INTRODUCTION
Gout is a kind of inflammatory arthritis in which uric acid crystals accumulate in the joints, especially in the knee, ankle, wrist, finger, and elbow [1]. Xanthine oxidase (XO), a key enzyme in purine catabolism, catalyzes the oxidation of xanthine to uric acid in the body, but overformulation of uric acid may lead to hyperuricemia [2]. Late complications of long-term acute gout may induce poly-articular or oligo-articular gout, which is one of the most throbbing and painful conditions in humans [3]. Furthermore, gout patients have a higher risk of cardiovascular disorders as well [4,5]. One of the major strategies in the control of uric acid overproduction in gout treatment and its complications is that many new antihyperuricemic drugs have been synthesized and invented recently. However, some uric acid-lowering drugs have clinically toxic side effects. Hence, natural products have been considered to investigate their beneficial promotion. Bioactive natural chemical components are potential candidates with a safe, effective, and potential inhibitory effect on XO activity that stimulates uric acid production. Normally, there is a lack of systematic reviews about medicinal herbs and their chemical compounds with antihyperuricemic and anti-gout valuables. In this work, we attempted to review and summarize (1) the XO inhibitory capacity of herbal crude extracts, (2) the antihyperuricemic and antigout effects of purified chemical compounds in vitro and vivo, and (3) the molecular docking mechanism of the active chemical compounds and their derivatives focusing on XO inhibitory activities. Further research strategy for gout treatment therapy is still recommended.
MATERIALS AND METHODOLOGY
In this work, we used XO, uric acid, and gout as the keywords to collect information related to gout investigations from Web of Science, Science Direct, Springer, Google Scholar, PubMed, and other professional websites. This review summarizes and evaluates the gout treatment properties of medicinal herbs reported in the literature.
IN VITRO STUDIES
XO inhibitory capacity of herbal crude extracts
Investigations of medicinal plants have uncovered a number of anti-gouts. González et al. [6] reported the XO inhibitory activity of 34 crude extracts from species belonging to the Celastraceae and Lamiaceae. The 26 species from 18 families utilized for gout treatment in northeastern North America have been shown to have XO inhibitory capacity [7]. Over a hundred Chinese medicinal plants have been evaluated for antigout [8]. In other works, a number of herbal medicines have also been reported for XO inhibitory potency [9–23]. Interestingly, in all candidates, 46 herbs with outstanding XO inhibitory potency have been organized and listed in Table 1. However, many herbs in this group had not been investigated on pharmacological mechanisms, kinetics, in vivo and in silico, and clinically related to anti-gout activity.
XO inhibitory capacity of chemical compounds from herbal medicines
The chemical composition of herbal medicines for gout treatment has been studied for some recent decades. The phytochemical studies on XO inhibitory capacity have resulted in the isolation of hundreds of compounds. In all candidates, 85 chemical compounds with the lowest haft maximal inhibitory concentration (IC50) of XO inhibitory activity from a series of studies have been displayed in Table 2. The chemical compound group exhibited the highest potency with an IC50 < 1 μM. It included 2′,4′-dimethoxy-4,5′,6′-trihydroxychalcone (IC50 0.21 μM), neotaiwanensol B (IC50 0.28 μM), eupatilin (IC50 0.37 μM), chrysoeriol (IC50 0.5 μM), hyprhombin C (IC50 0.6 μM), apparicine (IC50 0.65 μM), and luteolin (IC50 1.2 μM). The isolated compound chemical structures are shown in Figure 1.
In serial other studies, XO inhibitory activity has also been evaluated. Baicalin and baicalein are the key XO inhibitory compounds of scutellariae radix [60]. The total alkaloids of nelumbinis folium inhibited XO with an IC50 of 3.313 μg/ml [61]. Flavonol glycosides of Allium cepa L. displayed XO inhibitory activity with an IC50 from 10.5 to 20.8 μg/ml [62]. Hoshani et al. [63] reported that leaf extracts of Physalis alkekengi at the green fruit stage exhibited higher XO inhibitory efficacy compared to the vegetative stage (86.86% and 45% at the concentration of 0.3 mg/ml, respectively). The underlying mechanisms of curcumin in preventing XO have been elucidated via studies on the molecular docking simulations [64]. The XO inhibitory effects of the main phenols of pickled radish have been characterized by molecular docking stimulated by hydrophobic interactions and hydrogen bonds and elucidated by molecular dynamics [65]. Betacyanin from Hylocereus undatus rind exhibited an XO inhibitory effect with an IC50 of 9 mM. Kinetics study and docking analysis for the XO inhibitory mechanism of betacyanin were also proved [66]. Du and Li [67] revealed that porphyra polysaccharide is capable of XO inhibitory activity through study on enzyme kinetics and molecular docking. The XO inhibitory mechanism of other natural products had also been evidenced revealed via fluorescence titration, molecular level interaction of chemical compounds with the amino acid residues, such as black rice anthocyanins [68]; chrysoerial [69]; monoterpenoids and flavonoid aglycones of Chrysanthemum morifolium [70]; flavonoids of Gardenia oudiepe [71]; Chrysanthemum moriforlium [72]; Quercetin-3-O-rhamnoside and chlorogenic acid obtained from Smilax china L. exhibited strong XO inhibitory capacity through kinetics and mechanism analysis [73]; luteolin [74]; Genistein from soybean [75]; atherospermidine and cyathocaline extracted from Alphonsea cylindrica and Alphonsea elliptica [76]; malic acid [77]; Eugenol, a marker component of clove [78]; benzofuran from Viburnum grandiflorum with an IC50 value of 0.59 μM) [79]; quercetin from Erodium birandianum [80]; catechin, epicatechin, gallic acid, and ellagic acid from acetone extract of Vicia faba L. seeds [81]; and 6-(3-methylbut-1-enyl)-5,7-dimethoxy-4′-hydroxy flavone from Spilanthes calva [82].
