INTRODUCTION
Terfez or Terfass or Kamés (“desert truffles”) are seasonal edible hypogeal fungi (Ascomycetes, Pezizales) that have been exclusively harvested in wild areas for hundreds of years [1]. They live in symbiotic mycorrhizal associations [2] with herbaceous or shrubby plants of the Cistaceae family, especially of the genus Helianthemum. These fungi grow in all arid and semi-arid regions around the Mediterranean (Maghreb, southern Italy, southern Spain, and Portugal) and in the Middle East (Iran, Iraq, Syria, Palestine, Kuwait, and Saudi Arabia) [3–11]. The most common method of harvesting desert truffles is observing the ground, as it is often swollen and cracked at the base of the host plant [9].
In Algeria, it is represented by the three genera: Terfezia (“Red Terfez”), Tirmania (“White Terfez”), and Picoa (“Jouaber”); 11 species are known so far in our country [12,13]. These species are distributed in the steppe and Saharan areas. In these regions, Terfez are appreciated for their edible value and properties in traditional medicine: their effects against eye diseases of bacterial origin have been reported for several centuries by the Arabs of ancient times [14] and by a Muslim religious Hadith. “Truffles are like Manna, and their water heals eye diseases” (PBUH Sahih al-Bukhari 5708). By identifying their bioactive components, such as phenolics, terpenoids, polysaccharides, fatty acids, and ergosterols, researchers have paid attention to their biological activities, including antioxidant, antibacterial, and antifungal activities [15]. The chemical content of Terfez has so far been studied with regard to the dietary elements [16–19], demonstrating that this fungus provides an excellent dietary protein intake, it is rich in essential amino acids, various fatty acids and, vitamins [20,21]. On the other hand, to the best of our knowledge, despite repeated attempts at isolation, identification, and evaluation of mycelium-active ingredients responsible for the bioactivity of Terfezia claveryi from Algeria, a few works have been done [22–26].
Therefore, to increase the knowledge about the bioactivity potential of T. claveryi harvested for the first time from the Tindouf area, the current study aims to present experimental optimization of extraction and fractionation of raw extracts from fruit body flesh, mycelium, and its culture medium, and then, the demonstration of antimicrobial active ingredients by in vitro tests on several plant-pathogenic fungi and bacteria through mycelium isolation from ascospores of T. claveryi, highlighting the antibacterial and antifungal activities of mycelium extracts, culture filtrate, and flesh; investigating the in vitro antagonism phenomenon between Terfez and plant-pathogenic fungi; and finally we present organic extracts fractionating process using various chromatographic methods, to achieve active components isolation and chemical characterization.
MATERIALS AND METHODS
Chemicals and instruments
Organic solvents were purchased from SDS (F-Asnières sur Seine) and distilled before use. For thin layer chromatography (TLC), we used a chromatographic tank and Ultra Violet (UV) cabinet with a Camag UV lamp De Luxe 29000 (254 and 366 nm) was used. The thin-layer system was silica gel with UV indicator (Si 60 F 254, Merk). The high-performance liquid chromatography (HPLC) apparatus included a Constrametric III (LDC/Milton Roy) pump, a Rheodyne Inc. injector, and Spectro-Monitor D (LDC) UV modules.
Samples collection
Desert truffle fruiting bodies were collected from the region of Tindouf city situated in the Western South desert of Algeria (27°40′33.964″ N 8°7′39.549″ W) (Fig. 1) during the spring season (February–April). As soon as they arrived at the laboratory after 24 transportation hours, samples were gently cleaned to take off sand particles, and their surface was rapidly flamed with ethyl alcohol for disinfection. Truffle samples were separated into two batches, the first to be tested directly as “fresh gleba” and the second for storage for 2 months at room temperature in a dry and dark place, disposed separately on absorbent paper to avoid wilting, to be explored as “dried gleba” samples.
T. claveryi identification
After macroscopic observation of the fruit bodies’ aspect, light microscopy (LM) and scan electron microscopy (SEM) were performed to identify ascomycetes and spores’ characteristic features. A small amount of desiccated Terfezia ascocarp fragment was deposited in a drop of water between the slide and cover slip. For SEM, powder from grounded desiccated ascocarp section was fixed on double-sided tape, gold coated, and kept under vacuum overnight, then observed in a SEM GEOL JSM 6300 at Nantes University, Faculty of Medicine, Sciences and techniques, Odontology, CHU, Service Commun de Microscopie Electronique, Microcaractérisation et Morpho-histologie-imagerie fonctionnelle (SC3M).
Monoclonal mycelium isolation of T. claveryi
Mycelia were isolated, according to Chevalier [27]. The method consisted of taking fragments of gleba from previously alcohol-disinfected dried ascocarps. The fragments were rehydrated in sterile distilled water and ground to obtain a suspension of ascospores. This was later stirred using a vortex. Three to four drops of the suspension were spread on the surface of 1% Malt extract agar poured into Petri dishes, which were firmly sealed with adhesive tape and incubated at 25°C. The ascospores were regularly observed under LM to follow germination stages. Germinated spores were localized under LM and delicately collected to be transferred separately in fresh Malt extract Agar medium Petri dishes to obtain monoclonal strains. Incubation was carried out at 25°C.
Flasks containing 150 ml of 1% Malt extract liquid medium were seeded with a 15 mm square implant collected from T. claveryi monoclonal pre-cultures aged 60 days. Once sealed, flasks were incubated on a rotary stirrer (50 rpm) at 25°C.
Bioactivity exploration
To be compared, bioactive metabolites of T. claveryi have been prospected in different extracts obtained with other methods. “Soxhlet gleba” extract, “Culture broth” extract, and “Mycelium” extract are symbolized respectively hereafter “Soxhlet, Culture medium, Mycelium (SCM)” extracts for text lecture simplification.
