarrhea score. The results indicated less toxicities and more anti-cancer efficacy in the treatment of CRC. The combination of standard and test drugs (high dosage) yielded the most notable results when compared to the test group alone. Histological evaluation revealed that the polyherbal combination was effective in reducing DMH-induced inflammation and dysplasia. According to the findings of the current investigation, the polyherbal combination can help to minimize DMH-induced dysplasia and prevent CRC progression. A rare research study is available that aims to evaluate herbals with first-line chemotherapy for CRC. Further investigation of Caspase2/3/9, inflammatory indicators, such as interleukins, KRAS, MutL protein homolog 1 and carcinoembryonic antigen, can assist in determining the molecular mechanism of polyherbal combination. In this study, we evaluated the effects of herbal medicines and 5-FU for the initial stages of CRC, but its evaluation in advanced stages of CRC and associated comorbidities need to be evaluated in appropriate animal models.
ACKNOWLEDGEMENT
This Research work was supported by the Entrepreneurship Development & Incubation Cell (EDIC) under the Student Startup and Innovation Policy (SSIP, 2017-22) under protocol number SSIP-RPCP-2021-14.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The animal experiment protocol (RPCP/IAEC/2021-22/R8) was approved by the Institutional Animal Ethics Committee. The study was conducted as per the CPCSEA & ARRIVE guidelines.
DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49. CrossRef
2. Zhang M, Li T, Zhu J, Tuo B, Liu X. Physiological and pathophysiological role of ion channels and transporters in the colorectum and colorectal cancer. J Cell Mol Med. 2020;24:9486–94. CrossRef
3. Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, et al. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr. 2018;108:363–70. CrossRef
4. Hagland HR, Berg M, Jolma IW, Carlsen A, Søreide K. Molecular pathways and cellular metabolism in colorectal cancer. Dig Surg. 2013;30:12–25. CrossRef
5. Hamiza OO, Rehman MU, Khan R, Tahir M, Khan AQ, Lateef A, et al. Chemopreventive effects of aloin against 1, 2-dimethylhydrazine-induced preneoplastic lesions in the colon of Wistar rats. Hum Exp Toxicol. 2014;33:148–63. CrossRef
6. Little J, Faivre J. Family history, metabolic gene polymorphism, diet and risk of colorectal cancer. Eur J Cancer Prev. 1999;8:S61–72.
7. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Gastroenterol Rev/Prz Gastroenterol. 2019;14:89–103. CrossRef
8. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. CrossRef
9. Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8:57–84. CrossRef
10. Shang S, Hua F, Hu ZW. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8:33972. CrossRef
11. Kucukler S, Benzer F, y?ld?r?m S, Gür C, Kandemir F, Bengü A, et al. Protective effects of chrysin against oxidative stress and inflammation induced by lead acetate in rat kidneys: a biochemical and histopathological approach. Biol Trace Elem Res. 2021;199(4):1501–14. CrossRef
12. Özbolat SN, Ayna A. Chrysin suppresses HT-29 cell death induced by diclofenac through apoptosis and oxidative damage. Nutr Cancer. 2021;73:1419–28. CrossRef
13. Emre K?z?l H, Gür C, Ayna A, Darendelio?lu E, Küçükler S, Sa? S. Contribution of oxidative stress, apoptosis, endoplasmic reticulum stress and autophagy pathways to the ameliorative effects of hesperidin in NaF-induced testicular toxicity. Chem Biodivers. 2023;20:e202200982. CrossRef
14. Zhao P, Zhang Z. TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncol Lett. 2018;15:3820–7. CrossRef
15. de Leon MP, Roncucci L. The cause of colorectal cancer. Dig Liver Dis. 2000;32:426–39. CrossRef
16. Nyeem MAB, Rashid AMU, Nowrose M, Hossain MA. Solanum nigrum (Maku): a review of pharmacological activities and clinical effects. IJAR. 2017;3:12–7.
