INTRODUCTION
A wholesome (balanced) diet epitomizes excellent physical condition [1] and often leads to healthy buccal health and a beautiful smile (Fig. 1) [2,3]. A vital aspect of long-term health, growth, and metabolic consequences in the deterrence and therapeutic intervention of disease hinges on nutrition [4–6]. Nutrition is the biochemical and physiological activity by which living creatures utilize food components [7] to sprout, mature, sustain themselves, and reproduce [7,8]. The influence of nutrition on the body’s tissues and organs exists locally and systemically [9–11]. Nutrition strongly affects the integrity of the periodontium [12,13].
The availability of a sufficient supply of essential nutrients to the host significantly impacts the viability of the periodontal tissues in both health and disease [14,15].
Nutrients are generally categorized into micronutrients, macronutrients, and essential nourishments [16]. A range of nutrients can significantly influence periodontal health [12,17]. Macronutrients comprise proteins, lipids, and carbohydrates, indispensable nutrition constituents [18]. However, micronutrients include water and fat-soluble vitamins, trace minerals, minerals, and organic acids, frequently necessitated to conserve vital physiological functions [19]. Essential nutrients are biological molecules and embrace selected amino and fatty acids, sterols, and vitamins [20]. Essential nutrients are expressed as those that cannot be produced or are insufficiently made from the beginning by animals, including human beings, to accommodate their physiological need [16].
 | Figure 1. Schematic representation showing dental, especially periodontal-health-friendly diets. This figure has been drawn with the premium version of BioRender (https://biorender.com/ accessed on 30th December 2023) with the license number NX269Y56XP. Image Credit: Susmita Sinha. [Click here to view] |
Micronutrients are the constituents of food that are needed in small or negligible quantities [21,22]. Carotenoids and carotene (vitamin A), ascorbic acid (vitamin C), tocopherol (vitamin E), folate and folic acid (vitamin B9), iron, zinc, copper, phosphorus, calcium, glutathione, quercetin, tannic acid, N-acetyl cysteine (NAC), and melatonin are typical examples of micronutrients and trace elements and act as antioxidants [23–26]. If not consumed at the apposite level, these micronutrients are often reported to provoke inflammatory disorders in periodontal tissues [17,21,27–29].
In concomitance with oxygen and water, macronutrients such as minerals, proteins, carbohydrates, and fats must be consumed appropriately [30]. High carbohydrate (HC) [31], especially sugar-sweetened beverages [32,33], saturated fat with low fiber [17,34], and scarce protein, vitamin C, and B12 [35–37] consumption has been attributed to gingival bleeding, gingivitis dental cavities, periodontal disease, overall poor oral health [17,38].
Typical cellular metabolism yields reactive oxygen species (ROS). After that, ROS performs several physiological activities [39]. Nevertheless, excessive synthesis of ROS starts exhibiting injurious or pathological outcomes by oxidizing nucleic acids such as deoxyribonucleic acid (DNA), lipid peroxidation, protein carbonylation, and ultimately infuriating tissue impairment, including cellular aging and death [40–42]. Multiple studies reported that periodontal disorders generate ROS in very high amounts from inflammatory cells. The extra quantity of ROS diffuses out and ultimately damages vital organs, causing systematic diseases, such as cataracts, malignancy, and cardiovascular disease [43]. It also denotes individuals suffering from chronic periodontitis possess threatened antioxidant competence [44–48]. It has been reported that ROS increases osteoclast growth while suffering from periodontal disorders, leading to growth deterioration of maxillary and mandibular bone, thereby damaging periodontal anatomy and loss of tooth [49,50].
It has been reported that antioxidants’ pharmacodynamic properties possess the potential to alleviate the irreparable teeth-supportive anatomical mutilation triggered by unrestrained amounts of ROS [44,51,52]. Antioxidants’ pharmacological potential includes several vibrant belongings [53]. Free radicals neutralizing molecules scavenge free radicals and confiscate changeover metal ions, disintegrating hydrogen peroxide or hydroperoxides. It also extinguishes operational pro-oxidants and augments endogenic antioxidant protection; nevertheless, it repairs damaged cells through ROS [54,55].
Antioxidants archetypally differed principally into two forms: endogenous and exogenous [56,57]. Endogenous antioxidants signify that these molecules can be formed by the human body [58]. Common endogenic enzymatic antioxidant particles include “superoxide dismutase, catalase, glutathione peroxidase, and reduced glutathione or glutathione-reductase” [59–62].
Endogenous antioxidants, accompanied by vitamins and minerals, are the most functional and competent enzymatic set-up at odds with oxidative stress under physiological equilibrium [63,64]. Exogenous antioxidants denote that the human system is incompetent to produce these molecules of their own. They are usually naturally derived from fruits, vegetables, meat, and fish. These days, exogenous antioxidants are manufactured in laboratories [65,66]. Frequently utilized exogenous antioxidants are “ascorbic acid (vitamin C), tocopherol (vitamin E), quercetin, tannic acid, and NAC” in dental issues [51].
PROBLEM STATEMENT OF THE STUDY
The research question evaluates the effect of micronutrient and macronutrient fortification on periodontal parameters in healthy people with healthy periodontium.
OBJECTIVES OF THE STUDY
This review’s primary objective is to assess micronutrients’ impact on periodontal health by consulting the accessible substantiation from pertinent research results and inspecting the effect of different doses of micronutrients on periodontal health outcomes. To help formulate public strategies for public health workers, to restrict the gap between the prevailing scientific papers, and to highlight areas where further research is required.
MATERIALS AND METHODS
A wide-ranging literature review was steered to congregate applicable information on the impact of macronutrients and micronutrients on periodontal health. Databases such as PubMed and Scopus were systematically searched for articles published up to 2023. Key words included were “periodontal disease,” “macronutrients,” “micronutrients,” and specific nutrients such as carbohydrates, proteins, lipids, and vitamins A, D, E, K, C, B complex, and minerals. The articles included were based on their relevance to the connection between nutrition and periodontal health. Studies focusing on the impact of macronutrients and micronutrients on periodontal tissues were selected. Studies investigating dietary patterns, nutritional intake, and their association with periodontal diseases were prioritized (Fig. 2). Information regarding the recommended daily doses for various was also collected. Special attention was given to the quality of evidence, study designs, and methodologies for assessing the rapport concerning nutrients and periodontal health.
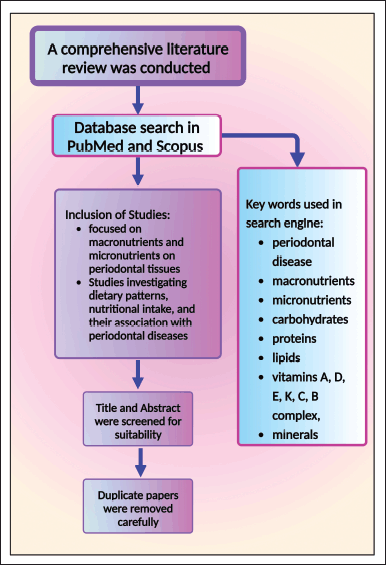 | Figure 2. The materials and method section of the current study was portrayed. This figure has been drawn with the premium version of BioRender (https://biorender.com/ Accessed on 21st December 2023) with the license number BI268O2W3Q. Image Credit: Susmita Sinha. [Click here to view] |
IMPACT OF MACRONUTRIENTS ON PERIODONTAL DISEASE
Carbohydrates
Multiple studies reported no clear-cut or comprehensive explanation regarding the terms HC and low-carbohydrate (LC)-containing diet. This issue is raised as the paramount impediment to deducing the research findings. After that, the HC or LC diet is a comparative notion [67–71]. However, it has been broadly reported that it is considered an HC diet when an individual derives over 45% of total energy from carbohydrates [67–71].
Carbohydrates are a crucial energy resource and contribute to fat metabolism [72–74]. Incorporating low-glycemic, unprocessed, and fiber-rich complex carbohydrates containing phytochemicals such as fruits, whole grains, or coarsely milled grains, vegetables, and legumes into the diet is generally considered healthy. These foods possess conceivable anti-inflammatory and antioxidant assets [75–78]. Conversely, high-glycemic, processed, and low-fiber carbohydrates such as refined sugar, white wheat flour, and sugary drinks can potentially contribute to several chronic inflammatory diseases [79–81]. Diet, including fermentable carbohydrates, can impact oral biofilm composition and potentially contribute to evolving oral conditions such as dental caries and periodontitis [82–84]. Contrary to expectations, it has been noted that incorporating raw vegetables into one’s diet can positively impact periodontal health [17,31,85].
Chewing raw vegetables has been recognized for supporting natural oral detoxification processes, leading to reduced plaque accumulation on the tooth surface, decreased periodontal inflammation, and a lower risk of developing tooth decay. This practice can contribute to improved periodontal and dental health [17,86].
Recent evidence indicates that consuming high-glycemic foods on their own may contribute to increased inflammation through activating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), high-sensitivity C-reactive protein, and oxidative stress [87–89]. Thereby, causing bleeding of the gums, as well as an elevated risk of gingivitis and periodontitis [90–92]. On the other hand, a diet copious in complex carbohydrates, without a corresponding increase in overall energy intake, can potentially decrease the risk of gingivitis and periodontitis [12,91–94].
Carbohydrates, because of their numerous functions, are present in all life forms [95]. Carbohydrates make up most of the organic stuff on earth [96,97]. Carbohydrates provide fuels, energy reserves, and metabolic intermediaries [74,98,99]. Plants and bacteria have polysaccharides as structural components in their cell walls [100–103] (Fig. 3). One of the most prevalent organic chemicals in the biosphere is cellulose, the primary component of plant cell walls [104,105]. Ribonucleic acid (RNA) and DNA are constructed from nucleobases related to a phosphate sugar, which remains the mainstay [106]. Ribose and deoxyribose sugars form part of the physical skeleton of RNA and DNA, respectively [107,108].
 | Figure 3. Schematic representation of cellulose chains in plant cell walls. This figure has been drawn with the premium version of BioRender (https://biorender.com/ accessed on 21st December 2023) with the license number EY268O3CFY. Image Credit: Susmita Sinha. [Click here to view] |
Periodontal implication of carbohydrates
The presence of considerable portions of carbohydrates in individuals’ diets, especially in the form of refined crystallized sugar and refined carbs (devoid of fiber, minerals, vitamins, and so on), encouraged microbioma (microbiome) dysbacteriosis (an imbalance in the microbiota community) in the buccal cavity. This oral dysbiosis possesses the ultimate potential to cause an inflammatory process, pursuing periodontal diseases with pocket formation extending severe clinical manifestation [109–112].
Glycosaminoglycans (GAGs) are exceedingly negative-charged polysaccharides (carbohydrates) constituted of reiterating disaccharide molecules [113]. GAGs are cataloged into four different clusters grounded on their carbohydrate remnants, such as hyaluronic acid (HA), chondroitin sulfate (CS), dermatan sulfate (DS), and heparan sulfate (HS), and keratan sulfate (KS) [114,115]. Thereby, carbohydrates are vital in synthesizing chondroitin, KS, and DSs and are essential for developing connective tissue components. After that, these molecules are extensively found on the cell membrane, in the extracellular matrix, and in connective tissue [116–118]. It has been reported that one of the chief extracellular matrix constituents is chondroitin sulfate proteoglycan (CSPG). CSPG performs an imperative role in organogenesis [119,120].
CS is involved in adding cells to dental tissue. CS furthermore determines the functional distinction of vital cells of the dental pulp. CS improves vascular cells, enhancing dental pulp tissue’s restorative process after tooth damage [120].
Morquio syndrome [Mucopolysaccharidosis (MPS) type IV A)] is a sparse congenital (autosomal recessive) metabolic (lysosomal storage) disease instigated by the insufficiencies in the metabolism and dilapidation of unique enzymes named GAGs [121–125]. Morquio syndrome resulted in diverse continuing and long-lasting skeletal deformities. It also involves enamel defects of teeth and “broad mouth, unerupted, malposition, and spaced permanent teeth” [121]. The diagnosed cases of MPS between 1982 and 2009 were 1.53 per 100,000 live births were reported [126,127].
KS is considered the most novel GAG from the evolutionary perspective; little is known about this carbohydrate-derived new compound [128,129]. KS dispersed throughout the length of collagen (an unsolvable fiber composed of protein among all animals having a bony structure and vertebral column) fibrils in the predentin extracellular matrix to preserve constant texture. It has been reported that KS possesses properties to deflect collagen fibrillogenesis [130]. KS and other glycoproteins had a biological half-life (t½) of 6.8 days [131]. The principal action of proteoglycan GAGs, especially KS and CS, is tissue hydration and organization [128].
Proteoglycan KS and DS disseminated in the predentin impede the calcification activity of collagen fibrils; those are not calcified extracellular matrix and halt the calcification process [130,132]. There has been reported to be an intensification in KS immune-related activity around the wound area along with macrophages, reactive microglia, and oligodendrocyte precursors [128,133–135]. It has been reported that due to hereditary causes, KS can be deficient; nevertheless, little is known about the detailed reasons for deficiency [136]. One more study revealed that Morquio syndrome, or MPS, is primarily an inherited disease instigated by the insufficiency of the N-acetylglucosamine-6-sulfate sulfatase enzyme [137].
Archetypally, diminished biosynthesis KS is concomitant with inflammation, signifying proinflammatory cytokines downregulated ion of KS formation, thereby causing delayed wound healing [138].
HA is a carbohydrate-containing polysaccharide (GAG). It is considered a unique biomolecule serving multiple purposes, such as osteoarthritis, vesicoureteral reflux, urinary incontinence, skin wrinkles, dry eyes, and interstitial cystitis. HA also addresses pharmaceutical, aesthetic, and cosmetic issues through wound restoration and tissue rejuvenation [139–142].
HA GAG is a foremost and organic constituent of the extracellular matrix. However, HA contributes to diverse living cellular accomplishments by incorporating growth factors and comparative receptors. Nevertheless, the controlling physiological role of HS on the vasculogenesis of mesenchymal stem cells remains obscure [143]. Hayano et al. [144] first reported the “functional roles of HS proteoglycans’ sulfation in dentin formation.” Duplancic et al. [145] said that consuming manmade derivatives of heparan sulfate glycosaminoglycan (HS GAG) and blemished extracellular matrix (ECM) with therapeutic intention effectively regenerates periodontal tissue among animal models suffering from periodontal diseases. However, the pharmacodynamics of HS GAG therapeutic intervention for periodontal disease in humans is still undetermined [145].
