INTRODUCTION
Bacterial resistance has become a worldwide problem that endangers the efficacy of existing antibiotics to treat infections, especially with the emergence of multidrug-resistant (MDR) strains of bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) and MDR strains of Escherichia coli. Antimicrobial resistance may be attributed to several factors, including the formation of biofilms. Biofilms are complex communities of microorganisms that produce a polymeric extracellular matrix on the surfaces that can inhibit the penetration of antibiotics, making them more resistant than planktonic cells [1–3]. In addition to modifying existing drugs, researchers are looking for new antibiotics by exploring marine organisms. Since the ocean covers about 70% of the earth, it is undeniable that marine environments have more enormous biodiversity compared to terrestrial creatures and contain very promising bioactive compounds to explore. The life of marine organisms varies depending on their surrounding environmental conditions, such as temperature, light, salinity, pressure, and depth of their habitat. They have different evolutionary systems, metabolic pathways, and ecology from terrestrial creatures [4,5], which leads to distinctive chemical composition, complexity, and biological efficacy [6,7].
Marine sponges are known to serve as a source of food and are commonly found throughout the world’s oceans. Sponges are soft, sessile, and able to survive in water with strong currents, since the current provides food and oxygen for its life. They can live in various marine habitats, from shallow to deep seas [8,9]. Sponges are immobile and vulnerable to predators, producing metabolite compounds in self-defense [10]. Many studies report that metabolite compounds contained in sponges have therapeutic activities, including cytotoxic activity [11,12], antimicrobial activity [13,14], antiplasmodial activity [15], and other activities. Given the large potential of sponges as food and medical resources, especially by way of isolating their active compounds, which makes it is necessary to pay attention to the survival of organisms and their environment. Interestingly, sponges can associate with various microbes, both fungi and bacteria. These associated microbes produce metabolites similar to their host but may also create novel active metabolites [16–18]. Some metabolites of sponge-associated microbes have chemical structures similar to terrestrial species, making them a great resource for further exploration, especially as antibacterial agents [19–21].
This review aims to examine isolated metabolite compounds from sponge-associated fungi with antibacterial and biofilm inhibitor activity, including the genus that produces the most active compounds, their antibacterial and antibiofilm potential, and to find out the relationship between antibacterial and biofilm inhibitor activity. The original article used various potential values as a way to compare their antibacterial potential by way of changing its μg/ml units based on the molecular weight. However, the difficult conversion forces it to preserve its original value. This review is expected to assist many researchers in exploring compounds with potential development as new antibacterial and antibiofilm to that “supply issues” to avoid sponge exploitation.
MATERIAL AND METHODS
This review highlights metabolite compounds from fungi associated with sponges exhibiting antibacterial and antibiofilm activity. The data presented were derived from primary articles in English related to keywords “marine” OR “sea” AND “sponge-associated fungi” OR “marine-derive fungi” AND “antibacterial” AND “antibiofilm,” which was discovered in 2002–2022 from Scopus and Pubmed. Variables discussed include genus/species of fungi associated with sponges, species of sponges, metabolite compounds, their potential value, and the type of bacteria being inhibited. The minimum inhibitory concentration (MIC) (units μg/ml or μM) or the diameter of inhibition/DOI (unit mm) of metabolites indicates their ability to inhibit bacterial growth. The potential of antibiofilms is shown by their ability to inhibit the formation of biofilms (% inhibition of biofilms).
Sponge-associated fungi as a source of antibacterial
Many studies reported the activity of sponges, including antimicrobial, cytotoxic, anti-inflammatory, antidiabetic, and antimalarial [22–26]. Microorganisms are closely related to sponge life because of the nature of sea sponges as feeder fillers; their body tissues are related to these microorganisms. These associated microorganisms provide host advantages regarding nutrient supply, skeletal stabilization, regulating waste, and secondary metabolite production [27–30]. Fungi associated with sponges have shown the ability to produce unique and biologically active structures. On this basis, utilizing appropriate biotechnology to isolate metabolite compounds from fungi associated with sponges is likely to be developed as new drugs. Until 2022, there had been 55 articles related to fungi associated with sponges with antibacterial and antibiofilm activity. During these two decades (2002–2022), there was a notable increase in the number of publications (Fig. 1).
The highest publication was found in the 2017–2021 period with 27 articles, which constantly escalated in terms of quantity. Countless scientists were found to show a growing interested in studying sponges associated with microbes, thus indicating a trend considering the need to maintain the existence of sponges. We identified that the most frequently studied families were Trichocomaceae, Hypocreaceae, Herpotrichiellaceae, and Pleosporaceae (Fig. 2), and the genus most commonly studied were Aspergillus, Penicillium, Trichoderma, and Neosartorya (Fig. 3A and B). Some genus were the most prolific sources of antibiotics [31,32].
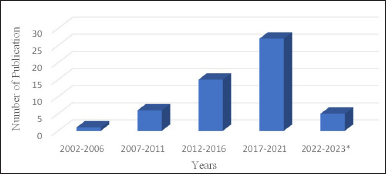 | Figure 1. An overview of research related to sponge-associated fungi with antibacterial and anti-biofilm activity. January 2023*. [Click here to view] |
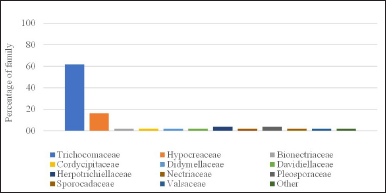 | Figure 2. The distribution of the family of sponge-associated fungi with antibacterial and biofilm inhibitors activity (N = 55). [Click here to view] |
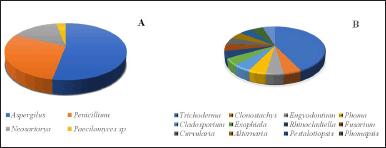 | Figure 3. The distribution of the genus sponge-associated fungi with antibacterial and antibiofilm activity. (A) Family Trichocomaceae (B) Other than Trichocomaceae. [Click here to view] |
Genus Aspergillus
Aspergillus contains various chemical metabolites likely to be developed into new drugs, including antimicrobial properties. Marine life contains abundant resources, which thus caught the attention of many researchers. Studies on antibacterial chemicals generated by Aspergillus fungi associated with sponges are presented in Table 1. The compounds of averantine (1) and nidurufin (2) are anthraquinones isolated from the fungus Aspergillus versicolor associated with the sponge Petrosia sp. in the Jeju Sea, Korea. Both of them showed antibacterial activity against gram-positive clinical isolate bacteria, including Staphylococcus aureus SG511, S. aureus 285, S. aureus 503, and most sensitive to Streptococcus pyogenes 308A. Other compounds, including sterigmatocystin, which is a xantone, and methyl-averantin showed potential as potent antitumors against human tumor cells A-549, SK-OV-3, SK-MEL-2, XF-498, HCT-15 indicated by IC50 values ranging from 0.41 to 4.61 μg/ml [33]. The cytotoxic activity of the averantin compound is likely due to the presence of free hydroxyl derivatives, which affect its polarity.
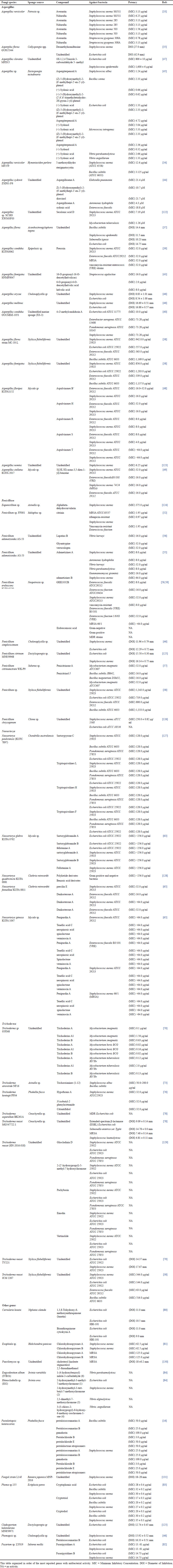 | Table 1. Collected data from sponge-associated fungi with antibacterial activity. [Click here to view] |
Another strain from A. versicolor is MF359, derived from the sponge Hymeniacidon perleve in the Bohai Sea, China, isolated 5-methoxydihydrosterigmatocystin (3) and showed antibacterial activity against S. aureus ATCC 6538 and Bacillus subtilis ATCC 6633, which are better than the extract by the Minimum Inhibitory Concentration (MIC) values. In terms of its ability as an antibacterial against MRSA (clinical isolate) and Pseudomonas aeruginosa ATCC 15692, the MIC value was >100 μg/ml. This study also has been able to isolate hemiacetal sterigmatocystin, acyl-hemiacetal sterigmatocystin, and aversin [34]. The fungus Aspergilus flavus GU815344 derived from the sponge Callyspongia spp. in the sea of Kovalam, India, generates desmethylnomifensine (GC-MS verified) to serve as an antibacterial against S. aureus and E. coli. The inhibitory power of extracts derived from biomass is smaller than extracts from supernatants based on the diameter clear zone against E. coli and S. aureus bacteria [35]. Similar to the research by Samirana et al. [36], the supernatant extract from Trichoderma reesei TV 221 associated sponge Stylissa flabelliformis showed antibacterial activity better than the biomass extract. Aspergillus flavus IBI 141 and Aspergillus sp IBI 151 associated with the sponge Acanthostrongylophora ingens from Mendeh Island, south coast of West Sumatra, Indonesia, showed antibacterial activity against bacteria B. subtilis, Staphylococcus epidemidis, Salmonella typosa, and E. coli. It also has cytotoxic activity against shrimp larvae, with an LC50 value of 58.56 ppm. The compound class of the fungus A. flavus IBI 141 is phenolics and terpenoids [37].
