INTRODUCTION
Substance use disorders (SUDs) pose a considerable public health problem, with an estimated 284 million individuals worldwide reported to have experimented with drugs in 2020 [1] despite evidence highlighting the negative influence of substance abuse on both individuals and the broader society [2]. Illicit drug use remains widespread, contributing significantly to the total load of diseases. Still, the underlying factors that contribute to vulnerability to dependence are still completely noncomprehended, and there is a lack of effective treatments available [3].
Benzodiazepines such as Clonazepam are considered the most used psychotropic medications in psychiatry for treating different disorders such as epilepsy, depression, anxiety, and sleep disorders [4,5]. However, benzodiazepines are also highly addictive and can lead to dependence, withdrawal symptoms, and other adverse effects [6]. Despite these risks, benzodiazepines continue to be widely prescribed, and their misuse remains a major public health concern.
Clonazepam addiction is a major health problem; around 20%–30% of people become addicted after 1 month of use [3,7]. The withdrawal syndrome associated with Clonazepam dependence can be severe and may include symptoms such as seizures, anxiety, insomnia, and tremors [8]. The management of this withdrawal syndrome is difficult, and there is currently no consensus on the optimal therapeutic approach [9].
Persistent utilization of benzodiazepines, including Clonazepam, has been consistently linked to modifications within several neurotransmitter systems encompassing gamma-aminobutyric acid (GABA), serotonin, and dopamine, ultimately exerting profound repercussions on the structure and functioning of the brain. Noteworthy, the disrupted equilibrium of these neurotransmitters has been implicated in the observed alterations occurring in key brain regions that regulate emotional responses and cognitive processes, notably the prefrontal cortex, hippocampus, and amygdala. These regions play pivotal roles in emotion regulation, memory consolidation, and decision-making, and any disturbances in neurotransmitter function can substantially impact their normal functioning [9,10]. In addition to these neurotransmitter systems, emerging research suggests that the role of glutamate receptors should be considered in understanding the effects of benzodiazepine addiction on the brain. Glutamate is a primary excitatory neurotransmitter in the central nervous system (CNS) that plays a critical role in synaptic plasticity, learning, and memory. Dysregulation of glutamate receptors, particularly the N-methyl-D-aspartate (NMDA) receptor subtype, has been implicated in the pathophysiology of addiction and withdrawal [11].
The oxidative stress associated with benzodiazepine dependence and Clonazepam use may further impact glutamate receptor function and contribute to neuronal damage and dysfunction [12]. Oxidative stress, characterized by an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense systems, has been linked to anxiety and depressive disorders frequently observed in individuals with benzodiazepine dependence [13]. The interplay between oxidative stress and glutamate receptors suggests a potential mechanistic pathway that underlies the adverse effects of Clonazepam addiction on brain function. Oxidative stress-induced modifications of glutamate receptors, particularly NMDA receptors, can lead to dysregulated glutamatergic neurotransmission, excitotoxicity, and neuronal damage. These changes may contribute to the development of withdrawal symptoms and exacerbate the overall impact of Clonazepam dependence on brain function [14].
Hence, continued research of novel features to prevent, predict, and treat the stages of drug abuse and dependence are necessary. Since an important rate of relapse is noticed among patients suffering from SUD, even after prolonged abstinence, it is a crucial challenge to expand potent therapeutic procedures to control SUD affecting the brain and to attenuate withdrawal syndrome [15].
The utilization of natural products, including medicinal plants, as a means of obtaining therapeutic agents is experiencing a surge in popularity. This is primarily due to their potential effectiveness and comparatively lower toxicity when compared to synthetic drugs. Natural products are increasingly recognized as promising alternatives for treating and/or preventing neuropsychiatric conditions such as SUD [15].
Anacyclus pyrethrum (A. pyrethrum) is a plant belonging to the Asteraceae family and to the Anacyclus genus. It is commonly known as pellitory, Aqar Qarha, Oud El Attas, and tigandizt. Anacyclus pyrethrum is a medicinal plant that has been used for various ailments, including pain, inflammation, and fever. However, its potential utility as a therapeutic agent for managing the adverse effects of Clonazepam dependence has not been investigated. Given the association between Clonazepam dependence and oxidative stress, it is possible that targeting oxidative stress may represent a novel therapeutic approach for managing the adverse effects of Clonazepam dependence. Therefore, the proposed study could contribute to a good comprehension of the role of oxidative stress in the adverse effects of Clonazepam dependence, as well as the potential utility of A. pyrethrum as a therapeutic agent for managing these effects.
MATERIAL AND METHODS
Plant material
The collection of A. pyrethrum roots took place in Bin El Ouidan, located near Marrakech, Morocco (32° 7′48? latitude N/6° 27′36? longitude W). The roots were first verified for authenticity by Pr. Chait and then stored under the MARK-1003 voucher in the plant herbarium of the Faculty of Sciences Semlalia, Marrakech. The extraction process of the plant involved the use of distilled water, with minor adjustments made to a previously described method [16]. After 24 hours of stirring, the aqueous extract was first filtered, then the powder form was obtained after lyophilization, and stored at 4°C. The final yield of this extraction was 17.1%.
During this investigation, the toxicity of the aqueous extract of Anacyclus pyrethrum (AEAP) was assessed using doses of 1,000, 2,000, and 5,000 mg/kg. The results indicated that these doses were safe, as there were no instances of mortality nor alterations in body and organ weights after a 14-day administration period. Furthermore, the LD50 value of the AEAP was determined to be >5,000 mg/kg, showing the extract’s nontoxicity [17].
Animals
Male Sprague-Dawley rats, weighing 190 ± 15 g, were housed in transparent cages in a controlled environment. The temperature was maintained at 22°C ± 2°C, while the humidity was kept at 50% ± 10%. A 12:12-hour light/dark cycle was followed. The rats had unrestricted access to both water and food, which were provided ad libitum. Before the commencement of the experiments, the rats were acclimatized to the laboratory environment for a period of 7 days. All procedures involving animals were carried out in compliance with the guidelines outlined by the European Council Directive for Care and Use of Laboratory Animals (EU2010/63). Before the commencement, the Institutional Local Review Board approved the study protocol for animal experimentation, with the protocol code CA965/07/22 and an approval date of October 2022.
Drugs administration for physical dependence
The Clonazepam used in this study was commercially available. It was administered intraperitoneally (i.p) in an escalating dose regimen, starting with 1.5 mg/kg/day and gradually increasing by 10% each day, up to a maximum dose of 6 mg/kg/day. The Clonazepam was solubilized in a saline solution and administered daily for a period of 30 days. This dosing protocol aimed to mimic the progressive increases in benzodiazepine consumption observed in human addiction, as supported by previous research studies [5,18].