HA is essential in maintaining the appropriate physiology of extracellular matrices in both pulpy and solid constituents of gum and tooth [146]. HA possesses anti-inflammatory, anti-edematous, osteoinductive, pro-angiogenetic properties, and antimicrobial effective pharmacodynamics, especially for periodontal diseases in managing gum infection and hemorrhage [140,141,147]. It has been reported that topical (gel formulation) HA has been documented as an accessory medication to improve long-lasting inflammatory illnesses like periodontitis, especially after dental surgical intervention through reducing inflammatory intercessors and improving healing and tissue regeneration [148–151] and oral lichen planus (topical 0.2% HA) [152–154]. HA also potentiates the periodontal wound healing process [150]. It has been reported that HA 0.8% and chlorhexidine digluconate 0.05%–0.2% avert the growth and development of dental biofilm [155–158]. High molecular weight HA and chlorhexidine thoroughly fine-tune the gums and humidify, dampen, and rinse the buccal mucus-secreting membrane [158–160]. Therefore, it revealed day-to-day oral cleanliness after periodontal therapeutic intervention, oral, or peri-implant surgery [158,161,162].
Proteins
The specific role of dietary proteins in systemic inflammation has not been elucidated [163]. It has been reported that animal and plant proteins generate insulin-like growth factors (IGFs) equally [164,165].
Furthermore, reducing the animal protein-rich diet decreases all-embracing and all forms of cardiovascular death compared to plant protein-based food [166,167]. IGFs are mitogens that promote the carcinogenesis process. These IGFs are indispensable components for developing diverse cancers and their metastatic activity. IGF biomolecules surmount normal apoptosis and promote cell cycle progression and angiogenesis process. The IGF events are arbitrated and completed by the IGF-1 receptor involved in cell transformation provoked by carcinoma [168–172].
Conversely, the ingesting of a high vegetable-proteins-based diet has been associated with a decreased risk of cardiovascular disease, type 2 diabetes mellitus, and chronic kidney disease [173–175]. In a study by Staufenbiel et al. [176], a comparison was made between the periodontal health of 100 vegetarians and 100 nonvegetarians. The findings revealed that the vegetarian group exhibited considerably shallower periodontal pockets, less bleeding upon probing, and better oral hygiene than the nonvegetarian participants. Therefore, the vegetarian diet appears to exert a beneficial impact on periodontal health [176].
A randomized clinical trial was conducted for 4 weeks to determine the relationship between dietary vegetables (vegetables half in quantity with a high-fat diet) and animal protein and periodontal health [177]. A vegetable-rich diet containing, e.g., seeds, nuts, cereals, marine vegetables, extra-virgin olive oil, cocoa, legumes, tomatoes, and so on, contain Mg2+ was more favorable toward oral health when compared with a diet rich in HC and animal protein [12,177]. Relevant studies also found that nutritional supplementation, especially protein, minimizes periodontal probing depth, reduces clinical attachment of teeth salvage, which was about to be lost, and curtails inflammatory oozing fluid caused by periodontitis. In addition, the hemorrhagic tendency was reduced on probing 2–6 months later while conduction surgical intervention was conducted for check-ups [177,178]. Multiple studies reported that individuals consuming a diet containing plenty of vegetables, fruits, soya containing various foods, and fish get more micronutrients and antioxidants [179]; after that, these folks suffer much less from periodontal diseases and dental carries than a diet rich in animal protein (Fig. 4) [12,17,38,179]. One more study revealed that a substantial portion of red meat consumption was expectantly connected with vaster periodontal probing depth and hemorrhage on probing. Correspondingly, a small amount of red and/or processed meat intake was linked to bringing down the likelihood of emerging periodontal diseases [180,181].
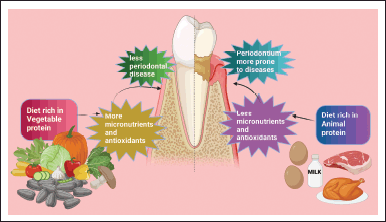 | Figure 4. Illustrates the beneficial effect of a diet containing high vegetable protein on periodontal health compared to consuming high animal protein. This figure has been drawn with the premium version of BioRender (https://biorender.com/ accessed on 30th December 2023) with the license number NU269YDO3T. Image Credit: Rahnuma Ahmad. [Click here to view] |
Function of proteins over periodontal health
We receive amino acids [small carbon-based particles that contain an α (central) carbon atom linked to an amino, and a carboxyl group, atomic hydrogen, and a wavering constituent termed a side chain] from proteins, which are the building blocks of protein [182]. It has been revealed that protein is responsible for nearly all physiological commitments and rheostats gene countenance. A considerable portion of the essential constituents of every single cell of a living creature are made with protein [183,184]. Around 50% of the body’s dry weight is made up of protein [185,186]; it is the second most prevalent material after water. The intracellular protein network’s cytoskeleton preserves cells’ physical integrity and shape [187]. The contractile components of muscle are made up of actin and myosin filaments [188].
Periodontal implication
Proteins are necessary for effective host defenses [189]. They are elements of protective molecules and barriers that aid in limiting the spread of disease [190]. The complement system, innate immunity, antibody or humoral immunity, and cell-mediated immunity are all part of the periodontal defenses [191]. The junctional and crevicular epithelia provide an epithelial barrier function against invaders for periodontium [192–194]. This epithelium surface, which has a rapid turnover, is a significant defense barrier against invading microorganisms, poisonous substances, and antigens [195]. The epithelial defense barrier needs enough protein, Zn2+, folic acid, iron, vitamin A, and vitamin C to maintain good periodontal health [21,196,197].
Osteoporosis of the alveolar, supporting bone, and removing fibroblasts and connective tissue fibers in the periodontal membrane are degenerative lesions created in the periodontium [198,199]. Protein deficiency hinders periodontal wound healing by preventing angiogenesis, fibroblast growth, and collagen production, build-up, and remodeling [200–202].
The mechanisms by which protein deficiency enhances periodontal disease
The salivary antimicrobial peptides (AMPs) act as natural primary protective strategies for the oral and gastrointestinal anatomical structures from pathogenic microbes [203]. Oral saliva contains AMPs such as Mucin-7 and lactoferrin [204]. These salivary AMPs consent to beneficial microbes (microbiota) colony formation of the buccal space. However, microbiota avert wide-ranging pathogenic microbial settlement and prevent infection. Subsequently, maintain stability and composure around the mouth and oropharyngeal anatomical structure. Thus, it prevents infectious disorders in and around the mouth cavity [203,205]. Patients admitted to the intensive care unit with ventilators often produce minimal salivary secretion, enhancing poor oral hygiene [206,207]. These issues raise the possibility of developing pathogenic microbes [208,209]. Therefore, these pathogens cause oral infective diseases, including periodontitis [203–209].
Individuals consuming protein more than 1 g/kg/day is related to a diminution of periodontal infective and inflammatory disorders [35,210], and dietary Ca2+, principally adequate amount drinking milk also shows defensive activities against gum disorders [211]. Periodontitis is highly prevalent in the elderly community (82%) and older adults [211–213]. It can be assumed that the elderly community regularly does not consume enough amount protein and maintain oral hygiene properly, causing periodontal diseases [35,214]
Lipids
Lipids are essential energy sources and are crucial structural and metabolic components [215–217]. Numerous studies have demonstrated that consuming unhealthy saturated fats, including trans fats and omega-6 fatty acids, significantly promotes inflammation [218–221]. Saturated fats are common in animal products, e.g., fowls with skin, fatty meats (beef and pork), pork white fat, and full-cream milk products (butter, cheese, and whole milk) [222,223]; vegetable oils (coconut and palm) [224–226]; and prepackaged or fast foods such as pastries and biscuits [227,228]. Trans fats are a type of unhealthy fat formed through industrial hydrogenation, which converts liquid oils into solid fats. Consuming trans fats has increased the risk of noncommunicable diseases (NCDs), such as heart disease and other health tribulations [221,229–232].
Multiple research projects observed a significant positive correlation between the intake of saturated fatty acids and the prevalence of periodontal lesions [17,181,233–236]. In recent years, there has been considerable research interest in omega-3 fatty acids due to their potential association with reduced systemic inflammation [237–240]. This concept is commonly known as the resoleomics theory, initially introduced in the study by Serhan and his team [17,241–247]. Serhan and associates initiated the term resoleomics in 1996 [248,249]. It is the course of decreasing inflammation. Resoleomics can be cogitated as the evolution set-up for renovating homeostatic equilibriums following infection, injury, and inflammation [248,249]. Numerous clinical studies in periodontology have shown that supplementing with omega-3 fatty acids, whether through fish oil supplements, consumption of fatty fish, or plant-based sources, can decrease periodontal inflammation and/or pocket depth [14,250–254]. Researchers chose imperative Randomized Controlled Trials Regarding Diet and Periodontal Diseases Published in the last 5 years, depicted in Table 1.
IMPACT OF MICRONUTRIENTS ON PERIODONTAL DISEASE
Healthy, nutritious food-consuming habits and regular exercise with strict avoidance of principal risk factors of NCDs such as chronic alcohol, cigarette, processed food intake, and mainly sedentary life eventually prevent or decrease the rate of NCDs [261–265]. Furthermore, overuse of proton pump inhibitors, antimicrobials, chelating agents, and nonsteroidal anti-inflammatory drugs leads to micronutrient deficiencies [266–268]. Systemic conditions such as pregnancy, breastfeeding, and physical/mental stress require increased nutrient consumption [269–272]. Vitamins are commonly classified as fat-soluble [273] and water-soluble [274]. Fat-soluble vitamins comprise vitamins A, D, E, and K [275]. Fat-soluble vitamins cannot be excreted by the kidneys through urine [275] and stored in the tissues [276]. However, most water-soluble vitamins, except vitamins B6 and B12, get easily excreted via the kidneys [277].
Vitamin A
Vitamin A preserves the integrity of epithelial cells’ gene transcription and remains a de rigueur constituent all through life [278–280]. Also, it helps in the growth, differentiation, and maintenance of epithelial tissues, bone growth, and embryonic development [281–286]. A case study of a 20-year-old lady suggested that excessive vitamin A consumption leads to gingival attritions, ulcers, hemorrhage, puffiness, gum inflammation, and forfeiture of cornification. She consumed 200,000 International Units (IUs) of vitamin A every day for 6 months to decrease pimples with skin inflammation. Discontinuation of added vitamin A exhibited gingival upgrading within 7 days. A downright typical manifestation of oral tissues was detected by the end of 60 days [287]. Multiple studies reported that excessive consumption of vitamin A affects periodontal tissues and several vital structures of the human body [288–290].
Vitamin D
Vitamin D maintains normal blood Ca2+ levels and metabolism of skeletal issues [291–296]. It also regulates calcium absorption from the intestines [297]. A blood Ca2+ deficiency leads to inadequate osseous tissue calcification, causing rickets and osteomalacia [298,299]. Fortified milk stands to be the primary dietary source for vitamin D. Loss of periodontal attachment has been reported with vitamin D deficiency [300]. Several studies concluded that the anti-inflammatory outcomes of vitamin D diminish the proneness of gum and periodontal diseases [301–303].
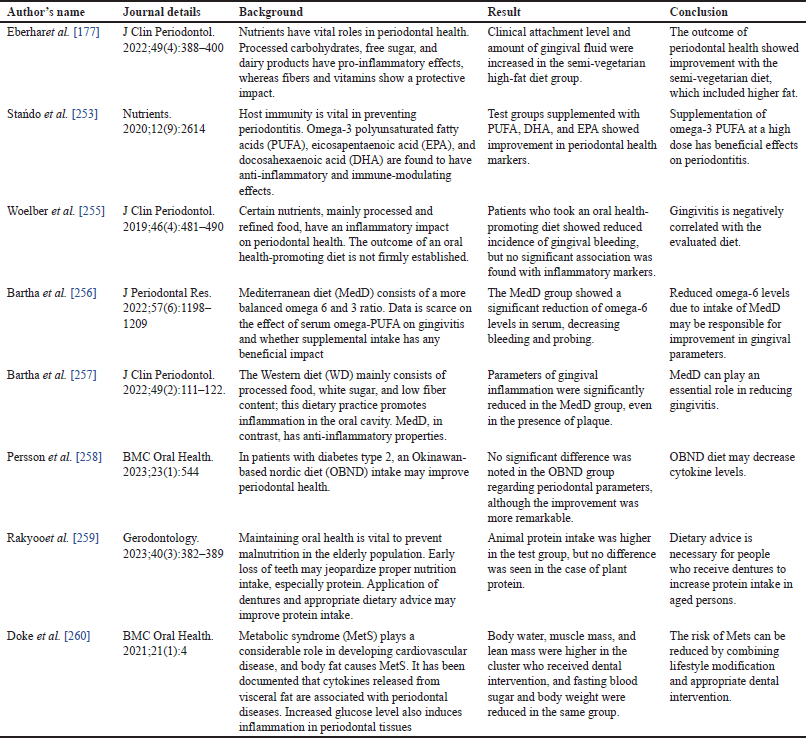 | Table 1. Certain critical randomized controlled trials regarding diet and periodontal diseases were published in the last 5 years [Accessed on December 9, 2023] in the PubMed database. [Click here to view] |
Vitamin K
Producing osteonectin and “matrix gla protein” requires vitamin K [304,305]. Intestinal microbiomes synthesize a considerable amount of vitamin K in the body [306]. Deficiency of it leads to poor bone density [307,308]. In addition, vitamin K deficiency often leads to periodontal disorders such as gum hemorrhage and tooth clinical attachment loss [31,309].