IN VIVO STUDIES
Moringa oleifera hydrolysate at doses of 200 and 500 mg/kg significantly reduced the serum uric acid level of hyperuricemic rats by regulating serum XO activity [83]. For Paeonia suffruticosa leaf extract, it effectively decreased increased serum uric acid in hyperuricemic mice. Insure evidence indicated the effects of protecting against renal damage and oxidative stress induced by hyperuricemia of apigenin 7-O-glucoside in mouse models [84]. It has been reported that extract of Rhizoma Alpiniae officinarum has hypouricemic and renal protective effects on hyperuricemic mice by XO inhibitory activity, down-regulating URAT1 and GLUT9, which is similar to the study on XO inhibitory activity of Saengmaeksan formulation including of Panax ginseng reported by Sung et al. [85]. Galangin, kaempferide, and 3-methoxyl-glangin are its marker XO inhibitors [86]. The mixture of methanol extracts of Euonymus laxiflorus, Rubia lanceolata, and Gardenia jasminosides reduced serum urate levels in hyperuricemic mice [87]. Interestingly, Huang et al. [88] reported that genistein, apigenin, quercetin, rutin, and astilbin exhibited insignificant effects on XO activity in vitro, but these compounds decreased serum uric acid levels in mice. The XO inhibitory effect of Lobetyolin, being a main bioactive chemical compound of Codonopnis plants, had been reported by Yoon and Cho [89]. It is revealed that lobetyolin exhibited weekly inhibitory XO capacity through a mixed-type mechanism, but it significantly decreased liver XO activity with a dose of 50 mg/kg in rats. The ethanol extract of Campomanesia velutina and its isolated myricitrin were demonstrated to be able to decrease serum uric acid levels and inhibit hepatic XO activity [90]. The Christia vespertilionis leaf aqueous extract induces a decrease in uric acid levels (31.95%) in mice at a dose of 200 mg/kg [22]. Many other studies on antigout activity in in vivo models of medicinal herbs and phytochemical compounds resulted in strong antigout benefits. All results indicated that evaluated herbal extracts exhibited no damage to the liver and kidney in hyperuricemic rats and inhibited excessive uric acid levels, which includes Artemisia selengensis leaf extracts [91]; theaflavin with an IC50 of 63.17 μM [92]; lemon-peel extract [93]; and green tea polyphenols [94], which may suggest an attractive strategy for antigout therapy.
SCIENCE OPINION AND RESEARCH STRATEGY
Former studies have shown that the pathogenesis of hyperuricemia in the blood is closely related to metabolism, immunity, and inflammation. Traditional medicine considers weaknesses in the liver, spleen, and kidneys as the principal causes of an increase in uric acid. In addition, the “military prieshood theory” is the primary principle of the control composition, which is modeled following the rule of the ancient monarchy system. In traditional medicine, this principle is applied, and each ingredient plays a particular role in treating the whole harmony and balance. The use of medicinal herbs, herbal extracts, and chemical compounds may contribute to the lower incidence of hyperuricemia. However, the fundamental principle in the treatment therapy of gout is to improve and restore liver, renal, and spleen function. Although kinetic studies and molecular docking analysis have been evidenced, there have not been any investigations in vivo on pharmacokinetics or internal metabolism in humans in a long time. Based on the above arguments, there is an urgent need to establish a comprehensive strategy for preventing and treating gout disease, which includes additional clinical trials with longer study periods on humans to certify the anti-gout potential of herbal medicine and case studies are also encouraged and called for in the future.