Fresh gleba Soxhlet extract (S extract)
A mass of 15 g of fresh gleba taken from ascocarps of T. claveryi was cut into small fragments and put in a Soxhlet cartridge. The Soxhlet flask, containing 250 ml of EtOAc, was heated at 50°C for 3 hours. The solvent was removed under vacuum using a Rotavapor at 50°C. The remaining crude extract was dissolved in 2 ml of EtOAc and weighed after complete solvent evaporation.
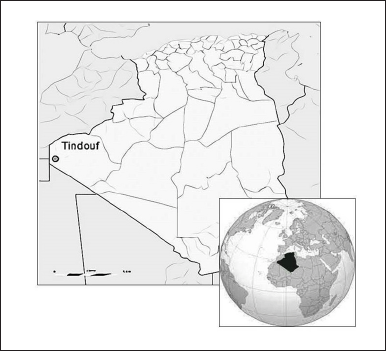 | Figure 1. Geographical position of Tindouf city (Algeria). [Click here to view] |
Culture broth extract (C extract)
After 8 weeks of incubation, filtration through a sintered glass funnel containing filter paper separated mycelial monoclonal culture in a liquid medium from the culture broth. The mycelial mass was drained and weighed. The broth was collected directly into a flask and extracted with EtOAc. One liter of culture filtrate and 150 ml of EtOAc were introduced into a separating funnel, and the mixture was slowly stirred (the funnel valve must be open to escape gases). After decantation, the organic phase was collected in anhydrous sodium sulfate (Na2SO4). The aqueous phase was extracted two more times with 150 ml of EtOAc. The three organic phase fractions were mixed, and EtOAc was removed using a Rotavapor at 50°C. The remaining crude extract was dissolved in 2 ml of EtOAc and weighed after the solvent’s total evaporation.
Mycelium extract (M extract)
The recovered mycelium was put in EtOAc and ground for 5 minutes in an electric mixer, then filtered through cotton and filter paper. The final filtrate was collected in a flask containing a few grams of Na2SO4. After Rotavapor concentration at 50°C, the brownish residue was taken up in a few milliliters of EtOAc and weighed after solvent total evaporation.
Antibacterial activity
Nine bacterial strains were tested: Escherichia coli ATCC 25922; Pseudomonas aeruginosa ATCC 27853; Streptococcus ATCC 29212; Staphylococcus aureus ATCC 25923; S. aureus Rubber Oxygenases A (ROXA) 97009108; Pseudomonas fluorescens; Salmonella typhi; Klebsiella sp. and Shigella sp. This was performed using the disc Agar diffusion method. One milliliter of standardized bacterial suspensions at 106 CFU/ml was spread out over the surface of Mueller-Hinton Agar 4 mm depth poured in Petri plates. The latter was left close to a Bunsen burner for about 20 mn to dry the medium surface. Sterilized discs of 6 or 10 mm made of Whatman n°1 paper were impregnated separately with SCM extracts. They were aseptically applied to the surface of Agar plates at well-spaced intervals. Control discs were impregnated with a sterile 1% Malt extract medium. The plates were incubated at 37°C for 16 hours.
Border-bioactivity assessment of T. claveryi EtOAc mycelium extract
Serial dilutions of bioactive EtOAc crude were made to obtain decreasing concentrations as follows:
 | [Click here to view] |
The inhibitory effect of each concentration was tested on five bacterial ATCC and ROXA species (Section “Antibacterial activity”) by disc diffusion method. A sterile paper disc was impregnated with 40 μl of one of the dilutions, dried, and then put on the surface of Mueller Hinton agar medium previously inoculated with 1 ml of standardized bacterial suspension (106 CFU) in Petri dishes. Incubation was achieved for 16 hours at 37°C.
Antifungal activity tests
Six fungal plant-pathogen strains were tested: Sclerotinia sclerotiorum, Phytophtora sp., Aschochyta rabiei, Fusarium oxysporum f. sp. lycopersici, F. oxysporum f. sp. aalbedinis, F. oxysporum f. sp. lentii and one yeast human pathogen strain Candida albicans. The testing method used for C. albicans was previously described for bacterial tests (Section “Antibacterial activity”), replacing the Mueller-Hinton medium with Sabouraud agar medium [28]. Incubation lasts 24 and 48 hours at 25°C [29].
Dennis and Webster method I
Consisted of digging an 8 mm well in the center of 1% Malt Agar medium in Petri dishes, then filling it with 0.5 ml of SCM extracts. Afterward, the well was covered with an 8 mm fresh active growing mycelium implant of one of the fungal plant-pathogen strains to be tested. Controls were made by filing wells with 0.5 ml of 1% sterile Malt medium or EtOAc before placing the fungal plant-pathogen implant. Petri plates were incubated at 25°C for 7 days.
Dennis and Webster method II
A droplet of 500 μl of one of the SCM extracts was deposited on the surface of the 1% Malt Agar medium poured into Petri dishes. After solvent evaporation, a circular implant of the plant pathogen fungus to be tested (8 mm φ) was deposited 1 cm from the concerned extract. For controls, extracts were replaced by 1% sterile Malt medium or EtOAc. Incubation was at 25°C.
Culture broth mixture test
A mycelial T. claveryi culture broth aged at 8 weeks was sterilized by microfiltration on a sterile 0.45 μm Millipore membrane and then incorporated (volume/volume) into Malt (1%) Agar (4%) medium, kept supercooled at 40°C. After homogenization, the mixture was poured into Petri dishes. Succeeding Agar solidification, Petri dishes were inoculated with a circular implant cut from young cultures of one of the six plant-pathogen fungal species tested and incubated at 25°C.