Vitamin C
The ability of vitamin C to cure scurvy gave it a name as ascorbic acid [310]. Three critical signs of inflammation, such as redness, swelling, and edema, regulated by regional blood flow, are controlled by histamine [311]. Vitamin C renders significant antihistamine properties [312] and maintains healthy periodontium [15,36,313,314]. Rebound or conditional scurvy was described in a case report by Siegel et al. [315], suggesting that withdrawal from chronic consumption of high doses of vitamin C leads to the development of oral symptoms of scurvy. Clinical features of scurvy are often observed within 8 to 12 weeks of sporadic or insufficient vitamin C. Canadian health authorities recommend vitamin C 75 and 90 mg daily either with food or as a supplement [316]. Another study revealed that if a patient develops obvious insufficiency symptoms because of poor consumption of vitamin C. Around 10 mg/day is recommended for many weeks [317].
Vitamin B complex
The consumption of processed food made using refined grains has increased, a significant cause of vitamin B complex deficiency with global modernization [318–320]. Energy production in the human body is mainly regulated by thiamin (vitamin B1), which is necessary to metabolize branched-chain amino acids and carbohydrates [321–323]. The classic form of thiamin-deficient disease known as Beri-Beri affects the muscular, neurological, cardiovascular, and gastrointestinal systems [322–326]. Consuming an imbalanced diet deficient in thiamin can lead to Beri-Beri within 7–10 days. Primary dietary sources for thiamin are “whole grains, cereals, nuts, seeds, meat (especially pork, with much less in fish, poultry, beef, and lamb), round beans, peas, lentils, soy and Marmite, Vegemite, Bovril,” fortified bread, and orange juice [327]. Oral manifestations of thiamin insufficiency, such as glossitis, angular cheilosis, stomatitis, burning tongue, loss of taste, and hypersensitivity to the oral mucosa, are often associated with riboflavin scarcity [328].
Pellagra is a sporadic systemic disorder caused by an acute paucity of niacin (vitamin B3) in the human body [329–330]. Pellagra comprises the troika of “dermatitis, dementia, and diarrhea” and can result in fatal outcomes [324]. Pellagra was eradicated in the USA due to the fortification of the finest type of wheat flour with niacin in the preliminary days of the 1940s [331]. Niacin is synthesized from tryptophan [332] and is involved in several cellular pathways, which include the energy-building process for the coalescences and metabolism of fatty acids [333–338]. Elevated levels of cholesterol can be treated with niacin [332]. One more study reported that Vitamin B3 is associated with hepatic toxicity and failure when regularly consumed over 2 g daily [339]. “Pyridoxal, pyridoxine, and pyridoxamine;” these 03 compounds are formed from vitamin B6 [340]. Its primary function is to synthesize heme [341]. In addition, carpal tunnel syndrome can be treated with vitamin B6 [342] with varying degrees of long-term success. However, high doses stored in the body lead to irreversible nerve damage [343].
Folate or folic acid is the most integral vitamin for DNA synthesis [344]. Folic acid closely works with vitamin B12 to regenerate folate coenzyme [345,346]. The synthesis process of folate coenzyme without vitamin B12 remains “either from a failure to provide the proper substrate for polyglutamate synthesis or to a direct requirement for vitamin B12 for polyglutamate synthesis” [347]. Megaloblastic anemia with considerably greater size immature red blood cells is evident because of vitamin B12 (cobalamin) and folate insufficiencies, which are obligatory components for the generation of DNA [348]. In a study performed using data from NHANES 2001/02, where subjects underwent periodontal health checkups and serum folate level ascertainment, it was concluded that low serum folate level was independent of periodontal disease in the issues. Hence, a statistically significant negative association had been established [349]. Multiple studies reported that low-level serum folic acid promotes periodontal diseases [29,350,351]. Animal-derived foods, the primary source of vitamin B12 [352], are often deficient in strict vegetarians and vegans [353–355]. Thus, supplemental cyanocobalamin is a must for them to maintain folate and homocysteine metabolism. Pernicious anemia is the prime disorder linked with vitamin B12 deficiency [356,357]. The characteristic features of pernicious anemia include tiredness, feebleness, wishy-washy look, changes to visualization and odor, prickling feeling, overactive bladder, melancholy, unhappiness, and psychotic attacks [358]. Researchers selected an imperative Systematic Review and Meta-analysis Regarding Diet and Periodontal Diseases Published in the last 5 years depicted in Table 2.
Minerals
Minerals comprise 4% to 5% of the body weight [369,370]. Minerals maintain “heart rhythm” [371], “muscle contraction” [372], “nerve conduction” [373], and “acid-base balance” [374]. Minerals required in the amount of >100 mg/day are called significant minerals [375], and those needed in the amounts of <100 mg/day are termed trace minerals [376]. Leading minerals include “Na+, K+, Ca2+, Mg2+, P3−, and S2-” for maintaining human physiology [377]. In contrast, silhouette amounts of elements include “Fe3+, Zn2+, I- , Se+4, F−, Cu2+, Cu3+, Co+3, Cr+3, Mn2+, Mn3+, and Mo3+” [378,379].
Na+ is the primary electrolyte in the body, constituting a significant part of extracellular fluid [380]. The body’s fluid balance and nerve conduction are maintained by Na+ [381]. Ha reported in 2014 that 5 to 6 g/day is the set daily requirement of Na+ hypertensive patients [382]. K+ is the notable cation for intracellular fluid and functions similarly to sodium but intracellularly [381]. Low K+ levels are more common as it is not often added as a dietary supplement, resulting in muscle cramps, confusion, irregular heartbeats, and life-threatening situations (Fig. 5) [381].
Research by Nishida et al. [383] recommended that inadequate food available calcium intake resulted in severe periodontal diseases. Shimazaki et al. [384] suggested an optimistic relationship between the intake of lactic acid-comprehending foods and the inhibition of the development of periodontal diseases. The electron transport functions of mitochondria are regulated by coenzyme Q10, which is dispersed from end to end of human tissues [385]. Treatment of periodontally compromised patients with this coenzyme has significantly improved in periodontal conditions [386,387]. Zinc acts on ROS and neutralizes, hence reducing the chronic inflammatory process and improving periodontal health [388–392].
Table 3 [19] was created summarizing the recommended daily doses for essential nutrients and their importance in periodontal health. References supporting the recommended doses were added to enhance the credibility of the information. The reliability and validity of selected studies were critically appraised to ensure the robustness of the evidence. Potential biases, limitations, and gaps in the existing literature were acknowledged and discussed.
 | Table 2. Several analytical, systematic reviews and meta-analyses regarding diet and periodontal diseases were published in the last 5 years [Accessed December 9, 2023] in the PubMed database. [Click here to view] |
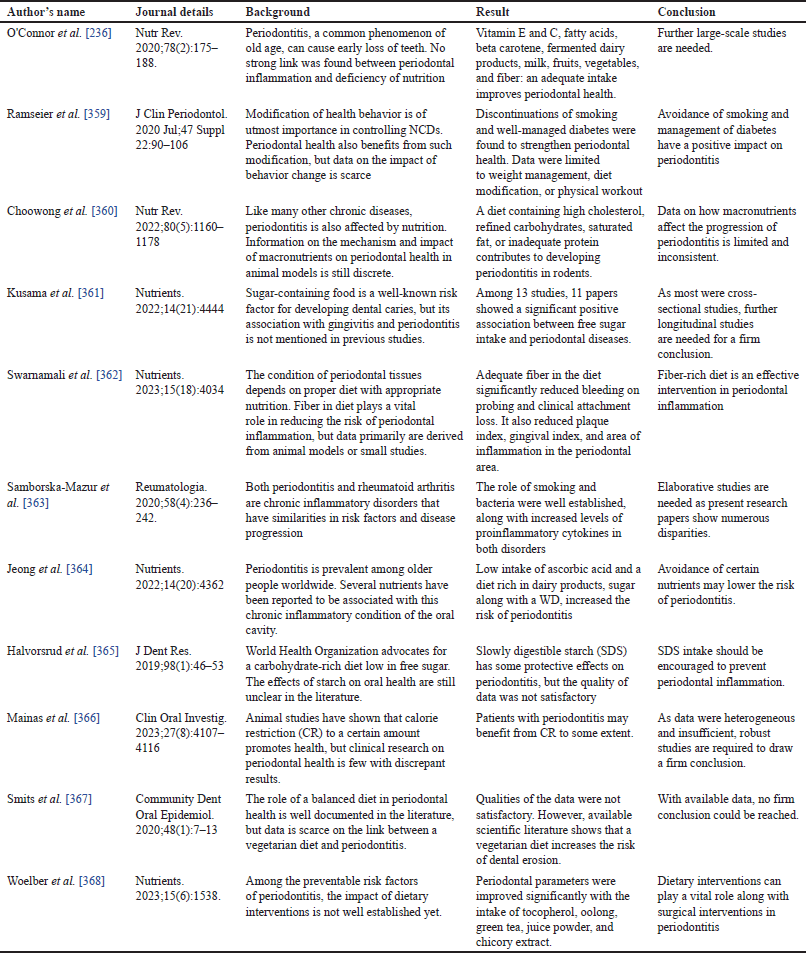
 | Figure 5. Illustrates the impact of micronutrients on periodontal disease. This figure has been drawn with the premium version of BioRender (https://biorender.com/ accessed on 21st December 2023) with the license number IS268O3U4S. Image credit: Susmita Sinha. [Click here to view] |
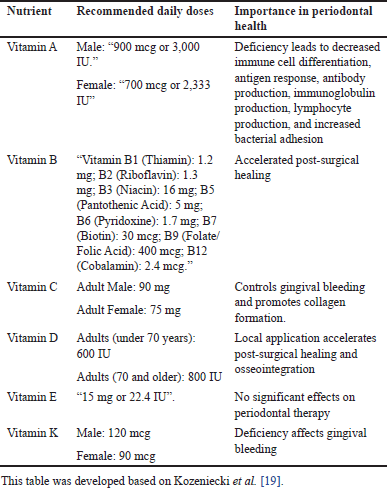 | Table 3. Recommended daily doses to maintain periodontal health. [Click here to view] |
LIMITATIONS OF THIS REVIEW
The limitation of this review was the complete reliance on the previously published manuscript. Only PubMed, Medline, and Scopus databases were consulted. We may have missed the research work published in Web of Science and other smaller databases. In addition, multiple studies reported that narrative review possesses integral limitations such as substandard research paper exploration [393], scarcity of explicit substantiation of search strategy [394], imaginable preferential in the evaluation of selected scientific articles [395,396], according to SANRA scale score below four denotes poor quality of the paper [397], and not appropriate specific clinical situation [398]. These traditional reviews help as potential resources for the speedy accessibility of recent references for precise arenas of interest of authors [399–401]. However, it has been reported that narrative reviews postulate better explanation and appraisal; the strategic impact of these old-style review papers extends comprehension level [402–404].
CONCLUSION
As is observed throughout this review, nutrition plays a vital role in oral cavity health. Each nutrient has its own function, and nutrients also interact to boost and sustain a healthy periodontium. Nutrients such as carbohydrates in the form of GAG are essential for extracellular matrix formation, proteins contribute to host immunity, and lipids like omega-3 fatty acids may decrease the chronic inflammatory process. Vitamin C is needed to avoid gum bleeding, and vitamin K deficiency leads to periodontal disorders such as gum hemorrhage and tooth clinical attachment. Mineral deficiency, like calcium deficiency, causes periodontal disease, and zinc neutralizes ROS. Poor oral health may hamper physical, mental, and social well-being. To maintain oral health, it is, therefore, vital to know about the benefits of these nutrients. It is essential to spread this knowledge among the general population. Dentists, other health workers, and policymakers need to work together to ensure the dissemination of knowledge regarding proper balanced diet consumption in adequate amounts, sources of various nutrients, and harmful effects of an unhealthy diet, as well as a diet deficient in essential nutrients on oral health. Research in this field of study must continue to build awareness and prevent damage to oral health so that the global population can enjoy a healthy smile.
ACKNOWLEDGMENT
The graphical abstract has been drawn with the premium version of BioRender (https://biorender.com/ accessed on 29th December 2023) with the license number MF269TMZ3R. Image Credit: Rahnuma Ahmad.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research received no external funding.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gantenbein KV, Kanaka-Gantenbein C. Mediterranean diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients. 2021;13:1951. CrossRef
2. Chan AKY, Tsang YC, Jiang CM, Leung KCM, Lo ECM, Chu CH. Diet, nutrition, and oral health in older adults: a review of the literature. Dent J (Basel). 2023;11:222. CrossRef
3. Watanabe Y, Okada K, Kondo M, Matsushita T, Nakazawa S, Yamazaki Y. Oral health for achieving longevity. Geriatr Gerontol Int. 2020;20:526–38. CrossRef
4. Ohlhorst SD, Russell R, Bier D, Klurfeld DM, Li Z, Mein JR, et al. Nutrition research to affect food and a healthy life span. Am J Clin Nutr. 2013;98(2):620–5. CrossRef
5. Martín-Rodríguez A, Bustamante-Sánchez Á, Martínez-Guardado I, Navarro-Jiménez E, Plata-SanJuan E, Tornero-Aguilera JF, et al. Infancy dietary patterns, development, and health: an extensive narrative review. Children (Basel). 2022;9:1072. CrossRef
6. Robinson SM. Infant nutrition and lifelong health: current perspectives and future challenges. J Dev Orig Health Dis. 2015;6:384–9. CrossRef
7. Gupta A. Nutrition. In: Comprehensive biochemistry for dentistry. Gateway East, Singapore: Springer Nature Singapore Private Limited; 2019. 533–56 pp.