Treatment and grouping
Following the acclimatization period to the laboratory environment, the animals were then assigned into four different groups, with each group consisting of six animals:
(1) Vehicle (control; saline solution 0.9%).
(2) Clonazepam dependent group: 30 days of daily administration of Clonazepam followed by a withdrawal phase (07 days).
(3) AEAP treatment group (200 mg/kg; orally), the choice of that dose was based on our prior studies [19–21].
(4) Clonazepam + AEAP group; Clonazepam-dependent treated group after 30 days of daily administration of Clonazepam followed with AEAP treatment (200 mg/kg; orally) for 1 week from day 34 to 40.
Conditioned place preference (CPP)
The Clonazepam-induced CPP test was conducted following a previously established protocol [19,22]. The CPP apparatus consisted of three Polyvinyl chloride compartments: two larger conditioning side chambers (30 × 25 × 30 cm) with distinct somatosensory cues such as colored walls (white or zebra) and different floor surfaces and a middle neutral chamber (11 × 25 × 30 cm). The CPP procedure consisted of three phases: preconditioning, conditioning, and postconditioning (dependence). During the preconditioning (days 1–3), the rats were put in the middle chamber with opened doors, allowing them unrestricted access to both compartments. Their behavior and preferences were observed and recorded for 15 minutes to establish the baseline preference for each chamber. Rats showing a clear preference for one side compartment over the others were excluded from the study. In the second phase (conditioning: days 4–9), rats received alternating injections of either Clonazepam or saline solution two times per day: in the morning (10:00 a.m), and evening (8:00 p.m.) for 6 days. After receiving Clonazepam, the rats were confined to the zebra compartment for 45 minutes, while after saline injection, they were confined to the white compartment. The control group received saline injections during the rotated sessions throughout the conditioning and postconditioning phases. In the postconditioning phase (days 10–33), the rats underwent re-testing for Clonazepam-CPP. They were given free access to both the white and zebra chambers for 15 minutes, and their behavior was monitored using a camera connected to a computer interface. The number of entries to the Clonazepam-paired chamber and the total entries were recorded to calculate the CPP score. The time spent in each compartment was also measured. At the end of the withdrawal phase (day 40), the rats were allowed unrestricted entry to all apparatus, and their behavior was observed for 15 minutes.
Behavioral tests
On days 1 and 40, behavioral tests were carried out between 09:00 and 15:00 in a soundproof room with the experimental rats.
Elevated plus maze (EPM)
To assess anxiety-like behaviors, the EPM test was conducted on both day 1 and 40 during the preconditioning and withdrawal phases, respectively. These tests were performed during the light phase of the light/dark cycle. The EPM apparatus was situated in a separate room at a height of 100 cm above the floor. The maze comprised two open arms and two enclosed arms, each with dimensions of 50 × 10 cm. The enclosed arms were equipped with walls measuring 40 cm in height, whilst the open arms were wall-less. The EPM also included a central zone of 10 × 10 cm. At the beginning of each test, the rat was placed in the central zone, facing the intersection of the maze, and observed for exploratory behavior for 10 minutes. In both open and closed arms (defined as having all four legs on the arm) ,the number of entries and the time spent were recorded as the dependent measures [23]. Following each test, the EPM apparatus was cleaned using a 10% ethanol solution to minimize the introduction of pheromonal cues.
Forced swim test (FST)
The FST is a behavioral test commonly used to assess depression-like behavior in rats [24]. Each rat was placed individually in a transparent cylinder measuring 21 cm in diameter and 60 cm in height. The cylinder was filled with water up to a height of 40 cm and maintained at a temperature of 25°C ± 1°C. The duration of immobility was then recorded for a period of 10 minutes. Immobility was characterized as the duration in which the rats displayed minimal movement in the water, demonstrating an inactive attitude such as limited swimming, diving, and jumping, with the primary objective of keeping their heads above water. An elevation in immobility time signifies the presence of a depressive-like effect in the rats’ behavioral patterns.
Open field test (OFT)
To assess exploratory behavior and locomotor activity, the OFT was conducted in a brightly lit room. The animals were individually placed in the center of a white arena measuring 80 × 80 × 40 cm. The arena was divided into 25 equal squares. The rats were given 10 minutes to freely explore the unfamiliar environment. During the observation period, the number of squares crossed by the rats using all four legs and the number of instances where the animals stood on their hind legs (rearing behavior) to explore their environment were recorded [25]. After each test, the OFT apparatus was thoroughly cleaned using a 10% ethanol solution to eliminate any potential olfactory cues that could influence subsequent testing sessions.
Biochemical analyses
Blood samples were collected into centrifugal tubes that were precooled on ice. The samples were allowed to clot for 30 minutes at 25°C, followed by centrifugation at 1,500 × g for 15 minutes. This centrifugation step was performed to separate the serum from the rest of the blood components. The collected serum was then stored at −20°C for later biochemical analysis. The biochemical analysis included quantifying levels of cholesterol, C-reactive protein (CRP), triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and cortisol. The biochemical analyses were performed using a standard technique with a Cobas 6,000 machine from Roche.
Blood collection and preparation of brain tissue samples
After 40 days, the animals were sacrificed by decapitation, and the blood was drawn for biochemical analyses. Later, the rats’ brains were immediately removed and cooled on dry ice. Then, the hippocampus was dissected on ice, with reference to a rat cerebral atlas in order to carry out an enzymatic assay of oxidative stress [26]. The hippocampus was chosen since it is considered the most important brain region in relation to oxidative stress and neurodegeneration associated with Clonazepam dependence; the hippocampus is highly sensitive to oxidative stress-induced damage [27,28].
Oxidative stress assessment
The hippocampus tissues were homogenized in 20-mM Tris-HCl buffer (pH 7.4) on ice, and were subsequently used to determine the levels of lipid peroxidation (LPO), the activities of catalase (CAT) and superoxide dismutase (SOD).
LPO assay
The level of LPO was assessed by measuring the thiobarbituric acid-reacting substances (TBARSs) in the hippocampus homogenates, following a previous protocol [29]. In brief, a portion of the crude homogenate from the hippocampus weighing 100 mg was subjected to centrifugation at 4°C (1,000 × g for 10 minutes). The resulting supernatant was then combined with 1 ml of 10% trichloroacetic acid and 1 ml of 0.67% thiobarbituric acid. The mixture was heated in a boiling water bath for 15 minutes, followed by the addition of butanol (2:1, v/v) to the solution. After another round of centrifugation (800 × g for 05 minutes), the absorbance at 535 nm was measured to determine the concentration of TBARS. The outcomes were expressed as nanomoles of malondialdehyde (MDA) per gram of wet tissue.