Antagonism of T. claveryi mycelium against plant-pathogen fungi
Confrontation on solid medium
Terfezia claveryi mycelium was confronted with F.o.l., F.o.a., F.o.le., Phytophthora sp., A. rabiei, and S. sclerotiorum. An 8 mm φ implant of T. claveryi mycelium was put on the surface of 1% Malt extract Agar medium and incubated at 25°C. One month later, an 8 mm plant-pathogen fungal strain implant to be tested was placed 3 cm away from the mycelial implant of T. claveryi. The control plates were inoculated with just an implant of the fungal plant-pathogen fungal species to be tested. Dishes were sealed with tape and incubated at 25°C.
Kope and Fortin confrontation method
Malt extract Agar medium poured into Petri dishes was covered with a sterile Millipore membrane (0.45 μm, 5 cm in diameter). An 8 mm φ T. claveryi mycelium implant was placed on the membrane. Dishes were sealed with tape and incubated at 25°C. After 3–4 weeks of incubation, the Millipore membrane invaded by Terfez mycelium was aseptically removed and replaced by an implant of one of the plant-pathogen fungal strains tested. For controls, Malt Agar medium dishes were directly inoculated with An 8 mm φ implant of the plant-pathogen fungal species. Petri dishes were incubated for a week at 25°C.
Double layer confrontation method
A mycelial 8 mm implant of T. claveryi was placed on 1% Malt extract Agar medium in Petri dishes, which were sealed with tape and incubated at 25°C. One month later, the mycelium was covered with a sterile Millipore membrane (0.45 μm, 5 cm φ), and then 15 ml of supercooled Malt extract Agar medium at 40°C was poured on it. An 8 mm implant of a plant-pathogen fungal species was then placed on the freshly poured medium layer after its solidification. Concerning controls, no Terfez mycelial implant was used first. The sterile Millipore membrane was put on a Malt extract Agar medium layer just before adding the second one, on which the corresponding plant pathogen fungal strain 8 mm implant was placed. Dishes were sealed with tape and incubated at 25°C for a week.
Measurement methods
Antibacterial tests
Bacterial sensitivity to the culture filtrates or to the various Terfez extracts (flesh, culture broth, or mycelium) was appreciated by inhibition zone formation. The inhibition zone diameter around the disk was measured after 24 hours of incubation.
Antifungal tests
Yeast strain C. albicans sensitivity was evaluated by measuring the inhibition zone diameter after 24 hours. Plant-pathogen fungal strains: for the tests carried out with Dennis and Webster [30] method I, the mixing broth culture to Malt extract agar medium method, Kope and Fortin [31] method, Millipore membrane double layer method, and the antagonism tests (direct confrontation), the growth of the plant-pathogen fungus was evaluated daily by measuring cultures diameters around the circular implant in two perpendicular directions. Concerning the confrontation tests, the fungal species growth was monitored daily and stopped as soon as the two mycelial fronts came into contact.
For tests performed with SCM extracts of T. claveryi by method II of Dennis and Webster [30], the inhibition was evaluated by mycelial growth stopping of plant-pathogen fungus. The inhibition zone diameter was measured in two perpendicular directions. In both cases, the results represent the average of three repetitions, and the percentage of inhibition was calculated according to the following formula [32]: I = (Dt − Dm)/Dt.100 where I: % mycelial growth inhibition; Dt: mycelial culture control mean diameter; Dm: mycelial culture mean diameter in the presence of Terfez. Measurements ended when mycelial control cultures covered the entire surface of the medium.
Chemical bioactive compounds characterization
Throughout the fractionation optimization process, antibiogram tests using P. aeruginosa ATCC 27853 were performed on all issued fractions to track the bioactive molecules’ progression. The following methods were successively used: TLC, liquid/liquid polarity partition, open column chromatography on silica or Sephadex LH-20 column, HPLC, and finally gas chromatography-mass spectrometry (GC-MS). Analytic TLC mode allowed the first extract exploration step. Otherwise, it was used parallel to other techniques to appreciate their efficiency. All obtained fractions were inspected for their chemical composition (TLC) and their biological activity (antibiogram on ATCC bacteria).
TLC on silica gel
Extraction migration was performed in the CH2Cl2/MeOH (90:10) mixture and revealed by UV.
Liquid/liquid polarity partition (Kupchan method)
A solution of the initial mixture (Terfez extract) was extracted using a separating funnel. 5 mg of mycelium crude was dissolved in 20 ml of MeOH: H2O (90:10), and 20 ml of hexane was added. The mixture was carefully stirred and allowed to settle until the appearance of two phases. The methanol phase was put aside, and the hexane phase was extracted twice again as precedent to obtain “Hexane extract.” The methanol phase was adjusted with water to 70:30 MeOH: H2O and extracted 3 times using 20 ml of CH2Cl2, which gives the “CH2Cl2 extract.” Extracts were dried over Na2SO4, filtered, evaporated, and weighed.
Open chromatography column
Silica chromatography “step gradient mode”
We used a 1.5 cm φ column filled with 6 g of silica gel to a height of 10 cm (Kiesel gel 60 types, 40–63 μm). 5 mg of extract was deposited at the top of the column. The elution takes place in successive stages with the following solvents (20 ml for each mixture) as follows:
 | [Click here to view] |
Obtained fractions were dried over Na2SO4, filtered, evaporated, and weighed.
Chromatography on silica “flash” mode
We used the same conditions as above but with a solvent mixture volume of 50 ml and applied a vacuum at the column outlet to accelerate the flow elution rate. Fractions were dried over Na2SO4, filtered, evaporated, and weighed.
Sephadex LH-20 chromatography (Pharmacia)
This open-column chromatography requires special conditions without a vacuum. A 2.5 cm φ column was filled to a height of 10 cm with 7.5 g of LH-20 gel previously swollen in the first elution solvent for 2 hours. It must always be soaked in solvent once introduced into the column to prevent it from cracking in the column, which would disturb the fractionation process. 50 mg of crude extract were delicately deposited at the head of the column. The solvent was slowly added. The elution was performed with 60 ml of each of the following solvents as follows:
 | [Click here to view] |
Fractions were dried over Na2SO4, filtered, evaporated, and weighed.