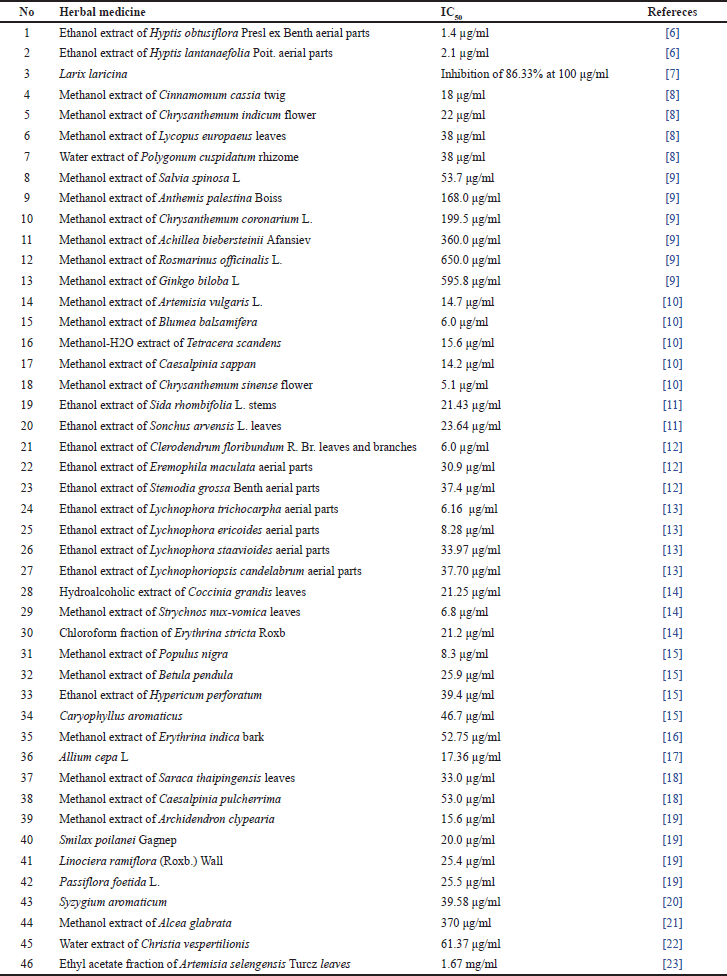 | Table 1. XO inhibitory capacity of herbal crude extracts. [Click here to view] |
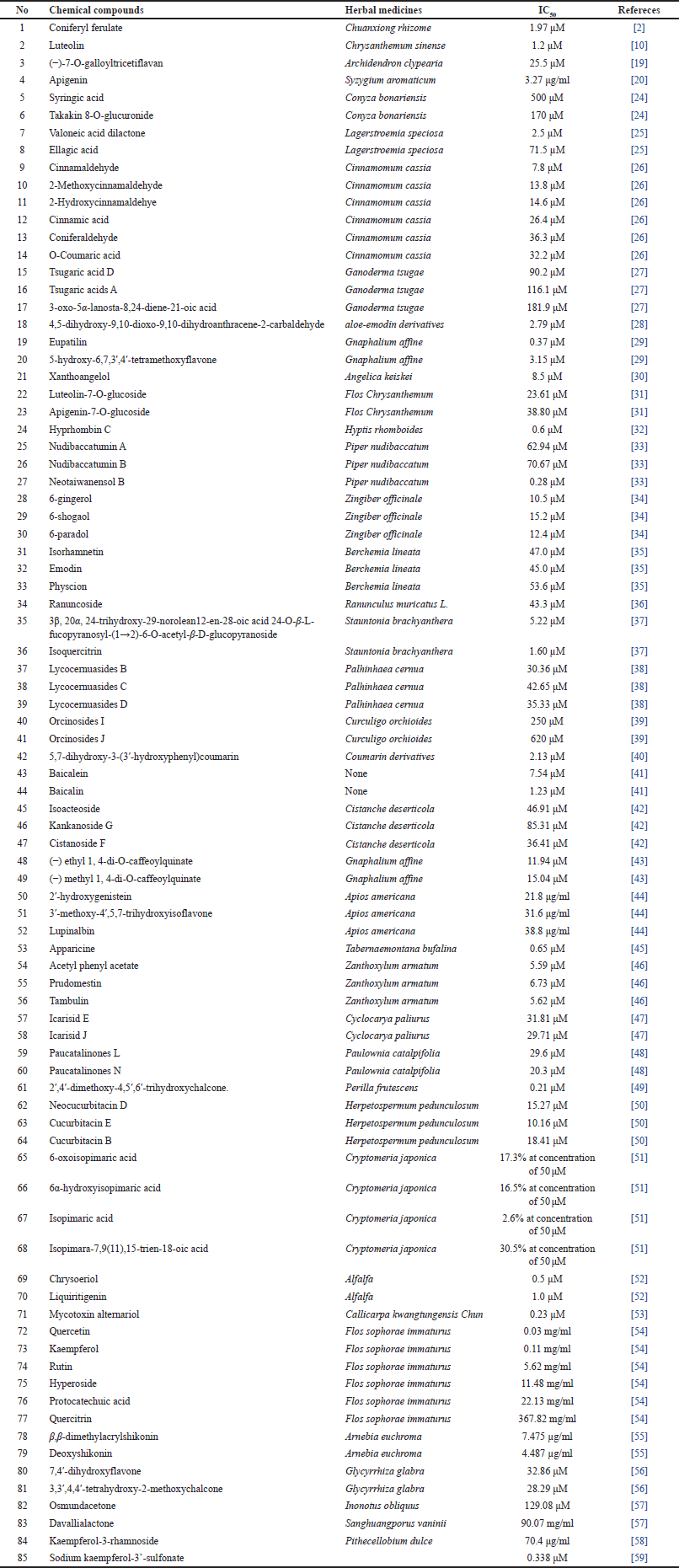 | Table 2. XO inhibitory capacity of chemical compounds from herbal medicines. [Click here to view] |
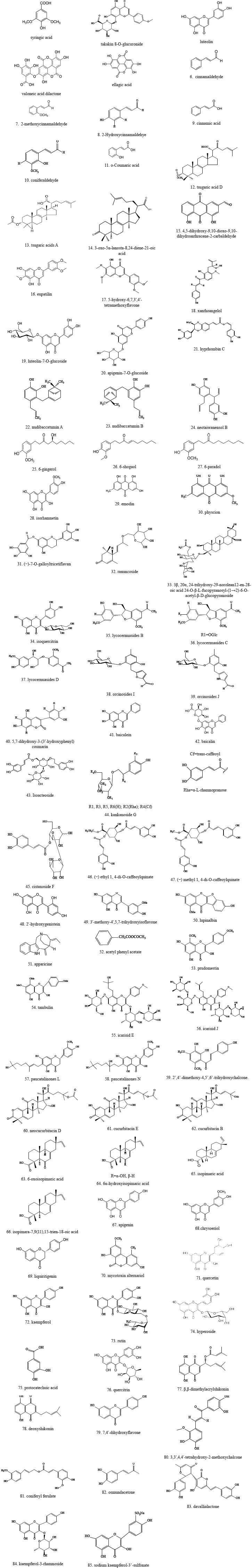 | Figure 1. Chemical structures of compounds. [Click here to view] |
CONCLUSION
Gout has attracted considerable attention because it causes serious health damage and affects human life quality. Serial pharmacological studies have been investigated in vitro and also developed in vivo in rat models. Though several pharmacological mechanisms and kinetics-related XO inhibitory activities of independent herbal derivative extracts and chemical compounds have already been achieved as emerging evidence, the more comprehensive pharmacological mechanisms of synergistic combinations of herbs and chemical components with each other need to be elucidated. Moreover, we are concerned that the medical resistance phenomenon is very likely when used for a long time; therefore, firm evidence for more clinical studies and applications needs to be elucidated in order to form an effective gout therapeutic formula of herbal medicine.
ACKNOWLEDGMENT
This work was supported by a project belonging to the Science and Technology program of the Ministry of Education and Training (No. CT2020.03.TNA 04).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This word was supported by the project (No. CT2020.03.TNA.04).
CONFLICTS OF INTEREST
The author has no relevant financial or nonfinancial interests to disclose.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda J, et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. 2020;79(1):31–8. CrossRef
