INTRODUCTION
The oral route is the most popular and convenient route of drug administration due to good patient compliance, safety, and versatility in the dosage form design. However, the issue with oral drug delivery is with those drugs that exhibit poor aqueous solubility, resulting in low dissolution rate and reduced bioavailability. One of the main challenges of the pharmaceutical industry is concerned with the strategies to enhance the aqueous solubility of poorly soluble drugs [1].
The biopharmaceutical classification system (BCS) acts as a guide for the classification of drugs according to their aqueous solubility and intestinal permeability into four classes. Thus, the in vivo drug bioavailability can be predicted by using BCS. Poorly soluble drugs are classified into Class II and Class IV according to their permeability. BCS class II drugs are poorly water-soluble but highly permeable; thus, they exhibit dissolution rate-limited absorption [2]. To increase the bioavailability of BCS class II drugs, the aqueous solubility and dissolution rate of the drug should be enhanced.
Improvement of drug solubility and dissolution can be accomplished by various techniques, such as particle size reduction, salt formation, complexation, crystal engineering, and so on. Amorphous solid dispersion is one of the most effective pharmaceutical strategies for improving the drug solubility, dissolution rate, and absorption of poorly water-soluble drugs. Thus, the bioavailability of poorly soluble drugs can be enhanced by formulating into an amorphous solid dispersion. Amorphous solid dispersion reduces drug particle size and incorporates the poorly water-soluble drug into a hydrophilic carrier to enhance the wettability, deagglomeration, and micellization of the drug. By formulating solid dispersion with high-energy amorphous drugs, the dissolution rate and bioavailability of poorly soluble drugs can be improved significantly. Finally, highly porous particles in the solid dispersion can enhance the drug release profile as well [3].
Solid dispersions can be manufactured by several methods, which include a kneading method, melting method, melting-solvent method, and solvent evaporation method. Electrospinning is one of the solvent evaporation methods to prepare solid dispersion, which is the combination of solid dispersion technology and nanotechnology. By using the electrospinning technique, the drug-loaded microfiber can be produced from a polymeric fluid melt or stream, which is delivered through a millimeter-scale nozzle. Electrospinning is ideal for formulating controlled-release medicines and microfibers that can improve both drug solubility and bioavailability. The solubility and bioavailability of BCS Class II drugs can, therefore, be enhanced with the use of the electrospun microfiber method [4].
Olmesartan medoxomil (OLM) is a selective AT-1 subtype angiotensin II receptor antagonist, which is used to treat hypertension. The chemical structure of OLM is shown in Figure 1. OLM is categorized as a BCS Class II drug which has low water solubility and high intestinal permeability. OLM’s oral bioavailability is restricted by its poor water solubility, resulting in a reduced antihypertensive effect. Since the antihypertensive effect of OLM is dependent on the oral dosage, the enhancement of OLM’s oral bioavailability can improve the therapeutic efficacy and reduce the oral dosage needed to achieve the same antihypertensive effect. Hence, OLM’s water solubility should be enhanced in order to increase the oral bioavailability and clinical efficacy of OLM [5]. Enhancing the solubility of OLM can significantly improve its therapeutic potential, leading to better treatment outcomes for hypertension and potentially other medical conditions. Several researchers have attempted to increase the solubility of OLM via PLGA nanoparticles [6,7], self-emulsifying drug delivery systems (SEDDSs) [8,9], nanosuspension [10], nanocrystal [11], nanosponge [12], and other conventional and novel drug delivery systems [13,14]. Enhancing the drug solubility with microfiber technology is a promising approach [15]. However, there are limited studies focusing on the enhancement of solubility of OLM through microfibers. Hence, we have tried to incorporate OLM into microfibers to explore its solubility enhancement.
