INTRODUCTION
Asthma and COPD are considered to be the most prevalent obstructive pulmonary diseases [1,2]. The prevalence of COPD among smokers was estimated to be 20%, whereas asthma diagnosis is prevalent in 9.8% of people aged 5–69 years [3,4]. Asthma and COPD are essential contributors to morbidity and mortality in both developed and developing countries [5]. Although they represent two separate diseases, there is also significant overlap between them in many cases [6,7]. Asthma is defined as an airway inflammation, usually characterized by a heterogeneous clinical set of characters as shortness of breath, wheezes, and coughing that vary in intensity over time [8]. COPD is a widespread disease with features that consist of constant airway obstruction that is incompletely reversible and treatable [9].
Smoking is a global health problem. A high rate of smoking pandemic (50% smoking rate) is detected in Jordan [10,11]. Smoking is the major cause of obstructive airflow diseases [1,2]. The decline of lung function is accelerated by smoking by up to 50 ml per year [12–14], where there is an established dose–response relationship [12,14,15]. Asthmatic subjects are more likely to have greater loss of their lung function and to develop COPD if they are smokers [16]. ACOS represents a special form of a spectrum of chronic obstructive airway diseases. ACOS can present in three types: childhood asthma and adult smoking; severe asthma with airway remodeling (incompletely reversible airflow obstruction); and eosinophilic COPD [17]. Gibson and McDonald [17] emphasized that the management approach should address the specific phenotypic features of the disease. Since no particular biomarkers distinguish ACOS from asthma or COPD, ACOS is considered a diagnostic challenge for physicians [18]. The prevalence of ACOS varies significantly due to differing criteria for diagnosis.
Quality of life (QoL) in subjects diagnosed with ACOS will likely be negatively impacted [19,20]. Treatment adherence may potentially impact the QoL among ACOS subjects. Adherence encompasses both treatment and other treatment modalities like peak flow monitoring and compliance with smoking discontinuation [21]. It has even been reported that smoking is an important behavioral risk factor associated with poor treatment adherence [22,23].
Subjects suffering from ACOS are expected to have higher economic and clinical burdens than subjects diagnosed with asthma or COPD alone [24]. In Jordan, ACOS was shown to be a risk factor for incurring higher health expenditures [25]. Still, data are limited about ACOS in Jordan, as well as in other Middle Eastern countries.
This study was designed to investigate ACOS prevalence among the Jordanian asthmatic smoker population and to evaluate the QoL and adherence to treatments among the study sample and by ACOS status.
MATERIALS AND METHODS
Design and settings
This was a cross-sectional study in which participants were recruited from the public healthcare sector (governmental hospitals) in Jordan. The study was conducted over 2 months at outpatient respiratory clinics from September to October 2018. Governmental hospitals are distributed in the north, south, and middle of Jordan and are attended by all population segments.
Study sample and recruitment
Participants were eligible if they met the criteria of age as 18 years or above, had a physician-diagnosed asthma, and were currently smokers or ex-smokers with 10 or more pack-years (one pack daily for 1 year for 10 years). A physician-diagnosed asthma is usually based on a detailed medical history, a physical exam, symptoms, and test results (spirometry and challenge tests). The study sample mostly represents ACOS phenotypes of severe asthma with incomplete airflow reversibility or childhood asthma and adult smoking, as defined by Gibson and McDonald [17]. Subjects were recruited by convenience sampling. Participants were approached nonsystematically in the waiting room at the clinic before or after seeing the physician. The researcher, an experienced clinical pharmacist in the area of respiratory diseases, interviewed the patients and completed the study questionnaires. With the assistance of the respiratory therapist at each hospital, the researcher instructed the subjects on how to perform the spirometry test. Figure 1 summarizes the protocol followed in collecting the data for this study. The sample size was calculated using G* power 3.1 software considering a 95% confidence interval and the margin error was 5%.
Study instruments
A three-part questionnaire and a spirometry test were used in this study. The first part of the questionnaire was designed to collect data regarding the sample sociodemographics and other patient characteristics: age, gender, education, income, smoking history (pack-year: less than 10 years, 10–30 years, and more than 30 years), body mass index (BMI: less than 20, 20–25, 26–30, more than 30) [26], and duration of disease (<5, 5–10, 11–15, ≥15 years).