2. Wang H, Zhang H, Zhang X, Yin Y, Ding G, Tang X, et al. Identification of coniferyl ferulate as the bioactive compound behind the xanthine oxidase inhibitory activity of chuanxiong rhizome. J Funct Foods. 2023a;100:105378. CrossRef
3. Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Int Med. 2005;143(7):499–516. CrossRef
4. Nawaz MZ, Ain QU, Zahid S, Zulfiqar T, Attique SA, Bilal M, et al. Physicochemical features and structural analysis of xanthine oxidase as a potential therapeutic target to prevent gout. J Radiat Res Appl Sci. 2020;13(1):616–28. CrossRef
5. Desideri G, Borghi C. Xanthine oxidase inhibition and cardiovascular protection: don’t shoot in the dark. Eur J Int Med. 2023;113:10–2. CrossRef
6. González AG, Bazzocchi IL, Moujir L, Ravelo AG, Correa MD, Gupta M P. Xanthine oxidase inhibitory activity of some Panamanian plants from Celastraceae and Lamiaceae. J Ethnopharmacol. 1995;46(1):25–9. CrossRef
7. Owen PL, Johns T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J Ethnopharmacol. 1999;64(2):149–60. CrossRef
8. Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73(1–2):199–207. CrossRef
9. Hudaib MM, Tawaha KA, Mohammad MK, Assaf AM, Issa AY, Alali FQ, et al. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Pharmacogn Mag. 2011;7(28):320. CrossRef
10. Nguyen MTT, Awale S, Tezuka Y, Le TQ, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull. 2004;27(9):1414–21. CrossRef
11. Hendriani R, Sukandar EY, Anggadiredja KS, Sukrasno S. In vitro evaluation of xanthine oxidase inhibitory activity of selected medicinal plants. Int J Pharm Clin Res. 2016;8(4):235–8.