In this study, two polymeric carriers, Eudragit RS 100 and Soluplus, were used to prepare microfibers. Eudragit RS100 is a copolymer of poly (ethylacrylate, methyl-methacrylate) and chloro trimethyl-ammonium methyl methacrylate. Although Eudragit RS100 is insoluble at physiologic pH, it can swell at this pH due to its hydrophilic characteristics. In addition, because it can adhere to negatively charged cells, this polymer becomes an ideal carrier for the targeted and sustained delivery of the drugs [16,17]. Soluplus is a polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer with amphiphilic properties that is used to enhance the solubility and bioavailability of poorly water-soluble drugs. Soluplus, with excellent extrudability and a simple processing nature, is generally recommended to develop a drug-carrier system via the hot-melt extrusion method [18]. Due to its excellent extrudability nature, Soluplus is an ideal polymer for preparing microfibers. Hence, this study is planned to enhance the solubility of OLM by formulating its amorphous solid dispersions by electrospinning technique using Eudragit RS 100 and Soluplus and evaluate in-vitro characteristics of the resultant OLM microfibers.
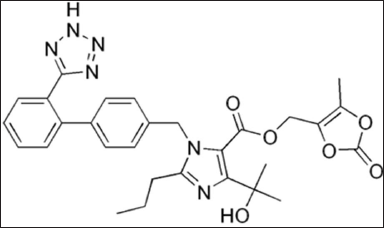 | Figure 1. Chemical structure of OLM. [Click here to view] |
MATERIAL AND METHODS
Materials
The materials used were as follows: OLM (Nivon specialties, India), Eudragit RS 100 (Colorcon), Soluplus (BASF, Germany), Ethanol (Merck, Germany), and Milli Q-system derived water. All other chemicals and reagents used were of analytical reagent grade.
Preparation of OLM electrospun microfibers
The required amount of polymers and drugs were weighed accurately relative to 5% w/w, 7.5% w/w, and 10% w/w of OLM. Based on the preliminary trials, the polymer concentration was set at 25% w/v for Eudragti RS 100 and 40% w/v for Soluplus for them to be sprayed into microfibers using electrospinning technology. The accurately weighed drug and polymer were dissolved in a beaker containing 5 ml ethanol, and the beaker was then placed in a bath sonicator for 10–15 minutes to complete the dissolution process [18]. The prepared electrospinning solutions were loaded in a 5 ml syringe with a 23 gauge needle. Based on the trial runs, the flow rate for Eudragti RS 100 was fixed at 0.2 ml/hour, whereas for Soluplus, the flow rate was fixed at 1.0 ml/hour. The flow rates were controlled by using a syringe pump. A high voltage supply between 10 kV (for 40% w/v Soluplus) and 15 kV (for 25% w/v Eudragit RS 100) was applied to the metallic needle. A grounded aluminum plate, which was covered with aluminum foil, acted as a microfiber collector. The microfiber collector was placed at a horizontal distance of 12 (for 25% w/v Eudragit RS 100) to 15 cm (for 40% w/v Soluplus) from the needle tip. The electrospun microfibers were then collected and stored in the desiccator until further analysis [18].
In vitro evaluation
Scanning electron microscopy (SEM)
Hitachi TM 3000 Tabletop Scanning Electron Microscope was used to examine the morphology of the OLM-loaded electrospun microfibers. Copper stubs, which act as metal carriers, were used to fix the electrospun microfiber samples by using double-sided conductive tape. Then, the samples were coated with gold and palladium by using SC7620 Mini Sputter Coater. A Digimizer image analysis software was used to define the mean fiber diameter, and the mean fiber diameter was measured using the SEM images produced (±standard deviation; SD). The measurements of mean fiber diameters were done by drawing straight lines that were perpendicular to the axis of the selected fiber. The pixel values of the straight lines were converted into diameter values [19].
Fourier transform infrared spectroscopy (FT-IR)
PerkinElmer Spectrum 100 FT-IR Spectrometer was used to record the FT-IR spectra of pure OLM, pure polymers (Soluplus & Eudragit RS 100), physical mixtures, the empty microfibers, and OLM-loaded microfibers. FT-IR analysis was done to qualitatively characterize the interaction and compatibility between the drug and polymer. The spectra were recorded with a resolution of 4 cm−1 and within the angle of 600–4,000 cm−1. Each recorded FT-IR spectrum was the mean of 32 scans [20].