CAT activity
CAT activity was determined using an H2O2-dependent method to measure the production of H2O and O2 [30]. In brief, 0.05 ml of the sample was mixed with 1 ml of H2O2 solution (0.019 M) and 1.95 ml of 50-mM phosphate buffer in a 3-ml quartz cuvette. The absorbance of the mixture was measured at 240 nm at time 0 (T0) and then at 30-second intervals for 2 minutes.
SOD activity
SOD activity was determined by measuring its ability to inhibit the photoreduction of nitro blue tetrazolium (NBT) using spectrophotometric methods [31]. The assay systems were prepared by combining 2.4 × 10−6 M riboflavin, 0.01 M methionine, 1.67 × 10−4 M NBT, and 0.05 M potassium phosphate buffer at pH 7.4 and 25°C. This reaction mixture, under aerobic conditions, resulted in a blue color, and its optical density was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme protein that caused a 50% reduction in the rate of NBT reduction.
Statistical analysis
Statistical analysis of the data was conducted using GraphPad Prism Software version 9.00 (San Diego, California, USA). The results are presented as the mean ± SEM. One-way analysis of variance (ANOVA) was performed, followed by post-hoc Tukey’s tests, to assess the differences between groups. A significance level of p < 0.05 was considered statistically significant in determining the observed group differences.
RESULTS
Clonazepam-induced CPP
Clonazepam preference was measured using the CPP test, where rats were administered a daily escalating dose of Clonazepam, starting at 1.5 mg/kg/day during the initial phase. No significant differences were observed in the time spent and percentage of entries to the Clonazepam-paired chamber among the groups (Fig. 1A and B). However, the daily administration of Clonazepam significantly increased the time spent and percentage of entries to the Clonazepam-paired chamber compared to the vehicle group (p < 0.01) and the initial phase (p < 0.01), indicating the establishment of CPP for Clonazepam. The rats spent 235 seconds in the Clonazepam-compartment. In the Clonazepam-dependent withdrawal group treated with AEAP, a decrease was noted in the time spent (121 seconds) and percentage of entries to the drug-paired chamber compared to the Clonazepam-dependent rats (Fig. 1A and B).
Effects of AEAP on rats withdrawn from Clonazepam on behavioral parameters
To evaluate withdrawal symptoms, anxiety-like and depression-like behaviors were assessed by measuring the time spent and the percentage of entries to the open arms in the EPM
test (Fig. 2). During the preconditioning phase, the results showed that there were no significant differences across the groups for EPM and % of entries to the open arms. However, during the withdrawal phase, the Clonazepam withdrawn group exhibited a significant decrease in both the time spent and the percentage of entries to the open arms compared to the vehicle group (p < 0.001) and the preconditioning phase (p < 0.001), indicating the presence of anxiety-like behavior during withdrawal (Fig. 2A and B). On the other hand, both parameters increased in the Clonazepam withdrawn group treated with AEAP versus Clonazepam withdrawn group (p < 0.001), expressing the anxiolytic effect of AEAP (Fig. 2A and B).
As illustrated in Figure 3, a decrease in the number of rearing (Fig. 3A), and number of crossed lines (Fig. 3B) were significantly noticed in Clonazepam-induced rats during the withdrawal phase than vehicles (p < 0.001) and initial phase (p < 0.001), and those traits were mitigated in the Clonazepam-exposed rats treated with AEAP compared to the Clonazepam withdrawn group (p < 0.001). Rats in the AEAP group yielded a significant increase in the number of rearings (Fig. 3A) and the number of lines crossed (Fig. 3B) in the OFT as compared to the normal control rats. Significant differences in the immobility time were observed among the various groups in the FST, which is commonly used to assess depression-like behavior (Fig. 3C), according to ANOVA. Data showed a significant increase in immobility time in Clonazepam withdrawn rats (p < 0.001) than in control rats and the preconditioning phase. However, treatment with AEAP for the Clonazepam-withdrawn group decreased the immobility time as compared to the Clonazepam withdrawn group (p < 0.001), confirming the anti-depressant effect.
Effects of AEAP on rats withdrawn from Clonazepam on biochemical markers
As the withdrawal phase induces stress, cortisol levels were measured as a biochemical marker to assess the stress response. The Clonazepam group showed higher cortisol levels compared to the vehicle group, with values of 13.54 (g/L) ± 1.68 and 6.4 (g/l) ± 2.23, respectively, indicating a significant difference (p < 0.001; Fig. 4F). However, in the Clonazepam-dependent group treated with AEAP, the cortisol level significantly decreased compared to the Clonazepam-dependent group (p < 0.01; Fig. 4F). In addition, CRP, a marker of inflammation produced by the liver, showed a significant increase in the Clonazepam-dependent group compared to the vehicle group (p < 0.001). However, post-treatment with AEAP reduced CRP levels (p < 0.001; Fig. 4E). On the other hand, there were no significant differences in the levels of other biochemical markers, including total cholesterol, triglycerides, HDL, and LDL, among all groups (p > 0.05; Fig. 4A–D).
Effects of AEAP on rats withdrawn from Clonazepam on oxidative stress markers
Figure 5A illustrates the MDA measurement as a means of evaluating LPO due to oxidative stress. The results demonstrated a notable increase in the Clonazepam withdrawal group (35.21 ± 2.43) compared to the vehicle group (11.01 ± 0.30). However, post-treatment with AEAP in the Clonazepam withdrawal group produced a significant reduction in MDA levels (p < 0.001; Fig. 5A), suggesting a positive effect on oxidative stress. In addition, CAT and SOD levels significantly decreased in the Clonazepam withdrawal group compared to the vehicle group (p < 0.001). However, post-treatment with AEAP in the Clonazepam withdrawal rats significantly increased CAT and SOD levels (Fig. 5B and C), further elucidating the beneficial effect of AEAP on oxidative stress.
DISCUSSION
This study aimed to investigate behavioral, biochemical, and oxidative stress response to chronic administration of Clonazepam, and treatment with the AEAP in rats. The current study exhibited that oral administration of A. pyrethrum attenuated withdrawal syndrome in Clonazepam-dependent rats, as shown by decreased preference in the Clonazepam-paired chamber in the CPP test, decreased immobility time in the FST, increased locomotor activity, rearing in the OFT compared to Clonazepam-dependent rats and mitigated Clonazepam-induced oxidative stress.