12. Sweeney AP, Wyllie SG, Shalliker RA, Markham JL. Xanthine oxidase inhibitory activity of selected Australian native plants. J Ethnopharmacol. 2001;75(2–3):273–7. CrossRef
13. Ferraz Filha ZS, Vitolo I, Fietto LG, Lombardi JA. Xanthine oxidase inhibitory activity of Lychnophora species from Brazil (“Arnica”). J Ethnopharmacol. 2006;107(1):79–82. CrossRef
14. Umamaheswari M, AsokKumar K, Somasundaram A, Sivashanmugam T, Subhadradevi V, Ravi TK. Xanthine oxidase inhibitory activity of some Indian medical plants. J Ethnopharmacol. 2007;109(3):547–51. CrossRef
15. Havlik J, de la Huebra RG, Hejtmankova K, Fernandez J, Simonova J, Melich M, et al. Xanthine oxidase inhibitory properties of Czech medicinal plants. J Ethnopharmacol. 2010;132(2):461–5. CrossRef
16. Sowndhararajan K, Joseph JM, Rajendrakumaran D. In vitro xanthine oxidase inhibitory activity of methanol extracts of Erythrina indica Lam. leaves and stem bark. Asian Pac J Trop Biomed. 2012;2(3):S1415–7. CrossRef
17. Shin YJ, Hwang JM, Lee SC. Antioxidant and xanthine oxidase inhibitory activities of hot water extracts of medicinal herbs. J Korean Soc Food Sci Nutr. 2013;42(10):1712–6. CrossRef
18. Argulla LE, Chichioco-Hernandez CL. Xanthine oxidase inhibitory activity of some Leguminosae plants. Asian Pac J Trop Dis. 2014;4(6):438–41. CrossRef
19. Duong NT, Vinh PD, Thuong PT, Hoai NT, Bach TT, Nam NH, et al. Xanthine oxidase inhibitors from Archidendron clypearia (Jack.) IC Nielsen: results from systematic screening of Vietnamese medicinal plants. Asian Pac J Trop Med. 2017;10(6):549–56. CrossRef
20. Ooi KL, Zakaria R, Tan ML, Sulaiman SF. The influence of chemical composition of potent inhibitors in the hydrolyzed extracts of anti-hyperuricemic plants to their xanthine oxidase activities. J Ethnopharmacol. 2021;278:114294. CrossRef
21. Pirmohammadi Y, Asnaashari S, Nazemiyeh H, Hamedeyazdan S. Bioactivity assays and phytochemical analysis upon Alcea glabrata; focusing on xanthine oxidase inhibitory and antimalarial properties. Toxicon. 2023;229:107140. CrossRef
22. Endrini S, Bakar FIA, Bakar MFA, Abdullah N, Marsiati H. Phytochemical profiling, in vitro and in vivo xanthine oxidase inhibition and antihyperuricemic activity of Christia vespertilionis leaf. Biocatal Agric Biotechnol. 2023;48:102645. CrossRef
23. Li Y, Wan Y, Li R, Xu L, Xie M, Fu G. Solvent extraction of caffeoylquinic acids from Artemisia selengensis Turcz leaves and their in vitro inhibitory activities on xanthine oxidase. Ind Crops Prod. 2018;118:296–301. CrossRef
24. Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CHK, Tan RX. Glycosides and xanthine oxidase inhibitors from Conyza bonariensis. Phytochemistry. 2001;58(4):645–51. CrossRef
25. Unno T, Sugimoto A, Kakuda T. Xanthine oxidase inhibitors from the leaves of Lagerstroemia speciosa (L.) Pers. J Ethnopharmacol. 2004;93(2–3):391–5. CrossRef
26. Tran MN, Nguyen MK, Do TH, Nguyen XN, Bui HT, Dao VD, et al. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorg Med Chem Lett. 2012;22(14):4625–8. CrossRef
27. Lin KW, Chen YT, Yang SC, Wei BL, Hung CF, Lin CN. Xanthine oxidase inhibitory lanostanoids from Ganoderma tsugae. Fitoterapia. 2013;89:231–8. CrossRef
28. Shi DH, Huang W, Li C, Liu YW, Wang SF. Design, synthesis and molecular modeling of aloe-emodin derivatives as potent xanthine oxidase inhibitors. Eur J Med Chem. 2014;75:289–96. CrossRef
29. Lin WQ, Xie JX, Wu XM, Yang L, Wang HD. Inhibition of xanthine oxidase activity by Gnaphalium affine extract. Chin Med Sci J. 2014;29(4):225–30. CrossRef
30. Kim DW, Curtis-Long MJ, Yuk HJ, Wang Y, Song YH, Jeong SH, et al. Quantitative analysis of phenolic metabolites from different parts of Angelica keiskei by HPLC–ESI MS/MS and their xanthine oxidase inhibition. Food Chem. 2014;153:20–7. CrossRef
31. Song HP, Zhang H, Fu Y, Mo HY, Zhang M, Chen J, et al. Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC–MS combined with enzyme channel blocking. J Chromatogr B. 2014;961:56–61. CrossRef
32. Tsai SF, Lee SS. Neolignans as xanthine oxidase inhibitors from Hyptis rhomboides. Phytochemistry. 2014;101:121–7. CrossRef
33. Liu HX, He MT, Tan HB, Gu W, Yang SX, Wang YH, et al. Xanthine oxidase inhibitors isolated from Piper nudibaccatum. Phytochem Lett. 2015;12:133–7. CrossRef
34. Nile SH, Park SW. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind Crops Prod. 2015;70:238–44. CrossRef