The second part involved the Arabic version of the Mini Asthma QoL questionnaire (MiniAQLQ) developed by Juniper et al. [27]. MiniAQLQ is a 15-item questionnaire classified into four domains (symptoms, emotions, environment, and activities). For symptoms, emotions, and environment domains, the responses are presented on a 7-point Likert scale, where “1” indicates “all of the time” and “7” indicates “none of the time.” The other four questions deal with activity limitations, where responses are available also on a 7-point Likert scale where “1” indicates “totally limited” and “7” indicates “not at all limited.”
The average Mini-AQLQ score was calculated by the total score/the number of items, and the domain scores (symptoms, emotions, environment, and activities) were calculated as the total score/the number of items for respective domains.
The third part of the questionnaire is a five-question adherence assessment questionnaire, developed and validated by Weinstein et al. [28]. It assessed the adherence status of the participants with responses provided as a six-point Likert scale as follows: 1 indicated (I completely agree), 2 (I mostly agree), 3 (I somewhat agree), 4 (I somewhat disagree), 5 (I mostly disagree), and 6 (I completely disagree). This part of the questionnaire has two subscales: general adherence (the first question) and specific barriers (the next four questions). The respondent was considered as having possible adherence problems if their score was >1 for question 1 (the general adherence question) and probable specific barriers if his/her score was 3 or below for the other questions (questions 2, 4, and 5) and 4 or below for question 3.
The final assessment in this study was the spirometry test. Spirometry was performed according to the guidelines set by Miller et al. [29] prior to and upon the administration of 400 μg of inhaled salbutamol while the patient was taking their usual asthma medication/s. The patient was considered to have ACOS if their post-bronchodilator FEV1/FVC ratio (the forced expiratory volume in 1 second divided by the forced vital capacity) was less than 0.70. The prevalence of ACOS (expressed as %) was calculated using the following formula: “The prevalence of ACOS (%) = (the number of subjects identified to have ACOS/number of people evaluated) * 100” [30].
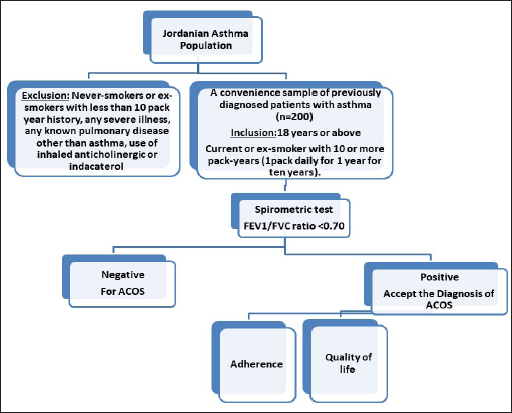 | Figure 1. Study protocol. QoL: Quality of life assessment. Adherence: Adherence questionnaire completion for adherence assessment. [Click here to view] |
Statistical analysis
The analysis was performed using IBM SPSS Statistics for Windows (version 25.0, Armonk, NY, IBM Corp.). Descriptive statistics were used to describe patient sociodemographic characteristics. The Chi-square test and the exact Fisher’s test, when appropriate, were used to compare demographic categorical variables by ACOS status [31]. The independent t-test or nonparametric Mann–Whitney U-test was used to compare the total MiniAQLQ score, and the domain scores (symptoms, emotions, environment, and activities) between the two study groups (with and without ACOS) [32]. Spearman’s rank correlation (Rho) was used to evaluate the association between adherence to treatment and MiniAQLQ score among subjects with and without ACOS [33]. Linear regression analysis was used to determine factors associated with MiniAQLQ scores, and logistic regression analysis was used to determine factors associated with a higher likelihood of possible adherence problems [34,35]. Variables included in all mentioned regression models were selected using the backward stepwise process with p < 0.2 to stay from the following variables: gender, age, current smoking, pack-years, BMI, disease group (asthma only and ACOS), and duration of disease years [36]; p-values of less than 0.05 were considered statistically significant.
Ethical approval
This study was approved by the Ministry of Health and the Ethical Committee at the Applied Science Private University (Ref number: 2018-PHA-1). A written informed consent was obtained from all the participants.
RESULTS
General characteristics of the sample in general and by ACOS status
A total of 200 subjects with asthma were recruited by the principal investigator. The response rate was 88.1%; out of 227 patients that were approached, 200 patients gave consent and participated in the study. As shown in Table 1, 66.0% of participants were males, the mean age was 54.9 ±14.82, and 61.5% were of less than high school education (n = 123). Of the total sample, 87.5% had less than 500 JOD (Jordanian dinar) monthly income (n = 175), 49.0% had a smoking history of more than 30 years, 31.5% had a disease history of above 15 years (n = 63), and 31.0% patients’ BMI ranged between 20 and 25 (n = 62).