8. Cena H, Calder PC. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients. 2020;12:334. CrossRef
9. Barchitta M, Maugeri A, Favara G, Magnano San Lio R, Evola G, Agodi A, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int J Mol Sci. 2019;20:1119. CrossRef
10. Álvaro Sanz E, Garrido Siles M, Rey Fernández L, Villatoro Roldán R, Rueda Domínguez A, Abilés J. Nutritional risk and malnutrition rates at diagnosis of cancer in patients treated in outpatient settings: early intervention protocol. Nutrition. 2019;57:148–53. CrossRef
11. Barge-Caballero E, Crespo-Leiro MG. Nutritional risk in patients with advanced heart failure. We know how to detect it, but can we correct it? Rev Esp Cardiol (Engl Ed). 2019;72(8):601–3. CrossRef
12. Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. Nutrition as a key modifiable factor for periodontitis and main chronic diseases. J Clin Med. 2021;10:197. CrossRef
13. Casarin M, da Silveira TM, Bezerra B, Pirih FQ, Pola NM. Association between different dietary patterns and eating disorders and periodontal diseases. Front Oral Health. 2023;4:1152031. CrossRef
14. Spahr A, Divnic-Resnik T. Impact of health and lifestyle food supplements on periodontal tissues and health. Periodontol 2000. 2022;90(1):146–75. CrossRef
15. Ustianowski ?, Ustianowska K, Gurazda K, Rusi?ski M, Ostrowski P, Pawlik A. The role of vitamin C and vitamin D in the pathogenesis and therapy of periodontitis-narrative review. Int J Mol Sci. 2023;24:6774. CrossRef
16. Warne RW. The micro and macro of nutrients across biological scales. Integr Comp Biol. 2014;54:864–72. CrossRef
17. Santonocito S, Polizzi A, Palazzo G, Indelicato F, Isola G. Dietary factors affecting the prevalence and impact of periodontal disease. Clin Cosmet Investig Dent. 2021;13:283–92. CrossRef
18. Venn BJ. Macronutrients and human health for the 21st century. Nutrients. 2020;12:2363. CrossRef
19. Kozeniecki M, Ludke R, Kerner J, Patterson B. Micronutrients in liver disease: roles, risk factors for deficiency, and recommendations for supplementation. Nutr Clin Pract. 2020;35:50–62. CrossRef
20. Gladyshev MI. Fatty acids: essential nutrients and important biomarkers. Biomolecules. 2022;12:1250. CrossRef
21. Dommisch H, Kuzmanova D, Jönsson D, Grant M, Chapple I. Effect of micronutrient malnutrition on periodontal disease and periodontal therapy. Periodontol 2000. 2018;8:129–53. CrossRef
22. Savarino G, Corsello A, Corsello G. Macronutrient balance and micronutrient amounts through growth and development. Ital J Pediatr. 2021;47:109. CrossRef
23. Lahaye C, Parant F, Haesebaert J, Goldet K, Bendimred L, Henaff L, et al. Minerals and antioxidant micronutrients levels and clinical outcome in older patients hospitalized for COVID-19 during the first wave of the pandemic. Nutrients. 2023;15:1516. CrossRef
24. Gîlc?-Blanariu GE, Diaconescu S, Ciocoiu M, ?tef?nescu G. New insights into the role of trace elements in IBD. Biomed Res Int. 2018;2018:1813047. CrossRef
25. Galloway P, McMillan DC, Sattar N. Effect of the inflammatory response on trace element and vitamin status. Ann Clin Biochem. 2000;37(Pt.3):289–97. CrossRef
26. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. CrossRef
27. Li W, Shang Q, Yang D, Peng J, Zhao H, Xu H, et al. Abnormal micronutrient intake is associated with the risk of periodontitis: a dose-response association study based on NHANES 2009-2014. Nutrients. 2022;14:2466. CrossRef
28. Luo PP, Xu HS, Chen YW, Wu SP. Periodontal disease severity is associated with micronutrient intake. Aust Dent J. 2018;63:193–201. CrossRef
29. Hans M, Malik PK, Hans VM, Chug A, Kumar M. Serum levels of various vitamins in periodontal health and disease- a cross-sectional study. J Oral Biol Craniofac Res. 2023;13:471–5. CrossRef
30. Carreiro AL, Dhillon J, Gordon S, Higgins KA, Jacobs AG, McArthur BM, et al. The macronutrients, appetite, and energy intake. Annu Rev Nutr. 2016;36:73–103. CrossRef
31. Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: an update. Nutrients. 2016;8:530. CrossRef
32. Gupta V, Dawar A, Bhadauria US, Purohit BM, Nilima N. Sugar-sweetened beverages and periodontal disease: a systematic review. Oral Dis. 2022;29(8):3078–90. CrossRef
33. Haque M, McKimm J, Sartelli M, Samad N, Haque SZ, Bakar MA. A narrative review of the effects of sugar-sweetened beverages on human health: a key global health issue. J Popul Ther Clin Pharmacol. 2020;27:76–103. CrossRef
34. Khan TZ, Mobin T. Unraveling the link between periodontal disease and high cholesterol: a cross- sectional study. Cureus. 2023;15:43463. CrossRef
35. Jayasinghe TN, Harrass S, Erdrich S, King S, Eberhard J. Protein intake and oral health in older adults-a narrative review. Nutrients. 2022;14:4478. CrossRef
36. Murererehe J, Uwitonze AM, Nikuze P, Patel J, Razzaque MS. Beneficial effects of vitamin C in maintaining optimal oral health. Front Nutr. 2022;8:805809. CrossRef
37. Zong G, Holtfreter B, Scott AE, Völzke H, Petersmann A, Dietrich T, et al. Serum vitamin B12 is inversely associated with periodontal progression and risk of tooth loss: a prospective cohort study. J Clin Periodontol. 2016;43:2–9. CrossRef
38. Hujoel PP, Lingstrom P. Nutrition, dental caries, and periodontal disease: a narrative review. J Clin Periodontol. 2017;44:79–84. CrossRef
39. Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM. Reactive oxygen species: the dual role in physiological and pathological conditions of the human body. Eurasian J Med. 2018;50:193–201. CrossRef
40. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. CrossRef
41. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int J Mol Sci. 2021;22:4642. CrossRef
42. Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792. CrossRef
43. Moore A, Khanna D. The role of vitamin C in human immunity and its treatment potential against COVID-19: a review article. Cureus. 2023;15:33740. CrossRef
44. Shang J, Liu H, Zheng Y, Zhang Z. Role of oxidative stress in the relationship between periodontitis and systemic diseases. Front Physiol. 2023;14:1210449. CrossRef
45. Wang Y, Andrukhov O, Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. 2017;8:910. CrossRef
46. Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl 1):S237–48. CrossRef
47. Patil VS, Patil VP, Gokhale N, Acharya A, Kangokar P. Chronic periodontitis in type 2 diabetes mellitus: oxidative stress as a common factor in periodontal tissue injury. J Clin Diagn Res. 2016;10:12–6. CrossRef
48. Tóthová L, Celec P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front Physiol. 2017;8:1055. CrossRef
49. Palathingal P, Mahendra J, Annamalai PT, Varma SS, Mahendra L, Thomas L, et al. A cross-sectional study of serum glutathione peroxidase: an antioxidative marker in chronic periodontitis and chronic kidney disease. Cureus. 2022;14:22016. CrossRef
50. Sui L, Wang J, Xiao Z, Yang Y, Yang Z, Ai K. ROS-scavenging nanomaterials to treat periodontitis. Front Chem. 2020;8:595530. CrossRef
51. Qi F, Huang H, Wang M, Rong W, Wang J. Applications of antioxidants in dental procedures. Antioxidants (Basel). 2022;11:2492. CrossRef
52. Olmedo DERP, Kury M, Resende BA, Cavalli V. Use of antioxidants to restore bond strength after tooth bleaching with peroxides. Eur J Oral Sci. 2021;129:12773. CrossRef
53. Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of natural plant origins: from sources to food industry applications. Molecules. 2019;24:4132. CrossRef
54. Flieger J, Flieger W, Baj J, Maciejewski R. Antioxidants: classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials (Basel). 2021;14:4135. CrossRef
55. Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Abdull Razis AF, Modu B, et al. Oxidative stress, free radicals, and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;11:1158198. CrossRef
56. Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 2010;3:228–37. CrossRef
57. Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–97. CrossRef
58. Carvalho RH, Ida EI, Madruga MS, Shimokomaki M, Estévez M. Collapse of the endogenous antioxidant enzymes in post-mortem broiler thigh muscles triggers oxidative stress and impairs water-holding capacity. J Food Sci Technol. 2019;56:1371–9. CrossRef
59. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–54. CrossRef
60. Gusti AMT, Qusti SY, Alshammari EM, Toraih EA, Fawzy MS. Antioxidants-related superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferase (GST), and nitric oxide synthase (NOS) gene variants analysis in an obese population: a preliminary case-control study. Antioxidants (Basel). 2021;10:595. CrossRef
61. Jena AB, Samal RR, Bhol NK, Duttaroy AK. Cellular red-Ox system in health and disease: the latest update. Biomed Pharmacother. 2023;162:114606. CrossRef
62. Korczowska-??cka I, S?owikowski B, Piekut T, Hur?a M, Banaszek N, Szymanowicz O, et al. Disorders of endogenous and exogenous antioxidants in neurological diseases. Antioxidants (Basel). 2023;12:1811. CrossRef
63. Niu ZY, Min YN, Liu FZ. Dietary vitamin E improves meat quality and antioxidant capacity in broilers by upregulating the expression of antioxidant enzyme genes. J Appl Anim Res. 2017;2119:1–5. CrossRef
64. Hooda R, Madke B, Choudhary A. Photoaging: reversal of the oxidative stress through dietary changes and plant-based products. Cureus. 2023;15:37321. CrossRef
65. Walke G, Gaurkar SS, Prasad R, Lohakare T, Wanjari M. The impact of oxidative stress on male reproductive function: exploring the role of antioxidant supplementation. Cureus. 2023;15:42583. CrossRef
66. Panova IG, Tatikolov AS. Endogenous and exogenous antioxidants as agents preventing the negative effects of contrast media (Contrast-Induced Nephropathy). Pharmaceuticals (Basel). 2023;16:1077. CrossRef
67. Jung CH, Choi KM. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients. 2017;9:322. CrossRef
68. Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. CrossRef
69. Accurso A, Bernstein RK, Dahlqvist A, Draznin B, Feinman RD, Fine EJ, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond). 2008;5:9. CrossRef
70. Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One. 2014;9(7):e100652. CrossRef
71. Liebman M. When and why carbohydrate restriction can be a viable option. Nutrition. 2014;30(7–8):748–54. CrossRef
72. Cocinero EJ, Çarçabal P. Carbohydrates. Top Curr Chem. 2015;364:299–333. CrossRef
73. Spriet LL. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44(Suppl 1):S87–96. CrossRef
74. Su L, Feng Y, Wei K, Xu X, Liu R, Chen G. Carbohydrate-based macromolecular biomaterials. Chem Rev. 2021;121(18):10950–1029. CrossRef
75. Zazpe I, Santiago S, Gea A, Ruiz-Canela M, Carlos S, Bes-Rastrollo M, et al. Association between a dietary carbohydrate index and cardiovascular disease in the SUN (Seguimiento Universidad de Navarra) project. Nutr Metab Cardiovasc Dis. 2016;26(11):1048–56. CrossRef
76. Ventriglio A, Sancassiani F, Contu MP, Latorre M, Di Slavatore M, Fornaro M, et al. Mediterranean diet and its benefits on health and mental health: a literature review. Clin Pract Epidemiol Ment Health. 2020;16:156–64. CrossRef
77. Hemler EC, Hu FB. Plant-based diets for cardiovascular disease prevention: all plant foods are not created equal. Curr Atheroscler Rep. 2019;21:18. CrossRef
78. Ludwig DS, Hu FB, Tappy L, Brand-Miller J. Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ. 2018;361:2340. CrossRef
79. Ma X, Nan F, Liang H, Shu P, Fan X, Song X, et al. Excessive intake of sugar: an accomplice of inflammation. Front Immunol. 2022;13:988481. CrossRef
80. Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr. 2014;100:959–67. CrossRef
81. Clemente-Suárez VJ, Mielgo-Ayuso J, Martín-Rodríguez A, Ramos-Campo DJ, Redondo-Flórez L, Tornero-Aguilera JF. The burden of carbohydrates in health and disease . Nutrients. 2022;14:3809. CrossRef
82. Rezende G, Arthur RA, Lamers ML, Hashizume LN. Structural organization of dental biofilm formed in situ in the presence of sucrose associated to maltodextrin. Braz Dent J. 2019;30:36–42. CrossRef
83. Rezende G, Arthur RA, Grando D, Hashizume LN. Cariogenic potential of sucrose associated with maltodextrin on dental enamel. Caries Res. 2017;51:129–35. CrossRef
84. Bertolini M, Costa RC, Barão VAR, Cunha Villar C, Retamal-Valdes B, Feres M, et al. Oral microorganisms and biofilms: new insights to defeat the main etiologic factor of oral diseases. Microorganisms. 2022;10:2413. CrossRef
85. Skoczek-Rubi?ska A, Bajerska J, Menclewicz K. Effects of fruit and vegetables intake in periodontal diseases: a systematic review. Dent Med Probl. 2018;55:431–9. CrossRef
86. Mazur M, Bietolini S, Bellardini D, Lussi A, Corridore D, Maruotti A, et al. Oral health in a cohort of individuals on a plant-based diet: a pilot study. Clin Ter. 2020;171:142–8. CrossRef
87. Dickinson S, Hancock DP, Petocz P, Ceriello A, Brand-Miller J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean, healthy subjects. Am J Clin Nutr. 2008;87:1188–93. CrossRef
88. Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr. 2006;84(1):70–6. CrossRef
89. Huffman KM, Orenduff MC, Samsa GP, Houmard JA, Kraus WE, Bales CW. Dietary carbohydrate intake and high-sensitivity C-reactive protein in at-risk women and men. Am Heart J. 2007;154:962–8. CrossRef