High-performance liquid chromatography
Sephadex LH-20 issued fractions were analyzed under the following conditions: Diol columns (4,6 × 300 mm Interchim, France) in normal phase, solvent CH2Cl2-MeOH 95:5, then 97:3, flow rate 1 ml/mn, injection of 2.5 mg in 500 μl of solvent (5 mg/ml), UV detection at 230 nm.
Gas chromatography-mass spectrometry analysis
GC-MS was carried out on an Agilent 6890 gas chromatograph equipped with a splitless capillary inlet system and an Optima 5 MS fused-silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) (Macherey-Nagel, Düren, Germany). The carrier gas was helium at an inlet pressure of 2.48 psi. A Hewlett-Packard 7673A liquid autosampler, operated in the fast mode for splitless injection, was used in conjunction with the gas chromatograph. The volume of sample injections was 2 μl. A Hewlett-Packard 5973 mass spectrometer, used as a detector, was operated in electron impact mode at 70 eV in the full-scan mode and directly interfaced with the chromatograph by the capillary column. The oven temperature was started at 50°C (held for 3.0 minutes), followed by a 3°C/minute ramp to 130°C and a second 10°C/minute ramp to 240°C. The injector and transfer line temperatures were maintained at 250°C.
RESULTS
T. claveryi identification
The hypogeous fruiting body of T. claveryi, also called ascocarp, have a rounded mass shape, fleshy like a tubercle enveloped by a peridium of reddish-brown color, thin, continuous with a smooth surface with several slits (Fig. 2a). The homogeneous, firm flesh, also called gleba (or glebe), is white in color, tinged with yellow.
As reported by Janex-Favre et al. [33], asci contain 3–8 ascospores club-shaped (Fig. 2b). The ascospores of T. claveryi measure 16–17 μm in diameter (Fig. 2c); they are spherical, hyaline, and provided with ornamentation in the form of simple nipples with loosely organized contents (Fig. 2d) as described by Malençon [34].
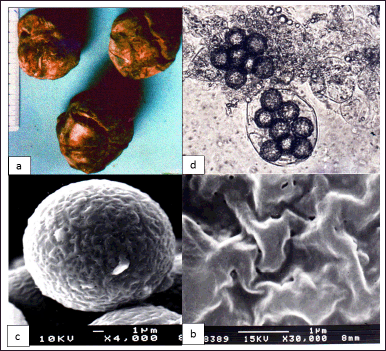 | Figure 2. (a) Dark-colored T. claveryi ascocarps with small shallow slits. (b) T. claveryi asci with eight ornamented ascospores (×1,600). (c) Cross-linked ascospore of T. claveryi observed with SEM (×4,000). (d) Details of alveolar ornamentations of T. claveryi ascospore (×30,000). [Click here to view] |
Monoclonal mycelium isolation of T. claveryi
The mycelium culture of the hypogeous Ascomycete Terfezia is hard to obtain [35]. Spore germination of T claveryi ascospores, as for other species of the same genus, is preceded by a latency phase of variable duration (3 months in our study).
Initially, each ascospore releases a large, highly refractive lipid globule (Fig. 3a). We observed the extrusion of one or two germ tubes that emerge from any point of the ascospore (Fig. 3b), which elongate and ramify after that (Fig. 3c). The hyphae of T. claveryi are septate and have many branches (Fig. 3d and e).
The mycelium is characterized by slow rate growth and is intra-matrix organized on Malt extract Agar medium. After 6 weeks of culture, its color varies from yellow to brown (Fig. 4a). In a shacked liquid culture medium, the mycelium makes a submerged compact and dense mass of brown color with a shallow supernatant face of the same feature as that grown on a solid medium (Fig. 4b).
Bioactivity exploration of different T. claveryi extracts
Fresh gleba Soxhlet extract (S extract)
Fresh EtOAc gleba S extracts were tested on three bacterial strains (P. fluorescens, S. aureus, and S. typhi). P. fluorescents were insensitive, while S. typhi and S. aureus were slightly sensitive (Fig. 5a and b). The inhibition zone diameters were 15 and 17 mm, respectively. Candida albicans was also faintly sensitive to this extract (Fig. 5c).
Culture broth extract (C extract)
The crude extract is brown, turning to very dark pink. The four bacterial tested strains, P. fluorescens, S. aureus, E. coli, and S. typhi, were sensitive to this extract (Fig. 6a–c). The inhibition diameter zones ranged from 20 to 29 mm (with 10 mm φ paper test discs). Terfezia claveryi culture filtrate extract affects the growth of C. albicans. The inhibition zone diameter was 23 mm (with test discs of 10 mm φ) (Fig. 6d).
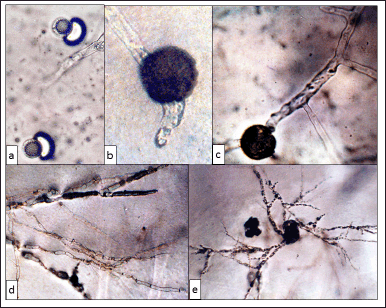 | Figure 3. (a) Ascospores of T. claveryi in early germination stages, showing the release of refractive lipid globules (×1,000). (b) Ascospore germination with two germinative tubes (×750). (c)–(e) First T. claveryi hyphae branching (×1,000), (×100), and (×10). [Click here to view] |
For the plant-pathogen fungal strains bioactivity tested with Dennis and Webster method I, three species tested by the well method, F.o.l., F.o.a, and F.o.le, were sensitive to the culture filtrate extract with respective inhibition percentages of 22.3%, 21.7%, and 24.3%. Phytophthora sp. was strongly inhibited by this extract, with a percentage of inhibition exceeding 89%. The other fungal species tested (S. sclerotiorum and A. rabiei) did not show sensitivity to this extract. Tested with method II of Dennis and Webster, only three fungal species (F.o.a., F.o.l., and F.o.le.) are sensitive to the extract of the culture filtrate deposited near the implant; the inhibition percentages were 14.6%, 15%, and 16.3%, respectively. At the same time, the greatest inhibition was observed for Phytophthora sp. with a percentage inhibition of 48.3%.