About one-third of the patients (32.5%, n = 65) had FEV1/FVC1 values lower than 0.7, which indicates the presence of ACOS.
Results showed that subjects with ACOS were significantly older than the nonACOS group (p-value = 0.043). Smoking history of more than 30 years was significantly more prevalent among ACOS subjects (67.7% versus 40%, p-value < 0.001). The average total score of the MiniAQLQ was low in the total sample (3.23 ± 1.01) and was not significantly different between ACOS and nonACOS groups (3.16 in ACOS group compared to 3.3 in the nonACOS group; p-value = 0.702). The average scores for the symptoms, emotions, and environment domains were also not significantly different between ACOS and nonACOS groups (2.81 versus 2.68, 2.11 versus 2.45, and 3.26 versus 3.11, respectively; p values were >0.05). However, the average score for the activity domain was higher in the nonACOS group (4.7) compared to the ACOS group (4.27); the p-value was marginally significant (0.055). The mean total general adherence score was also low (1.76 ± 1.2), indicating that patients in this study were minimally adherent to their treatment modalities [28]. Tables 2 and 3 provide a detailed description of MiniAQLQ and adherence results for the total sample and by ACOS status.
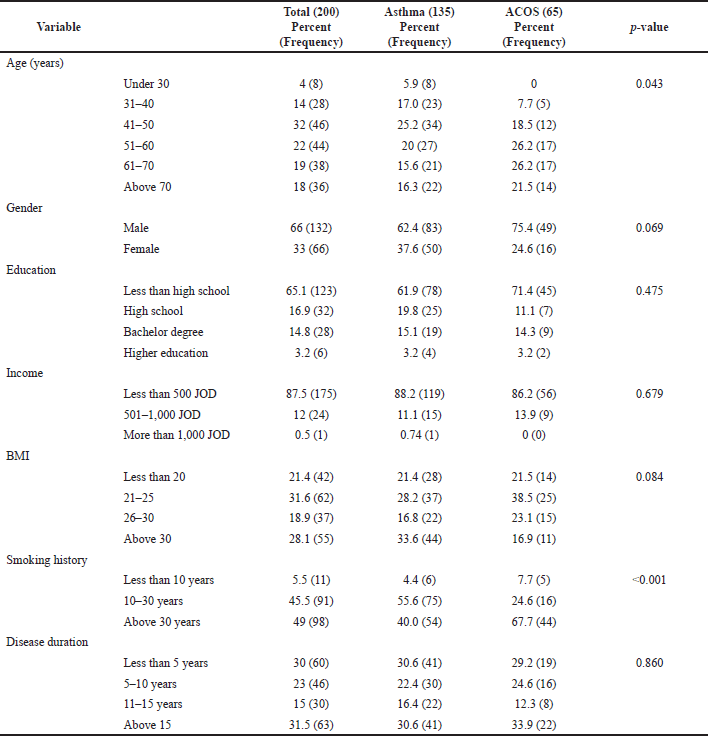 | Table 1. Study sample of demographic characteristics. [Click here to view] |
Correlation between the level of adherence to treatment and MiniAQLQ score in subjects with and without ACOS
Results showed a statistically significant positive relationship between adherence to treatment and AQLQ scores (coefficient = 0.171, significant level/2-tailed = 0.018). On the other hand, no significant correlation was found in patients without ACOS (coefficient = 0.069, significant level/2-tailed = 0.427).
Predictors of MiniAQLQ score and adherence in the study sample
Age (increase by 1 year) and female gender were significantly associated with lower MiniAQLQ scores adjusting for potential confounders [coefficients were −0.015 (p-value = 0.004) and −0.356 (p-value = 0.028), respectively]. On the other hand, high school education (versus less than high school education) and monthly income of 500–1,000 JOD (versus <500 JOD) were associated with higher MiniAQLQ scores adjusting for potential confounders [coefficients were 0.511 (p-value = 0.011) and 0.579 (p-value = 0.022), respectively]. Only smoking history for more than 30 years (versus <10 years) was significantly associated with a higher likelihood of having adherence problems adjusting for potential confounders (OR = 5.99; p-value = 0.041), as shown in Table 4.