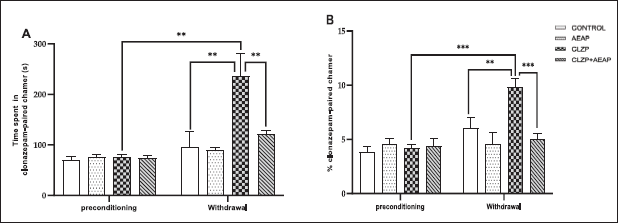 | Figure 1. Effects of A. pyrethrum aqueous extract on rats withdrawn from Clonazepam on (A) time spent and (B) % of entries to the Clonazepam-paired chamber during the CPP test. Data represent mean ± SEM (n = 6 per group). Statistical analyses were done according to one-way ANOVA, followed by Tuckey post hoc test, *** p < 0.0001, **p < 0.01. ns p > 0.5. [Click here to view] |
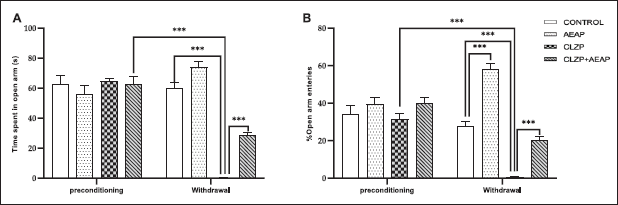 | Figure 2. Effects of A. pyrethrum aqueous extract on rats withdrawn from Clonazepam on (A) time spent in open arm and (B) % of open arm entries in the EPM test. Data represent mean ± SEM (n = 6 per group). Statistical analyses were done according to one-way ANOVA, followed by Tuckey post hoc test, *** p < 0.0001, **p < 0.01. ns p > 0.5. [Click here to view] |
In this study, CPP was utilized to evaluate preference-like behavior in rats. Through this paradigm, the rats learned to associate a specific chamber with Clonazepam administration and, during withdrawal, displayed a preference for the Clonazepam-paired chamber. This current research provides insight into the incentive-motivational value of Clonazepam for dependent rats, particularly when Clonazepam is abruptly stopped after 30 days of continuous administration with an escalating dose regimen designed to mimic the drug consumption pattern observed in humans. Similarly, another study found that rats exhibited a preference for a Clonazepam-associated environment during the acute withdrawal phase, suggesting that the rewarding effects of Clonazepam may contribute to the development of dependence [32]. The resulting brain concentrations were comparable to those that would be expected in humans receiving usual therapeutic doses [33]. The chronic administration of Clonazepam leads to tolerance related to changes in the density and function of GABA type A receptors in the brain. Specifically, chronic activation of these receptors by benzodiazepines leads to a downregulation of receptor density, resulting in a decreased sensitivity to the drug. This downregulation occurs after approximately 7 days of continuous benzodiazepine use and can be observed in various regions of the brain, including the hippocampus, amygdala, and cortex. The downregulation of GABA type A receptors is thought to be a compensatory response to the chronic presence of benzodiazepines, and it can contribute to the development of tolerance and dependence [5,34].
Furthermore, in the current study, increased stress sensitization was observed following Clonazepam dependence. This was assessed by measuring cortisol levels in the blood as an indicator of anxiety-like behavior, which is known to be mediated by the corticotrophin-releasing factor (CRF) system. Chronic administration of benzodiazepines has been shown to affect the functioning of the CRF system, which plays a key role in the regulation of the stress response. Specifically, benzodiazepine use has been associated with changes in the density and function of CRF receptors, as well as alterations in gene expression [34]. These changes can contribute to the development of anxiety and stress-related disorders and may also contribute to the withdrawal syndrome that occurs following benzodiazepine cessation [35].
The current study revealed behavioral impairments in the Clonazepam-dependent group, which included a decrease in the number of crossed lines and rearing in open-field activity. These findings are consistent with previous research that has reported reduced exploratory activity and hypolocomotion in rodents following chronic benzodiazepine administration [36,37]. In addition, the current study demonstrated an increase in stress sensitization in the Clonazepam-dependent rats, as evidenced by elevated cortisol levels in the blood. This finding is consistent with previous research that has linked CRF to anxiety-like behavior during drug withdrawal [38]. Specifically, CRF has been identified as a crucial factor in the manifestation of anxiety-like symptoms during alcohol and benzodiazepine withdrawal. Studies have demonstrated that CRF levels increase in response to stress and are associated with anxiety, depression, and other mood disorders. In the context of substance withdrawal, the release of CRF in the brain has been linked to the onset of withdrawal symptoms such as anxiety, agitation, and dysphoria [39,40].
The current study revealed that daily administration of Clonazepam caused an increase in depressive-like behavior during the withdrawal phase. Prolonged activation of GABAergic receptors by Clonazepam leads to a decrease in serotonin levels; this reduction in serotonin availability subsequently influences neural plasticity and structural modifications, along with CNS hyperexcitability. In the CNS, inhibitory synapses play a crucial role in regulating the number of principal neurons, thereby impacting overall CNS functioning. Moreover, the activation of μ-opioid receptors on GABAergic interneurons within the ventral tegmental area plays a significant role in this context. When these μ-opioid receptors are activated, they inhibit GABAergic interneurons, leading to disinhibition of dopaminergic neurons. Consequently, the release of dopamine in the nucleus accumbens is heightened. These interconnected mechanisms contribute to the complex effects of Clonazepam use on neural function and the development of addictive behaviors [6,41,42].
Oxidative stress is known to play a critical role in various pathological conditions, including drug dependence and withdrawal. In this study, we found that Clonazepam withdrawal resulted in a significant increase in LPO, as evidenced by elevated MDA levels. This finding is consistent with previous studies that have shown a correlation between LPO and drug withdrawal syndrome [43,44]. The observed decrease in CAT and SOD levels further supports the involvement of oxidative stress in Clonazepam withdrawal [45,46]. During chronic exposure to Clonazepam, the continuous activation of GABA receptors leads to a decrease in serotonin levels, which in turn causes a decrease in the activity of antioxidant enzymes [47,48]. This imbalance leads to an accumulation of ROS and oxidative damage to cellular structures, including lipids, proteins, and DNA [49]. Furthermore, dopamine, as a prominent neurotransmitter that is involved in reward and addiction processes, has also been implicated in the interplay between oxidative stress and Clonazepam withdrawal. Dopamine metabolism can generate ROS as byproducts, thereby contributing to oxidative stress [50–52].