Mycelium extract (M extract)
EtOAc extract had an inhibitory effect on the growth of all the bacterial species tested: P. aeruginosa ATCC 27853, S. aureus ATCC 25923, S. aureus ROXA 27009108, E. coli ATCC 25922, Streptococcus ATCC 29212, and S. typhi (Fig. 7a–e). Its activity on bacteria was maintained even after a freezing period of more than 20 days. Candida albicans was sensitive to this extract, too, with an 18 mm inhibition zone average diameter. Concerning its effect on plant pathogen fungal species, with method I of Dennis and Webster, the growth of the three special forms of F. oxysporum was slowed down in contact with the mycelial extract of T. claveryi. The inhibition percentage was 23% for F.o.l, 22.2% for F.o.le, and 25% for F.o.a. Phytophtora sp. was also susceptible to this extract; its growth was inhibited by 86.6%. The remaining fungal strains tested had an average growth rate. But using method II of Dennis and Webster, the mycelium extract had a weak inhibitory effect on the growth of F.o.l., F.o.le., and F.o.a., where percentage inhibitions of 6.3%, 6.13%, and 7% were recorded, respectively. Its effect was nil on the growth of S. sclerotiorum and A. rabiei. The strongest growth inhibition effect was observed on Phytophthora sp. with a percentage of 39%.
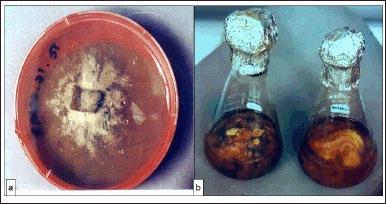 | Figure 4. (a) Macroscopic aspect of T. claveryi mycelium grown on Malt extract agar-based medium. (b) Static (left) and agitated (right) cultures of T. claveryi in liquid Malt extract-based medium. [Click here to view] |
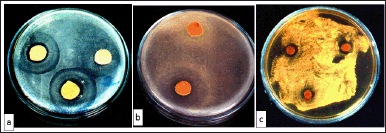 | Figure 5. (a) Growth inhibition of S. typhi in the presence of T. claveryi flesh Soxhlet extract. (b) Inhibition of S. aureus growth in the presence of T. claveryi flesh Soxhlet extract. (c) Inhibition of C. albicans growth in the presence of T. claveryi flesh Soxhlet extract. [Click here to view] |
 | Figure 6. (a) Growth inhibition of P. fluorescens in the presence of T. claveryi EtOAc culture medium extract. (b) Growth inhibition of S. aureus in the presence of T. claveryi EtOAc culture medium extract. (c) Growth inhibition of S. typhi in the presence of T. claveryi EtOAc culture medium extract. (d) Growth inhibition of C. albicans in the presence of T. claveryi EtOAc culture medium extract. [Click here to view] |
Culture broth mixture test
Concerning the method of mixing T. claveryi culture broth to 1% Malt extract Agar medium, among the six fungal species tested, only F.o.l., F.o.le., and F.o.a. showed growth slowdown compared to control cultures. The inhibition percentages for each species were 18.5%, 19%, and 20.7%, respectively.
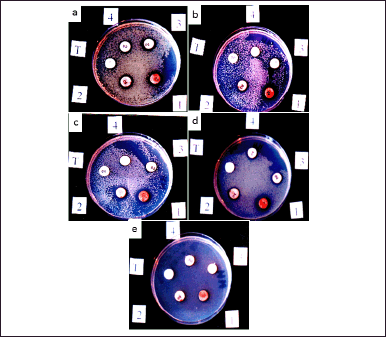 | Figure 7. (a) Pseudomonas aeruginosa American Type Culture Collection (ATTC) 27853 growth inhibition and bioactivity borderline of EtOAc T. claveryi mycelium extract: 1 = 20 mg/ml; 2 = 10; 3 = 05; 4 = 02.5, (b) S. aureus ATCC 25923 growth inhibition and bioactivity borderline of EtOAc T. claveryi mycelium extract: 1 = 20 mg/ml; 2 = 10; 3 = 05; 4 = 02.5, (c) S. aureus ROXA97009108 growth inhibition and bioactivity borderline of EtOAc T. claveryi mycelium extract: 1 = 20 mg/ml; 2 = 10; 3 = 05; 4 = 02.5, (d) E. coli ATCC 25923 growth inhibition and bioactivity borderline of EtOAc T. claveryi mycelium extract: 1 = 20 mg/ml; 2 = 10; 3 = 05; 4 = 02.5, and (e) Streptococcus ATCC 29212 growth inhibition and bioactivity borderline of EtOAc T. claveryi mycelium extract: 1 = 20 mg/ml; 2 = 10; 3 = 05; 4 = 02.5. T = controls. [Click here to view] |
Border bioactivity assessment of T. claveryi EtOAc extract
The five tested bacterial strains were sensitive to mycelium organic extract. Pseudomonas aeruginosa ATCC27853 and E. coli ATCC 25922 were inhibited by concentrations below 2.5 mg/ml (Fig. 7a and d). Streptococcus ATCC 29212, a very resistant strain to many antibiotics, was the less sensitive, with a limited dose of 10 mg/ml (Fig. 7e). Staphylococcus aureus ATCC 25923 and S. aureus ROXA 97009108 had a moderate sensitivity compared to the other bacterial tested strains (Fig. 7b and c).