Differential scanning calorimetry
Perkin Elmer differential scanning calorimetry (DSC) with HyperDSC DSC 8500 was used to evaluate the degree of crystallinity of OLM-loaded microfibers and the physical mixture of the drug and polymers. 5 mg of samples were precisely weighed in 40 μl aluminum pans, and the samples were closed with a press. A calorimeter was used to record the resultant DSC curves. After that, the heating rate was fixed at 10°C/minute; a temperature range from 30°C to 200°C was used to heat the samples. The purge rate of nitrogen gas was fixed at 20 ml/minute [21].
Drug content uniformity
For drug content estimation, electrospun microfibers equivalent to 5 mg OLM were weighed. The weighed microfibers (n = 3) were dissolved in the beakers, each containing 10 ml ethanol. The solutions were sonicated to ensure microfibers were completely dissolved. 1 ml of solution was diluted with phosphate-buffered saline (PBS) solution to make up the total volume of 10 ml. Then, 1 ml of diluted microfiber solution was further diluted with PBS solution to make up a volume of 10 ml, to obtain a concentration of 5 μg/ml. The actual amounts of OLM in the resultant microfiber solutions were measured by using a UV-Vis spectrometer at 257 nm. The actual amounts of OLM contained in the test samples were back-calculated from the obtained data against a predetermined calibration curve of pure OLM. The calibration curves of pure OLM were carried out in concentrations ranging from 5 to 30 μg/ml.
In vitro drug dissolution studies
The in-vitro dissolutions of pure OLM and OLM from the electrospun microfibers were performed using USP dissolution apparatus 2 (paddle method). 5 mg of pure OLM and microfibers containing 5 mg OLM were precisely weighed. Microfibers were gently rolled into a dialysis membrane, which was then tied tightly by using a string. Precautions were taken to minimize any possible influence that may disrupt the integrity of the microfiber structures. The samples within the dialysis membrane were tied on the paddle and placed into a dissolution vessel containing 900 ml of pH of 6.8 PBS solution. The experimental conditions were fixed at a temperature of 37 ± 0.5°C and stirred at a rotation speed of 50 rpm. 5 ml of the samples were collected at predetermined time intervals (0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 hours), and the samples were filtered through a 0.45 μm membrane filter. The concentrations of OLM presented in each of the drawn samples were analyzed by using the UV-vis spectrophotometer (λmax = 257 nm). For each predetermined time interval, the samples were drawn and replaced with an equal volume of fresh dissolution medium to maintain the sink conditions [22].
Drug release kinetics
To study the drug release kinetics and the mechanism of drug release, in vitro release data were fitted to various mathematical models. The models that were employed in this study included the zero order, first order, Higuchi, and Peppas–Korsemeyer models represented as follows:
Zero order kinetics: Q = K0t
First order kinetics: ln (1 − Q) = −K1t
Higuchi model: Q = Kht1/2
Korsmeyer–Peppas model: Mt/M∞ = Ktn
where Q is the fractional amount of drug release at time t; “K0” is the zero-order release constant; “K1” is the first-order release constant; Kh is the Higuchi kinetic constant; Mt/M∞ is the fractional amount of drug release at time t; and K is the kinetic constant and n is the diffusion exponent which is indicative of the drug release mechanism.
The best-fit kinetic model and the mechanism of the drug release were identified using the correlation coefficient (R2) and the release exponent (n) [22,23]. When n is less than or equal to 0.45, the drug is released through the Fickian diffusion mechanism. However, when n is between 0.45 and 0.89, the non-Fickian diffusion mechanism is considered as the release mechanism, in which the drug is released through a combination of diffusion and surface erosion of the fibers.
RESULT AND DISCUSSION
Scanning electron microscope
SEM analysis was used to evaluate the morphology and size of microfibers. The morphology and size of microfibers could affect the capability of microfibers to improve the physicochemical and in vivo characteristics. There were several parameters that determined the morphology and size of microfibers, including the nature of the polymer, polymer concentration, and solvent properties. Other critical parameters are operational factors such as the flow rate of the electrospinning solution, applied voltage, and distance between the needle tip and the grounded collector. Optimization of these parameters could assure the formation of smooth microfiber [16,24].