17. Davoodvandi A, Sadeghi S, Alavi SMA, Alavi SS, Jafari A, Khan H, et al. The therapeutic effects of berberine for gastrointestinal cancers. Asia Pac J Clin Oncol. 2023:1–16. CrossRef
18. Davoodvandi A, Shabani Varkani M, Clark CCT, Jafarnejad S. Quercetin as an anticancer agent: focus on esophageal cancer. J Food Biochem. 2020;44:e13374. CrossRef
19. Davoodvandi A, Mahdavi Sharif P, Maleki Dana P, Asemi Z. Resveratrol effects on molecular pathways and microRNAs in gastrointestinal cancers. Curr Med Chem. 2022;29:820–40. CrossRef
20. Davoodvandi A, Farshadi M, Zare N, Akhlagh SA, Alipour Nosrani E, Mahjoubin-Tehran M, et al. Antimetastatic effects of curcumin in oral and gastrointestinal cancers. Front Pharmacol. 2021;12:1836. CrossRef
21. Dalli M, Bekkouch O, Azizi S, Azghar A, Gseyra N, Kim B. Nigella sativa L. phytochemistry and pharmacological activities: a review (2019–2021). Biomolecules. 2021;12:20. CrossRef
22. Lim SH, Lee HS, Lee CH, Choi CI. Pharmacological activity of Garcinia indica (Kokum): an updated review. Pharmaceuticals. 2021;14:1338. CrossRef
23. El-Saber Batiha G, Magdy Beshbishy A, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, et al. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12:872. CrossRef
24. Patel A, Biswas S, Shoja MH, Ramalingayya GV, Nandakumar K. Protective effects of aqueous extract of Solanum nigrum Linn. leaves in rat models of oral mucositis. Sci World J. 2014;2014:345939. CrossRef
25. Balaji C, Muthukumaran J, Nalini N. Chemopreventive effect of sinapic acid on 1, 2-dimethylhydrazine-induced experimental rat colon carcinogenesis. Hum Exp Toxicol. 2014;33:1253–68. CrossRef
26. Tanwar L, Vaish V, Sanyal SN. Chemoprevention of 1, 2-dimethylhydrazine-induced colon carcinogenesis by a non-steroidal anti-inflammatory drug, etoricoxib, in rats: inhibition of nuclear factor kappaB. Asian Pac J Cancer Prev. 2009;10:6.
27. Jain R, Sharma A, Gupta S, Sarethy IP, Gabrani R. Solanum nigrum: current perspectives on therapeutic properties. Altern Med Rev. 2011;16:78–85.
28. Salim EI, Fukushima S. Chemopreventive potential of volatile oil from black cumin (Nigella sativa L.) seeds against rat colon carcinogenesis. Nutr Cancer. 2003;45:195–202. CrossRef
29. Salim EI. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L., in a rat multi-organ carcinogenesis bioassay. Oncol Lett. 2010;1:913–24. CrossRef
30. Panda VS, Ashar HD. Antioxidant and hepatoprotective effects of Garcinia indica choisy fruits in carbon tetrachloride-induced liver injury in rats. J Food Biochem. 2012;36:240–7. CrossRef
31. Panda VS, Khambat PD. Antiulcer activity of Garcinia indica fruit rind (kokum berry) in rats. Biomed Aging Pathol. 2014;4:309–16. CrossRef
32. Xian-kun W, Xue W, Jian H. Effects of allicin on experimental colorectal cancer in rats and its mechanism. Nat Prod Res Dev. 2016;28:943. CrossRef
33. Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–62. CrossRef
34. Morris GM, Goodsell DS, Huey R, Olson AJ. Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J Comput Aided Mol Des. 1996;10:293–304. CrossRef
35. Morris GM, Lim-Wilby M. Molecular docking. In: Kukol A, editor. Molecular modeling of proteins. Totowa, NJ: Humana Press; 2008. pp. 365–82. CrossRef
36. Mariyappan P, Kalaiyarasu T, Manju V. Effect of eriodictyol on preneoplastic lesions, oxidative stress and bacterial enzymes in 1, 2-dimethyl hydrazine-induced colon carcinogenesis. Toxicol Res (Camb). 2017;6:678–92. CrossRef