Several fungi were isolated from the sponge S. flabelliformis, including T. reesei strain JCM 2267, A. flavus strain MC-10-L, Penicillium sp, and Aspergillus fumigatus [38]. Extracts from the fungus were tested for antibacterial ability against S. aureus ATCC 25923, E. coli ATCC 25922, Enterococcus faecalis ATCC 29212, and B. subtilis ATCC 6633. The MIC values of A. flavus fungal extract strain MC-10-L and A. fumigatus are presented in Table 1. The antibacterial activity of A. flavus and A. fumigatus fungi is found to be moderate. The cytotoxic ability of A. flavus strain MC-10-L extract against T47D tumor cells was shown with an IC50 value of 550 (mg/ml). Based on analysis with GC-MS, ethyl acetate extract from the fungus contains cyclohexanone, which requires further research.
The compound preussin (4), as a class of hydroxy pyrrolidine alkaloids, was isolated from the fungus Aspergillus candidus KUFA 0062 associated with the sponge Epipolasis sp. originates from coral reefs in Similan Island National Park in Phang-Nga province, Southern Thailand. This preussin showed better inhibitory activity than its analogues, namely preussin C against bacteria S. aureus ATCC 29213, E. faecalis ATCC 29212, MRSA, and vancomycin-resistant enterococci (VRE). Another activity shown by preussin was inhibited by almost 50% biofilm formation from E. coli ATCC 25922 (42.8% ± 32.7% control), S. aureus ATCC 29213, and E. faecalis ATCC 29212 at concentrations equal to above MIC (Table 2), when preussin was combined with vancomycin antibiotics, oxacillin, and colistin showed a relatively strong synergistic effect. The cytotoxic effect of preussin can reduce the viability of colon cancer cells (6.4% and 8.6% in HCT116 and HT29). The presence of the N-methyl group on the pyrrolidine ring in preussin provides an antibacterial effect and cytotoxic activity. It can serve as a candidate for new antibiotics and anticancers. Other compounds isolated from this fungus are chrysophanic acid and petromurin C [39].
The p-terphenyl alcohol group, 4-O-methylcandidusin A, which was isolated from the fungus A. candidus OUCMDZ-1051, showed a potent and selective cytotoxic effect against MDA-MB-468, BT474, and A431 cancer cells. Its ability to inhibit the growth of E. coli bacteria is demonstrated by the MIC value of 10.0 μg/ml using the dilution method. Hence, it can potentially be developed as an anticancer and antibacterial [40]. Natural p-terphenyls have been widely reported, where the structural diversity is due to variations in ring B, position, and number of hydroxy groups [41,42]. The 16-O-propionyl-16-O-deacetylhelvolic acid (5), 6-O-propionyl-6-O-deacetylhelvolic acid (6), and helvolic acid (7) isolated from the fungus A. fumigatus HNMF0047 effective against Streptococcus agalactiae even though the MIC value was lower than tobramycin as a control. The 6-O-propionyl-6-O-deacetylhelvolic acid has more potent antimicrobial effects than helvolic acid because propionyloxy replaces the acetoxy group at C-6 in helvolic acid [43]. Ethyl acetate extract from the fungus Aspergillus sydowii ZSDS1-F6 fermented using oligotropic medium reported contain sesquiterpenes of bisabolen groups including aspergillusene A, (Z)-5-(Hydroxymenthyl)-2-(6′)-methylhept-2′-en-2′-yl)-phenol, diorcinol, and sydonic acid [44]. Aspergillusene A, (Z)-5-(Hydroxymenthyl)-2-(6′-methylhept-2′-en-2′-yl)-phenol, and diorcinol inhibit the bacterial growth of Klebsiella pneumonia and Aeromonas hydrophila. Except for aspergillusene, both compounds were against H3N2 with IC50 of 57.4 and 66.5 μM. Compounds (Z)-5-(Hydroxymethyl)-2-(6′-methylhept-2′-en-2′-yl)-phenol and (+)-sydonic acid were also found in the fungus Aspergillus sp. associated with the sponge Xestospongia testudinaria in the South China Sea [45]. Based on the identification and elucidation structure, the fungus also contains (−)5-(hydroxymethyl)-2-(2′,6′,6′-trimethyltetrahydro-2H-pyran-2-yl)-phenol, Aspergiterpenoid A, and (+)-sydonol. These compounds show antibacterial activity with MIC values ranging from 1.25 to >20 μM against some bacteria (Table 1). All compounds showed weak cytotoxic effects (IC50 >50 μg/ml) against human promyelocytic leukemia HL-60 and human lung carcinoma cell A-549. None of the compounds showed cholinesterase inhibitory activity.
Sponge Chelonaplysilla sp., which was isolated from 12 fungi-associated were Aspergillus oryzae, Phomompsis sp., Penicillium simplicissimum, Beauveria bassiana, and Aspergillus mellinus [46]. The extracts of A. oryzae and A. mellinus were more sensitive to S. aureus and E. coli, demonstrated by a diameter clear zone ranging from 8.54 to 16.88 mm. The cytotoxic effect is shown by extracts from the fungus Phomompsis sp. with an IC50 value of 83.69 μg/ml. It was concluded that the fungus associated with the sponge Chelonaplysilla sp. can be utilized as a novel antibacterial and anticancer therapeutic agent. The fungal strain Aspergillus clavatus MFD15 yielded the compound 1H-1,2,4 Triazole 3-carboxaldehyde 5-methyl, which exhibited antibacterial properties against Staphylococcus epidermidis and E. coli [47] with the MIC values of more than 500 μg/ml The strains showing MIC values exceeding 500 μg/ml indicated low efficacy.
Prenylated phenylbutyrolactone group compounds, namely aspulvinone R, S, T, isolated from the fungus Aspergillus flavipes KUFA1152 associated with the sponge Mycale sp. from Samasean Island, Gulf of Thailand, Chonburi Province. Aspulvinone R (8) and aspulvinone S (9) exhibit potential as novel drugs for their antibacterial activity against E. faecalis ATCC 29212, S. aureus ATCC 29213, E. faecalis B3/101 (VRE), and S. aureus 66/1 (MRSA) evidenced by their MIC values ranging from 4.0 to 16.0 μg/ml [48]. Aspergillus stellatus KUFA 2017 was also reported to be associated with the Mycale sp. The presence of 5[(3E,5E)-nona-3,5-dien-1-yl]-benzene (10) in the fungal extract has antibacterial activity against E. faecalis ATCC 29212, E. faecalis B3/101 (VRE), S. aureus ATCC 29213, and S. aureus 74/24 (MRSA) with MIC values ranging from 16.0 to 64.0 μg/ml. It also significantly inhibited nitric oxide production in lipopolysaccharide-induced raurine macrophage RAW264.7 cells and was found to contain antibiofilm activity [49].