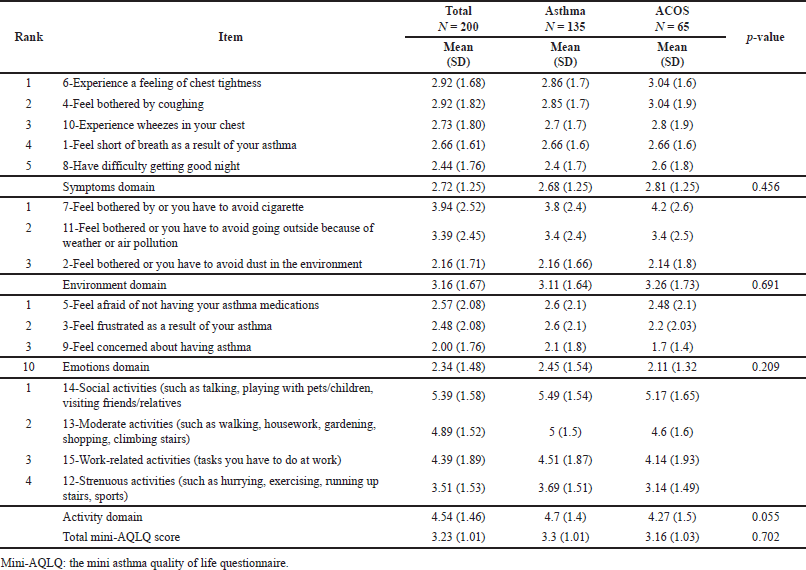 | Table 2. Average MiniAQLQ scores for the total sample and by ACOS status. [Click here to view] |
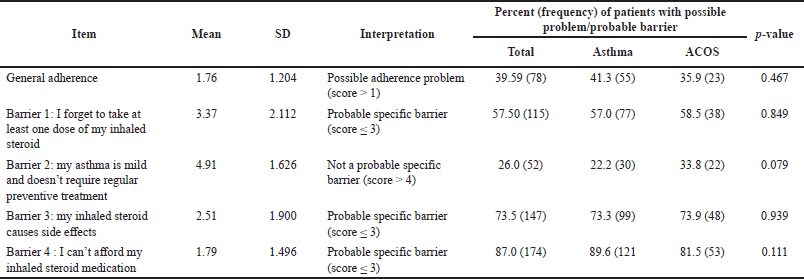 | Table 3. Average patients’ response to general adherence and specific barriers questionnaire. [Click here to view] |
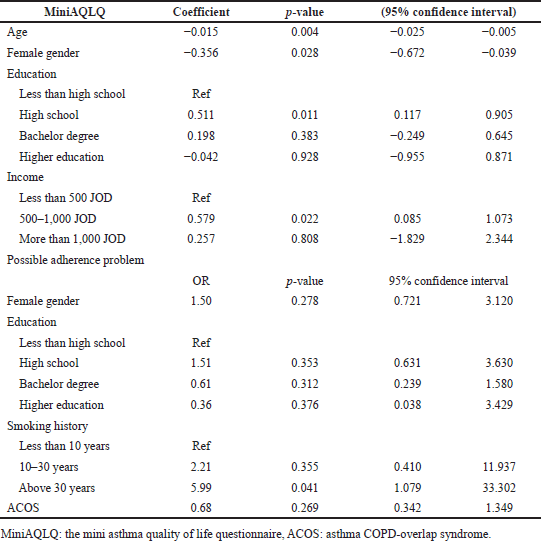 | Table 4. Predictors of MiniAQLQ score and possible adherence problem. [Click here to view] |
DISCUSSION
This study investigated the prevalence of ACOS among Jordanian smokers diagnosed previously with bronchial asthma. Findings showed that ACOS was moderately prevalent among Jordanian asthmatic smokers (33%). ACOS was more frequent among patients of older ages and those with a longer smoking history. The QoL and adherence to treatment were both low and correlated significantly among ACOS subjects.