Antagonism of T. claveryi mycelium against plant-pathogen fungi
Confrontation on solid medium method
After 15 days of incubation, the growth of F.o.a., F.o.l. (Fig. 8), F.o.le. and Phytophthora sp. confronted with T. claveryi were completely inhibited throughout the experimentation period. The percentage of inhibition was 100% for the four plant-pathogen fungal species. However, S. sclerotiorum and A. rabiei had an average growth rate, and the percentage of inhibition was trivial.
Kope and Fortin confrontation method
The growth of F.o.l (Fig. 9), F.o.a., F.o.le., and Phytophthora sp. was totally inhibited by diffusible metabolites through the Millipore membrane; the inhibitions percentage was 100%. Those metabolites didn’t affect the growth of A. rabiei or S. sclerotiorum; the inhibition percentage was 0% for these fungal species.
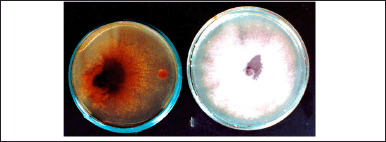 | Figure 8. Confrontation of T. claveryi mycelium with F. oxysporum f.sp. lycopersici showing total growth inhibition of the fungal plant-pathogen strain. Dish on the right = control. [Click here to view] |
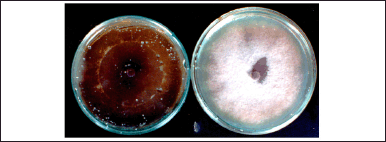 | Figure 9. Growth Inhibition effect of T. claveryi on the growth of F. oxysporum f.sp. lycopersici tested by [30] . Dish on the right = control. [Click here to view] |
Double layer confrontation method
Phytophthora sp. growth was totally inhibited throughout the incubation period by 100%.
The metabolites of T. claveryi were active on the growth of F.o.l., F.o.a., and F.o.le, but the percentage of inhibitions were, respectively, 52.9%, 47.2%, and 49%. The other fungal species tested had normal rate growth on this test.
Chemical bioactive compounds characterization
TLC on silica gel
The EtOAc culture broth and mycelium extracts of T. claveryi were completely comparable in TLC: two main compound clusters visible under UV (360 nm). Phosphor-molybdenum reagent spraying revealed a red spot followed by a blue one and 6 other brown spots. It is very probable that T claveryi mycelium synthesizes and releases some of these products in his proximate environment. These substances were more present in the mycelium than in the culture broth: the broth extract was entirely used for the TLC, while we put aside a significant amount of mycelium extract, allowing further fractionation investigations.
Liquid/liquid polarity partition
We obtained middling results. A weak activity was found in the two extracts, “CH2Cl2” and “hexane,” with the same inhibitory action on P. aeruginosa ATCC 27853 and E. coli 25922.
Open chromatography column
Silica chromatography “step gradient mode”
From the first column, three fractions were collected corresponding to metabolite clusters. In the two first fractions, we detected the same metabolite clusters visible under UV in TLC; they were free of bioactivity. The third eluted fraction was invisible under UV and bioactive on P. aeruginosa and S. typhi. Fractions 2 and 3, collected from the second column, were bioactive on P. aeruginosa ATCC 27853.
Chromatography on silica “flash” mode
The first three fractions were bioactive on P. aeruginosa ATCC 27853. This column was qualitatively correct, and there were no product losses.
Sephadex LH-20 chromatography
Fractions 1 and especially 3 and 4 were active against P. aeruginosa ATCC 27853. The LH-20 allowed us to recover good bioactivity; its resolving power was higher than that of silica. According to TLC analysis and the biological test results, we selected the last two methods, leading to a good bioactivity recovery. However, the LH-20 method leads to a better repartition of the metabolites in the fractions, the raison, because we choose it to improve the fractionation protocol. We noticed at least two different active ingredients or two clusters of active ingredients. Based on the eluting solvent nature, these active principles were relatively polar since they migrate only with high percentages of MeOH in the solvent. The subsequent fractionation steps require a more resolving method: HPLC, which we applied to fractions 3 and 4 from the LH-20 column (these fractions were extracted with 2% and 5% MeOH in CH2Cl2).
High-performance liquid chromatography
A first attempt (CH2Cl2:MeOH 95:5) showed that this mixture is too eluent. However, with the proportions 97:3, we obtained a suitable peak spread (Fig. 10). The recordings show the presence of major products: two in fraction number 3 (LH-20) and three in fraction number 4. It is early to declare that they correspond to the active ingredients, especially since the highest UV peaks do not necessarily compare to the most abundant products. But we now have a fractionation method that is settled and, therefore, applicable on a larger scale.
At the end of this characterization process, we attempted GC-MS analysis to characterize the chemical nature of the bioactive T. claveryi compounds. Unfortunately, we recovered only the HPLC fraction of three prominent peaks in tiny amounts because of successive extract depletion. In addition, HPLC fraction 4 amounts were inoperative.
DISCUSSION
Terfezia claveryi ascospores have very weak germination ability, characterized by a latency phase of unknown nature. This dormancy ascospores phenomenon could be of constitutive origin (spore’s wall thickness preventing nutrient penetration) or exogenous (lack of specific growing factors) [36]. Terfez spores’ germination factors are not understood [5,37]. The germinative bud of T. claveryi emerges from no particular area of the spore, as noted by Awameh and Alsheikh [38] and Fortas and Chevalier [5], unlike the ascospores of the genus Tuber, which have a particular germinative pore [39–43]. We also noticed that the Terfezia spores’ suspension used for the mycelium isolation was free of contaminating germs (bacteria, yeasts, and fungi), comforting the hypothesis that Terfez could therefore contain antibiotic material. Nevertheless, given the low yields of mycelia obtained using traditional culture methods of this truffle [44], it remains crucial to find an adequate culture medium and to improve the culture conditions to solve this drawback. In this aim, the work carried out by López-Nicolás et al. [45] confirmed the ability of cyclodextrins to stimulate the growth of T. claveryi mycelium when present in the culture medium, while Navarro-Ródenas et al. [46] reported that slight water stress (−0.45 MPa) could improve mycelial inoculum production of two desert truffles strains, T. claveryi and Picoa lefebvrei.