Genus Penicillium
Penicillium has been widely acknowledged and proven as an antibiotic since its discovery by Fleming [50]. Research on this genus has continued to expand the understanding of its potential benefits on medication. Penicillium species provide a wide range of metabolite compounds and their biological activities either from terrestrial or marine environments [51]. Several publications regarding the antibacterial activity of the genus Penicillium fungi associated with sponges are listed in Table 1. Some studies summarized in this review did not isolate and identify the structure. Hence, which chemicals are involved in the pharmacological activity is still unclear, and additional investigation is required.
 | Table 2. Summary of research on sponge-associated fungi and their antibiofilm activity. [Click here to view] |
The citrinin (11) was isolated from the fungus Penicillium sp. FF001 association with sponge Melophus sp., showing potent antibacterial activity against antibiotic-resistant bacteria, such as MRSA ATCC 10537, rifampicin-resistant S. aureus, and Vancomycin-resistant Enterococcus faecium [52]. Another potential of citrinin is antifungal activity against Cryptococcus neoformans (MIC 3.90 μg/ml) and cytotoxic to shrimp larvae (LD50 96 μg/ml [52]. Penicillium crysogenum also produces citrinin and shows potent antibacterial against gram-positive and Gram-negative bacteria, and antifungal activity [53,54]. The fungal strain Penicillium adametzioides AS-53 was cultivated using Potato Dextrose Agar, resulting in two novel bisthiodiketopiperazine compounds, namely adametizines A (12) and B (13). Concurrently, the metabolites isolated from cultivation in rice media showed novel acorane sesquiterpenes, namely adametacorenols A and B. Adametizines A potential were developed as a novel antibacterial activity based on the MIC values, which were less than 16 μg/ml against bacteria S. aureus, Aeromonas hydrophilia, Vibrio harveyi, Vibrio parahaemolyticus, and Gaeumannomyces graminis. Cl substitution in C-7 enhances the cytotoxic effect on shrimp larvae and their antimicrobial activity [55]. Quinazolin group compounds, including Lapatins B, glyantrypine, and verruculogen were also isolated from these fungi [56].
Two citrinin-derived compounds, namely Penicitrinone A (14), were isolated and identified from Suberea sp. and found to be inhibited by Mycobacterium smegmatic ATCC607 bacteria (MIC 32 μg/ml). In contrast, Penicitrinol J (15) indicated the MIC values 16, 16, and 32 μg/ml for B. subtilis JH642, Bacillus megaterium DSM32, M. smegmatic ATCC607 [57]. Based on the MIC values, all compounds were classified as moderate. The presence of phenolics in the structure of penicitrinol increases its potential as an antibacterial. Penicillium erubescens KUFA0220 associated with sponge Neopetrosia sp. contains several metabolites including GKK1032B, citromycin, 12-methoxycitromycin, myxotrichin D, 12-methoxycitromycetin, anhydrofulvic acid, myxotrichin C, penialidin D, penialidin F, SPF-3059-30, and secalonic acid A. Only the GKK1032B demonstrated the potential to serve as bacteriostatic against E. faecalis ATCC 29212 vancomycin-resistant E. faecalis (VRE) B3/101, E. faecium ATCC 19434, and E. faecium 1/6/63 (VRE) with the MIC values between 8 and 16 mg/ml. A novel polyketide, namely erubescensoic acid, was also isolated from this fungus. The compound was also tested for inhibiting bacterial growth against Gram-negative, Gram-positive, and MDR strains from the environment. The results showed that this compound had no activity. This compound was also unable to inhibit biofilm formation against P. aeruginosa ATCC 27853, S. aureus ATCC 29213, E. faecalis ATCC 29212 (Table 2) [58,59].
Genus Neosartorya
Fungi belonging to the Neosartorya genus contain numerous metabolites, such as indole alkaloids, peptides, meroterpenes, and polyketides, with various biological activities, such as antimicrobial, anticancer, and so on [60,61]. Below is an overview of a study on the Neosartorya fungus and its antibacterial effect (Table 1). Mycale sp. sponge-associated with the fungus Neosartorya glabra KUFA 0702 originating from Samasean Island, Gulf of Thailand, contains cyclotetrapeptides group compounds, namely sartoryglabramides A (16), sartoryglabramides B (17), and new fellutanine A. In addition, this research reported that the compounds have never been isolated before from the genus Neosartorya associated with a sponge. Despite the lack of antibacterial activity against E. coli and S. aureus (MIC >256.0 μg/ml), it is important to note that these compounds may possess other significant biological properties. Several cyclopeptides have been shown to have antifungal and antibacterial activities. However, their potencies depend on the stereochemical configurations of the amino acid constituents [62,63].
Another fungus associated with Mycale sp. is Neosartorya spinosa KUFA1047 reported by de Sá [64] contains penipurdin A, tenellic acid C, neospinosic acid, spinolactone (18), vermixocin (the anthraquinones group). However, only spinolactone showed a bacteriostatic effect against E. faecalis B3/101, although lower than ceftazidime antibiotics. Paecilin E (19) and dankasterone A (20) compounds were isolated from supernatant extracts of the fungus Neosartorya fennelliae KUFA 0811 in association with the sponge Clathria reinwardtii. Both showed antibacterial activity against S. aureus ATCC 29213 and E. faecalis ATCC 29212, although the antibacterial effect of paecilin E was better than dankasterone A based on the MIC values. This study also assessed the impact of these two compounds on biofilm formation. The findings indicated that neither of these compounds exhibited an inhibitory effect on biomass production in the tested bacterial strains. In the future, it is necessary to test other biological activities to determine the pharmacological effects of these two compounds [65].
Genus Trichoderma
Many species of Trichoderma produce metabolite compounds with various activities depending on the strain. This genus is widely recognized as a biological control agent against microorganisms either directly or indirectly. The volatile compounds, such as 1,2-Benzenedicarboxylic acid dibutyl ester, 2H-Pyran-2-one, and palmitic acid produced by Trichoderma asperellum are known to serve as effective pesticides [66]. Another potential of Trichoderma species is for enhanced growth of plants, which is commonly used for agricultural purposes [67–69]. Trichoderin A (21), A1 (22), and B (23) (new amino lipopeptide) isolated from Trichoderma sp. 05FI48 exhibited potent antimycobacterial against Mycobacterium smegmatis, Mycobacterium bovis BCG, and Mycobacterium tuberculosis H37Rv according to the MIC values (under aerobic and hypoxic conditions). The mechanism of trichoderin A inhibits Adenosine triphosphate (ATP) production of bacteria [70,71], which may serve as a promising antidormant mycobacterial drug. The study conducted on Trichoderma atroviride NF16 associated with the sponge Axinella sp. from the eastern Mediterranean coast, Israel, showed the antibacterial potential of Trichorzianines (peptaibol group) against Staphylococcus albus and B. subtilis. Peptaibol is a linear peptide consisting of 5–20 amino acids, such as alpha-aminoisobutyric acid. A large amount of peptide is found in the genus of Trichoderma and plays an essential role in the activity of Trichoderma spp as a biocontrol agent [72–75]. The furan derivatives, namely hypofurans A (24) and B, together with cyclopentanon derivatives, namely N-isobutyl-2-phenylacetamide (25), citrantifidiol (26) were extracted and isolated from the fungus Trichoderma koningii PF04 associated with the sponge Phakellia fusca originating Yongxing Island in the South China. Except for hypofurans B, all substances showed antibacterial activity with moderate potential against S. aureus ATCC25923 [76].
Sponge Cinachyrella sp. from the Panjang island, Central Java, Indonesia, was found to be associated with the fungus T. asperellum BK261A and T. reesei KU377472.1 showed antibacterial activity against MDR E. coli based on the diameter inhibition zone. Another sponge Cinachyrella sp., collected from Pandang Island, North Sumatra, Indonesia, associated with nine fungi, one of which is T. reesei MG547722.1 showed its potential in inhibiting clinical isolate bacteria, including ESBL E. coli, Staphylococcus haemolyticus strain MDR, MRSA and the highest antibacterial potential against Salmonella enterica ser. Typhi [77,78]. Some fungi from the genus of Trichoderma, such as T. reesei TV221 and T. reesei JCM 2267, associated with sponge S. flabelliformis showed antibacterial activity with various categories from moderate to weak based on MIC and DOI values [38,79] (Table 1). Unfortunately, all the above-mentioned research did not report the isolated compounds responsible for the antibacterial activities.
Another genus
The screening articles related to sponge-associated fungi reported that fungi from genera other than those mentioned above showed antibacterial activity (Table 1). Ethyl acetate extract from Curvularia lunata associated with Niphates olemda sponge from Bali Bata National Park, Indonesia, produced a potent antrakinon, namely lunatin (32) and cytoskyrin A (bisanthraquinone) to serve as antibacterial activity against S. aureus, E. coli, and B. subtilis although it may not show potential antifungal activity against Candida albicans [80]. The aspiron-derived compounds known as Chlorohydroaspyrones A (27) and B (28) were proven to inhibit the growth of S. aureus and MRSA bacteria with MIC values of 62.5 and 125 μg/ml [81]. This fungal organism also produces polyketide substances such as aspirin, asperlactone, and penicillin acid, although their activity is not well reported. Fusaripyridines A and B (dimeric alkaloids) isolated from Fusarium sp. LY019 denoted antifungal activity against C. albicans at MIC 8.0 μM, but their antibacterial activity was classified as moderate which could serve as a guide for finding new drugs [82].