35. Li J, Deng GR, Cheng W, He B, Zhang GL, Huang BS, et al. Chemical constituents of Berchemia lineata. Medicine and Biopharmaceutical: Proceedings of the 2015 International Conference; 2016. pp 1140–8. CrossRef
36. Raziq N, Saeed M, Ali MS, Zafar S, Shahid M, Lateef M. A new glycosidic antioxidant from Ranunculus muricatus L. (Ranunculaceae) exhibited lipoxygenasae and xanthine oxidase inhibition properties. Nat Prod Res. 2017;31(11):1251–7. CrossRef
37. Liu D, Wang D, Yang, W, Meng D. Potential anti-gout constituents as xanthine oxidase inhibitor from the fruits of Stauntonia brachyanthera. Bioorg Med Chem. 2017;25(13):3562–6. CrossRef
38. Li J, Xu PS, Tan LH, Zou ZX, Wang YK, Long HP, et al. Neolignans and serratane triterpenoids with inhibitory effects on xanthine oxidase from Palhinhaea cernua. Fitoterapia. 2017;119:45–50. CrossRef
39. Chen X, Zuo A, Deng Z, Huang X, Zhang X, Geng C, et al. New phenolic glycosides from Curculigo orchioides and their xanthine oxidase inhibitory activities. Fitoterapia. 2017;122:144–9. CrossRef
40. Fais A, Era B, Asthana S, Sogos V, Medda R, Santana L, et al. Coumarin derivatives as promising xanthine oxidase inhibitors. Int J Biol Macromol. 2018;120:1286–93. CrossRef
41. Zeng N, Zhang G, Hu X, Pan J, Zhou Z, Gong D. Inhibition mechanism of baicalein and baicalin on xanthine oxidase and their synergistic effect with allopurinol. J Funct Foods. 2018;50:172–82. CrossRef
42. Chen X, Deng Z, Huang X, Geng C, Chen J. Liquid chromatography–mass spectrometry combined with xanthine oxidase inhibition pro?ling for identifying the bioactive constituents from Cistanche deserticola. Int J Mass Spectrom. 2018;430:1–7. CrossRef
43. Zhang W, Chun-Zhen WU, Si-Yang FAN. Chemical constituents from Gnaphalium affine and their xanthine oxidase inhibitory activity. Chin J Nat Med. 2018;16(5):347–53. CrossRef
44. Kim JH, Jin CH. Xanthine oxidase inhibitory activity of isoflavonoids from Apios americana. Comput Biol Chem. 2019;83:107137. CrossRef
45. Shi BB, Chen J, Bao MF, Zeng Y, Cai XH. Alkaloids isolated from Tabernaemontana bufalina display xanthine oxidase inhibitory activity. Phytochemistry. 2019;166:112060. CrossRef
46. Nooreen Z, Bushra U, Bawankule DU, Shanker K, Ahmad A, Tandon S. Standardization and xanthine oxidase inhibitory potential of Zanthoxylum armatum fruits. J Ethnopharmacol. 2019;230:1–8. CrossRef
47. Ye ZJ, He XA, Wu JP, Li J, Chang XW, Tan J, et al. New prenylflavonol glycosides with xanthine oxidase inhibitory activity from the leaves of Cyclocarya paliurus. Bioorg Chem. 2020;101:104018. CrossRef
48. Xiao CM, Jia XH, Du HF, Zhao HX, Du CL, Tang WZ, et al. Three new C-geranylated flavonoids from Paulownia catalpifolia T. Gong ex DY Hong seeds with their inhibitory effects on xanthine oxidase. Phytochem Lett. 2020;36:162–5. CrossRef
49. Liu Y, Hou Y, Si Y, Wang W, Zhang S, Sun S, et al. Isolation, characterization, and xanthine oxidase inhibitory activities of flavonoids from the leaves of Perilla frutescens. Nat Prod Res. 2020;34(18):2566–72. CrossRef
50. Jiang HZ, Hu S, Tan RX, Tan R, Jiao RH. Neocucurbitacin D, a novel lactone-type norcucurbitacin as xanthine oxidase inhibitor from Herpetospermum pedunculosum. Nat Prod Res. 2020;34(12):1728–34. CrossRef
51. Chang CI, Chen CC, Wang SY, Chen JJ, Chen CR, Chao CY, et al. Three new isopimaric acid diterpenoids from the bark of Cryptomeria japonica and their xanthine oxidase inhibitory activity. Phytochem Lett. 2021;46:61–5. CrossRef
52. Hsu SJ, Verpoorte R, Lin SM, Lee CK. Fast dereplication of xanthine oxidase-inhibiting compounds in alfalfa using comparative metabolomics. Food Res Int. 2021;141:110170. CrossRef
53. Fan J, Sun S, Lv C, Li Z, Guo M, Yin Y, et al. Discovery of mycotoxin alternariol as a potential lead compound targeting xanthine oxidase. Chem Biol Interact. 2022;360:109948. CrossRef
54. Li J, Gong Y, Li J, Fan L. In vitro xanthine oxidase inhibitory properties of Flos Sophorae immaturus and potential mechanisms. Food Biosci. 2022a;47:101711. CrossRef
55. Kumar N, Rajput A, Kaur H, Sharma A, Bhagat K, Singh JV, et al. Shikonin derivatives as potent xanthine oxidase inhibitors: in-vitro study. Nat Prod Res. 2022;37(16): 2795–800 CrossRef