Medicinal plants have been extensively used due to their antioxidant potential, safe for long-term use, and being considered an alternative treatment option due to their availability, low cost, and lack of side effects [53]. Anacyclus pyrethrum is a medicinal plant commonly used in traditional medicine to treat various ailments such as rheumatism, toothache, and dyspepsia [54]. It is known to possess several bioactive compounds, including flavonoids, phenols, and terpenoids, which exhibit potent antioxidant and anti-inflammatory activities.
In our preliminary investigation, we conducted a systematic evaluation of AEAP’s pharmacological effects using various doses (100, 200, 400, and 800 mg/kg). Notably, our initial findings highlighted the 200 mg/kg dose as particularly effective in producing therapeutic effects. This pivotal outcome informed our deliberate choice of the 200 mg/kg dose for further exploration in our study [19]. Before addressing the effects of A. pyrethrum on withdrawal, it is crucial to highlight that the current study demonstrated significant anxiolytic and antidepressant activity of the AEAP. In FST, AEAP reduced immobility times compared to the control group, indicating strong antidepressant activity. Similar findings have been reported [55,56], indicating that the ethanolic extract of A. pyrethrum also acts as an antidepressant in mice. The observed decrease in motility is comparable to the effects observed with a reference antidepressant, further supporting the consistency of our results with other studies. In addition, it was proposed that the root extract of A. pyrethrum may exert an antidepressant effect by interacting with the adrenergic or dopaminergic system, resulting in elevated levels of norepinephrine and dopamine [57].
Moreover, in behavioral tests such as the OFT and the EPM, AEAP displayed anxiolytic potential by increasing exploratory and locomotor activities, as evidenced by elevated central crossed lines, peripheral crossed lines, and rearing behavior in the OFT, as well as increased exploration of open arms and higher open arm entries in the EPM. These observed anxiolytic effects are consistent with the results from another study [58] investigating the effects of the ethanol extract of A. pyrethrum in mice, which revealed an increase in the time spent in the light compartment and alterations in the number of shuttle crossings, indicating its anxiolytic activity. The anxiolytic effects observed may be attributed to the compound’s agonistic effects on the GABA/benzodiazepine receptor complex, the 5-HT1A receptor, or its ability to antagonize the 5-HT1B receptor [59,60].
Furthermore, the potential therapeutic value of A. pyrethrum for managing anxiety and depression can be attributed to the presence of bioactive compounds, particularly alkylamides. These compounds are known to increase the level of GABA in the brain, leading to reduced anxiety and promotion of relaxation [19].
Moving on to the effects of A. pyrethrum during Clonazepam withdrawal, our study revealed promising outcomes in mitigating withdrawal syndrome, akin to the observed anxiolytic, antidepressant effects, as well as cortisol and oxidative stress reduction. Upon discontinuation of Clonazepam, animals typically exhibit withdrawal symptoms, which include heightened anxiety, depression, and increased cortisol levels indicative of stress response. However, AEAP treatment displayed a noteworthy alleviation of withdrawal symptoms.
Moreover, in light of the dysregulation of dopamine homeostasis observed during Clonazepam withdrawal, the noteworthy alleviation of withdrawal symptoms demonstrated by treatment with AEAP highlights the potential interplay between neurotransmitter function, oxidative stress, and the observed beneficial effects during Clonazepam withdrawal. The dysregulation of both serotonin and dopamine systems during Clonazepam withdrawal likely contributes to the generation of oxidative stress, further exacerbating cellular damage. This complex relationship between neurotransmitters, GABA receptors, oxidative stress, and the observed alleviation of withdrawal symptoms underscores the potential therapeutic value of A. pyrethrum in managing Clonazepam withdrawal [61,62].
Anacyclus pyrethrum has been shown to have a protective effect against several neurological disorders, such as Alzheimer’s and Parkinson’s diseases; these disorders are also associated with oxidative stress. The neuroprotective properties of A. pyrethrum can be attributed to its antioxidant and anti-inflammatory activities [63]. The phytochemical screening of A. pyrethrum revealed the presence of saponins, terpenoids, flavonoids, tannins, and alkaloids, and those secondary metabolites enhance GABA transmission [21,64,65]. Moreover, the biochemical screening of the roots of A. pyrethrum, showed they contain principally pellitorine as a main active constituent, which is a N-isobutyl amide alkaloid [66]. Alkaloids, including pellitorine, exhibit antioxidant properties and can contribute to the reduction of oxidative stress through multiple mechanisms. These alkaloids can directly scavenge ROS, neutralizing them and preventing oxidative damage to cells. In addition, alkaloids have been shown to stimulate the activity or expression of endogenous antioxidant enzymes, which play a vital role in protecting cells from oxidative stress. Moreover, alkaloids can modulate signaling pathways, including those involving dopamine and GABA, which are neurotransmitters involved in various physiological functions. Dopamine and GABA have been implicated in antioxidant defenses and neuroprotection, and their interaction with alkaloids may further enhance the cellular antioxidant response. Furthermore, alkaloids may influence the balance of GABAergic neurotransmission, which can impact oxidative stress levels. By reducing oxidative stress, alkaloids, in combination with dopamine and GABA, have the potential to support cellular well-being and protect against oxidative damage [67,68].
In summary, the combination of A. pyrethrum’s anxiolytic and antidepressant properties, along with its antioxidant and anti-inflammatory activities, holds promise for the management of various aspects of Clonazepam dependence. The present study provides valuable insights into the behavioral, biochemical, and oxidative stress responses associated with A. pyrethrum and Clonazepam withdrawal. However, it is important to acknowledge the study’s limitations. The focus was primarily on withdrawal syndrome and oxidative stress without delving extensively into other potential mechanisms or pathways involved in these processes. Future investigations should explore additional molecular and cellular mechanisms to further elucidate the observed effects.
CONCLUSION
In conclusion, our study provides compelling evidence that exposure to Clonazepam leads to depression-like behavior and biochemical alterations accompanied by increased oxidative stress levels. However, the administration of A. pyrethrum aqueous extract effectively mitigated the dependence-like behavior induced by Clonazepam. Importantly, the ability of AEAP to modulate oxidative stress is considered one of the contributing factors to its protective effects on Clonazepam-exposed rat stress in the brain. These findings highlight the potential of A. pyrethrum as a promising therapeutic approach for managing the adverse effects of Clonazepam dependence, by attenuating oxidative stress-induced damage; it represents a novel and natural alternative for alleviating the unfavorable consequences associated with prolonged Clonazepam use. Further investigation is required to gain a deeper understanding of the molecular mechanisms responsible for the observed effects and to fully explore the therapeutic potential of A. pyrethrum in addressing SUD.