Other compounds that develop potential as antibacterial agents are cryptophomic acid (29), crytotriol (30), and cryptodiol (31) isolated from Phoma sp.135 [83]. The indole alkaloid compound, namely 1-(4-hydroxybenzoyl)indole-3-carbaldehyde isolated from the fungus Engyodontium album (IVB1b) associated with the sponge Ircinia variabilis [84]. The isocoumarin derivative, known as 3-(3-chloro-2-hydroxypropyl)-8-hydroxy-6-methoxy-isochromen-1-one was also isolated from the fungus Rhinocladiella sp. (IO2) with association with the sponge Ircinia oros. However, none of above mentioned showed antibacterial activity against E. coli, S. aureus, Vibrio alginolyticus, Vibrio anguillarum, V. harveyi, V. parahaemolyticus, Alternaria brassicae, and Fusarium graminearum. Inactive compounds against certain bacteria may have a different effect on other bacteria, which could be attributed to the compound’s ability to provide antibacterial effects due to many factors, such as genetic differences between every bacteria, concentration, and polarity of the compound tested [85,86].
Data from Table 1 indicates that the bacteria used in this study to determine the antibacterial effect of sponge-associated fungi were Gram-positive and negative bacteria, both from reference strains and clinical isolates. Staphylococcus aureus (Gram-positive bacteria) and E. coli (Gram-negative) were the most common bacteria, which mainly caused nosocomial and many infectious diseases [87,88]. Antibiotic-resistant microorganisms, such as MRSA, multidrug-resistant E. coli, and vancomycin-resistant enteroccocus (VRE) are known to increase the incidence of infections. This condition encourages researchers to look for new antibiotic agents using these bacterial species to solve the resistance problem.
Some methods are commonly used to examine antibacterial agent potential, such as dilution, diffusion, or others [89,90]. Each method has its own advantages and limitations, thus making it necessary to determine the most appropriate to use, depending on various factors, such as the availability of equipment and facilities, amount, and characteristics of the samples. The data on the diameter of the inhibition zone obtained from the diffusion method may not be able to determine the bactericidal and bacteriostatic effects compared to the microdilution method. However, it is revealed to be more convenient, more affordable, more applicable to many different microorganisms, and more easily interpreted [91,92]. It is necessary to recognize that different types of bacteria in bacterial testing can affect antibacterial potential, which is normally categorized based on the MIC value or inhibition diameter marked by the appearance of a clear zone. MIC is the lowest concentration that may inhibit any growth of microorganisms. On the basis of these values, the antibacterial activity is categorized as, i.e., 50–500 μg/ml is vigorous; 600–1,500 μg/ml is moderate; and >1,500 μg/ml indicates weak or inactivity [93,94]. Based on diameter inhibition, there are several categories of antibacterial strength. If the diameter is less than 8 mm, the organism is resistant. The diameter zone between 9 and 14 mm is classified as sensitive, 15 to 19 mm is very sensitive, and more than 20 mm is deemed as extremely sensitive [95,96]. The compounds showed potent antibacterial activity from marine sponges associated with fungi shown in Figure 4. Figure 4 shows that marine derive-fungi contain various compounds and have potential as antibacterial agents.
Sponge-associated fungi as a source of antibiofilm
Superbugs have become a global health issue and contribute to morbidity and mortality rates. Superbugs are described by the increased immunity of microbes to several types of antibiotics. It may occur naturally in bacteria when their genes evolve. Another reason for increasing resistance is biofilm formation, which makes treating infectious diseases more difficult, and thus makes it necessary to develop strategies to eradicate superbugs effectively [97–100]. Biofilm is microorganism communities that adhere to the surface and form an extracellular matrix known as extracellular polymeric substance (EPS) consisting of polysaccharides, various proteins, lipids, and DNA [101–103, 1]. Biofilm morphology depends on its constituent bacteria and conditions for biofilm formation.
The cycle growth of biofilm formation begins with the initial attachment phases. Planktonic bacteria attached to a surface or devices initiate reversible or irreversible attachment. Microorganisms have specific signals to start biofilm formation. They prefer hydrophobic surfaces because they reduce the repulsive force between the bacteria and the surface, resulting in stronger adhesion. The next phase is microcolony formation, characterized by bacteria multiplying and biofilm EPSs production [2,104–106]. In the mature phase, biofilms develop entirely and, eventually multilayers. The cell density reaches a peak and forms a typical 3-D biofilm structure. After the biofilm reaches complete maturity, the dispersal step begins by releasing bacteria into the environment. The dispersal stage is essential in the biofilm life cycle because it promotes new biofilm initiation at other sites [1,107]. The biofilm life cycle is presented in Figure 5. In recent years, biofilm-related research has attracted many researchers, although studies on exploring marine-associated fungi and their potential as antibiofilm are still rare. The summarized data of sponge-derived fungi and their potential to serve as antibiofilm is shown in Table 2. A cis-cyclo (Leucyl-Tyro-syl) dipeptide was successfully isolated from Penicillium sp. associated with the sponge Axinella corrugata. The compound effectively inhibited biofilm formation of S. epidermidis ATCC 35984 up to 85% at a concentration of 1 mg/ml and fully inhibited at 2 mg/ml. Observation of the biofilm structure by Scanning Electron Microscope (SEM) showed that the sample group treated with 1.0 mg ml dipeptide showed less intense EPS formation [108]. Diketopiperazines have many biological activities, such as antitumor and antiviral. Bacterial signaling systems, including quorum-sensing (QS), can be influenced by diketopiperazine [109,110]. Secalonic acid D (SAD) and B isolated from Penicillium sp. SCSGAF 0023 effectively inhibits biofilm formation >90% at 6.25 μg/ml against S. aureus ATCC 6538, although there is no effect on the P. aeruginosa PAO1 on the concentration up 100%. SAD showed a biofilm eradication effect at level 12.5 μg/ml. The biofilm architecture of S. aureus, also influenced by SAD, showed a decrease in biofilm biomass and thickness by the dose-dependent. SAD can regulate the transcription level of biofilm-related genes. Nonetheless, other genetic studies are needed to clarify the mechanism of SAD inhibition of S. aureus biofilm formation [111], which concludes that SAD has the potential to develop as an antibiofilm agent.
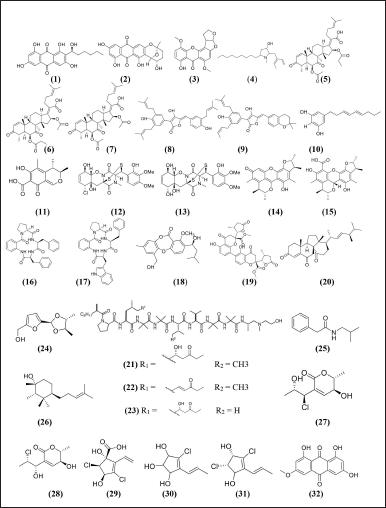 | Figure 4. Structure compounds from marine sponge derived-fungi with potent antibacterial activity [33,34,70,76,80,81,83,65,72,39,43,49,52,55,57,62,65]. [Click here to view] |
Tenellic acid C and neospinosic acid, both isolated from N. spinosa KUFA 1047, are potentially developed as antibiofilm agents due to their ability to suppress biofilm production of E. coli ATCC 25922 and S. aureus ATCC 29213. Neospinosic acid particularly showed the most potent inhibition, up to 44% for S. aureus ATCC 29213 biofilm formation (Table 2), and the viability of biofilm decreased by 98% after 8 hours of incubation, even though after 24 hours, the decrease in viability was only 10%. This phenomenon may occur because bacteria adapt genetically and phenotypically [65]. The prenylated phenylbutyrolactones, identified as Aspulvinones B’, Aspulvinones H, Aspulvinones R, Aspulvinones S, and Aspulvinones T isolated from A. flavipes KUFA1152 showed antibiofilm activity to E. faecalis ATCC 29212 and S. aureus ATCC 29213 bacteria (Table 2). The presence of prenyl substituents in ring A of compounds Aspulvinones R and Aspulvinones S may be responsible for antibacterial activity and inhibition of biofilm formation. Nevertheless, none of the compounds exhibited synergistic effects with antibiotics against multidrug-resistant bacteria.
Another strain was isolated from Mycale sp, A. stellatus KUFA 2017, contains 5[(3E,5E)-nona-3,5-dien-1-yl]benzene can inhibit biofilm formation almost 99% on E. faecalis ATCC 29212 and S. aureus ATCC 2921 [48,49] (Table 2). These compounds may develop as new drugs for antibacterial and biofilm inhibitors. Several compounds isolated from fungi associated with marine sponges may not show antibiofilm activities, such as erubescenoic acid (Table 2), which was isolated from P. erubescens KUFA0220 [58,59]. However, it does not mean they have no biological effects, thus making it crucial to conduct further investigation in other bioassays to examine what these compounds can potentially accomplish. Biofilms are formed not only of monospecies but also of polymicrobial (including Gram-positive and Gram-negative bacteria) or biofilm complexes of bacteria and fungi. Each Gram-positive or Gram-negative bacteria has its mechanism for biofilm formation [112], but generally, the stages of biofilm formation are illustrated in Figure 5. Staphylococcus aureus is a Gram-positive bacteria contributing to most biofilm-associated infections, including biofilm on medical devices. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 are Gram-negative bacteria that produce strong biofilms [113–115].