56. Mahrous RS, Fathy HM, Ibrahim RS. Metabolic bioprofiling for discovering xanthine oxidase inhibitors from Glycyrrhiza glabra root solvents fractions using orthogonal separation modalities coupled with chemometry. S Afr J Bot. 2023;154:251–9. CrossRef
57. Song J, Wang Z, Chi Y, Zhang Y, Fang C, Shu Y, et al. Anti-gout activity and the interaction mechanisms between Sanghuangporus vaninii active components and xanthine oxidase. Bioorg Chem. 2023;133:106394. CrossRef
58. Wichaidit W, Thongyoo P. A novel γ-lactone isolated from the leaves of Pithecellobium dulce (Roxb.) Benth. and its xanthine oxidase activity. Nat Prod Res. 2023;37(7):1168–76. CrossRef
59. Wang X, Cui Z, Luo Y, Huang Y, Yang X. In vitro xanthine oxidase inhibitory and in vivo anti-hyperuricemic properties of sodium kaempferol-3′-sulfonate. Food Chem Toxicol. 2023b;177:113854. CrossRef
60. Yan Z, Liqiong S, Yingduo Y, Jin Q, Boyang Y. Application of multi-dimensional and multi-informational (MD-MI) integrated xanthine oxidase and superoxide anion fingerprint in quality evaluation of Scutellariae radix. J Pharm Biomed Anal. 2020;191:113595. CrossRef
61. Sang M, Du G, Hao J, Wang L, Liu E, Zhang Y, et al. Modeling and optimizing inhibitory activities of Nelumbinis folium extract on xanthine oxidase using response surface methodology. J Pharm Biomed Anal. 2017;139:37–43. CrossRef
62. Nile SH, Nile AS, Keum YS, Sharma K. Utilization of quercetin and quercetin glycosides from onion Allium cepa (L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017;235:119–26. CrossRef
63. Hoshani M, Mianabadi M, Aghdasi M, Azim-Mohseni M. Inhibition effects of Physalis alkekengi extract on xanthine oxidase activity in different phenological stages. Clin Biochem. 2011;13(44):S343. CrossRef
64. Shen L, Ji HF. Insights into the inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett. 2009;19(21):5990–3. CrossRef
65. Liu X, Wu D, Liu J, Li G, Zhang Z, Chen C, et al. Characterization of xanthine oxidase inhibitory activities of phenols from pickled radish with molecular simulation. Food Chem. 2022;X(14):100343. CrossRef
66. Dey D, Hemachandran H, Doss GP, Priyadarshini R, Siva R. Accumulation of betacyanin in Hylocereus undatus rind: pigment stability analysis and its role in xanthine oxidase inhibition. Phytomed Plus. 2022;2(1):100197. CrossRef
67. Du H, Li SJ. Inhibition of porphyra polysaccharide on xanthine oxidase activity and its inhibition mechanism. Spectrochim Acta Part A Mol Biomol Spectrosc. 2022;266:120446. CrossRef
68. Feng LJ, Ou WW, Yang YB, Qi Y, Qi Z, Zhang JL. Black rice anthocyanins alleviate hyperuricemia in mice: possible inhibitory effects on xanthine oxidase activity by cyanidin 3-O-glucoside. J Cereal Sci. 2022;104:103406. CrossRef
69. Liu Y, Han C, Lu T, Liu Y, Chen H, Yang C, et al. Investigation of the interaction between chrysoeriol and xanthine oxidase using computational and in vitro approaches. Int J Biol Macromol. 2021;190:463–73. CrossRef
70. Peng AN, Lianzhu L, Mouming Z. Screening of key flavonoids and monoterpenoids for xanthine oxidase inhibitory activity-oriented quality control of Chrysanthemum morifolium Ramat. ‘Boju’based on spectrum-effect relationship coupled with UPLC-TOF-MS and HS-SPME-GC/MS. Food Res Int. 2020;137:109448. CrossRef
71. Santi MD, Zunini MP, Vera B, Bouzidi C, Dumontet V, Abin-Carriquiry A, et al. Xanthine oxidase inhibitory activity of natural and hemisynthetic flavonoids from Gardenia oudiepe (Rubiaceae) in vitro and molecular docking studies. Eur J Med Chem. 2018;143:577–82. CrossRef
72. Li X, Yang W, Chen H, Pan F, Liu W, Qi D, et al. Rapid screening and in vivo target occupancy quantitative evaluation of xanthine oxidase inhibitors based on drug-target binding kinetics research strategy: a case study of Chrysanthemum morifolium Ramat. Biomed Pharmacother. 2023;161:114379. CrossRef
73. Li X, Jin W, Zhang W, Zheng G. The inhibitory kinetics and mechanism of quercetin-3-O-rhamnoside and chlorogenic acid derived from Smilax china L. EtOAc fraction on xanthine oxidase. Int J Biol Macromol. 2022b;213:447–55. CrossRef
74. Yan J, Zhang G, Hu Y, Ma Y. Effect of luteolin on xanthine oxidase: inhibition kinetics and interaction mechanism merging with docking simulation. Food Chem. 2013;141(4):3766–73. CrossRef
75. Lin S, Zhang G, Pan J, Gong D. Deciphering the inhibitory mechanism of genistein on xanthine oxidase in vitro. J Photochem Photobiol B Biol. 2015;153:463–72. CrossRef