37. Jrah-Harzallah H, Ben-Hadj-Khalifa S, Almawi WY, Maaloul A, Houas Z, Mahjoub T. Effect of thymoquinone on 1, 2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur J Cancer. 2013;49:1127–35. CrossRef
38. Mi H, Dong Y, Zhang B, Wang H, Peter CCK, Gao P, et al. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal mucositis via regulating T cell immunity in colorectal cancer rats. Cell Physiol Biochem. 2017;42:2330–41. CrossRef
39. Bowen JM, Stringer AM, Gibson RJ, Yeoh ASJ, Hannam S, Keefe DMK. VSL# 3 probiotic treatment reduces chemotherapy-induced diarrhoea and weight loss. Cancer Biol Ther. 2007;6:1445–50. CrossRef
40. Huang L, Chiau JSC, Cheng ML, Chan WT, Jiang CB, Chang SW, et al. SCID/NOD mice model for 5-FU induced intestinal mucositis: safety and effects of probiotics as therapy. Pediatr Neonatol. 2019;60:252–60. CrossRef
41. Chari KY, Polu PR, Shenoy RR. An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine induced colon cancer in Wistar rats. J Toxicol. 2018;2018:6086490. CrossRef
42. Prasad VG, Reddy N, Francis A, Nayak PG, Kishore A, Nandakumar K, et al. Sambar, an Indian dish prevents the development of dimethyl hydrazine–induced colon cancer: a preclinical study. Pharmacogn Mag. 2016;12:S441. CrossRef
43. Khan N, Kumar N, Ballal A, Datta D, Belle VS. Unveiling antioxidant and anti-cancer potentials of characterized Annona reticulata leaf extract in 1, 2-dimethylhydrazine-induced colorectal cancer in Wistar rats. J Ayurveda Integr Med. 2021;12:579–89. CrossRef
44. Liu FD, Tam K, Pishesha N, Poon Z, Van Vliet KJ. Improving hematopoietic recovery through modeling and modulation of the mesenchymal stromal cell secretome. Stem Cell Res Ther. 2018;9:1–14. CrossRef
45. Silva-Reis R, Faustino-Rocha AI, Gonçalves M, Ribeiro CC, Ferreira T, Ribeiro-Silva C, et al. Refinement of animal model of colorectal carcinogenesis through the definition of novel humane endpoints. Animals. 2021;11:985. CrossRef
46. Svitina H, Skrypkina I, Areshkov P, Kyryk V, Bukreieva T, Klymenko P, et al. Transplantation of placenta?derived multipotent cells in rats with dimethylhydrazine?induced colon cancer decreases survival rate. Oncol Lett. 2018;15:5034–42. CrossRef
47. Cao D, Ziemba A, McCabe J, Yan R, Wan L, Kim B, et al. Differential expression of uridine phosphorylase in tumors contributes to an improved fluoropyrimidine therapeutic activityuridine phosphorylase and fluoropyrimidine activity. Mol Cancer Ther. 2011;10:2330–9. CrossRef
48. Roubert A, Gregory K, Li Y, Pfalzer AC, Li J, Schneider SS, et al. The influence of tumor necrosis factor-α on the tumorigenic Wnt-signaling pathway in human mammary tissue from obese women. Oncotarget. 2017;8:36127. CrossRef
49. Yusoff AAM, Khair SZNM, Abdullah WSW, Abd Radzak SM, Abdullah JM. Somatic mitochondrial DNA D-loop mutations in meningioma discovered: a preliminary data. J Cancer Res Ther. 2020;16:1517. CrossRef
50. Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8. CrossRef
51. Shameer PS, Sabu T, Mohanan NN. Garcinia gamblei (Clusiaceae), a new species from the southern Western Ghats, India. Phytotaxa. 2017;297:71–6. CrossRef
52. Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med. 2016;14:732–45. CrossRef
53. Lee DY, Song MY, Kim EH. Role of oxidative stress and Nrf2/keap1 signaling in colorectal cancer: mechanisms and therapeutic perspectives with phytochemicals. Antioxidants. 2021;10:743. CrossRef
54. Czigle S, Bittner Fialová S, Tóth J, Mu?aji P, Nagy M, OEMONOM. Treatment of gastrointestinal disorders—plants and potential mechanisms of action of their constituents. Molecules. 2022;27:2881. CrossRef