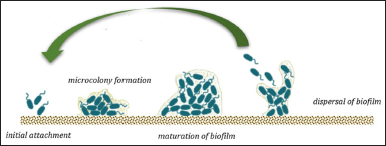 | Figure 5. Biofilm life cycle. [Click here to view] |
Biofilm formation is a complicated process, and improving our understanding of biofilms molecular composition and function will greatly assist in identifying and developing effective strategies for preventing and treating infections caused by these complex multicellular communities. Several feasible strategies are applicable for biofilm prevention and therapy, including biofilm formation inhibition and eradicating existing biofilms. Inhibition of biofilm formation by preventing bacteria from attaching to a surface and inhibiting the formation of EPS [101] inhibits cell-to-cell communication by anti-QS [116], modify surfaces to make it harder for bacteria to attach and grow on surfaces, especially on medical devices [117,118]. Physical and chemical methods also can eradicate biofilm [119–121].
This review highlighted that some compounds isolated from sponge-associated fungi showed antibacterial and antibiofilm activity, but some were only active as antibacterial or antibiofilm. This phenomenon may occur because the mechanism of action of antibiofilm may differ from that of antibacterials, especially if polymicrobials cause biofilms. Some studies did not report the compound mechanism as antibacterial or antibiofilm. Future research is expected to observe the compound mechanism thoroughly and may lead to the development of antibiofilm agents for specific targets.
CONCLUSION
Currently, the efficacy of conventional antibiotics is especially limited to biofilm-related infections. The marine environment offers a rich opportunity to discover novel, highly effective antibacterial, and antibiofilm chemicals. This review summarized the isolated compounds from marine derive-fungi with promising potential to develop as antibacterial or antibiofilm, including anthraquinones, sterigmatocystin analog, hydroxy pyrrolidine alkaloids, helvolic acid derivatives, lactones, prenylated phenylbutyrolactones, citrinin and derivatives, bisthiodiketopiperazine, cyclotetrapeptides, dihydrochromone dimer, amino lipopeptide, furan derivatives, aspiron-derivatives, halogenated metabolites, and alkaloids. Thorough research is required to determine the reliable mechanism of bioactive compounds, considering that the interaction between bacteria in biofilms is challenging to treat. Hence, exploring new antibacterial and antibiofilm agents derived from marine natural products has considerable value to drug development as a way to solve problems related to sponge availability.
ACKNOWLEDGMENTS
The author would like to acknowledge to the Center For Higher Education Funding and Indonesia Endowment Fund for Education for their funding support as Doctoral Scholarship.
AUTHOR CONTRIBUTION
All authors contributed to the conception and design, acquisition of data, analysis, and interpretation of data, drafting the article, or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the published version; and agreed to be accountable for all aspects of the work. According to the guidelines defined by the International Committee of Medical Journal Editors (ICMJEs), all authors are eligible to be authors.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVALS
This study does not involve animal or human subjects for experimental purposes.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Rabin N, Zheng Y, Opoku-Temeng C, Du Y, Bonsu E, Sintim HO. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med Chem. 2015;7(4):493–512. CrossRef
2. Goel N, Fatima SW, Kumar S, Sinha R, Khare SK. Antimicrobial resistance in biofilms: exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol Rep. 2021;30:e00613.
3. Flemming HC, Wuertz S. Bacteria and archaea on Earth and their abundance in biofilms. Nat Rev Microbiol. 2019;17(4):247–60. CrossRef
4. Hanif N, Murni A, Tanaka C, Tanaka J. Marine natural products from Indonesian waters. Mar Drugs. 2019;17(6):364. CrossRef
5. Mostafa O, Al-Shehri M, Moustafa M. Promising antiparasitic agents from marine sponges. Saudi J Biol Sci. 2022;29(1):217–27. CrossRef
6. Bertrand B, Munoz-Garay C. Marine antimicrobial peptides: a promising source of new generation antibiotics and other bio-active molecules. Int J Pept Res Ther. 2019;25(4):1441–50. CrossRef
7. Kiuru P, Valeria D?Auria M, Muller CD, Tammela P, Vuorela H, Yli-Kauhaluoma J. Exploring marine resources for bioactive compounds. Planta Med. 2014;80(14):1234–46. CrossRef
8. Renard E, Gazave E, Fierro-Constain L, Schenkelaars Q, Ereskovsky A, Vacelet J, et al. Porifera (Sponges): recent knowledge and new perspectives. eLS. 2013.p1–8.
9. Hajdu E, Fonseca CA, Schories D, Kohlberg G. Sponges, porifera. In: Schories D, Kohlberg G, editors. Marine WildlifeKing George Island Antarctica. 1st ed. Rostock, German: Dirk Schories Publications; 2016. p. 348.
10. Anjum K, Abbas SQ, Shah SAA, Akhter N, Batool S, Hassan SSU. Marine sponges as a drug treasure. Biomol Ther. 2016;24(4):347–62. CrossRef
11. El-Naggar HA, Bashar MAE, Rady I, El-Wetidy MS, Suleiman WB, Al-Otibi FO, et al. Two red sea sponge extracts (Negombata magnifica and Callyspongia siphonella) induced anticancer and antimicrobial activity. Appl Sci. 2022;12(3):1–23. CrossRef
12. Filho JAC. Endophytic microbes as a novel source for producing anticancer compounds as multidrug resistance modulators. In: Akhtar MS, Swamy MK, editors. Anticancer plants: natural products and biotechnological implements. Singapore: Springer; 2018. pp. 343–81.
13. Ahmad I, Althubiani AS, Dar MS, Samreen, Qais FA, Abulreesh HH, et al. Actinomycetes as continued source of new antibacterial leads. In: Ahmad I, Ahmad S, Rumbaugh K, editors. Antibacterial drug discovery to combat MDR. Singapore: Springer; 2019. pp. 327–49.
14. Setyowati EP, Pratiwi SUT, Purwantiningsih, Samirana PO. Antimicrobial activity and identification of fungus associated Stylissa flabelliformis sponge collected from Menjangan Island West Bali National Park, Indonesia. Indones J Pharm. 2018;29(2):66–73. CrossRef
15. Hikmawan BD, Wahyuono S, Setyowati EP. Marine sponge compounds with antiplasmodial properties: focus on in vitro study against Plasmodium falciparum. J Appl Pharm Sci. 2020;10(5):142–57. CrossRef
16. Lei H, Lin X, Han L, Ma J, Ma Q, Zhong J, et al. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar Drugs. 2017;15(3):69. CrossRef
17. Fuerst JA. Diversity and biotechnological potential of microorganisms associated with marine sponges. Appl Microbiol Biotechnol. 2014;98(17):7331–47. CrossRef
18. Kiran GS, Sekar S, Ramasamy P, Thinesh T, Hassan S, Lipton AN, et al. Marine sponge microbial association: towards disclosing unique symbiotic interactions. Mar Environ Res. 2018;140:169–79. CrossRef