76. Sidik MN, Bakri YM, Azziz SSSA, Aldulaimi AKO, Wong CF, Ibrahim M. In silico xanthine oxidase inhibitory activities of alkaloids isolated from Alphonsea sp. S Afr J Bot. 2022;147:820–5. CrossRef
77. Vijeesh V, Vysakh, A, Jisha N, Latha MS. Multispectroscopic binding studies and in silico docking analysis of interactions of malic acid with xanthine oxidase. J Mol Struct. 2022;1268:133621. CrossRef
78. Vijeesh V, Jisha N, Vysakh A, Latha MS. Interaction of eugenol with xanthine oxidase: multi spectroscopic and in silico modelling approach. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021;258:119843. CrossRef
79. Alam M, Uddin G, Rashid U, Rauf A, Raza M, Shah SMM, et al. In vitro and in silico xanthine oxidase inhibitory potential of benzofuran isolated from Viburnum grandiflorum Wall. Ex DC. S Afr J Bot. 2021;143:359–62. CrossRef
80. Kekilli EB, Orhan IE, Deniz FSS, Eren G, Emerce E, Kahraman A, et al. Erodium birandianum Ilarslan & Yurdak. shows anti-gout effect through xanthine oxidase inhibition: combination of in vitro and in silico techniques and profiling of main components by LC-Q-ToF-MS. Phytochem Lett. 2021;43:80–7. CrossRef
81. Choudhary DK, Mishra A. In vitro and in silico interaction of faba bean (Vicia faba L.) seed extract with xanthine oxidase and evaluation of antioxidant activity as a therapeutic potential. Nat Prod Res. 2019;33(18):2689–93. CrossRef
82. Jayaraj P, Mathew B, Parimaladevi B, Ramani VA, Govindarajan R. Isolation of a bioactive flavonoid from Spilanthes calva DC in vitro xanthine oxidase assay and in silico study. Biomed Prev Nutr. 2014;4(4):481–4. CrossRef
83. Tian Y, Lin L, Zhao M, Peng A, Zhao K. Xanthine oxidase inhibitory activity and antihyperuricemic effect of Moringa oleifera Lam. leaf hydrolysate rich in phenolics and peptides. J Ethnopharmacol. 2021;270:113808. CrossRef
84. Zhang Y, Li Y, Li C, Zhao Y, Xu L, Ma S, et al. Paeonia suffruticosa Andrews leaf extract and its main component apigenin 7-O-glucoside ameliorate hyperuricemia by inhibiting xanthine oxidase activity and regulating renal urate transporters. Phytomedicine. 2023;118:154957. CrossRef
85. Sung YY, Yuk HJ, Kim DS. Saengmaeksan, a traditional herbal formulation consisting of Panax ginseng, ameliorates hyperuricemia by inhibiting xanthine oxidase activity and enhancing urate excretion in rats. J Ginseng Res. 2021;45(5):565–74. CrossRef
86. Lin L, Xuemei L, Mouming Z. Screening of xanthine oxidase inhibitor from selected edible plants and hypouricemic effect of Rhizoma Alpiniae officinarum extract on hyperuricemic rats. J Funct Foods. 2018;50:26–36. CrossRef
87. Liu LM, Cheng SF, Shieh PC, Lee JC, Chen JJ, Ho CT, et al. The methanol extract of Euonymus laxiflorus, Rubia lanceolata and Gardenia jasminoides inhibits xanthine oxidase and reduce serum uric acid level in rats. Food Chem Toxicol. 2014;70:179–84. CrossRef
88. Huang J, Wang S, Zhu M, Chen J, Zhu X. Effects of genistein, apigenin, quercetin, rutin and astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem Toxicol. 2011;49(9):1943–7. CrossRef
89. Yoon IS, Cho SS. Effects of lobetyolin on xanthine oxidase activity in vitro and in vivo: weak and mixed inhibition. Nat Prod Res. 2021;5(10):1667–70. CrossRef
90. Araújo MC, Ferraz-Filha ZS, Ferrari FC. Campomanesia velutina leaves extracts exert hypouricemic effects through inhibition of xanthine oxidase and ameliorate inflammatory response triggered by MSU crystals. Rev Bras Farmacogn. 2016;26:720–7. CrossRef
91. Xiang L, Huang Y, Li R, Tao Y, Wu T, Pan S, et al. Artemisia selengensis Turcz. leaves extract ameliorates hyperuricemia in mice by inhibiting hepatic xanthine oxidase activity, modulating renal uric acid transporters, and improving metabolic disorders. Food Biosci. 2023;56:102639. CrossRef
92. Chen J, Li Q, Ye Y, Ran M, Ruan Z, Jin N. Inhibition of xanthine oxidase by theaflavin: possible mechanism for anti-hyperuricaemia effect in mice. Process Biochem. 2020;97:11–8. CrossRef
93. Zou GS, Li SJ, Zheng SL, Pan X, Huang ZP. Lemon-Peel extract ameliorates rheumatoid arthritis by reducing xanthine oxidase and inflammatory cytokine levels. J Taiwan Inst Chem Eng. 2018;93:54–62. CrossRef
94. Chen G, Tan ML, Li KK, Leung PC, Ko CH. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J Ethnopharmacol. 2015;175:14–20. CrossRef