Chronic respiratory diseases are a common health problem worldwide. The prevalence of respiratory diseases in Arab countries is increasing due to the outgrowing of industrialization acts [14]. These diseases are becoming serious health problems in Arab countries and represent a major health burden [14]. Jordan and the nearby Arabic countries lack previous statistics and baseline data to compare the prevalence and burden of ACOS. In the current study, the prevalence of ACOS was 33% among 200 asthmatic smokers. Compared to previous literature, ACOS prevalence was lower, parallel, or higher than estimated in this study. Menezes et al. [37] and Miravitlles et al. [38] reported 1.8% and 6.5% prevalence, respectively, while Milanese et al. [39] and Andersen et al. [40] reported a higher prevalence of 29% and 16.1%, respectively. de Marco et al. [41] reported an ACOS prevalence of 16%–61% among people of 20–84 years. In addition, Fu et al. [42] and Marsh et al. [43] identified a prevalence of 56% among subjects with asthma aged 55 years and older. This variability of the reported prevalence among different studies might be explained by the use of different diagnostic criteria for ACOS. A standardized method of diagnosing ACOS and using consistent criteria in the studies would help researchers identify an accurate ACOS prevalence comparable across different populations. Nevertheless, Gibson and Simpson [44] have concluded that ACOS prevalence in older adults is significant regardless of the diagnosis criteria. In addition, using different inclusion and exclusion criteria in different studies might also contribute to the variability of reported prevalence, especially considering the different age ranges included in different studies. Findings from the current study showed that ACOS prevalence was significantly different between age groups. It was evident that ACOS was more prevalent in people above 60 years of age. Age is reported to be a risk factor for the development of obstructive lung diseases and incomplete reversibility of airflow obstruction; a decline in the lung function parameters is associated with age [45]. In a previous evaluation of 44 older adults (>55 years) with stable obstructive airway disease (asthma or COPD), 65% were diagnosed with ACOS [44]. In another large population study, it was found that ACOS increased in prevalence with increasing age [46]. In addition to the aging effect, an accelerated decline in lung function is more concerning, particularly in those with asthma who smoke [12,13]. Indeed, a dose–response relationship between smoking and lung function decline was well established in previous literature [13,45]. Smoking for ≥20 packs per year and age of ≥60 years were identified as major predictors of ACOS diagnosis [47].
Patients with ACOS are exposed to multiple clinical problems that may adversely influence their health and sense of well-being. ACOS is typically associated with more intense and frequent respiratory symptoms such as dyspnea and wheezing, more frequent recurrence and exacerbations, a lower respiratory-speci?c QoL, and lower physical activity levels than asthma alone or COPD alone [38,48,49]. In one of the studies conducted to assess QoL among three patient groups, asthma alone, COPD alone, and ACOS patients, ACOS patients were found to have the poorest QoL among the three groups [19]. Findings from the current study showed predominately low QoL scores for both asthmatic and ACOS patients. The clinically significant difference in QoL between asthmatic and ACOS patients was unclear in the current study. However, a sample of only 65 ACOS patients might not be enough to reveal the difference. Indeed, a marginal difference was observed in the activity-related items, suggesting that activity limitations caused by ACOS potentially mediate the ACOS effect on QoL.
Findings from the current study showed that older ages and female gender were negatively associated with QoL. In contrast, higher income and higher educational levels were shown to predict better QoL. In fact, deterioration in health-related QoL with increasing age was shown in previous literature [19,50]. Changes in lung function, changes in the immune system, comorbidities, and lower adherence levels to treatments due to deterioration in sight, hearing, motor abilities, and cognitive functions were the major explanations in previous studies [51,52]. Consistent with our findings, female patients with asthma were shown in previous literature to have poorer QoL as compared to males [53]. Such gender differences in QoL are suggested to be related to subjective differences in perceptions, emotional factors, and coping skills [53]. Similar to our findings, low education level was shown in previous studies to be a predictive factor for poorer health-related QoL in asthmatic patients, which might be explained by lower health literacy, delayed diagnosis of asthma, and incompliance with healthy lifestyles [50,54,55].
Adherence level in this study was found to be low. Nonadherence to medications is reported to be a global and significant health problem [56]. It has also been emphasized that at least 50% of patients with chronic illnesses do not adhere to their prescribed therapy [56]. Nonadherence is reported to be a significant health problem worldwide [56] and in Jordan [57]. Consistently, in a Poland study of 63,000 participants suffering from various chronic diseases, it was found that 83.8% claimed to be nonadherent to their prescribed therapeutic regimens [58]. In this study, asthma patients took an average of 65.4% ± 17.1% of doses of their prescribed drugs, whereas COPD patients took an average of 61.6% ± 24.2%. In the same study, more than 20% of asthma and COPD patients discontinued their therapy. Reasons behind drug discontinuation were patient lack of motivation (41.6%) and lack of knowledge relevant to the disease (19.3%) [58].