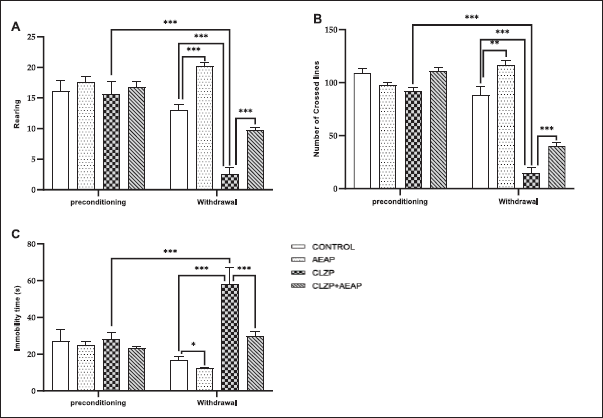 | Figure 3. Effects of A. pyrethrum aqueous extract on rats withdrawn from Clonazepam on (A) the number of rearing, (B) the number of crossed lines in EPM, and (C) the immobility time in FST. Data represent mean ± SEM (n = 6 per group). Statistical analyses were done according to one-way ANOVA, followed by Tuckey post hoc test, *** p < 0.0001, **p < 0.01. ns p > 0.5. [Click here to view] |
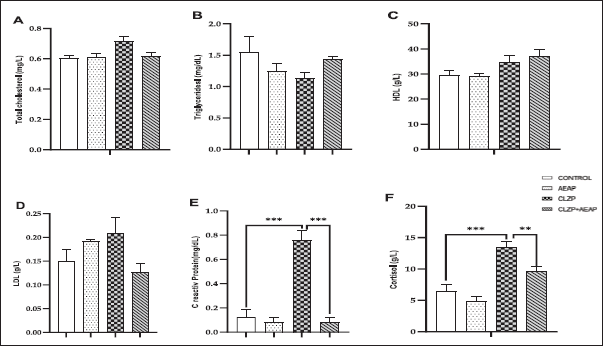 | Figure 4. Effects of A. pyrethrum aqueous extract on rats withdrawn from Clonazepam on the biochemical analyses (A) total cholesterol, (B) Triglycerides, (C) HDL, (d) LDL, (e) C reactive protein, and (f) Cortisol during the withdrawal phase. Data represent mean ± SEM (n = 6 per group). Statistical analyses were done according to one-way ANOVA, followed by Tuckey post hoc test, *** p < 0.0001, **p < 0.01. ns p > 0.5. [Click here to view] |
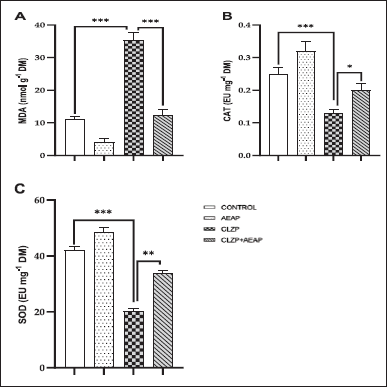 | Figure 5. Effects of A. pyrethrum aqueous extract on rats withdrawn from Clonazepam on oxidative stress: (A) MDA, (B) CAT, (C) SOD during the withdrawal phase. Statistical analyses were done according to one-way ANOVA, followed by Tuckey post hoc test, *** p < 0.0001, **p < 0.01. ns p > 0.5. [Click here to view] |
ACKNOWLEDGMENT
The authors express their gratitude to Abderrazak Regragui for his assistance in animal handling during the study. The authors also extend their appreciation to Hamdaz Ilham, Sali Siham, Mouaniss Hayat, and Jouhafa Saloua for their valuable contributions in conducting the biochemical analyses.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work
ETHICAL APPROVALS
All procedures involving animals were carried out in compliance with the guidelines outlined by the European Council Directive for Care and Use of Laboratory Animals (EU2010/63). Before the commencement, the Institutional Local Review Board approved the study protocol for animal experimentation, with the protocol code CA965/07/22 and an approval date of October 2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. UNODC. UNODC World Drug Report 2022 highlights trends on cannabis post-legalization, environmental impacts of illicit drugs, and drug use among women and youth. Vienna, Austria. UNODC [cited 2023 Mar 18]. Available from: https://www.unodc.org/unodc/en/frontpage/2022/June/unodc-world-drug-report-2022-highlights-trends-on-cannabis-post-legalization--environmental-impacts-of-illicit-drugs--and-drug- use-among-women-and-youth.html
2. Volkow ND, Boyle M. Neuroscience of addiction: relevance to prevention and treatment. Am J Psychiatry. 2018 Aug 1;175(8):729–40. CrossRef
3. Olfson M, Wall MM, Liu SM, Blanco C. Cannabis use and risk of prescription opioid use disorder in the United States. Am J Psychiatry. 2018 Jan;175(1):47–53. CrossRef
4. de la Iglesia-Larrad JI, Barral C, Casado-Espada NM, de Alarcón R, Maciá-Casas A, Vicente Hernandez B, et al. Benzodiazepine abuse, misuse, dependence, and withdrawal among schizophrenic patients: a review of the literature. Psychiatry Res. 2020 Feb;284:112660. CrossRef
5. Galpern WR, Lumpkin M, Greenblatt DJ, Shader RI, Miller LG. Chronic benzodiazepine administration. VII. Behavioral tolerance and withdrawal and receptor alterations associated with clonazepam administration. Psychopharmacology (Berl). 1991;104(2):225–30. CrossRef
6. Longo LP, Johnson B. Addiction: part I. Benzodiazepines—side effects, abuse risk and alternatives. Am Fam Physician. 2000 Apr 1;61(7):2121–8.
7. Riss J, Cloyd J, Gates J, Collins S. Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008 Aug;118(2):69–86. CrossRef
8. Sadock B, Sadock VA, Sussman N. Kaplan & Sadock’s pocket handbook of psychiatric drug treatment. Philadelphia, PA: Lippincott Williams & Wilkins; 2017.