The SEM images of OLM-loaded microfibers with Eudragit RS 100 and Soluplus are shown in Figure 2A–F. According to Table 1, the diameter of OLM-loaded Eudragit RS 100 microfibers increased as the drug content increased, with values of 7.57 ± 0.37, 10.3 ± 0.5, and 18.67 ± 1.7 μm. A similar result could be observed in the OLM-loaded Soluplus microfibers, with mean diameter values of 9.2 ± 0.50, 13.57 ± 0.70, and 18.77 ± 0.78 μm.
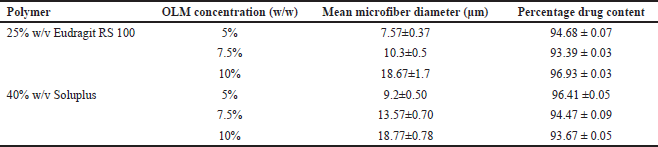 | Table 1. Mean microfiber diameters and percentage drug content of six OLM-loaded microfibers. [Click here to view] |
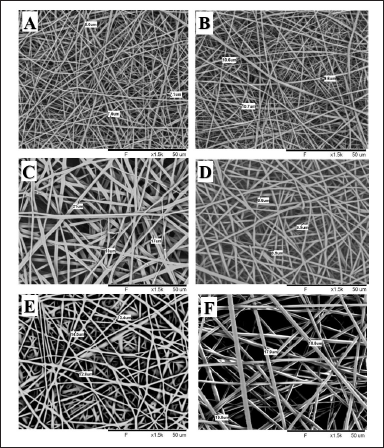 | Figure 2. (A) SEM image of 25% Eudragit RS 100-5% OLM microfiber, (B) SEM image of 25% Eudragit RS 100-7.5% OLM microfiber, (C) SEM image of 25% Eudragit RS 100-10% OLM microfiber, (D) SEM image of 40% Soluplus 5% OLM microfiber, (E) SEM image of 40% Soluplus-7.5% OLM microfiber, and (F) SEM image of 40% Soluplus 10% OLM microfiber. [Click here to view] |
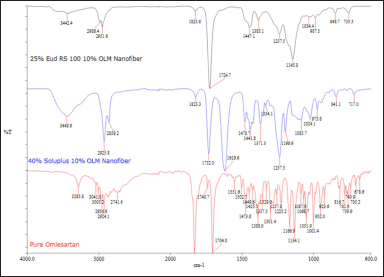 | Figure 3. FT-IR spectrum of pure OLM, 25% w/v Eud RS 100 10% w/w OLM microfiber, and 40% w/v Soluplus 10% w/w OLM. [Click here to view] |
It can be concluded that the higher polymer concentration resulted in a larger fiber diameter because of the increased viscoelastic forces. In contrast, low polymer concentration had higher surface tension that caused the formation of divided droplets from the fluid jet instead of fiber formation [16,17,25]. Hence, the microfiber formation was significantly influenced by the concentration of electrospinning solution.
As shown in Figure 2A–F, all microfibers were randomly spread and had a smooth surface without bead formation. The absence of visible particles on the surface of microfibers indicated the drug was homogeneously dispersed with the polymer and formed smooth microfibers. As the drug was completely dissolved in the solvent, the fast solvent evaporation rate during the electrospinning process prevented the crystallization of the drug and resulted in the amorphous drug content in the microfibers. The smooth microfiber surface also suggested that the drug was entrapped by the polymer and molecularly dispersed in the microfibers [18].