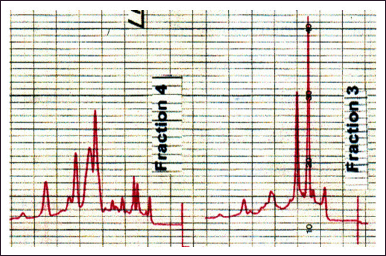 | Figure 10. HPLC spectrum of bioactive fractions 3 and 4. [Click here to view] |
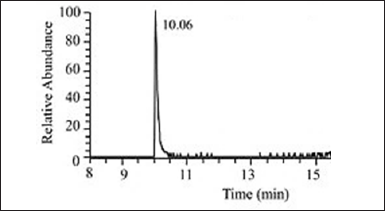 | Figure 11. GC analysis of HPLC issued fraction 3 main’s peak. [Click here to view] |
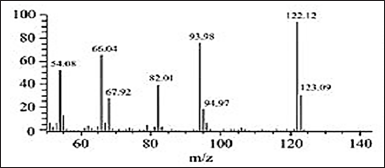 | Figure 12. Mass spectrum of GC peak at 10.06 of HPLC gave fraction 3 main’s peak. [Click here to view] |
The three special forms of F. oxysporum, F.o.l, F.o.le., and F.o.a., tested by mixing the culture broth filtrate with the culture medium of T. claveryi show growth slowdown compared to controls, hence a low sensitivity to the substances present in the culture broth. The other species were not inhibited. These results differ from those of Dennouni [25], who revealed that the culture broth of two Tirmania species (Tirmania nivea and Tirmania pinoyi) incorporated into the culture medium of plant-pathogen fungal species inhibits their growth. In her study, the percentage inhibition was 50% for S. sclerotiorum, but bacteria were weakly sensitive. Dried or fresh gleba extracts obtained by different solvent interactions had no effect on the growth of bacterial and fungal tested species. These results differ from those of Rougieux [22] with Terfezia boudieri and Dennouni [25] with T. nivea and T. pinoyi.
In contact with fresh gleba Soxhlet extract obtained, two bacterial species were slightly sensitive. Pseudomonas fluorescens, strongly inhibited by the extract of gleba from T. pinoyi and T. nivea when studied by Dennouni [25], was insensitive to the extract of fresh gleba of T. claveryi. The extract of dried gleba obtained using Soxhlet was inactive on E. coli, S. typhi, P. fluorescens, P. aeruginosa, Klebsiella sp., and Shigella sp., as well as on C. albicans. The T. claveryi fresh gleba, therefore, contains low concentrations of active products on tested bacteria and fungi. Similar results were obtained by Chellal and Lukasova [47] and Dennouni [25], who respectively tested the extracts of T. boudieri and T. nivea on the growth of various bacterial and fungal species. Conversely, the culture filtrate extract with EtOAc strongly inhibits bacterial and yeast-like tested species. Species susceptibility in descending order is scored as follows: E. coli > S. typhi P. fluorescens > S. aureus. Candida albicans was also inhibited by this extract.
Culture filtrate extract effect on plant-pathogen species growth was variable; Phytophthora sp. was strongly inhibited by methods I and II of Dennis and Webster [30]; on the other hand, F.o.l., F.o.a., and F.o.le. Showed a lower inhibitions percentage with method I than with method II. These results agreed with a [25] study that revealed that culture filtrate extract of T. nivea with EtOAc inhibits the mycelial growth of Phytophthora sp. Otherwise, EtOAc extract was active on all the bacterial strains tested and even on Streptococcus ATCC 29212, a hospital strain with several antibiotic resistance. The susceptibility of the bacteria tested, in decreasing order, was as follows: S. typhi > E. coli > P. fluorescens S. aureus > E. coli ATCC 25922 = S. aureus ATCC 25923 > P. aeruginosa ATCC 27853 = S. aureus ROXA 97009108 > Streptococcus ATCC 29212. With method 1 of Dennis and Webster [30], the mycelial extract had a strong inhibiting effect on Phytophthora sp., F.o.a., F.o.l. and F.o.le. growth. Method II of Dennis and Webster [30] gave similar results but lower inhibition percentages. The remaining plant-pathogen fungi tested show no sensitivity. However, the EtOAc mycelial extract had a variable inhibitory effect, depending on the fungal species tested and the techniques used. Kope and Fortin’s [31] confrontation methods result, and those of the double layer technique, showed that T. claveryi is antagonistic to most of the plant-pathogen fungal tested species. Regardless of the technique used, mycelial growth of the three special forms of F. oxysporum (F.o.l., F.o.a., and F.o.le.) and Phytophthora sp. were inhibited. The growth inhibition of A. rabiei and S. sclerotiorum is weak and almost missing. These results differ somewhat from those of Dennouni [25], who showed that T. nivea has a generally weak antagonistic power on plant-pathogen fungal species, particularly S. sclerotiorum; Phytophthora sp., and Phoma sp. However, a purified aqueous extract of T. claveryi presented a very good bioactivity against E. coli, S. aureus, and Staphylococcus epidermidis as reported by Casarica et al. [48], and that of Tirmania sp. had a promising antibacterial activity toward P. aeruginosa according to Owaid et al. [49].