9. Lader M, Kyriacou A. Withdrawing benzodiazepines in patients with anxiety disorders. Curr Psychiatry Rep. 2016 Jan 6;18(1):8. CrossRef
10. Vorspan F, Hjelmström P, Simon N, Benyamina A, Dervaux A, Brousse G, et al. What place for prolonged-release buprenorphine depot-formulation Buvidal® in the treatment arsenal of opioid dependence? Insights from the French experience on buprenorphine. Expert Opin Drug Deliv. 2019 Sep 2;16(9):907–14. CrossRef
11. Berríos-Cárcamo P, Quezada M, Quintanilla ME, Morales P, Ezquer M, Herrera-Marschitz M, et al. Oxidative stress and neuroinflammation as a pivot in drug abuse. A focus on the therapeutic potential of antioxidant and anti-inflammatory agents and biomolecules. Antioxidants. 2020 Sep;9(9):830. CrossRef
12. Sahoo AK, Dandapat J, Dash UC, Kanhar S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J Ethnopharmacol. 2018 Apr 6;215:42–73. CrossRef
13. Yazici S, Demirtas S, Guclu O, Karahan O, Yavuz C, Caliskan A, et al. Using oxidant and antioxidant levels to predict the duration of both acute peripheral and mesenteric ischemia. Perfusion. 2014 Sep 1;29(5):450–5. CrossRef
14. Gass JT, Foster Olive M. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008 Jan 1;75(1):218–65. CrossRef
15. Wiss DA, Avena N, Rada P. Sugar addiction: from evolution to revolution. Front Psychiatry [Internet]. 2018 [cited 2023 Apr 20];9:545. CrossRef
16. Pahuja M, Mehla J, Reeta KH, Joshi S, Gupta YK. Root extract of Anacyclus pyrethrum ameliorates seizures, seizure-induced oxidative stress and cognitive impairment in experimental animals. Epilepsy Res. 2012 Feb;98(2–3):157–65. CrossRef
17. OECD. Guidelines for the testing of chemicals. Acute oral toxicity—acute toxic Cl method test no-423. Paris, France: OECD; 2001.
18. Seip KM, Reed B, Ho A, Kreek MJ. Measuring the incentive value of escalating doses of heroin in heroin-dependent Fischer rats during acute spontaneous withdrawal. Psychopharmacology (Berl). 2012 Jan;219(1):59–72. CrossRef
19. Baslam A, Aitbaba A, Lamrani Hanchi A, Tazart Z, Aboufatima R, Soraa N, et al. Modulation of gut microbiome in ecstasy/MDMA-induced behavioral and biochemical impairment in rats and potential of post-treatment with Anacyclus pyrethrum L. Aqueous extract to mitigate adverse effects. Int J Mol Sci. 2023 Jan;24(10):9086. CrossRef
20. Bezza K, Laadraoui J, Gabbas ZE, Laaradia MA, Oufquir S, Aboufatima R, et al. Effects of Anacyclus pyrethrum on affective behaviors and memory during withdrawal from cigarette smoke exposure in rats. Pharmacogn Mag. 2020 Jan 3;16(68):123. CrossRef
21. Manouze H, Bouchatta O, Gadhi AC, Bennis M, Sokar Z, Ba-M’hamed S. Anti-inflammatory, antinociceptive, and antioxidant activities of methanol and aqueous extracts of Anacyclus pyrethrum roots. Front Pharmacol. 2017;8:598. CrossRef
22. Biswas A, Banerjee S. Effect of Valeriana wallichii on alcohol addiction in mice. Pharmacogn Mag. 2018 Jan 10;14(59):613. CrossRef
23. Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997 Nov 7;21(6):801–10. CrossRef
24. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978 Feb 15;47(4):379–91. CrossRef
25. Gould TD, Dao DT, Kovacsics CE. The open field test. In: Gould TD, editor. Mood and anxiety related phenotypes in mice: characterization using behavioral tests [Internet]. Totowa, NJ: Humana Press; 2009 [cited 2023 Apr 20]. pp. 1–20. CrossRef
26. Roland PE, Zilles K. Brain atlases—a new research tool. Trends Neurosci. 1994 Jan 1;17(11):458–67. CrossRef
27. Sinha JK, Ghosh S, Swain U, Giridharan NV, Raghunath M. Increased macromolecular damage due to oxidative stress in the neocortex and hippocampus of WNIN/Ob, a novel rat model of premature aging. Neuroscience. 2014 Jun 6;269:256–64. CrossRef
28. Hemati K, Pourhanifeh MH, Dehdashtian E, Fatemi I, Mehrzadi S, Reiter RJ, et al. Melatonin and morphine: potential beneficial effects of co-use. Fundam Clin Pharmacol. 2021 Feb;35(1):25–39. CrossRef
29. Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993 May 1;57(5):779S–86S. CrossRef
30. Aebi H. [13] Catalase in vitro. In: Methods in enzymology [Internet]. Cambridge, MA: Academic Press; 1984 [cited 2023 Apr 20]. 121–6 pp. CrossRef
31. Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov 1;44(1):276–87. CrossRef
32. McLelland AE, Martin-Iverson MT, Beninger RJ. The effect of quetiapine (SeroquelTM) on conditioned place preference and elevated plus maze tests in rats when administered alone and in combination with (+)-amphetamine. Psychopharmacology (Berl). 2014 Nov 1;231(22):4349–59. CrossRef
33. Miller FD, Tetzlaff W, Bisby MA, Fawcett JW, Milner RJ. Rapid induction of the major embryonic alpha-tubulin mRNA, T alpha 1, during nerve regeneration in adult rats. J Neurosci Off J Soc Neurosci. 1989 Apr;9(4):1452–63. CrossRef
34. Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PRA. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015 May;16(5):305–12. CrossRef
35. Friedman H, Abernethy DR, Greenblatt DJ, Shader RI. The pharmacokinetics of diazepam and desmethyldiazepam in rat brain and plasma. Psychopharmacology (Berl). 1986;88(3):267–70. CrossRef
36. Barry JM, Costall B, Kelly ME, Naylor RJ. Withdrawal syndrome following subchronic treatment with anxiolytic agents. Pharmacol Biochem Behav. 1987 Jun 1;27(2):239–45. CrossRef
37. Haider S, Nawaz A, Batool Z, Tabassum S, Perveen T. Alleviation of diazepam-induced conditioned place preference and its withdrawal-associated neurobehavioral deficits following pre-exposure to enriched environment in rats. Physiol Behav. 2019 Sep 1;208:112564. CrossRef
38. Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, et al. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010 Jan 1;332(1):298–307. CrossRef