FT-IR spectroscopy
FT-IR spectroscopy can detect any possible chemical interactions between the drug and polymer in the solid state. The FT-IR spectra of pure OLM and OLM-loaded microfibers with Eudragit RS 100 and Soluplus are presented in Figure 3. The FT-IR of pure OLM, which had a molecular formula of C29H30N6O6, showed the characteristic peaks of hydrogen at 3,285.8 cm−1 (N-H stretch), 3,005.2 cm−1 (sp2 C-H stretch), and 2,924.1 cm−1 (sp3 C-H stretch). The aromatic C=O group and specific C=O group of OLM were stretching at 1,831 and 1,704.8 cm−1, respectively. The absorption peaks at 1,473 and 1,388.9 cm−1 indicated the hydrogen of the CH3 group and C-N in pure OLM [9].
By comparing the FT-IR spectra of pure OLM with 10% w/w OLM-loaded microfibers with 25% w/v Eudragit RS 100 and 40% w/v Soluplus, most of the characteristic peaks of pure OLM could be seen in the spectra of both microfibers The sp3 C-H stretch was present in the FT-IR spectrums of both OLM-loaded microfibers at 1,825.6 and 1,823.3 cm−1, respectively, which meant both OLM-loaded microfibers possessed aliphatic C-H group. Similarly, the stretching of both aromatic and specific C=O groups could be found in the FT-IR spectrum of both OLM-loaded microfibers. The presence of peaks at 1,440 and 1,380 cm−1 showed that C-N and CH3 existed in both OLM-loaded microfibers. The presence of characteristic peaks in the spectra of both microfibers indicated that OLM drug was present in both microfibers.
Differential scanning calorimeter (DSC)
DSC was carried out for the pure OLM and OLM-loaded microfibers with 25% w/v Eudragit RS 100 and 40% w/v Soluplus. This analysis was carried out in order to evaluate the transformation of the OLM during the formation of OLM-loaded Eudragit RS 100 and Soluplus microfibers by using electrospinning technique. The DSC thermograms of pure OLM and both microfibers are shown in Figure 4A and B. The DSC of pure OLM showed a single sharp endothermic peak at 183.6°C, which represents the melting point of pure OLM and thus confirmed its crystalline state. No endothermic peaks were observed in the DSC thermograms of both microfibers, as reflected in Figure 4. These observations imply that OLM’s crystallinity has diminished and that the drug is present in both microfibers in an amorphous state. The results also demonstrate that OLM was uniformly distributed throughout the microfibers. Thus, the DSC thermogram studies signify that OLM which was incorporated in the formulated microfibers, was in an amorphous state instead of crystalline state.
Drug content uniformity
OLM content in the prepared OLM-loaded microfibers with Eudragit RS 100 and Soluplus were found to be 96.93 ± 0.03% and 93.67 ± 0.05%, respectively (Table 1). Drug content uniformity can be assured because all microfibers were prepared by completely dissolving OLM and polymers in the common solvent, which is ethanol, to form an electrospinning solution. Hence, these studies ensure that all microfibers contain at least 90% of OLM and prove that the OLM was homogeneously and uniformly dispersed in all microfibers.
In vitro drug dissolution studies
The drug release profile of pure OLM and the prepared microfibers are shown in Figure 5. The corresponding dissolution curves of prepared microfibers depict a biphasic release pattern composed of an initial burst release within the first 30 minutes and subsequent plateau release. This biphasic drug release profile was common in nano drug delivery systems [25]. The initial burst release could be attributed to the huge microfiber surface area along with the accumulation of drugs on the microfiber surface, whereas the subsequent plateau drug release pattern was explained by the drug dissolution and diffusion from the core of microfibers [17,25].
As shown in Figure 5, all six microfiber formulations achieved more than 13% drug release within the first 30 minutes compared with pure OLM, which released only 6.8% in 30 minutes. This result indicated electrospun microfiber formulations had markedly improved the dissolution of pure OLM. In addition, all microfibers exhibited more than 50% of drug release after 12 hours, whereas pure OLM revealed only 32% of drug release because of its poor solubility. Therefore, the improvement of the OLM release profile can be accomplished by formulating OLM-loaded microfiber with hydrophilic polymers.