90. Hujoel P. Dietary carbohydrates and dental-systemic diseases. J Dent Res. 2009;88:490–502. CrossRef
91. Woelber JP, Bremer K, Vach K, König D, Hellwig E, Ratka-Krüger P, et al. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—a randomized controlled pilot study. BMC Oral Health. 2016;17:28. CrossRef
92. Lula EC, Ribeiro CC, Hugo FN, Alves CM, Silva AA. Added sugars and periodontal disease in young adults: an analysis of NHANES III data. Am J Clin Nutr. 2014;100:1182–7. CrossRef
93. van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, Ferreira I, van Greevenbroek MM, van der Kallen CJ, et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr. 2013;98:1533–42. CrossRef
94. Rajaram SS, Nisha S, Ali NM, Shashikumar P, Karmakar S, Pandey V. Influence of a low-carbohydrate and rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fiber diet on clinical outcomes in patients with chronic gingivitis: a randomized controlled trial. J Int Soc Prev Community Dent. 2021;11:58–67. CrossRef
95. Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. CrossRef
96. Mikkola S. Nucleotide sugars in chemistry and biology. Molecules. 2020;6:5755. CrossRef
97. Seeberger PH. The logic of automated glycan assembly. Acc Chem Res. 2015;48:1450–63. CrossRef
98. Hochrein SM, Wu H, Eckstein M, Arrigoni L, Herman JS, Schumacher F, et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell Metab. 2022;34:516–32. CrossRef
99. Mesquita I, Rodrigues F. Cellular metabolism at a glance. Exp Suppl. 2018;109:3–27. CrossRef
100. Gow NAR, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5(3):10–128. CrossRef
101. Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol. 2008;11:266–77. CrossRef
102. Chapot-Chartier MP, Kulakauskas S. Cell wall structure and function in lactic acid bacteria. Microb Cell Factories. 2014;13(Suppl 1):S9. CrossRef
103. Christiaens S, Van Buggenhout S, Houben K, Jamsazzadeh Kermani Z, Moelants KR, Ngouémazong ED, et al. Process-structure-function relations of pectin in food. Crit Rev Food Sci Nutr. 2016;56:1021–42. CrossRef
104. Lankiewicz TS, Choudhary H, Gao Y, Amer B, Lillington SP, Leggieri PA, et al. Lignin deconstruction by anaerobic fungi. Nat Microbiol. 2023;8:596–6. CrossRef
105. Ali N, Zhang Q, Liu ZY, Li FL, Lu M, Fang XC. Emerging technologies for the pretreatment of lignocellulosic materials for bio-based products. Appl Microbiol Biotechnol. 2020;104:455–73. CrossRef
106. Minchin S, Lodge J. Understanding biochemistry: structure and function of nucleic acids. Essays Biochem. 2019;63:433–56. CrossRef
107. Dedon PC. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem Res Toxicol. 2008;21:206–19. CrossRef
108. Krogh N, Nielsen H. Sequencing-based methods for detection and quantitation of ribose methylations in RNA. Methods. 2019;156:5–15. CrossRef
109. Santonocito S, Giudice A, Polizzi A, Troiano G, Merlo EM, Sclafani R, et al. A crosstalk between diet and the oral microbiome: balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients. 2022;14:2426. CrossRef
110. Pang L, Zhi Q, Jian W, Liu Z, Lin H. The oral microbiome impacts the link between sugar consumption and caries: a preliminary study. Nutrients. 2022;14:3693. CrossRef
111. Li Y, Qian F, Cheng X, Wang D, Wang Y, Pan Y, et al. Dysbiosis of oral microbiota and metabolite profiles associated with type 2 diabetes mellitus. Microbiol Spectr. 2023;11:e0379622. CrossRef
112. Angarita-Díaz MDP, Fong C, Bedoya-Correa CM, Cabrera-Arango CL. Does high sugar intake really alter the oral microbiota? A systematic review. Clin Exp Dent Res. 2022;8:1376–90. CrossRef
113. Song Y, Zhang F, Linhardt RJ. Glycosaminoglycans. Adv Exp Med Biol. 2021;1325:103–16. CrossRef
114. Townley RA, Bülow HE. Deciphering functional glycosaminoglycan motifs in development. Curr Opin Struct Biol. 2018;50:144–54. CrossRef
115. Gowd V, Gurukar A, Chilkunda ND. Glycosaminoglycan remodeling during diabetes and the role of dietary factors in their modulation. World J Diabetes. 2016;7:67–73. CrossRef
116. Mah J. Histochemistry of the foetal human temporomandibular joint articular disc. Eur J Orthod. 2004;26:359–65. CrossRef
117. Nakao Y, Konno-Nagasaka M, Toriya N, Arakawa T, Kashio H, Takuma T, et al. Proteoglycan expression is influenced by mechanical load in TMJ discs. J Dent Res. 2015;94:93–100. CrossRef
118. Zhang B, Chi L. Chondroitin sulfate/dermatan sulfate-protein: interactions and their biological functions in human diseases: implications and analytical tools. Front Cell Dev Biol. 2021;9:693563. CrossRef
119. Avram S, Shaposhnikov S, Buiu C, Mernea M. Chondroitin sulfate proteoglycans: structure-function relationship with implication in neural development and brain disorders. Biomed Res Int. 2014;2014:642798. CrossRef
120. Ida-Yonemochi H, Takeuchi K, Ohshima H. Role of chondroitin sulfate in the developmental and healing process of the dental pulp in mice. Cell Tissue Res. 2022;388:133–48. CrossRef
121. Gómez-González A, Rosales-Berber MÁ, De Ávila-Rojas P, Pozos-Guillén A, Garrocho-Rangel A. Pediatric dental management of an uncommon case of mucopolysaccharidosis type IV A (Morquio A Syndrome). A case report of a three-year follow-up. Case Rep Dent. 2020;2020:2565486. CrossRef
122. Ramphul K, Mejias SG, Ramphul-Sicharam Y. Morquio syndrome: a case report. Cureus. 2018;10:2270. CrossRef
123. Colmenares-Bonilla D, Colin-Gonzalez C, Gonzalez-Segoviano A, Esquivel Garcia E, Vela-Huerta MM, Lopez-Gomez FG. Diagnosis of mucopolysaccharidosis based on history and clinical features: evidence from the Bajio Region of Mexico. Cureus. 2018;10:3617. CrossRef
124. Kiem Hao T, Diem Chi NT, Hong Duc NT, Kim Hoa NT. A case study of three patients with mucopolysaccharidoses in Hue Central Hospital. SAGE Open Med Case Rep. 2020;8:2050313–938245. CrossRef
125. Khan S, Alméciga-Díaz CJ, Sawamoto K, Mackenzie WG, Theroux MC, Pizarro C, et al. Mucopolysaccharidosis IVA and glycosaminoglycans. Mol Genet Metab. 2017;120:78–95. CrossRef
126. Çelik B, Tomatsu SC, Tomatsu S, Khan SA. Epidemiology of mucopolysaccharidoses update. Diagnostics (Basel). 2021;11:273. CrossRef
127. Khan SA, Peracha H, Ballhausen D, Wiesbauer A, Rohrbach M, Gautschi M, et al. Epidemiology of mucopolysaccharidoses. Mol Genet Metab. 2017;121:227–40. CrossRef
128. Caterson B, Melrose J. Keratan sulfate is a complex glycosaminoglycan with unique functional capability. Glycobiology. 2018;28:182–206. CrossRef
129. Song Y, Zhang F, Linhardt RJ. Analysis of the glycosaminoglycan chains of proteoglycans. J Histochem Cytochem. 2021;69:121–35. CrossRef
130. Moriguchi M, Yamada M, Yanagisawa T. Immunocytochemistry of keratan sulfate proteoglycan and dermatan sulfate proteoglycan in porcine tooth-germ dentin. Anat Sci Int. 2004;79:145–51. CrossRef
131. Linde A. Glycosaminoglycan turnover and synthesis in the rat incisor pulp. Eur J Oral Sci. 1973;81:145–54. CrossRef
132. Scott JE, Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985;5:71–81. CrossRef
133. Hering TM, Beller JA, Calulot CM, Snow DM. Contributions of chondroitin sulfate, keratan sulfate, and N- linked oligosaccharides to inhibition of neurite outgrowth by aggrecan. Biology (Basel). 2020;9:29. CrossRef
134. Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 2002;22:4611–24. CrossRef
135. Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z, et al. Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci. 2011;31:17091–102. CrossRef
136. Mizumoto S, Yamada S. Congenital disorders of deficiency in glycosaminoglycan biosynthesis. Front Genet. 2021;12:717535. CrossRef
137. Sawamoto K, Álvarez González JV, Piechnik M, Otero FJ, Couce ML, Suzuki Y, et al. Mucopolysaccharidosis IVA: diagnosis, treatment, and management. Int J Mol Sci. 2020;21:1517. CrossRef
138. Funderburgh JL. Keratan sulfate biosynthesis. IUBMB Life. 2002;54:187–94. CrossRef
139. Al-Khateeb R, Olszewska-Czyz I. Biological molecules in dental applications: hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon. 2020;6:03722. CrossRef
140. Faour NH, Dayoub S, Hajeer MY. Evaluation of the hyaluronic acid versus the injectable platelet-rich fibrin in the management of the thin gingival phenotype: a split-mouth randomized controlled clinical trial. Cureus. 2022;14:25104. CrossRef
141. Schmidt J, Pilbauerova N, Soukup T, Suchankova-Kleplova T, Suchanek J. Low molecular weight hyaluronic acid effect on dental pulp stem cells in vitro. Biomolecules. 2020;11:22. CrossRef
142. Prestwich GD, Kuo JW. Chemically modified HA for therapy and regenerative medicine. Curr Pharm Biotechnol. 2008;9:242–5. CrossRef
143. Li A, Sasaki JI, Inubushi T, Abe GL, Nör JE, Yamashiro T, et al. Role of heparan sulfate in vasculogenesis of dental pulp stem cells. J Dent Res. 2023;102:207–16. CrossRef
144. Hayano S, Kurosaka H, Yanagita T, Kalus I, Milz F, Ishihara Y, et al. Roles of heparan sulfate sulfation in dentinogenesis. J Biol Chem. 2012;287:12217–29. CrossRef
145. Duplancic R, Roguljic M, Bozic D, Kero D. Heparan sulfate glycosaminoglycan is predicted to stabilize inflammatory infiltrate formation and RANKL/OPG ratio in severe periodontitis in humans. Bioengineering (Basel). 2022;9:566. CrossRef
146. Gontiya G, Galgali SR. Effect of hyaluronan on periodontitis: a clinical and histological study. J Indian Soc Periodontol. 2012;16:184–92. CrossRef
147. Dahiya P, Kamal R. Hyaluronic acid: a boon in periodontal therapy. N Am J Med Sci. 2013;5:309–15. CrossRef
148. Casale M, Moffa A, Vella P, Sabatino L, Capuano F, Salvinelli B, et al. Hyaluronic acid: perspectives in dentistry. A systematic review. Int J Immunopathol Pharmacol. 2016;29:572–82. CrossRef
149. Bhati A, Fageeh H, Ibraheem W, Fageeh H, Chopra H, Panda S. Role of hyaluronic acid in periodontal therapy (Review). Biomed Rep. 2022;17:91. CrossRef
150. Mohammad CA, Mirza BA, Mahmood ZS, Zardawi FM. The effect of hyaluronic acid gel on periodontal parameters, pro-inflammatory cytokines and biochemical markers in periodontitis patients. Gels. 2023;9:325. CrossRef
151. Miglani A, Vishnani R, Reche A, Buldeo J, Wadher B. Hyaluronic acid: exploring its versatile applications in dentistry. Cureus. 2023;15:46349. CrossRef
152. Waingade M, Medikeri RS, Gaikwad S. Effectiveness of hyaluronic acid in managing oral lichen planus: a systematic review and meta-analysis. J Dent Anesth Pain Med. 2022;22:405–17. CrossRef
153. Hashem AS, Issrani R, Elsayed TEE, Prabhu N. Topical hyaluronic acid in the management of oral lichen planus: a comparative study. J Investig Clin Dent. 2019;10:12385. CrossRef
154. Shetty RR, Burde KN, Guttal KS. The efficacy of topical hyaluronic acid 0.2% in the management of symptomatic oral Lichen planus. J Clin Diagn Res. 2016;10:46–50. CrossRef
155. Sakaue Y, Takenaka S, Ohsumi T, Domon H, Terao Y, Noiri Y. The effect of chlorhexidine on dental calculus formation: an in vitro study. BMC Oral Health. 2018;18:52. CrossRef
156. Hope CK, Wilson M. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother. 2004;48:1461–8. CrossRef
157. Binshabaib M, Aabed K, Alotaibi F, Alwaqid M, Alfraidy A, Alharthi S. Antimicrobial efficacy of 0.8% hyaluronic acid and 0.2% chlorhexidine against Porphyromonas gingivalis strains: an in-vitro study. Pak J Med Sci. 2020;36:111–14. CrossRef
158. Poppolo Deus F, Ouanounou A. Chlorhexidine in dentistry: pharmacology, uses, and adverse effects. Int Dent J. 2022;72:269–77. CrossRef
159. Alharbi MS, Alshehri FA, Alobaidi AS, Alrowis R, Alshibani N, Niazy AA. High molecular weight hyaluronic acid reduces the growth and biofilm formation of the oral pathogen Porphyromonas gingivalis. Saudi Dent J. 2023;35:141–6. CrossRef
160. Alharbi MS, Alshehri FA. High molecular weight hyaluronic acid reduces the expression of virulence genes fimA, mfa1, hagA, rgpA, and kgp in the oral pathogen Porphyromonas gingivalis. Pharmaceutics. 2022;14:1628. CrossRef
161. James P, Worthington HV, Parnell C, Harding M, Lamont T, Cheung A, et al. Chlorhexidine mouth rinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev. 2017;3:008676. CrossRef
162. Boccalari E, Tadakamadla SK, Occhipinti C, Lanteri V, Maspero C. Evaluation of the effectiveness of a novel mouth rinse containing hyaluronic acid and hydrogen peroxide on gingivitis: a randomized pilot controlled trial. Clin Exp Dent Res. 2022;8:673–9. CrossRef
163. Talebi S, Zeraattalab-Motlagh S, Rahimlou M, Naeini F, Ranjbar M, Talebi A, et al. The association between total protein, animal protein, and animal protein sources with risk of inflammatory bowel diseases: a systematic review and meta-analysis of cohort studies. Adv Nutr. 2023;14:752–61. CrossRef
164. Schüler R, Markova M, Osterhoff MA, Arafat A, Pivovarova O, Machann J, et al. Similar dietary regulation of IGF-1- and IGF-binding proteins by animal and plant protein in subjects with type 2 diabetes. Eur J Nutr. 2021;60:3499–504. CrossRef
165. Lee DH, Tabung FK, Giovannucci EL. Association of animal and plant protein intakes with biomarkers of insulin and insulin-like growth factor axis. Clin Nutr. 2022;41:1272–80. CrossRef
166. Song M, Fung TT, Hu FB, Willett WC, Longo VD, Chan AT, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176:1453–63. CrossRef