39. Baldwin DC Jr, Hughes PH, Conard SE, Storr CL, Sheehan DV. Substance use among senior medical students: a survey of 23 medical schools. JAMA. 1991 Apr 24;265(16):2074–8. CrossRef
40. Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993 Mar 5;605(1):25–32. CrossRef
41. Anisman H, Merali Z, Poulte MO. Gamma-Aminobutyric acid involvement in depressive illness interactions with corticotropin-releasing hormone and serotonin. In: Dwivedi Y, editor. The neurobiological basis of suicide [Internet]. Boca Raton, FL: CRC Press/Taylor & Francis; 2012 [cited 2022 Dec 13]. CrossRef
42. Hossen MDA, Ali Reza ASM, Amin MDB, Nasrin MSTS, Khan TA, Rajib MDHR, et al. Bioactive metabolites of Blumea lacera attenuate anxiety and depression in rodents and computer-aided model. Food Sci Nutr. 2021 May 31;9(7):3836–51. CrossRef
43. Kannan N, Sakthivel KM, Guruvayoorappan C. Protective effect of Acacia nilotica (L.) against acetaminophen-induced hepatocellular damage in Wistar rats. Adv Pharmacol Sci. 2013;2013:987692. CrossRef
44. Kachungwa Lugata J, Ortega ADSV, Szabó C. The role of methionine supplementation on oxidative stress and antioxidant status of poultry-a review. Agriculture. 2022 Oct;12(10):1701. CrossRef
45. de Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, et al. Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res. 2014 Jan 1;258:52–64. CrossRef
46. Yasar H, Demirdogen F, Suleyman B, Mammadov R, Altuner D, Coban TA, et al. Effect of thiamine pyrophosphate upon oxidative brain injury induced by ischemia-reperfusion in rats. Int J Pharmacol. 2023 Mar 15;19(2):277–85. CrossRef
47. Wayhs CAY, Mescka CP, Vanzin CS, Ribas GS, Guerreiro G, Nin MS, et al. Brain effect of insulin and clonazepam in diabetic rats under depressive-like behavior. Metab Brain Dis. 2013 Dec 1;28(4):563–70. CrossRef
48. Luo W, Liu W, Huang Y, Deng X. Anticonvulsant and proconvulsant effects of trazodone in different seizure models. Int J Pharmacol. 2022 Sep 15;18(7):1474–81. CrossRef
49. Dinc K, Kiremitli T, Kiremitli S, Ozyurt R, Bulut B, Bulut S, et al. Effect of taxifolin on acrylamide-related oxidative ovarian damage, infertility and intrauterine growth retardation in female rats. Int J Pharmacol. 2023 Mar 15;19(2):244–52. CrossRef
50. Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018 May 1;15:490–503. CrossRef
51. Hsieh HL, Wang HH, Wu WB, Chu PJ, Yang CM. Transforming growth factor-β1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-κB pathways. J Neuroinflammation. 2010 Dec 6;7(1):88. CrossRef
52. Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS, et al. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci Lett. 2015 Jan 1;584:191–6. CrossRef
53. Nissen N. Practitioners of Western herbal medicine and their practice in the UK: beginning to sketch the profession. Complement Ther Clin Pract. 2010 Nov 1;16(4):181–6. CrossRef
54. Elazzouzi H, Fadili K, Cherrat A, Amalich S, Zekri N, Zerkani H, et al. Phytochemistry, biological and pharmacological activities of the Anacyclus pyrethrum (L.) Lag: a systematic review. Plants. 2022 Jan;11(19):2578. CrossRef
55. Usmani A, Khushtar M, Arif M, Siddiqui MA, Sing SP, Mujahid M. Pharmacognostic and phytopharmacology study of Anacyclus pyrethrum: an insight. J Appl Pharm Sci. 2016 Mar 30;6(3):144–50. CrossRef
56. Richelson E. Pharmacology of antidepressants. Mayo Clin Proc. 2001 May 1;76(5):511–27. CrossRef
57. Badhe SR, Badhe RV, Ghaisas MM, Chopade VV, Deshpande AD. Evaluations of antidepressant activity of Anacyclus pyrethrum root extract. Int J Green Pharm IJGP [Internet]. 2010 [cited 2023 Jun 17];4(2):79–82. Available from: CrossRef
58. Patil VP, Nanjappaiah HM, Chandrashekhar VM, Mucchandi IS, Hugar S, Kalyane NV. Evaluation of anti-anxiety activity of Anacyclus pyrethrum. Int Res J Pharm. 2018;8(12):46–9. CrossRef
59. Millan MJ, Hjorth S, Samanin R, Schreiber R, Jaffard R, De Ladonchamps B, et al. S 15535, a novel benzodioxopiperazine ligand of serotonin (5-HT)1A receptors: II. Modulation of hippocampal serotonin release in relation to potential anxiolytic properties. J Pharmacol Exp Ther. 1997 Jul;282(1):148–61.
60. Nishikawa H, Hata T, Itoh E, Funakami Y. A role for corticotropin-releasing factor in repeated cold stress-induced anxiety-like behavior during forced swimming and elevated plus-maze tests in mice. Biol Pharm Bull. 2004 Mar;27(3):352–6. CrossRef
61. Sarwar R, Farooq U, Naz S, Khan A, Bukhari SM, Khan H, et al. Isolation and characterization of two new secondary metabolites from Quercus incana and their antidepressant-and anxiolytic-like potential. Front Pharmacol. 2018 Apr 18;9:298. CrossRef
62. Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011 May 15;14(10):2013–54. CrossRef
63. Singh S, Singh TG, Rehni AK. An insight into molecular mechanisms and novel therapeutic approaches in Epileptogenesis. CNS Neurol Disord Drug Targets. 2020;19(10):750–79. CrossRef
64. Bezza K, Gabbas ZE, Laadraoui J, Laaradia MA, Oufquir S, Chait A. Ameliorative potential of Anacyclus pyrethrum extract in generalized seizures in rat: possible cholinergic mediated mechanism. Bangladesh J Pharmacol. 2019 Dec 14;14(4):188–95. CrossRef
65. Singh P, Singh D, Goel RK. Phytoflavonoids: antiepileptics for the future. Int J Pharm Pharm Sci. 2014 Aug 31;6(8)51–66.
66. Zaidi SMA, Pathan SA, Singh S, Jamil S, Ahmad FJ, Khar RK. Anticonvulsant, anxiolytic and neurotoxicity profile of Aqarqarha (Anacyclus pyrethrum) DC (Compositae) root ethanolic extract. Pharmacol Pharm. 2013 Oct 7;04(07):535. CrossRef
67. Macáková K, Afonso R, Saso L, Mlad?nka P. The influence of alkaloids on oxidative stress and related signaling pathways. Free Radic Biol Med. 2019 Apr 1;134:429–44. CrossRef
68. Taveira M, Sousa C, Valentão P, Ferreres F, Teixeira JP, Andrade PB. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J Steroid Biochem Mol Biol. 2014 Mar 1;140:106–15. CrossRef