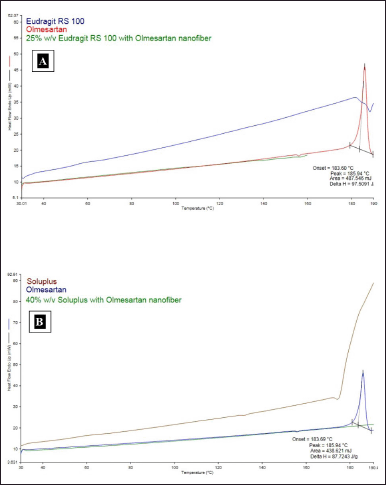 | Figure 4. (A) DSC thermograms combination of pure OLM, Eudragit RS 100, and OLM-loaded Eudragit RS 100 microfiber. (B) DSC thermograms combination of pure OLM, Soluplus, and OLM-loaded Soluplus microfibers. [Click here to view] |
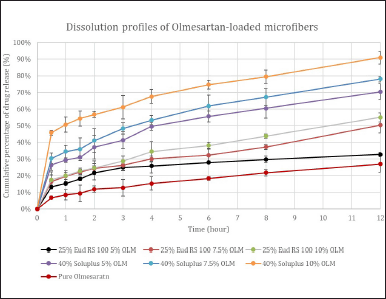 | Figure 5. In vitro dissolution curves of pure OLM and six OLM-loaded microfibers over 12 hours. [Click here to view] |
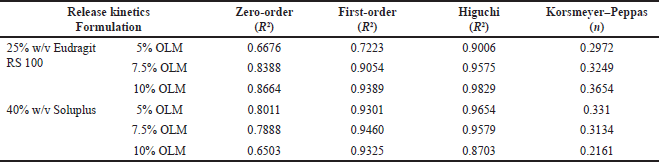 | Table 2. In vitro dissolution release kinetics from OLM-loaded microfibers over 12 hours. [Click here to view] |
Based on Figure 5, the higher drug release rate was observed in the microfibers with a higher drug: polymer ratio, i.e., 1:10. This might be explained by the drug being evenly and uniformly distributed throughout the polymer matrix. In contrast, the slower drug release rate in the drug: polymer ratio of 1:5 might result from the drug entrapment in the polymer matrix [16,24,25]. Increasing the polymer amount might form more homogenous drug dispersion in the polymer matrices, resulting in a higher drug release rate of the electrospun microfibers [17,25,26].
By comparing the drug dissolution rates between the Eudragit RS 100 microfiber and Soluplus microfiber, Soluplus microfibers showed a remarkably higher drug dissolution rate, which could be elucidated by its amphiphilic property that could further enhance the drug dissolution [18]. Eudragit RS 100 microfiber had a relatively slower drug release rate than Soluplus microfiber, and this could be explained by the pH-independent swelling properties of Eudragit RS 100. This swelling property resulted in the controlled release profile with a slower drug release rate. Based on the overall results, it can be said that Soluplus had increased the dissolution rate of OLM by three-fold when compared to Eudragit RS 100, which enhanced OLM’s dissolution rate by two-fold only.
Hence, this in vitro dissolution studies manifested that all microfibers had significantly higher drug dissolution rates than pure OLM, which confirmed that this microfiber formulation with hydrophilic polymer could enhance the bioavailability of BCS class II drugs.
Drug release kinetics
To examine the effectiveness of drug release, it is essential to understand the mechanism of drug release kinetics. As a result, four kinetic models (zero order, first order, Higuchi’s model, and Korsmeyer–Peppas plot) were used to fit the drug release data. The degree to which the release curve follows the kinetic model depends on the kinetic model’s R2 value. The R2 values for zero order, first order, and Higuchi’s model ranged from 0.667 to 0.8664, 0.7223 to 0.946, and 0.8703 to 0.9829 (Table 2). The R2 values for Higuchi’s model were higher and closer to 1, indicating that the drug release from OLM microfiber followed Higuchi’s model. The value of n dictates the drug release mechanism in drug-loaded microfibers. The diffusion exponent value (n) from the data fitting Korsmeyer–Peppas model used in the release of OLM was found to range from 0.2161 to 0.3654, all of which were less than 0.45. These results imply that release occurs through Fickian diffusion, indicating that OLM release is controlled by diffusion throughout the release process.