A smoking history of more than 30 years was associated with a higher probability of possible adherence problems with treatment modalities. Cigarette smoking was linked previously to undertreatment in asthmatic patients with a suggested link to patients’ perceptions of their overall asthma control [59,60]. Moreover, the number of cigarettes smoked each day was significantly associated with decreased asthma control, which might be explained by lower adherence levels [61].
A statistically significant positive relationship was identified in this study between adherence and QoL among ACOS patients. However, the clinical significance of this correlation needs to be further verified in larger samples. Nonadherence to a prescribed therapeutic plan for asthma can result in poor disease control and more frequent exacerbations [19]. The systematic exclusion of ACOS patients from asthma and COPD treatment clinical trials might contribute to underevaluating current treatments in ACOS patients. Ineffective or less effective treatments might be a possible explanation for nonadherence in this population. Moreover, nonadherence is expected to lead to poorer QoL, greater demand for medical services due to more frequent hospitalizations, and increased mortality [19]. This is the first study in Jordan and the Middle East to confirm a significant relationship between low adherence and low QoL scores among ACOS patients.
This study has some limitations. ACOS patients are usually manifested by dyspnea and other breathing difficulties, which may influence the cooperation of the patients with the researcher. In addition, the researcher accessed participants during their attendance at doctor clinic appointments, which might not be the optimal place to interview sick patients and collect research-oriented information. Indeed, the clinical pharmacist tried to maximize the response rate even for patients with severe symptoms to have a representative sample for a wide range of severity. Still, patients with very severe symptoms might be underrepresented in this study. We acknowledge that this study was limited to asthmatic patients with smoking exposure, so it might not represent the general asthmatic population. Indeed, this study was intended to highlight the issue of the overlap syndrome in the most vulnerable patients. Highlighting the needs of these patients is important to inform policymakers and healthcare providers in their decisions and interventions. Nevertheless, this selection bias impacts the generalizability of the study findings. Evaluating ACOS in a larger general asthmatic population is one of our future directions. Second, regarding the assessment criteria for ACOS, the literature reported multiple and various assessment tools and criteria for ACOS diagnosis assessment, which caused uncertainty as to which was more suitable to provide proper ACOS diagnosis. Future research using longitudinal study designs with larger sample sizes is warranted to further characterize this vulnerable population and provide more precise data. In addition, considering a standardized criterion or multiple assessment parameters for ACOS across different settings and populations is necessary.
CONCLUSION
This study identified a moderate prevalence of ACOS among Jordanian asthmatic smokers (33%). The prevalence of ACOS was the highest among older ages and those with longer smoking history. In addition, this study identified low levels of QoL and adherence to treatment among subjects with ACOS. Interestingly, a significant positive relationship between QoL and adherence to treatment was found. These findings can raise the attention of healthcare providers and policymakers regarding the presence of ACOS and the associated burden. Paying particular attention to groups of patients with certain identified risk factors can increase their chances of being diagnosed with ACOS.
IMPLICATIONS
Strategies to enhance QoL, treatment adherence, and identifying barriers toward treatment adherence should be involved in the future management plan for ACOS patients. It is also important to better involve healthcare workers, especially pharmacists, in ensuring that patients adhere to their prescribed regimen, including the correct use of devices and other long-term treatments [62]. Finally, including ACOS patients in randomized clinical trials is important to better evaluate and optimize treatment options in this population.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study is supported by the Deanship of Scientific Research at the Applied Science Private University. The funding agency was not involved in the study design, conduct, writing, or decision to submit the article for publication.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Please see the section 'Materials and Methods' sub section 'Ethical approval'.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2017. Available from: http://www.ginasthma.org