19. Thomas TRA, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association—a review. Mar Drugs. 2010;8(4):1417–68.
20. Anteneh YS, Yang Q, Brown MH, Franco CMM. Antimicrobial activities of marine sponge-associated bacteria. Microorganisms. 2021;9(1):1–19. CrossRef
21. Visamsetti A, Ramachandran SS, Kandasamy D. Penicillium chrysogenum DSOA associated with marine sponge (Tedania anhelans) exhibit antimycobacterial activity. Microbiol Res. 2016;185:55–60. CrossRef
22. Musa A, Abdelgawad MA, Shaker ME, El-Ghorab AH, Parambi DGT, Hamed AA, et al. Screening and molecular docking of bioactive metabolites of the red sea sponge Callyspongia siphonella as potential antimicrobial agents. Antibiotics. 2022;11(12):1682. CrossRef
23. Mazo RAM, Gelani CD, Lavilla CA, Uy MM, Tabugo SRM, Ohta E, et al. Antibacterial, anti-inflammatory, and antidiabetic studies of the amines isolated from the Philippine marine sponge Desmacella sp. Curr Bioact Compd. 2022;19(1):52–61. CrossRef
24. Stanojkovic TP, Filimonova M, Grozdanic N, Petovic S, Shitova A, Soldatova O, et al. Evaluation of in vitro cytotoxic potential of avarol towards human cancer cell lines and in vivo antitumor activity in solid tumor models. Molecules. 2022;27(24):9048. CrossRef
25. Mahfur, Wahyuono S, Purwantini I, Setyowati EP. In vitro antiplasmodial activities of the fractions of Hyrtios reticulatus sponge extract. J Appl Pharm Sci. 2022;12(9):114–20. CrossRef
26. Alves UV, Jardim e Silva E, dos Santos JG, Santos LO, Lanna E, de Souza Pinto AC, et al. Potent and selective antiplasmodial activity of marine sponges from Bahia state, Brazil. Int J Parasitol Drugs Drug Resist. 2021;17:80–3. CrossRef
27. Bergman O, Haber M, Mayzel B, Anderson MA, Shpigel M, Hill RT, et al. Marine-based cultivation of diacarnus sponges and the bacterial community composition of wild and maricultured sponges and their larvae. Mar Biotechnol. 2011;13(6):1169–82. CrossRef
28. Maldonado M, Cortadellas N, Trillas MI, Rützler K. Endosymbiotic yeast maternally transmitted in a marine sponge. Biol Bull. 2005;209(2):94–106. CrossRef
29. Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J, et al. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol. 2002;68(9):4431–40. CrossRef
30. Siegl A, Hentschel U. PKS and NRPS gene clusters from microbial symbiont cells of marine sponges by whole genome amplification. Environ Microbiol Rep. 2010;2(4):507–13. CrossRef
31. Indraningrat AAG, Smidt H, Sipkema D. Bioprospecting sponge-associated microbes for antimicrobial compounds. Mar Drugs. 2016;14(5):87. CrossRef
32. Cheng MM, Tang XL, Sun YT, Song DY, Cheng YJ, Liu H, et al. Biological and chemical diversity of marine sponge-derived microorganisms over the last two decades from 1998 to 2017. Molecules. 2020;25(4):853. CrossRef
33. Lee YM, Li H, Hong J, Cho HY, Bae KS, Kim MA, et al. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res. 2010;33(2):231–5. CrossRef
34. Song F, Ren B, Chen C, Yu K, Liu X, Zhang Y, et al. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl Microbiol Biotechnol. 2014;98(8):3753–8. CrossRef
35. Meenupriya J, Thangaraj M. Isolation and molecular characterization of bioactive secondary metabolites from Callyspongia spp. associated fungi. Asian Pac J Trop Med. 2010;3(9):738–40. CrossRef
36. Samirana PO, Murti YB, Jenie RI, Setyowati EP. Marine sponge-derived fungi: fermentation and cytotoxic activity. J Appl Pharm Sci. 2021;11(1):21–39. CrossRef
37. Handayani D, Aminah I. Antibacterial and cytotoxic activities of ethyl acetate extract of symbiotic fungi from West Sumatra marine sponge Acanthrongylophora ingens. J Appl Pharm Sci. 2017;7(2):237–40. CrossRef
38. Setyowati EP, Purwantiningsih, Erawan FMY, Rahmanti S, Hanum NR, Devi NCM. Cytotoxic and antimicrobial activities of ethyl acetate extract from fungus Trichoderma reesei strain jcm 2267, Aspergillus flavus strain mc-10-l, Penicillium sp, and Aspergillus fumigatus associated with marine sponge Stylissa flabelliformis. Res J Pharm Technol. 2021;14(10):5126–32. CrossRef
39. Buttachon S, Ramos AA, Inácio Â, Dethoup T, Gales L, Lee M, et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar Drugs. 2018;16(4):119. CrossRef
40. Wang D, Qu P, Zhou J, Wang Y, Wang L, Zhu W. p-Terphenyl alcohols from a marine sponge-derived fungus, Aspergillus candidus OUCMDZ-1051. Mar Life Sci Technol. 2020;2:262–7. CrossRef
41. Lin YK, Xie CL, Xing CP, Wang BQ, Tian XX, Xia JM, et al. Cytotoxic p-terphenyls from the deep-sea-derived Aspergillus candidus. 2019;35(10):1627–31. CrossRef
42. Sangsopha W, Lekphrom R, Schevenels FT, Saksirirat W, Bua-Art S, Kanokmedhakul K, et al. New p-terphenyl and benzoquinone metabolites from the bioluminescent mushroom Neonothopanus nambi. Nat Prod Res. 2019;34(15):2186–93. CrossRef
43. Kong FD, Huang XL, Ma QY, Xie QY, Wang P, Chen PW, et al. Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047. J Nat Prod. 2018;81(8):1869–76. CrossRef
44. Wang JF, Lin XP, Qin C, Liao SR, Wan JT, Zhang TY, et al. Antimicrobial and antiviral sesquiterpenoids from sponge-associated fungus, Aspergillus sydowii ZSDS1-F6. J Antibiot. 2014;67(8):581–3. CrossRef
45. Li D, Xu Y, Shao CL, Yang RY, Zheng CJ, Chen YY, et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar Drugs. 2012;10:234–41. CrossRef
46. Handayani D, Artasasta MA, Safirna N, Ayuni DF, Tallei TE, Hertiani T. Fungal isolates from marine sponge Chelonaplysilla sp.: diversity, antimicrobial and cytotoxic activities. Biodiversitas. 2020;21(5):1954–60. CrossRef
47. Manilal A, Sabarathnam B, Kiran GS, Sujith S, Shakir C, Selvin J. Antagonistic potentials of marine sponge associated fungi Aspergillus clavatus MFD15. Asian J Med Sci. 2010;2(4):195–200.
48. Machado FP, Kumla D, Pereira JA, Sousa E, Dethoup T, Freitas-Silva J, et al. Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus flavipes KUFA1152. Phytochemistry. 2021;185:1–11. CrossRef
49. Machado FP, Rodrigues IC, Gales L, Pereira JA, Costa PM, Dethoup T, et al. New alkylpyridinium anthraquinone, isocoumarin, C-glucosyl resorcinol derivative and prenylated pyranoxanthones from the culture of a marine sponge-associated fungus, Aspergillus stellatus KUFA 2017. Mar Drugs. 2022;20(11):672. CrossRef
50. Letek M. Alexander fleming, the discoverer of the antibiotic effects of penicillin. Front Young Minds. 2020;7(159):1–6. CrossRef
51. Park MS, Oh SY, Fong JJ, Houbraken J, Lim YW. The diversity and ecological roles of Penicillium in intertidal zones. Sci Rep. 2019;9(1):13540. CrossRef
52. Subramani R, Kumar R, Prasad P, Aalbersberg W. Cytotoxic and antibacterial substances against multi-drug resistant pathogens from marine sponge symbiont: citrinin, a secondary metabolite of Penicillium sp. Asian Pac J Trop Biomed. 2013;3(4):291–6. CrossRef
53. Devi P, D’Souza L, Kamat T, Rodrigues C, Naik CG. Batch culture fermentation of Penicillium chrysogenum and a report on the isolation, purification, identification and antibiotic activity of citrinin. Indian J Mar Sci. 2009;38(1):38–44.