Based on the above results, it can be concluded that Eudragit/Soluplus microfibers that have been loaded with OLM demonstrate the following mechanism. First, holes occur on the surface of the microfibers as OLM molecules diffuse from their surface into the PBS solution. The medium gradually enters the microfibers as pores begin to grow inside them as the release occurs. The OLM incorporated in the Eudragit/Soluplus microfibers then disintegrates gradually and eventually completely in the media, leaving the microfiber scaffolds.
CONCLUSION
Two polymeric carrier systems with Eudragit RS 100 and Soluplus were successfully prepared by electrospinning technique to formulate OLM-loaded microfibers to improve OLM’s aqueous solubility. SEM results showed that the microfiber with higher drug concentration resulted in a larger microfiber diameter. DSC studies revealed that the crystallinity of OLM was significantly decreased in all electrospun microfibers. In addition, in vitro dissolution studies concluded the electrospun microfibers with Eudragit RS 100 and Soluplus could remarkably enhance OLM’s dissolution rate by two- and three-fold, respectively. In conclusion, the electrospinning technique could effectively improve the water solubility of BCS class II drugs by formulating high surface area microfiber. The drug amorphization during rapid solvent evaporation and accumulation of the drug in the microfiber surface contributed to a higher drug dissolution rate as well. OLM’s dissolution rate was notably improved by the amphiphilic property of Soluplus and the controlled release property of Eudragit RS 100.
ACKNOWLEDGEMENTS
The authors would like to thank Taylor’s University for providing the necessary laboratory facilities for carrying out the research work.
AUTHOR CONTRIBUTIONS
Sreenivas Patro Sisinthy has conceived and designed the study and wrote the manuscript. Chai Siew En carried out the research work, contributed to data collection and wrote the manuscript. Nur Najihah Izzati Mat Rani has contributed to data analysis and interpretation. Shahnaz Majeed has reviewed and edited the manuscript for clarity, grammar, and consistency.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Viswanathan P, Muralidaran Y, Ragavan G. Challenges in oral drug delivery: a nano-based strategy to overcome. In: Nanostructures for oral medicine. Elsevier; 2017. pp. 173–201. CrossRef
2. Papich MG, Martinez MN. Applying biopharmaceutical classification system (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J. 2015;17(4):948–64.
3. Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Investig. 2012;2(1):12.
4. Tran P, Pyo YC, Kim DH, Lee SE, Kim JK, Park JS. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics. 2019;11(3):132.
5. Lee BS, Kang MJ, Choi WS, Choi YB, Kim HS, Lee SK, et al. Solubilized formulation of olmesartan medoxomil for enhancing oral bioavailability. Arch Pharm Res. 2009;32(11):1629–35.
6. Si S, Li H, Han X. Sustained release olmesartan medoxomil loaded PLGA nanoparticles with improved oral bioavailability to treat hypertension. J Drug Deliv Sci Technol. 2020;55:101422.
7. Anwer MK, Jamil S, Ansari MJ, Iqbal M, Imam F, Shakeel F. Development and evaluation of olmesartan medoxomil loaded PLGA nanoparticles. Mater Res Innov. 2016;20(3):193–7.
8. Sisinthy SP, Sarah CYL, Nalamolu KR. Optimization of coconut oil based self micro emulsifying drug delivery systems of olmesartan medoxomil by simplex centroid design. Int J Appl Pharm. 2016;8(4):47–52.
9. Prajapati ST, Joshi HA, Patel CN. Preparation and characterization of self-microemulsifying drug delivery system of olmesartan medoxomil for bioavailability improvement. J Pharm. 2013;2013:1–9.
10. Thakkar H, Patel B, Thakkar S. Development and characterization of nanosuspensions of olmesartan medoxomil for bioavailability enhancement. J Pharm Bioall Sci. 2011;3(3):426.