2. Global Initiative for Chronic Obstructive Lung Disease. Global initiative for chronic obstructive lung disease, management, and prevention of COPD. 2017. Available from: http://www.goldcopd.org
3. Song P, Adeloye D, Salim H, Dos Santos JP, Campbell H, Sheikh A, et al. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J Glob Health. 2022;12:04052. CrossRef
4. Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–92. CrossRef
5. Mannino D, Buist A. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–73. CrossRef
6. Reddel HK, Hurd SS, FitzGerald JM. World asthma day. GINA 2014: a global asthma strategy for a global problem. Int J Tuberculosis Lung Dis. 2014;18(5):505–6. CrossRef
7. Fabbri LM, Hurd S. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir Soc. 2003;22 (1):1–2. doi: 10.1183/09031936.03.00063703. PMID: 12882441. CrossRef
8. Reddel HK, Bateman ED, Becker A, Boulet L-P, Cruz AA, Drazen JM, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):622–39. CrossRef
9. Kim YS. Definition and epidemiology of COPD. In S.-D. Lee (Ed.), COPD: Heterogeneity and Personalized Treatment. Berlin, Heidelberg: Springer Berlin Heidelberg. pp 3–7. CrossRef
10. Barakat M, Assaf AM, Al-Qudah R, Thiab S, Alhamed M, Al-Obaidi HJ, et al. Perception of adults toward electronic cigarettes: a cross-sectional study from Jordan. Prim Health Care Res Dev. 2021;22:e3. CrossRef
11. Belbeisi A, Al Nsour M, Batieha A, Brown D, Walke H. A surveillance summary of smoking and review of tobacco control in Jordan. Global Health. 2009;1:5–18. CrossRef
12. James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, et al. Decline in lung function in the Busselton health study. Am J Respir Crit Care Med. 2005;171:109–14. CrossRef
13. Anthonisen N, Connett J, Murray R. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–9. CrossRef
14. Sweileh M, Al-Jabi S, Zyoud S, Sawalha A. Bronchial asthma and chronic obstructive pulmonary disease: research activity in Arab countries. Multidiscip Respir Med [Internet]. 2014;9:38. CrossRef
15. Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–52. CrossRef
16. Boulet LP, Boulay ME, Derival JL, Milot J, Lepage J, Bilodeau L, et al. Asthma-COPD overlap phenotypes and smoking: comparative features of asthma in smoking or non-smoking patients with an incomplete reversibility of airway obstruction. COPD. 2018;15(2):130–8. CrossRef
17. Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70(7):683–91. CrossRef
18. Rhee CK. Asthma-COPD overlap syndrome. COPD: Heterogeneity and Personalized Treatment. Berlin, Heidelberg: Springer Berlin Heidelberg. pp 189–93. CrossRef
19. Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpeläinen M, Kinnula V, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–85. CrossRef
20. Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12(1):127. CrossRef
21. National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute. Available from: https://www.ncbi.nlm.nih.gov/books/NBK7232/
22. Aggarwal B, Mosca L. Lifestyle and psychosocial risk factors predict nonadherence to medication. Ann Behav Med. 2010;40(2): 228–33. CrossRef
23. Liberman J, Lichtenfeld M, Galaznik A, Mastey V, Harnett J, Zou K, et al. Adherence to varenicline and associated smoking cessation in a community based setting. J Manag Pharm. 2013;19(2):125–31. CrossRef
24. Tho NV, Park HY, Nakano Y. Asthma–COPD overlap syndrome (ACOS): a diagnostic challenge. Respirology. 2016;21(3):410–8. CrossRef
25. Altawalbeh SM, Hijazi B, Kufoof L, Basheti IA. Health expenditures of asthma-COPD overlap in Northern Jordan. PLoS One. 2021;16(9):e0257566. CrossRef
26. NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda (MD): National Heart, Lung, and Blood Institute; 1998 Sep. Table IV-2, Classification of Overweight and Obesity by BMI, Waist Circumference and Associated Disease Risk. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2004/table/A242/
27. Juniper E, Guyatt G, Cox F, Ferrie P, King D. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14(1):32–8. CrossRef
28. Weinstein A, Schatz M, Laurenceau J. Preliminary analysis of an adherence survey for adult asthma patients. J Allergy Clin Immunol. 2009;123:s72. CrossRef
29. Miller M, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. CrossRef
30. Tenny S HM. Prevalence [Updated 2023 May 22]. Treasure Island, FL: StatPearls [Internet]; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430867/
31. Pearson K. X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Lond Edinb Dublin Philos Mag J Sci. 1900;50(302):157–75. CrossRef
32. McKnight PE, Najab J. Mann-Whitney U test. The Corsini encyclopedia of psychology. New York, NY: Wiley. pp 1–1.
33. Spearman rank correlation coefficient. The concise encyclopedia of statistics. New York, NY: Springer New York; 2008. pp 502–5.
34. Wright RE. Logistic regression. Reading and understanding multivariate statistics. Washington, DC: American Psychological Association; 1995. pp 217–44.