167. Mariotti F. Animal and plant protein sources and cardiometabolic health. Adv Nutr. 2019;10:351–66. CrossRef
168. Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. Biomed Res Int. 2015;2015:538019. CrossRef
169. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol. 2019;9:1399. CrossRef
170. Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25. CrossRef
171. Bowers LW, Rossi EL, O’Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol (Lausanne). 2015;6:77. CrossRef
172. Ikeda T, Waldbillig RJ, Puro DG. Truncation of IGF-I yields two mitogens for retinal Müller glial cells. Brain Res. 1995;686:87–92. CrossRef
173. Oosterwijk MM, Soedamah-Muthu SS, Geleijnse JM, Bakker SJL, Navis G, Binnenmars SH, et al. High dietary intake of vegetable protein is associated with lower prevalence of renal function impairment: results of the dutch DIALECT-1 cohort. Kidney Int Rep. 2019;4:710–9. CrossRef
174. Oosterwijk MM, Groothof D, Navis G, Bakker SJL, Laverman GD. High-normal protein intake is not associated with faster renal function deterioration in patients with type 2 diabetes: a prospective analysis in the DIALECT cohort. Diabetes Care. 2022;45:35–41. CrossRef
175. Jhee JH, Kee YK, Park JT, Chang TI, Kang EW, Yoo TH, et al. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. 2019;74:491–500. CrossRef
176. Staufenbiel I, Weinspach K, Förster G, Geurtsen W, Günay H. Periodontal conditions in vegetarians: a clinical study. Eur J Clin Nutr. 2013;67:836–40. CrossRef
177. Eberhard J, Ruiz K, Tan J, Jayasinghe TN, Khan S, Eroglu E, et al. A randomized clinical trial to investigate the effect of dietary protein sources on periodontal health. J Clin Periodontol. 2022;49(4):388–400. CrossRef
178. Né YGS, Martins BV, Castro MML, Alvarenga MOP, Fagundes NCF, Magno MB, et al. Is nutritional intervention an improvement factor in the management of periodontitis? A systematic review. Clin Nutr. 2020;39:2639–46. CrossRef
179. Harasym J, Oledzki R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30:511–7. CrossRef
180. Salazar CR, Laniado N, Mossavar-Rahmani Y, Borrell LN, Qi Q, Sotres-Alvarez D, et al. Better-quality diet is associated with lower odds of severe periodontitis in US Hispanics/Latinos. J Clin Periodontol. 2018;45:780–90. CrossRef
181. DeMayo F, Molinsky R, Tahir MJ, Roy S, Genkinger JM, Papapanou PN, et al. Diet quality and periodontal disease: results from the oral infections, glucose intolerance and insulin resistance study (ORIGINS). J Clin Periodontol. 2021;48:638–47. CrossRef
182. Safrhansova L, Hlozkova K, Starkova J. Targeting amino acid metabolism in cancer. Int Rev Cell Mol Biol. 2022;373:37–79. CrossRef
183. Institute of Medicine (US) Committee on Military Nutrition Research. The role of protein and amino acids in sustaining and enhancing performance. Washington, DC: National Academies Press (US); 1999 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK224623/; doi: https://doi.org/10.17226/9620
184. LaPelusa A, Kaushik R. Physiology, proteins [Updated 2022 Nov 14]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555990/
185. Rehman I, Farooq M, Botelho S. Biochemistry, secondary protein structure [Updated 2022 Dec 11]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470235/
186. Rehman I, Kerndt CC, Botelho S. Biochemistry, tertiary protein structure [Updated 2022 Sep 12]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470269/
187. Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–92. CrossRef
188. Sweeney HL, Hammers DW. Muscle contraction. Cold Spring Harb Perspect Biol. 2018;10:023200. CrossRef
189. Prasad SV, Fiedoruk K, Daniluk T, Piktel E, Bucki R. Expression and function of host defense peptides at inflammation sites. Int J Mol Sci. 2019;21:104. CrossRef
190. Kalló G, Kumar A, T?zsér J, Cs?sz É. Chemical barrier proteins in human body fluids. Biomedicines. 2022;10:1472. CrossRef
191. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000;64:57–80. CrossRef
192. Vitkov L, Singh J, Schauer C, Minnich B, Kruni? J, Oberthaler H, et al. Breaking the gingival barrier in periodontitis. Int J Mol Sci. 2023;24(5):4544. CrossRef
193. Fujita T, Yoshimoto T, Kajiya M, Ouhara K, Matsuda S, Takemura T, et al. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn Dent Sci Rev. 2018 54:66–75. CrossRef
194. Yamamoto M, Aizawa R. Maintaining a protective state for human periodontal tissue. Periodontol 2000. 2021;86(1):142–56. CrossRef
195. Takahashi N, Sulijaya B, Yamada-Hara M, Tsuzuno T, Tabeta K, Yamazaki K. Gingival epithelial barrier: regulation by beneficial and harmful microbes. Tissue Barriers. 2019;7:1651158. CrossRef
196. Scardina GA, Messina P. Good oral health and diet. J Biomed Biotechnol. 2012;2012:720692. CrossRef
197. Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(1):29–35. CrossRef
198. Zhu L, Zhou C, Chen S, Huang D, Jiang Y, Lan Y, et al. Osteoporosis and alveolar bone health in periodontitis niche: a predisposing factors-centered review. Cells. 2022;11:3380. CrossRef
199. Yu B, Wang CY. Osteoporosis and periodontal diseases—an update on their association and mechanistic links. Periodontol. 2000;89:99–113. CrossRef
200. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. CrossRef
201. Cho YD, Kim KH, Lee YM, Ku Y, Seol YJ. Periodontal wound healing and tissue regeneration: a narrative review. Pharmaceuticals (Basel). 2021;14(5):456. CrossRef
202. Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalized medicine. EPMA J. 2017;8:23–33. CrossRef
203. Grant M, Kilsgård O, Åkerman S, Klinge B, Demmer RT, Malmström J, et al. The human salivary antimicrobial peptide profile according to the oral microbiota in health, periodontitis, and smoking. J Innate Immun. 2019;11:432–44. CrossRef
204. Khurshid Z, Naseem M, Sheikh Z, Najeeb S, Shahab S, Zafar MS. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24:515–24. CrossRef
205. Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23:122–8. CrossRef
206. Dennesen P, van der Ven A, Vlasveld M, Lokker L, Ramsay G, Kessels A, et al. Inadequate salivary flow and poor oral mucosal status in intubated intensive care unit patients. Crit Care Med. 2003;31:781–6. CrossRef
207. Gupta A, Gupta A, Singh TK, Saxsena A. Role of oral care to prevent VAP in mechanically ventilated intensive care unit patients. Saudi J Anaesth. 2016;10:95–7. CrossRef
208. Winning L, Lundy FT, Blackwood B, McAuley DF, El Karim I. Oral health care for the critically ill: a narrative review. Crit Care. 2021;25:353. CrossRef
209. Takahama A Jr, de Sousa VI, Tanaka EE, Ono E, Ito FAN, Costa PP, et al. Analysis of oral risk factors for ventilator-associated pneumonia in critically ill patients. Clin Oral Investig. 2021;25:1217–22. CrossRef
210. Dodington DW, Young HE, Beaudette JR, Fritz PC, Ward WE. Improved healing after non-surgical periodontal therapy is associated with higher protein intake in patients who are non-smokers. Nutrients. 2021;13:3722. CrossRef
211. Adegboye AR, Christensen LB, Holm-Pedersen P, Avlund K, Boucher BJ, Heitmann BL. Intake of dairy products concerning periodontitis in older Danish adults. Nutrients. 2012;4:1219–29. CrossRef
212. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. Sci World J. 2020;2020:2146160. CrossRef
213. Clark D, Kotronia E, Ramsay SE. Frailty, aging, and periodontal disease: basic biologic considerations. Periodontol 2000. 2021;87(1):143–56. CrossRef
214. Kazemi S, Savabi G, Khazaei S, Savabi O, Esmaillzadeh A, Keshteli AH, et al. Association between food intake and oral health in elderly: SEPAHAN systematic review no. 8. Dent Res J (Isfahan). 2011 [cited 2023 Dec 26];8:15–20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3556278/pdf/DRJ-8-15.pdf
215. Jeon YG, Kim YY, Lee G, Kim JB. Physiological and pathological roles of lipogenesis. Nat Metab. 2023;5:735–59. CrossRef
216. Ahmed S, Shah P, Ahmed O. Biochemistry, lipids [Updated 2023 May 1]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525952/
217. Institute of Medicine (US) Committee on Military Nutrition Research; Marriott BM, editor. Food components to enhance performance: an evaluation of potential performance-enhancing food components for operational rations. Washington, DC: National Academies Press (US); 1994 [cited 2023 Dec 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK209056/; doi: https://doi.org/10.17226/4563
218. Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. CrossRef
219. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–8. CrossRef
220. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–70. CrossRef
221. Oteng AB, Kersten S. Mechanisms of action of trans-fatty acids. Adv Nutr. 2020;11:697–708. CrossRef
222. Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health, and disease—a review. Part 1: classification, dietary sources, and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:117–30. CrossRef
223. Kremmyda LS, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, health, and disease: a review. Part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:195–218. CrossRef
224. Boateng L, Ansong R, Owusu WB, Steiner-Asiedu M. Coconut oil and palm oil’s role in nutrition, health and national development: a review. Ghana Med J. 2016 [cited 2023 Dec 26];50:189–96. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5044790/pdf/GMJ5003-0189.pdf
225. Unhapipatpong C, Shantavasinkul PC, Kasemsup V, Siriyotha S, Warodomwichit D, Maneesuwannarat S, et al. Tropical oil consumption and cardiovascular disease: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2021;13:1549. CrossRef
226. DiNicolantonio JJ, O’Keefe JH. Good fats versus bad fats: a comparison of fatty acids in the promotion of insulin resistance, inflammation, and obesity. Mo Med. 2017 [cited 2023 Dec 26];114:303–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6140086/pdf/ms114_p0303.pdf
227. Martí Del Moral A, Calvo C, Martínez A. Consumo de alimentos ultraprocesados y obesidad: una revisión sistemática [Ultra-processed: food consumption and obesity-a systematic review]. Nutr Hosp. 2021;38:177–85. CrossRef
228. Martini D, Godos J, Bonaccio M, Vitaglione P, Grosso G. Ultra-processed foods and nutritional dietary profile: a meta-analysis of nationally representative samples. Nutrients. 2021;13:3390. CrossRef
229. Nagpal T, Sahu JK, Khare SK, Bashir K, Jan K. Trans fatty acids in food: a review on dietary intake, health impact, regulations and alternatives. J Food Sci. 2021;86:5159–74. CrossRef
230. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:3978. CrossRef
231. Mattei J, Sotres-Alvarez D, Daviglus ML, Gallo LC, Gellman M, Hu FB, et al. Diet quality and its association with cardiometabolic risk factors vary by hispanic and latino ethnic background in the hispanic community health study/study of latinos. J Nutr. 2016;146:2035–44. CrossRef
232. Islam MA, Amin MN, Siddiqui SA, Hossain MP, Sultana F, Kabir MR. Trans fatty acids and lipid profile: a serious risk factor to cardiovascular disease, cancer, and diabetes. Diabetes Metab Syndr. 2019;13:1643–7. CrossRef
233. Iwasaki M, Manz MC, Moynihan P, Yoshihara A, Muramatsu K, Watanabe R, et al. Relationship between saturated fatty acids and periodontal disease. J Dent Res. 2011;90:861–7. CrossRef
234. Canaan JCDR, Canaan MM, Costa PD, Pereira MA, Castelo PM, Pardi V, et al. Food preferences and periodontal status of adults assisted by a public health care system. PLoS One. 2023;18:0291878. CrossRef
235. Kruse AB, Gärtner M, Vach K, Grueninger D, Peikert SA, Ratka-Krüger P, et al. An exploratory study on the role of serum fatty acids in the short-term dietary therapy of gingivitis. Sci Rep. 2022;12:4022. CrossRef
236. O’Connor JP, Milledge KL, O’Leary F, Cumming R, Eberhard J, Hirani V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: a systematic literature review. Nutr Rev. 2020;78:175–88. CrossRef
237. Mohsen G, Stroemer A, Mayr A, Kunsorg A, Stoppe C, Wittmann M, et al. Effects of omega-3 fatty acids on postoperative inflammatory response: a systematic review and meta-analysis. Nutrients. 2023;15:3414. CrossRef
238. Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. 2019;9:18867. CrossRef
239. Albar SA. Dietary omega-6/omega-3 polyunsaturated fatty acid (PUFA) and omega-3 are associated with general and abdominal obesity in adults: UK National Diet and Nutritional Survey. Cureus. 2022;14:30209. CrossRef
240. Ramasamy S, Jain S, Kori R, Atri S, Singh CB. Role of omega-3 fatty acid infusion in surgical outcomes of perforation peritonitis patients: a randomized controlled trial. Cureus. 2022;14:23950. CrossRef
241. Lieske B, Moszka N, Borof K, Petersen EL, Jagemann B, Ebinghaus M, et al. Association between an anti-inflammatory dietary score and periodontitis-evidence from the population-based Hamburg city health study. Nutrients. 2023;15:3235. CrossRef
242. Elkhouli AM. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study). J Periodontal Res. 2011;46:261–8. CrossRef
243. Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. CrossRef
244. Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. CrossRef
245. Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and pro-resolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. CrossRef
246. Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9. CrossRef
247. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–15. CrossRef
248. Bosma-den Boer MM, van Wetten ML, Pruimboom L. Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels, and medication prevent our body from recovering. Nutr Metab (Lond). 2012;9:32. CrossRef
249. Serhan CN, Chiang N. Novel endogenous small molecules as the checkpoint controllers in inflammation and resolution: entrée for resoleomics. Rheum Dis Clin North Am. 2004;30:69–95. CrossRef
250. Kruse AB, Kowalski CD, Leuthold S, Vach K, Ratka-Krüger P, Woelber JP. What is the impact of the adjunctive use of omega-3 fatty acids in the treatment of periodontitis? A systematic review and meta-analysis. Lipids Health Dis. 2020;19:100. CrossRef
251. Saleh MHA, Decker A, Tattan M, Tattan O, Decker J, Alrmali A, et al. Supplement consumption and periodontal health: an exploratory survey using the bigMouth repository. Medicina (Kaunas). 2023;59:919. CrossRef
252. Sta?do-Retecka M, Piatek P, Namiecinska M, Bonikowski R, Lewkowicz P, Lewkowicz N. Clinical and microbiological outcomes of subgingival instrumentation supplemented with high-dose omega-3 polyunsaturated fatty acids in periodontal treatment–a randomized clinical trial. BMC Oral Health. 2023;23:290. CrossRef
253. Sta?do M, Piatek P, Namiecinska M, Lewkowicz P, Lewkowicz N. Omega-3 polyunsaturated fatty acids EPA, and DHA as an adjunct to non-surgical treatment of periodontitis: a randomized clinical trial. Nutrients. 2020;12:2614. CrossRef
254. Kujur SK, Goswami V, Nikunj AM, Singh G, Bandhe S, Ghritlahre H. Efficacy of omega 3 fatty acid as an adjunct in the management of chronic periodontitis: a randomized controlled trial. Indian J Dent Res. 2020;31:229–35. CrossRef
255. Woelber JP, Gärtner M, Breuninger L, Anderson A, König D, Hellwig E, et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J Clin Periodontol. 2019;46(4):481–90. CrossRef
256. Bartha V, Exner L, Basrai M, Bischoff SC, Schweikert D, Adolph M, et al. Changes in serum omega fatty acids on a Mediterranean diet intervention in patients with gingivitis: an exploratory study. J Periodontal Res. 2022;57(6):1198–209. CrossRef
257. Bartha V, Exner L, Schweikert D, Woelber JP, Vach K, Meyer AL, et al. Effect of the Mediterranean diet on gingivitis: a randomized controlled trial. J Clin Periodontol. 2022;49(2):111–22. CrossRef
258. Persson GR, Widén C, Wohlfart B, Sjöberg K, Steen S, Coleman M, et al. Impact of an Okinawa/Nordic based diet on endocrinological and periodontal conditions in individuals with type 2 diabetes. A randomized case-control study. BMC Oral Health. 2023;23(1):544. CrossRef
259. Rakyoo K, Vichayanrat T, Anunmana C, Kriengsinyos W, Gaewkhiew P. Effect of dentures and dietary advice on protein intake in older Thai adults with missing posterior occluding teeth. Gerodontology. 2023;40(3):382–9. CrossRef
260. Doke M, Komagamine Y, Kanazawa M, Iwaki M, Suzuki H, Miyazaki Y, et al. Effect of dental intervention on improvements in metabolic syndrome patients: a randomized controlled clinical trial. BMC Oral Health. 2021;21(1):4. CrossRef
261. Sadiq IZ. Lifestyle medicine as a modality for prevention and management of chronic diseases. J Taibah Univ Med Sci. 2023;18:1115–7. CrossRef
262. Alshammari SA, AlDhayan AZ, Saad Al-Essa OM, Alosaimi MM, Al-Badr BM, Ali AB, et al. Challenges to lifestyle modification of chronic disease patients attending primary health care centers in Riyadh. J Fam Med Prim Care. 2020;9:6186–93. CrossRef
263. Santos L. The impact of nutrition and lifestyle modification on health. Eur J Intern Med. 2022;97:18–25. CrossRef
264. Huston P. A sedentary and unhealthy lifestyle fuels chronic disease progression by changing interstitial cell behavior: a network analysis. Front Physiol. 2022;13:904107. CrossRef
265. Nyberg ST, Singh-Manoux A, Pentti J, Madsen IEH, Sabia S, Alfredsson L, et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern Med. 2020;180:760–8. CrossRef
266. Heidelbaugh JJ. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther Adv Drug Saf. 2013;4:125–33. CrossRef
267. Shobha. Antibiotics and nutritional implications- the drugs-nutrients interactions. Acta Sci Nutr Health. 2019 [cited 2023 Dec 5];3:51–4. Available from: https://actascientific.com/ASNH/pdf/ASNH-03-0285.pdf
268. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. CrossRef
269. Muscaritoli M. The impact of nutrients on mental health and well-being: insights from the literature. Front Nutr. 2021;8:656290. CrossRef
270. Chan LN. Drug-nutrient interactions. JPEN J Parenter Enteral Nutr. 2013;37:450–9. CrossRef
271. Marshall NE, Abrams B, Barbour LA, Catalano P, Christian P, Friedman JE, et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am J Obstet Gynecol. 2022;226:607–32. CrossRef
272. Kominiarek MA, Rajan P. Nutrition recommendations in pregnancy and lactation. Med Clin North Am. 2016;100:1199–215. CrossRef
273. Stevens SL. Fat-soluble vitamins. Nurs Clin North Am. 2021;56:33–45. CrossRef
274. Chawla J, Kvarnberg D. Hydrosoluble vitamins. Handb Clin Neurol. 2014;120:891–914. CrossRef
275. National Research Council (US) Committee on Diet and Health. Diet and health: implications for reducing chronic disease risk. Washington, DC: National Academies Press (US); 1989 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK218743/; doi: https://doi.org/10.17226/1222
276. Degrassi I, Leonardi I, Di Profio E, Montanari C, Zuccotti G, Verduci E. Fat-soluble vitamins deficiency in pediatric cholestasis: a scoping review. Nutrients. 2023;26:2491. CrossRef
277. Shibata K, Sugita C, Sano M, Fukuwatari T. Urinary excretion of B-group vitamins reflects the nutritional status of B-group vitamins in rats. J Nutr Sci. 2013;2:12. CrossRef
278. McCullough FS, Northrop-Clewes CA, Thurnham DI. The effect of vitamin A on epithelial integrity. Proc Nutr Soc. 1999;58:289–93. CrossRef
279. Bar-El Dadon S, Reifen R. Vitamin A and the epigenome. Crit Rev Food Sci Nutr. 2017;57:2404–11. CrossRef
280. DiKun KM, Gudas LJ. Vitamin A and retinoid signaling in the kidneys. Pharmacol Ther. 2023;248:108481. CrossRef
281. Zile MH. Vitamin A and embryonic development: an overview. J Nutr. 1998;128:455–8. CrossRef
282. Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. CrossRef
283. Huang Z, Liu Y, Qi G, Brand D, Zheng SG. Role of vitamin A in the immune system. J Clin Med. 2018;7:258. CrossRef
284. De Luca L, Maestri N, Bonanni F, Nelson D. Maintenance of epithelial cell differentiation: the mode of action of vitamin A. Cancer. 1972;30:5–1326. CrossRef
285. Yee MMF, Chin KY, Ima-Nirwana S, Wong SK. Vitamin A and bone health: a review on current evidence. Molecules. 2021;26:1757. CrossRef
286. Khojah Q, AlRumaihi S, AlRajeh G, Aburas A, AlOthman A, Ferwana M. Vitamin A and its derivatives effect on bone mineral density, a systematic review. J Fam Med Prim Care. 2021;10:4089–95. CrossRef
287. Schifferle RE. Periodontal disease and nutrition: separating the evidence from current fads. Periodontol 2000. 2009;50:78–89. CrossRef
288. de Menezes AC, Costa IM, El-Guindy MM. Clinical manifestations of hypervitaminosis A in human gingiva. A case report. J Periodontol. 1984;55:474–6. CrossRef
289. Borgan SM, Khan LZ, Makin V. Hypercalcemia and vitamin A: a vitamin to keep in mind. Cleve Clin J Med. 2022;89:99–105. CrossRef
290. LiverTox. Clinical and research information on drug-induced liver injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Vitamin A. [Updated 2020 Nov 4]. 2020 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548165/
291. Staud R. Vitamin D: more than just affecting calcium and bone. Curr Rheumatol Rep. 2005;7:356–64. CrossRef
292. Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–7. CrossRef
293. Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4:16041. CrossRef
294. Gil Á, Plaza-Diaz J, Mesa MD. Vitamin D: classic and novel actions. Ann Nutr Metab. 2018;72:87–95. CrossRef
295. Carmeliet G, Dermauw V, Bouillon R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Pract Res Clin Endocrinol Metab. 2015;29:621–31. CrossRef
296. Anderson PH, Turner AG, Morris HA. Vitamin D actions to regulate calcium and skeletal homeostasis. Clin Biochem. 2012;45:880–6. CrossRef
297. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347:25–9. CrossRef
298. Sahay M, Sahay R. Rickets-vitamin D deficiency and dependency. Indian J Endocrinol Metab. 2012;16:164–76. CrossRef
299. Cianferotti L. Osteomalacia is not a single disease. Int J Mol Sci. 2022;23:14896. CrossRef
300. Pellegrino L, Marangoni F, Muscogiuri G, D’Incecco P, Duval GT, Annweiler C, et al. Vitamin D fortification of consumption cow’s milk: health, nutritional and technological aspects. A multidisciplinary lecture of the recent scientific evidence. Molecules. 2021;26:5289. CrossRef
301. Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–80. CrossRef
302. Feng Y, Yang DS, Tang HB, Ding YS, Li XG. Effectiveness of vitamin D for adult patients with gingivitis. Medicine (Baltimore). 2020;99:18338. CrossRef
303. Meghil MM, Cutler CW. Influence of vitamin D on periodontal inflammation: a review. Pathogens. 2023;12:1180. CrossRef
304. Wei FF, Trenson S, Verhamme P, Vermeer C, Staessen JA. Vitamin K-dependent matrix gla protein as multifaceted protector of vascular and tissue integrity. Hypertension. 2019;73:1160–9. CrossRef
305. Shearer MJ. Role of vitamin K and gla proteins in the pathophysiology of osteoporosis and vascular calcification. Curr Opin Clin Nutr Metab Care. 2000;3:433–8. CrossRef
306. Smajdor J, Jedli?ska K, Porada R, Górska-Ratusznik A, Policht A, ?róttek M, et al. The impact of gut bacteria producing long chain homologs of vitamin K2 on colorectal carcinogenesis. Cancer Cell Int. 2023;23:268. CrossRef
307. Rodríguez-Olleros Rodríguez C, Díaz Curiel M. Vitamin K and bone health: a review on the effects of vitamin K deficiency and supplementation and the effect of non-vitamin K antagonist oral anticoagulants on different bone parameters. J Osteoporos. 2019;2019:2069176. CrossRef
308. Elshaikh AO, Shah L, Joy Mathew C, Lee R, Jose MT, Cancarevic I. Influence of vitamin K on bone mineral density and osteoporosis. Cureus. 2020;12:10816. CrossRef
309. Chuai Y, Dai B, Liu X, Hu M, Wang Y, Zhang H. Association of vitamin K, fiber intake and progression of periodontal attachment loss in American adults. BMC Oral Health. 2023;23:303. CrossRef
310. Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28:314–28. CrossRef
311. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–18. CrossRef
312. Johnston CS, Martin LJ, Cai X. Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis. J Am Coll Nutr. 1992;11:172–6. CrossRef
313. Yan Y, Zeng W, Song S, Zhang F, He W, Liang W, et al. Vitamin C induces periodontal ligament progenitor cell differentiation via activation of ERK pathway mediated by PELP1. Protein Cell. 2013;4:620–7. CrossRef
314. Tada A, Miura H. The relationship between vitamin C and periodontal diseases: a systematic review. Int J Environ Res Public Health. 2019;16:2472. CrossRef
315. Siegel C, Barker B, Kunstadter M. Conditioned oral scurvy due to megavitamin C withdrawal. J Periodontol. 1982;53:453–5. CrossRef
316. Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician. 2008 [cited 2023 Dec 25];54:1403–6. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2567249/pdf/0541403.pdf
317. National Institutes of Health. Office of the Dietary Supplements. Vitamin C. Fact sheet for consumers [Internet]. 2021 [cited 2023 Dec 8]. Available from: https://ods.od.nih.gov/factsheets/VitaminC-Consumer/
318. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3–21. CrossRef
319. Alessa M, Alarfaj MO, Albenayyan HA, Aleidan AA, Albahrani FA, Bokhuwah MA, et al. Awareness of the link between the consumption of processed food and colorectal cancer risk in Saudi Arabia. Cureus. 2023;15:33774. CrossRef
320. Falcão RCTMA, Lyra CO, Morais CMM, Pinheiro LGB, Pedrosa LFC, Lima SCVC, et al. Processed and ultra-processed foods are associated with high prevalence of inadequate selenium intake and low prevalence of vitamin B1 and zinc inadequacy in adolescents from public schools in an urban area of northeastern Brazil. PLoS One. 2019;14:0224984. CrossRef
321. Lonsdale D. A review of the biochemistry, metabolism, and clinical benefits of thiamin(e) and its derivatives. Evid Based Complement Alternat Med. 2006;3:49–59. CrossRef
322. Whitfield KC, Bourassa MW, Adamolekun B, Bergeron G, Bettendorff L, Brown KH, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann NY Acad Sci. 2018;430(1):3–43. CrossRef
323. Walker C, Ryu S, Giannone RJ, Garcia S, Trinh CT. Understanding and eliminating the detrimental effect of thiamine deficiency on the oleaginous yeast Yarrowia lipolytica. Appl Environ Microbiol. 2020;86:02299–19. CrossRef
324. Shible AA, Ramadurai D, Gergen D, Reynolds PM. Dry beriberi due to thiamine: deficiency associated with peripheral neuropathy and Wernicke’s encephalopathy mimicking guillain-barré syndrome: a case report and review of the literature. Am J Case Rep. 2019;20:330–4. CrossRef
325. Lei Y, Zheng MH, Huang W, Zhang J, Lu Y. Wet beriberi with multiple organ failure remarkably reversed by thiamine administration: a case report and literature review. Medicine (Baltimore). 2018;97(9):e0010. CrossRef
326. Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, Mynak ML, et al. Thiamine deficiency disorders: a clinical perspective. Ann N Y Acad Sci. 2021;1498(1):9–28. CrossRef
327. Tan ML, Willis CG. Beriberi: a reversible cause of acute severe pulmonary hypertension. Cureus. 2022;14:27376. CrossRef
328. Kaur K, Sculley D, Wallace J, Turner A, Ferraris C, Veysey M, et al. Micronutrients and bioactive compounds in oral inflammatory diseases. J Nutr Intermed Metab. 2019;18:100105. CrossRef
329. Viljoen M, Bipath P, Tosh C. Pellagra in South Africa from 1897 to 2019: a scoping review. Public Health Nutr. 2021;24:2062–76. CrossRef
330. Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1–5. CrossRef
331. Park YK, Sempos CT, Barton CN, Vanderveen JE, Yetley EA. Effectiveness of food fortification in the United States: the case of pellagra. Am J Public Health. 2000;90:727–38. CrossRef
332. Terakata M, Fukuwatari T, Kadota E, Sano M, Kanai M, Nakamura T, et al. The niacin required for optimum growth can be synthesized from L-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J Nutr. 2013;143:1046–51. CrossRef
333. Afzal M, Kuipers OP, Shafeeq S. Niacin-mediated gene expression and role of NiaR as a transcriptional repressor of niaX, nadC, and pnuC in Streptococcus pneumoniae. Front Cell Infect Microbiol. 2017;7:70. CrossRef
334. Lohani M, Dhasmana A, Haque S, Dar SA, Jawed A, Wahid M, et al. Niacin deficiency modulates genes involved in cancer: are smokers at higher risk? J Cell Biochem. 2019;120:232–42. CrossRef
335. Gasperi V, Sibilano M, Savini I, Catani MV. Niacin in the central nervous system: an update of biological aspects and clinical applications. Int J Mol Sci. 2019;20:974. CrossRef
336. Surjana D, Halliday GM, Damian DL. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J Nucleic Acids. 2010;2010:157591. CrossRef
337. Peechakara BV, Gupta M. Vitamin B3 [Updated 2022 Jun 11]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526107/
338. Zeman M, Vecka M, Perlík F, Hromádka R, Sta?ková B, Tvrzická E, et al. Niacin in the treatment of hyperlipidemias in light of new clinical trials: has niacin lost its place? Med Sci Monit. 2015;21:2156–62. CrossRef
339. Leung K, Quezada M, Chen Z, Kanel G, Kaplowitz N. Niacin-induced anicteric microvesicular steatotic acute liver failure. Hepatol Commun. 2018;2:1293–8. CrossRef
340. Abosamak NER, Gupta V. Vitamin B6 (Pyridoxine) [Updated 2023 Aug 17]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557436/
341. Parra M, Stahl S, Hellmann H. Vitamin B6 and its role in cell metabolism and physiology. Cells. 2018;22:84. CrossRef
342. Ryan-Harshman M, Aldoori W. Carpal tunnel syndrome and vitamin B6. Can Fam Physician. 2007;53:1161–2. Available from: https://www.aafp.org/pubs/afp/issues/2016/1215/p993.pdf
343. Bacharach R, Lowden M, Ahmed A. Pyridoxine toxicity small fiber neuropathy with dysautonomia: a case report. J Clin Neuromuscul Dis. 2017;19:43–6. CrossRef
344. Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011;4:52–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3218540/pdf/RIOG004002_0052.pdf
345. Maher A, Sobczy?ska-Malefora A. The relationship between folate, vitamin B12, and gestational diabetes mellitus with proposed mechanisms and foetal implications. J Fam Reprod Health. 2021;15:141–9. CrossRef
346. Huennekens FM, DiGirolamo PM, Fujii K, Henderson GB, Jacobsen DW, Neef VG, et al. Folic acid and vitamin B12: transport and conversion to coenzyme forms. Adv Enzyme Regul. 1974;12:131–53. CrossRef
347. Perry J, Lumb M, Laundy M, Reynolds EH, Chanarin I. Role of vitamin B12 in folate coenzyme synthesis. Br J Haematol. 1976;32:243–8. CrossRef
348. Sayar EH, Orhaner BB, Sayar E, NesrinTuran F, Küçük M. The frequency of vitamin B12, iron, and folic acid deficiency in the neonatal period and infancy and the relationship with maternal levels. Turk Pediatri Ars. 2020;55:139–48. CrossRef
349. Yu YH, Kuo HK, Lai YL. The association between serum folate levels and periodontal disease in older adults: data from the National Health and Nutrition Examination Survey 2001/02. J Am Geriatr Soc. 2007;55:108–13. CrossRef
350. Alkan D, Guven B, Turer CC, Balli U, Can M. Folate-receptor 1 level in periodontal disease: a pilot study. BMC Oral Health. 2019;19:218. CrossRef
351. Esaki M, Morita M, Akhter R, Akino K, Honda O. Relationship between folic acid intake and gingival health in non-smoking adults in Japan. Oral Dis. 2010;16:96–101. CrossRef
352. Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front Nutr. 2019;6:93. CrossRef
353. Niklewicz A, Smith AD, Smith A, Holzer A, Klein A, McCaddon A, et al. The importance of vitamin B12 for individuals choosing plant-based diets. Eur J Nutr. 2023;62:1551–9. CrossRef
354. Rizzo G, Laganà AS, Rapisarda AM, La Ferrera GM, Buscema M, Rossetti P, et al. Vitamin B12 among vegetarians: status, assessment, and supplementation. Nutrients. 2016;8:767. CrossRef
355. Herbert V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am J Clin Nutr. 1994;59:1213–22. CrossRef
356. Oo TH. Diagnostic difficulties in pernicious anemia. Discov Med. 2019 [cited 2023 Dec 25];28:247–53. Available from: https://www.discoverymedicine.com/Thein-H-Oo/2019/12/diagnostic-difficulties-in-pernicious-anemia/
357. Htut TW, Thein KZ, Oo TH. Pernicious anemia: pathophysiology and diagnostic difficulties. J Evid Based Med. 2021;14:161–9. CrossRef
358. Yocum AD, Patel M, Palocko B, Simon EL. Primary neurologic symptoms: have you considered pernicious anemia? J Emerg Med. 2023;64:217–9. CrossRef
359. Ramseier CA, Woelber JP, Kitzmann J, Detzen L, Carra MC, Bouchard P. Impact of risk factor control interventions for smoking cessation and promoting healthy lifestyles in patients with periodontitis: a systematic review. J Clin Periodontol. 2020 Jul;47 Suppl 22:90–106. CrossRef
360. 39.Choowong P, Wali JA, Nguyen ATM, Jayasinghe TN, Eberhard J. Macronutrient-induced modulation of periodontitis in rodents-a systematic review. Nutr Rev. 2022;80(5):1160–78. CrossRef
361. 40.Kusama T, Nakazawa N, Takeuchi K, Kiuchi S, Osaka K. Free sugar intake and periodontal diseases: a systematic review. Nutrients. 2022;14(21):4444. CrossRef
362. 41.Swarnamali H, Medara N, Chopra A, Spahr A, Jayasinghe TN. Role of dietary fibre in managing periodontal diseases-a systematic review and meta-analysis of human intervention studies. Nutrients. 2023;15(18):4034. CrossRef
363. 42.Samborska-Mazur J, Sikorska D, Wyganowska-?wi?tkowska M. The relationship between periodontal status and rheumatoid arthritis—systematic review. Reumatologia. 2020;58(4):236–42. CrossRef
364. 43.Jeong J, Kim HS, Lee D, Kim K, Kim YH. Association between four dietary patterns and the risk of periodontal diseases: a systematic review and meta-analysis. Nutrients. 2022;14(20):4362. CrossRef
365. 44.Halvorsrud K, Lewney J, Craig D, Moynihan PJ. Effects of starch on oral health: systematic review to inform WHO guideline. J Dent Res. 2019;98(1):46–53. CrossRef
366. 45.Mainas G, Santamaria P, Ide M, Longo V, Vinciguerra M, Nart J, et al. Could dietary restrictions affect periodontal disease? A systematic review. Clin Oral Investig. 2023;27(8):4107–16. CrossRef
367. 46.Smits KPJ, Listl S, Jevdjevic M. Vegetarian diet and its possible influence on dental health: a systematic literature review. Community Dent Oral Epidemiol. 2020;48(1):7–13. CrossRef
368. 47.Woelber JP, Reichenbächer K, Groß T, Vach K, Ratka-Krüger P, Bartha V. Dietary and nutraceutical interventions as an adjunct to non-surgical periodontal therapy-a systematic review. Nutrients. 2023;15(6):1538. CrossRef
369. Khayat S, Fanaei H, Ghanbarzehi A. Minerals in pregnancy and lactation: a review article. J Clin Diagn Res. 2017;11:01–5. CrossRef
370. King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71:1218–25. CrossRef
371. Volpe SL. Magnesium and the athlete. Curr Sports Med Rep. 2015;14:279–83. CrossRef
372. Prot-Bertoye C, Lievre L, Houillier P. The importance of kidney calcium handling in the homeostasis of extracellular fluid calcium. Pflugers Arch. 2022;474:885–900. CrossRef
373. Ali AAH. Overview of the vital roles of macro minerals in the human body. J Trace Elem Miner. 2023;4:100076. CrossRef
374. Sobh MM, Abdalbary M, Elnagar S, Nagy E, Elshabrawy N, Abdelsalam M, et al. Secondary osteoporosis and metabolic bone diseases. J Clin Med. 2022;11:2382. CrossRef
375. Morris AL, Mohiuddin SS. Biochemistry, nutrients [Updated 2023 May 1]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554545/
376. Tako E. Dietary trace minerals. Nutrients. 2019;11:2823. CrossRef
377. Farag MA, Abib B, Qin Z, Ze X, Ali SE. Dietary macrominerals: updated review of their role and orchestration in human nutrition throughout the life cycle with sex differences. Curr Res Food Sci. 2023;6:100450. CrossRef
378. Kim SY, Park JH, Kim EA, Lee-Kim YC. Longitudinal study on trace mineral compositions (selenium, zinc, copper, manganese) in Korean human preterm milk. J Korean Med Sci. 2012;27:532–6. CrossRef
379. Makwe CC, Soibi-Harry AP, Rimi GS, Ugwu OA, Ajayi AT, Adesina TA, et al. Micronutrient and trace element levels in serum of women with uterine fibroids in Lagos. Cureus. 2021;13:18638. CrossRef
380. Terry J. The major electrolytes: sodium, potassium, and chloride. J Intraven Nurs. 1994;17:240–7. CrossRef
381. Shrimanker I, Bhattarai S. Electrolytes [Updated 2023 Jul 24]. Treasure Island, FL: StatPearls Publishing; 2023 [cited 2023 Dec 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK541123/
382. Ha SK. Dietary salt intake and hypertension. Electrolyte Blood Press. 2014;12:7–18. CrossRef
383. Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Calcium and the risk for periodontal disease. J Periodontol. 2000;71:1057–66. CrossRef
384. Shimazaki Y, Shirota T, Uchida K, Yonemoto K, Kiyohara Y, Iida M, et al. Intake of dairy products and periodontal disease: the Hisayama study. J Periodontol. 2008;79:131–7. CrossRef
385. Varela-López A, Giampieri F, Battino M, Quiles JL. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21:373. CrossRef
386. Raut CP, Sethi KS, Kohale B, Mamajiwala A, Warang A. Subgingivally delivered coenzyme Q10 in the treatment of chronic periodontitis among smokers: a randomized, controlled clinical study. J Oral Biol Craniofac Res. 2019;9:204–8. CrossRef
387. Ghasemi S, Torab Z, Shirmohammadi A, Babaloo A, Johari R, Farhadi F, et al. Evaluation of the effect of coenzyme Q10 supplementation along with scaling and root planing (SRP) on periodontal and gingival indices in controlled diabetic patients. J Adv Periodontol Implant Dent. 2022;14:32–7. CrossRef
388. Singh R, Cheng S, Singh S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech. 2020;10:66. CrossRef
389. Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR. Zinc and oxidative stress: current mechanisms. Antioxidants (Basel). 2017;6:24. CrossRef
390. Xiang M, Pan Z, Hong S, Cao G, Feng B. Association of dietary zinc consumption with periodontitis in diabetes mellitus patients: a cross-sectional study of national health and nutrition examination surveys database (2009-2014). J Dent Sci. 2023. CrossRef
391. Rajendiran M, Trivedi HM, Chen D, Gajendrareddy P, Chen L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules. 2021;26:2001. CrossRef
392. Liu Y, Li X, Liu S, Du J, Xu J, Liu Y, et al. The changes and potential effects of zinc homeostasis in periodontitis microenvironment. Oral Dis. 2023;29(8):3063–77. CrossRef
393. Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. 2006;5(3):101–17. CrossRef
394. Collins JA, Fauser BC. Balancing the strengths of systematic and narrative reviews. Hum Reprod Update. 2005;11(2):103–4. CrossRef
395. 48.Siebert S, Machesky LM, Insall RH. Overflow in science and its implications for trust. Elife. 2015;4:e10825. CrossRef
396. 49.Byrne JA. Improving the peer review of narrative literature reviews. Res Integr Peer Rev. 2016;1:12. CrossRef
397. 50.Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;4:5. CrossRef
398. 51.Henry BM, Skinningsrud B, Vikse J, P?kala PA, Walocha JA, Loukas M, et al. Systematic reviews versus narrative reviews in clinical anatomy: methodological approaches in the era of evidence-based anatomy. Clin Anat. 2018;31(3):364–7. CrossRef
399. Basheer A. The art and science of writing narrative reviews. Int J Adv Med Health Res. 2022;9:124–6. CrossRef
400. Turnbull D, Chugh R, Luck J. Systematic-narrative hybrid literature review: a strategy for integrating a concise methodology into a manuscript. Soc Sci Hum Open. 2023;7:100381. CrossRef
401. Tulandi T, Suarthana E. Narrative reviews, systematic reviews, and scoping reviews. J Obstet Gynaecol Can. 2021;43:1355–6. CrossRef
402. Greenhalgh T, Thorne S, Malterud K. Time to challenge the spurious hierarchy of systematic over narrative reviews? Eur J Clin Invest. 2018;48:12931. CrossRef
403. Sukhera J. Narrative reviews: flexible, rigorous, and practical. J Grad Med Educ. 2022;14:414–7. CrossRef
404. Faggion CM Jr, Bakas NP, Wasiak J. A survey of prevalence of narrative and systematic reviews in five major medical journals. BMC Med Res Methodol. 2017;17(1):176. CrossRef