54. Lewis JA, Anderson N. Penicillium antibiotic effect. Am Biol Teach. 2018;80(7):530–5. CrossRef
55. Liu Y, Li XM, Meng LH, Jiang WL, Xu GM, Huang CG, et al. Bisthiodiketopiperazines and acorane sesquiterpenes produced by the marine-derived fungus Penicillium adametzioides AS-53 on different culture media. J Nat Prod. 2015;78(6):1294–9. CrossRef
56. Liu Y, Li XM, Meng LH, Wang BG. N-Formyllapatin A, a new N-formylspiroquinazoline derivative from the marine-derived fungus Penicillium adametzioides AS-53. Phytochem Lett. 2014;10:145–8. CrossRef
57. Sabdaningsih A, Liu Y, Mettal U, Heep J, Riyanti, Wang L, et al. A new citrinin derivative from the Indonesian marine sponge-associated fungus Penicillium citrinum. Mar Drugs. 2020;18(4):227. CrossRef
58. Kumla D, Pereira JA, Dethoup T, Gales L, Freitas-Silva J, Costa PM, et al. Chromone derivatives and other constituents from cultures of the marine sponge-associated fungus Penicillium erubescens KUFA0220 and their antibacterial activity. Mar Drugs. 2018;16(8):1–23. CrossRef
59. Kumla D, Dethoup T, Gales L, Pereira JA, Freitas-Silva J, Costa PM, et al. Erubescensoic acid, a new polyketide and a xanthonopyrone SPF-3059-26 from the culture of the marine sponge-associated fungus Penicillium erubescens KUFA 0220 and antibacterial activity evaluation of some of its constituents. Molecules. 2019;24(1):208. CrossRef
60. War May Zin W, Prompanya C, Buttachon S, Kijjoa A. Bioactive secondary metabolites from a Thai collection of soil and marine-derived fungi of the genera Neosartorya and Aspergillus. Curr Drug Deliv. 2016;13(3):378–88. CrossRef
61. de Sá JDM, Kumla D, Dethoup T, Kijjoa A. Bioactive compounds from terrestrial and marine-derived fungi of the genus Neosartorya †. Molecules. 2022;27(7):1–44. CrossRef
62. Xu L, Meng W, Cao C, Wang J, Shan W, Wang Q. Antibacterial and antifungal compounds from marine fungi. Mar Drugs. 2015;13(6):3479–513. CrossRef
63. Zin WWM, Buttachon S, Dethoup T, Fernandes C, Cravo S, Pinto MMM, et al. New cyclotetrapeptides and a new diketopiperzine derivative from the marine sponge-associated fungus Neosartorya glabra KUFA 0702. Mar Drugs. 2016;14(7):136. CrossRef
64. de Sá JDM, Pereira JA, Dethoup T, Cidade H, Sousa ME, Rodrigues IC, et al. Anthraquinones, diphenyl ethers, and their derivatives from the culture of the marine sponge-associated fungus Neosartorya spinosa kufa 1047†. Mar Drugs. 2021;19(8):457. CrossRef
65. Kumla D, Aung TS, Buttachon S, Dethoup T, Gales L, Pereira JA, et al. A new dihydrochromone dimer and other secondary metabolites from cultures of the marine sponge-associated fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9213. Mar Drugs. 2017;15(12):375. CrossRef
66. Bhardwaj NR, Kumar J. Characterization of volatile secondary metabolites from Trichoderma asperellum. J Appl Nat Sci. 2017;9(2):954–9. CrossRef
67. Tucci M, Ruocco M, De Masi L, De Palma M, Lorito M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol Plant Pathol. 2011;12(4):341–54. CrossRef
68. Daguerre Y, Siegel K, Edel-Hermann V, Steinberg C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: a review. Fungal Biol Rev. 2014;28(4):97–125. CrossRef
69. Jogaiah S, Abdelrahman M, Tran LSP, Ito SI. Different mechanisms of Trichoderma virens-mediated resistance in tomato against Fusarium wilt involve the jasmonic and salicylic acid pathways. Mol Plant Pathol. 2018;19(4):870–82. CrossRef
70. Pruksakorn P, Arai M, Kotoku N, Vilchze C, Baughn AD, Moodley P, et al. Trichoderins, novel aminolipopeptides from a marine sponge-derived Trichoderma sp., are active against dormant mycobacteria. Bioorg Med Chem Lett. 2010;20(12):3658–63. CrossRef
71. Pruksakorn P, Arai M, Liu L, Moodley P, Jacobs WR, Kobayashi M. Action-mechanism of trichoderin A, an anti-dormant mycobacterial aminolipopeptide from marine sponge-derived Trichoderma sp. Biol Pharm Bull. 2011;34(8):1287–90. CrossRef
72. Víglaš J, Dobiasová S, Viktorová J, Ruml T, Repiská V, Olejníková P, et al. Peptaibol-containing extracts of Trichoderma atroviride and the fight against resistant microorganisms and cancer cells. Molecules. 2021;26(19):6025. CrossRef
73. Panizel I, Yarden O, Ilan M, Carmeli S. Eight new peptaibols from sponge-associated Trichoderma atroviride. Mar Drugs. 2013;11(12):4937–60. CrossRef
74. Schuhmacher R, Stoppacher N, Zeilinger S. Peptaibols of Trichoderma atroviride?: screening, identification, and structure elucidation by liquid chromatography-tandem mass spectrometry. In: Méndez-Vilas A, editor. Communicating current research and educational topics and trends in applied microbiology. Badajoz, Spain: Formatex; 2007. pp. 609–17.
75. Stoppacher N, Reithner B, Omann M, Zeilinger S, Krska R, Schuhmacher R. Profiling of trichorzianines in culture samples of Trichoderma atroviride by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(24):3963–70. CrossRef
76. Ding LJ, Gu BB, Jiao WH, Yuan W, Li YX, Tang WZ, et al. New furan and cyclopentenone derivatives from the sponge-associated fungus Hypocrea koningii PF04. Mar Drugs. 2015;13(9):5579–92. CrossRef
77. Sibero MT, Sabdaningsih A, Cristianawati O, Nuryadi H, Radjasa OK, Sabdono A, et al. Isolation, identification and screening antibacterial activity from marine sponge-associated fungi against multidrug-resistant (MDR) Escherichia coli. IOP Conf Ser Earth Environ Sci. 2017;55(1):1–12. CrossRef
78. Sibero MT, Radjasa OK, Sabdono A, Trianto A, Triningsih DW, Hutagaol ID. Antibacterial activity of Indonesian sponge associated fungi against clinical pathogenic multidrug resistant bacteria. J Appl Pharm Sci. 2018;8(2):088–94. CrossRef
79. Samirana PO, Murti YB, Jenie RI, Setyowati EP. Antibacterial and cytotoxic activities of supernatant and mycelium extracts from fermentation of fungal symbiont Trichoderma reesei TV221. J Appl Pharm Sci. 2021;11(12):090–9. CrossRef
80. Jadulco R, Brauers G, Edrada RA, Ebel R, Wray V, Sudarsono, et al. New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J Nat Prod. 2002;65(5):730–3. CrossRef
81. Zhang D, Yang X, Jung SK, Hong DC, Byeng WS. Chlorohydroaspyrones A and B, antibacterial aspyrone derivatives from the marine-derived fungus Exophiala sp. J Nat Prod. 2008;71(8):1458–60. CrossRef
82. Shaala LA, Alzughaibi T, Genta-Jouve G, Youssef DTA. Fusaripyridines A and B; highly oxygenated antimicrobial alkaloid dimers featuring an unprecedented 1,4-bis(2-hydroxy-1,2-dihydropyridin-2-yl)butane-2,3-dione core from the marine fungus Fusarium sp. LY019. Mar Drugs. 2021;19(9):1–10. CrossRef
83. Elsebai MF, Ghabbour HA, Legrave N, Fontaine-Vive F, Mehiri M. New bioactive chlorinated cyclopentene derivatives from the marine-derived Fungus Phoma sp. Med Chem Res. 2018;27(5):1885–92. CrossRef
84. Meng LH, Chen HQ, Form I, Konuklugil B, Proksch P, Wang BG. New chromone, isocoumarin, and indole alkaloid derivatives from three sponge-derived fungal strains. Nat Prod Commun. 2016;11(9):1293–6. CrossRef
85. Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1639. CrossRef
86. de Dieu Tamokou J, Kuiate JR, Tene M, Nwemeguela TJK, Tane P. The antimicrobial activities of extract and compounds isolated from Brillantaisia lamium. Iran J Med Sci. 2011;36(1):24–31.
87. Inweregbu K, Dave J, Pittard A. Nosocomial infections. Contin Educ Anaesth Crit Care Pain. 2005;5(1):14–7. CrossRef
88. Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative Bacilli. Clin Infect Dis. 2005;41(6):848–54. CrossRef
89. Sasidharan S, Zuraini Z, Latha LY, Suryani S. Fungicidal effect and oral acute toxicity of Psophocarpus tetragonolobus root extract. Pharm Biol. 2008;46(4):261–5. CrossRef
90. Niyomkam P, Kaewbumrung S, Kaewnpparat S, Panichayupakaranant P. Antibacterial activity of Thai herbal extracts on acne involved microorganism. Pharm Biol. 2010;48(4):375–80. CrossRef
91. Arikan S, Paetznick V, Rex JH. Comparative evaluation of disk diffusion with microdilution assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. Antimicrob Agents Chemother. 2002;46(9):3084–7. CrossRef
92. Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6(2):71–9. CrossRef
93. Cos P, Vlietinck AJ, Vanden Berghe D, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro “proof-of-concept.” J Ethnopharmacol. 2006;106(3):290–302. CrossRef
94. De Oliveira AMA, Mesquita MDS, Da Silva GC, De Oliveira Lima E, De Medeiros PL, Paiva PMG, et al. Evaluation of toxicity and antimicrobial activity of an ethanolic extract from leaves of Morus alba L. (Moraceae). Evid-Based Complement Altern Med. 2015;2015:1–7. CrossRef
95. Yasir M, Nawaz A, Ghazanfar S, Okla MK, Chaudhary A, Al WH, et al. Anti-bacterial activity of essential oils against multidrug-resistant foodborne pathogens isolated from raw milk. Braz J Biol. 2024;84:e259449. CrossRef
96. Celikel N, Kavas G. Antimicrobial properties of some essential oils against some pathogenic microorganisms. 2008;26(3):174–81. CrossRef
97. Srinivasan R, Santhakumari S, Poonguzhali P, Geetha M, Dyavaiah M, Xiangmin L. Bacterial biofilm inhibition: a focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front Microbiol. 2021;12:676458. CrossRef
98. Ganewatta MS, Rahman MA, Tang C. Emerging antimicrobial research against superbugs: perspectives from a polymer laboratory. J South Carolina Acad Sci. 2017;15(1):3.