11. Jain S, Patel K, Arora S, Reddy VA, Dora CP. Formulation, optimization, and in vitro–in vivo evaluation of olmesartan medoxomil nanocrystals. Drug Deliv Transl Res. 2017;7(2):292–303.
12. Almutairy BK, Alshetaili A, Alali AS, Ahmed MM, Anwer MdK, Aboudzadeh MA. Design of olmesartan medoxomil-loaded nanosponges for hypertension and lung cancer treatments. Polymers. 2021;13(14):2272.
13. Prajapati ST, Bulchandani HH, Patel DM, Dumaniya SK, Patel CN. Formulation and evaluation of liquisolid compacts for olmesartan medoxomil. J Drug Deliv. 2013;2013:1–9.
14. Gunda RK, Manchineni PR, Dhachinamoorthi D. Design, development, and in vitro evaluation of sustained release tablet formulations of olmesartan medoxomil. MOJ Drug Des Develop Ther. 2018;7(3):164–9.
15. Bitay E, Gergely AL, Kántor J, Szabó ZI. Evaluation of lapatinib-loaded microfibers prepared by centrifugal spinning. Polymers. 2022;14(24):5557.
16. Jafari-Aghdam N, Adibkia K, Payab S, Barzegar-Jalali M, Parvizpur A, Mohammadi G, et al. Methylprednisolone acetate–Eudragit® RS100 electrospuns: preparation and physicochemical characterization. Artif Cells Nanomed Biotechnol. 2016;44(2):497–503.
17. Payab S, Jafari-Aghdam N, Barzegar-Jalali M, Mohammadi G, Lotfipour F, Gholikhani T, et al. Preparation and physicochemical characterization of the azithromycin-Eudragit RS100 nanobeads and nanofibers using electrospinning method. J Drug Deliv Sci Technol. 2014;24(6):585–90.
18. Bruni G, Maggi L, Tammaro L, Di Lorenzo R, Friuli V, Maietta M, et al. Electrospun fibers as potential carrier systems for enhanced drug release of perphenazine. Int J Pharm. 2016;511(1):190–7.
19. Mishra B, Saxena A, Tiwari A. Biosynthesis of silver nanoparticles from marine diatoms Chaetoceros sp., Skeletonema sp., Thalassiosira sp., and their antibacterial study. Biotechnol Rep. 2020;28:e00571.
20. Saxena A, Bhattacharya A, Kumar S, Epstein IR, Sahney R. Biopolymer matrix for nano-encapsulation of urease – a model protein and its application in urea detection. J Colloid Interface Sci. 2017;490:452–61.
21. Jeckson TA, Neo YP, Sisinthy SP, Foo JB, Choudhury H, Gorain B. Formulation and characterisation of deferoxamine nanofiber as potential wound dressing for the treatment of diabetic foot ulcer. J Drug Deliv Sci Technol. 2021;66:102751.
22. Qureshi MJ, Phin FF, Patro S. Enhanced Solubility and Dissolution Rate of Clopidogrel Nanosuspension: Formulation via High Pressure Homogenization Technique and Optimization Using Box Behnken Design Response Surface Methodology. J App Pharm Sci, 2017; 7 (02): 106–13.
23. Sisinthy SP, Rao NK, Rao MEB. Controlled release layered matrix tablets of itopride hydrochloride: in vitro and in vivo evaluation. Asian J Pharm Clin Res. 2015;8(6):130–5.
24. Nguyen DN, Clasen C, Van den Mooter G. Pharmaceutical applications of electrospraying. J Pharm Sci. 2016;105(9):2601–20.
25. Garjani A, Barzegar-Jalali M, Osouli-Bostanabad K, Ranjbar H, Adibkia K. Morphological and physicochemical evaluation of the propranolol HCl–Eudragit® RS100 electrosprayed nanoformulations. Artif Cells Nanomed Biotechnol. 2018;46(4):749–56.
26. Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J, et al. Inhibition of endotoxin-induced uveitis by methylprednisolone acetate nanosuspension in rabbits. J Ocul Pharmacol Ther. 2007;23(5):421–32.