35. Yan X, Su XG. Linear regression analysis: theory and computing. Singapore: World Scientific Publishing; 2009. CrossRef
36. Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med. 2016;4(7):136. CrossRef
37. Menezes AMB, de Oca MM, Pérez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. CHEST J. 2014;145(2):297–304. CrossRef
38. Miravitlles M, Soriano JB, Ancochea J, Munoz L, Duran-Tauleria E, Sánchez G, et al. Characterisation of the overlap COPD–asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–60. CrossRef
39. Milanese M, Di Marco F, Corsico AG, Rolla G, Sposato B, Chieco-Bianchi F, et al. ELSA study group asthma control in elderly asthmatics. An Italian observational study. Respir Med. 2014;108(8):1091–9. CrossRef
40. Andersen H, Lampela P, Nevanlinna A, Saynajakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7(4):342–6. CrossRef
41. de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8(5):e62985. CrossRef
42. Fu JJ, Gibson PG, Simpson JL, VM M. Longitudinal changes in clinical outcomes in older patients with asthma, COPD and asthma-COPD overlap syndrome. Respiration. 2014;87:63–74. CrossRef
43. Marsh S, Travers J, Weatherall M. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761–7. CrossRef
44. Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–35. CrossRef
45. James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, et al. Decline in lung function in the Busselton health study. Am J Respir Crit Care Med. 2005;171:109–14. CrossRef
46. Soriano J, Davis K, Coleman B, Visick G, Mannino D, Pride N. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124(2):474–81. CrossRef
47. Kiljander T, Helin T, Venho K, Jaakkola A, Lehtimäki L. Prevalence of asthma–COPD overlap syndrome among primary care asthmatics with a smoking history: a cross-sectional study. NPJ Prim Care Respir Med. 2015; 25:15047. CrossRef
48. Chung J, Kong K, Lee J, Lee S, Ryu Y, Chang J. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2014;9:795–804. CrossRef
49. Hardin M, Cho M, McDonald M-L, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44(2):341–50. CrossRef
50. Gonzalez-Barcala FJ, de la Fuente-Cid R, Tafalla M, Nuevo J, Caamano-Isorna F. Factors associated with health-related quality of life in adults with asthma. A cross-sectional study. Multidiscip Respir Med. 2012;7(1):32. CrossRef
51. Busse PJ, Mathur SK. Age-related changes in immune function: effect on airway inflammation. J Allergy Clin Immunol. 2010;126(4):690–9; quiz 700–1. CrossRef
52. Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376(9743):803–13. CrossRef
53. Chhabra SK, Chhabra P. Gender differences in perception of dyspnea, assessment of control, and quality of life in asthma. J Asthma. 2011;48(6):609–15. CrossRef
54. Mancuso CA, Rincon M. Impact of health literacy on longitudinal asthma outcomes. J Gen Intern Med. 2006;21(8):813–7. CrossRef
55. Apter AJ, Wang X, Bogen D, Bennett IM, Jennings RM, Garcia L, et al. Linking numeracy and asthma-related quality of life. Patient Educ Couns. 2009;75(3):386–91. CrossRef
56. WHO. Adherence to long-term therapies. Evidence for action. Geneva, Switzerland: World Health Organization; 2003.
57. Basheti IA, Hait SS, Qunaibi EA, Aburuz S, Bulatova N. Associations between patient factors and medication adherence: a Jordanian experience. Pharm Pract (Granada). 2016;14(1):639. CrossRef
58. Kardas P, Lewek P, Strzonda?a M. Adherence to treatment in asthma and COPD patients in their doctors’ assessment. Medica. 2015. Pneumonol Alergol Pol. 2015;83(6):436–44. doi: 10.5603/PiAP.2015.0072. PMID: 26559796. CrossRef
59. Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respir Med. 2006;100(2):354–62. CrossRef
60. Altawalbeh SM, Manoon NA, Ababneh MA, Basheti IA. Respiratory tract infection-induced asthma exacerbations in adults with asthma: assessing predictors and outcomes. J Asthma. 2020;57(3):231–40. CrossRef
61. Price D, Bjermer L, Popov TA, Chisholm A. Integrating evidence for managing asthma in patients who smoke. Allergy Asthma Immunol Res. 2014;6(2):114–20. CrossRef
62. Basheti I, Qunaibi E, Hamadi S, Abu-Gharbieh E, Saleh S, AbuRuz S, et al. Patient perspectives of the role of the community pharmacist in the middle east: Jordan, United Arab Emirates and Iraq. Pharmacol Pharm. 2014;5:588–99. CrossRef