99. Rajendran R. Superbug infection. J Drug Metab Toxicol. 2018;09(2):1–3. CrossRef
100. Khan SN, Khan AU. Breaking the spell: combating multidrug resistant “superbugs.” Front Microbiol. 2016;7:174662. CrossRef
101. Su Y, Yrastorza JT, Matis M, Cusick J, Zhao S, Wang G, et al. Biofilms: formation, research models, potential targets, and methods for prevention and treatment. Adv Sci. 2022;9(29):2203291. CrossRef
102. Neu TR, Lawrence JR. The extracellular matrix—an intractable part of biofilm systems. In: The Perfect Slime: Microbial Extracellular Polymeric Substances (EPS). Editors; H.-C. Flemming, T. R. Neu, and J. Wingender. London: IWA Publishing; 2017, p.25–60.
103. Flemming HC, Neu TR, Wozniak DJ. The EPS matrix: the “House of Biofilm Cells.” J Bacteriol. 2007;189(22):7945–7. CrossRef
104. Dewasthale S, Mani I, Vasdev K. Microbial biofilm: current challenges in health care industry. J Appl Biotechnol Bioeng. 2018;5(3):156–60. CrossRef
105. Jamal M, Bukhari SMAUS, Raza S, Shah L, Redaina, Ali N, et al. Microbial biofilms: properties, biodiversity, conservation and management in microbial biofilms. In: Abdul BAA, editor. Microbial biofilms. Boca Raton, FL: CRC Press; 2021. pp. 3–24.
106. Renner LD, Weibel DB. Physicochemical regulation of biofilm formation. MRS Bull. 2011;36(5):347–55. CrossRef
107. Stowe SD, Richards JJ, Tucker AT, Thompson R, Melander C, Cavanagh J. Anti-biofilm compounds derived from marine sponges. Mar Drugs. 2011;9(10):2010–35. CrossRef
108. Scopel M, Abraham WR, Henriques AT, MacEdo AJ. Dipeptide cis-cyclo(Leucyl-Tyrosyl) produced by sponge associated Penicillium sp. F37 inhibits biofilm formation of the pathogenic Staphylococcus epidermidis. Bioorg Med Chem Lett. 2013;23(3):624–6. CrossRef
109. Sinha S, Srivastava R, De Clercq E, Singh RK. Synthesis and antiviral properties of arabino and ribonucleosides of 1,3-dideazaadenine, 4-nitro-1,3-dideazapurine and diketopiperazine. Nucleosides Nucleotides Nucleic Acids. 2004;23(12):1815–24. CrossRef
110. P de Carvalho M, Abraham WR. Antimicrobial and biofilm inhibiting diketopiperazines. Curr Med Chem. 2012;19(21):3564–77. CrossRef
111. Wang J, Nong XH, Zhang XY, Xu XY, Amin M, Qi SH. Screening of anti-biofilm compounds from marine-derived fungi and the effects of secalonic acid D on Staphylococcus aureus biofilm. J Microbiol Biotechnol. 2017;27(6):1078–89. CrossRef
112. Ruhal R, Kataria R. Biofilm patterns in Gram-positive and Gram-negative bacteria. Microbiol Res. 2021;251:1–8. CrossRef
113. Papa R, Parrilli E, Sannino F, Barbato G, Tutino ML, Artini M, et al. Anti-biofilm activity of the antarctic marine bacterium Pseudoalteromonas haloplanktis TAC125. Res Microbiol. 2013;164(5):450–6. CrossRef
114. Moormeier DE, Bayles KW. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol. 2017;104(3):365–76. CrossRef
115. Rashiya N, Padmini N, Ajilda AAK, Prabakaran P, Durgadevi R, Veera Ravi A, et al. Inhibition of biofilm formation and quorum sensing mediated virulence in Pseudomonas aeruginosa by marine sponge symbiont Brevibacterium casei strain Alu 1. Microb Pathog. 2021;150:104693. CrossRef
116. Haque M, Islam S, Sheikh MA, Dhingra S, Uwambaye P, Labricciosa FM, et al. Quorum sensing: a new prospect for the management of antimicrobial-resistant infectious diseases. Expert Rev Anti Infect Ther. 2021;19:571–86. CrossRef
117. Jahanmard F, Croes M, Castilho M, Majed A, Steenbergen MJ, Lietaert K, et al. Bactericidal coating to prevent early and delayed implant-related infections. J Control Release. 2020;326:38–52. CrossRef
118. Hadjesfandiari N, Yu K, Mei Y, Kizhakkedathu JN. Polymer brush-based approaches for the development of infection-resistant surfaces. J Mater Chem B. 2014;2(31):4968–78. CrossRef
119. Xu J, Danehy R, Cai H, Ao Z, Pu M, Nusawardhana A, et al. Microneedle patch-mediated treatment of bacterial biofilms. ACS Appl Mater Interfaces. 2019;11(16):14640–6. CrossRef
120. Zoccolillo ML, Rogers SC, Mang TS. Antimicrobial photodynamic therapy of S. mutans biofilms attached to relevant dental materials. Lasers Surg Med. 2016;48(10):995–1005. CrossRef
121. Tkhilaishvili T, Wang L, Tavanti A, Trampuz A, Di Luca M. Antibacterial efficacy of two commercially available bacteriophage formulations, Staphylococcal bacteriophage and PYO bacteriophage, against methicillin-resistant Staphylococcus aureus: prevention and eradication of biofilm formation and control of a systemic infection of Galleria mellonella Larvae. Front Microbiol. 2020;11:1–15. CrossRef
122. Wang Y, Lin XP, Ju ZR, Liao XJ, Huang XJ, Zhang C, et al. Aspergchromones A and B, two new polyketides from the marine sponge-associated fungus Aspergillus sp. SCSIO XWS03F03. J Asian Nat Prod Res. 2017;19(7):684–90. CrossRef
123. Setiawan A, Lutfiah R, Juliasih NLGR, Setiawan WA, Hendri J, Arai M. Antibacterial activity of EtOAc extract from marine-derived fungus Aspergillus nomiae A12-RF against clinical pathogen bacteria, Staphylococcus aureus. AACL Bioflux. 2022;15(3):1413–21.
124. Xie LW, Ouyang YC, Zou K, Wang GH, Chen MJ, Sun HM, et al. Isolation and difference in anti-Staphylococcus aureus bioactivity of curvularin derivates from fungus Eupenicillium sp. Appl Biochem Biotechnol. 2009;159(1):284–93. CrossRef
125. Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, et al. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J Appl Pharm Sci. 2020;10(4):28–33. CrossRef
126. Al-Saleem MSM, Hassan WHB, El Sayed ZI, Abdel-Aal MM, Abdel-Mageed WM, Abdelsalam E, et al. Metabolic profiling and in vitro assessment of the biological activities of the ethyl acetate extract of Penicillium chrysogenum “endozoic of Cliona sp. marine sponge” from the red sea (Egypt). Mar Drugs. 2022;20(5):1–23. CrossRef
127. Gomes NM, Bessa LJ, Buttachon S, Costa PM, Buaruang J, Dethoup T, et al. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar Drugs. 2014;12(2):822–39. CrossRef
128. Prompanya C, Dethoup T, Gales L, Lee M, Pereira JAC, Silva AMS, et al. New polyketides and new benzoic acid derivatives from the marine sponge-associated fungus Neosartorya quadricincta KUFA 0081. Mar Drugs. 2016;14(7):134. CrossRef
129. Rehman SU, Wu JS, Yang LJ, Ting S, Shao CL, Wang CY. One new terphenyl glycoside from a sponge-derived fungus Trichoderma reesei (HN-2016-018). Nat Prod Commun. 2020;15(2):1–5. CrossRef
130. Mosadeghzad Z, Zuriati Z, Asmat A, Gires U, Wickneswari R, Pittayakhajonwut P, et al. Chemical components and bioactivity of the marine-derived fungus Paecilomyces sp. Collected from Tinggi Island, Malaysia. Chem Nat Compd. 2013;49(4):621–5. CrossRef
131. Bai X, Dong M, Lai T, Zhang H. Antimicrobial evaluation of the crude extract of symbiotic fungi from marine sponge Reniera japonica. Bangladesh J Pharmacol. 2018;13(1):53–6.