According to Shaw et al. [50], in in vitro interactions between ectomycorrhizal fungi and plant-pathogen fungi, the culture conditions, the nature of the nutrient medium, and its pH can either stimulate or inhibit fungal growth. For other authors [51], the inhibitory effect of ectomycorrhizal fungi against plant-pathogen fungi depends on factors combination, such as pH, hosting plant, ectomycorrhizal fungus, and the plant-pathogen fungus concerned. The inhibitory variation effect of Terfez against plant-pathogen fungi seems to depend on culture conditions, studied Terfez species, and tested plant-pathogen fungi. The antimicrobial activities of T. claveryi seem in vitro best demonstrated in culture broth and mycelium EtOAc extracts. Kope and Fortin [31] and double layer methods were the most antagonism displayed. The several biological tests explored herein show clearly that the mycelium of this fungus synthesizes and secretes antibacterial and antifungal substances in both liquid and solid culture media.
This phenomenon is rare [52], knowing that, in general, antibacterial agents are inactive on fungi because of structure differences, organization, and composition of the prokaryotic and eukaryotic cell walls. Our products are, therefore, able to cross a fungal chitin-cell wall and react on fungal cytoplasmic membranes containing sterols (absent in bacteria) [53]. This finding encourages us to try to identify these products; their presence seems important in the mycelium, the gleba, and at a minor level in the culture broth. Developing a fractionation process allowed us to observe that the active fractions were always colored (yellow to red) and of high polarity (solubility and chromatographic behavior). These characteristics, as well as the fungal origin of this species, oriented us to search in the bibliography if comparable substances had already been described, either in the genus Terfezia or, more generally, in the plant and fungal kingdoms. Chellal and Lukasova [47] performed EtOAc extractions from fresh T. boudieri and T. pinoyi gleba. Their results showed that the gleba of T. boudieri contains more antibiotic substances than that of T. pinoyi. The greatest antibiotic effect was obtained with a MeOH extract at 45°C on Gram-positive bacteria only; this extract was inactive on P. aeruginosa. Extraction at higher temperatures showed that these antibiotic substances, of a proteic nature, were thermolabile. The Retention Factor (RF) of the most active spot is very close to that of pristinamycin, suggesting that it is a streptogramin (also called synergistin) [24]. In the present study, the mycelial extract of T. claveryi was thermostable, and Soxhlet extraction and Rotavapor evaporation raised the temperature above 65°C. EtOAc allows antimicrobial substance extraction. The mycelial extract is active on Gram-positive and Gram-negative bacteria (including two strains of P. aeruginosa). This lets us suppose that the antimicrobial substances extracted from the gleba of T. boudieri and T. pinoyi by Chellal [24] are different from those that we extracted from the mycelium and the gleba of T. claveryi. Baute et al. [54] found a new antibacterial compound of metabolic origin in the gleba of Terfezia sp. This compound is only obtained after enzymatic activation and “activator” plasmolytic treatment (drying, freezing-thawing, grinding, and so on) of the α-D-1,4 glucans contained in the Terfez gleba glycogen and starch, resulting in pyrogenic T compounds that do not pre-exist in natural cells.
It seems logical to suppose that the presence or absence of antimicrobial compounds in Terfez could be inducible by aggression factors (cold and heat thermal shock alternations that characterize Terfez preferred regions climate). These molecules increase the intracellular molarity of the mycelium and could thus protect it against freezing [54]. These substances have no common point with ours that seem original and spontaneously present in Terfezia mycelium, culture broth, and fresh gleba. Very similar compounds were found [25] in the liquid culture broth of T. pinoyi and T. nivea. Moreover, by reviewing the main categories of fungal and plant metabolites, we realize the analogy between our products and those of the Quinone class. Quinones are ubiquitous biological pigments found in a range of living organisms (bacteria, fungi, higher plants, and a few animals) [55]. They are oxygenated aromatic compounds biosynthesized either in the polyacetate or shikinic acid pathway. There are several categories of Quinone compounds, mainly according to ring cycle number and oxidation degree:
- Antitumor tetracyclic Quinones in some Streptomyces.
- Tetracyclic Quinones: anthraquinones with a purgative action from Ramnaceae (Bourdaine, Cascara, and Rhubarb) and Caesalpinaceae (Senna).
- Bicyclic Quinones, or naphthoquinones, with antimicrobials and dyes with well-known properties, are present in Drosera, Jugians (Walnut), and Lawsonia (Henna).
- And finally, monocyclic Quinones or benzoquinones of yellow, orange, or red color are poorly soluble in water, well soluble in organic media of high polarity, and strongly antimicrobial. Only this group is stable and is often found in the plant kingdom.
All these Quinones are biologically very active products, acting mainly in biological oxido-reduction phenomena. Anthra and benzoquinones are especially active antibacterial by enzymatic inhibition (addition reaction with thiol and amine groups), antifungals, and sometimes dewormers [56]. Quinones can also be exfoliating and keratolytic, therefore, usable in specific skin disorders [57], which was also reported by Haloubi and Denizot [14], who mentioned that maceration water of Terfez cures alopecia areata. If the connection between our products and the general class of Quinones is clear, we can try to specify to which category they belong. Tetracyclic Quinones are rare and still undescribed in fungi. Anthraquinones are powerful purgatives, irritating the intestinal mucosa [57]. However, we know that excessive consumption of Terfez causes colic, urinary retention, paralysis, and even cardiac arrest [14]. However, Ahmed et al. [58] studying the chemical composition and toxicity of T. boudieri ascocarps Chat. from Libya did not find such products. Naphthoquinones are closer to the biological behavior of our products, but their colors show up more in an alkaline medium. Finally, there remain the benzoquinones, which are closest in their characteristics to the active principles of T. claveryi and are, in addition, widespread fungal pigments [57]. Because of tiny HPLC-issued fractions amounts, GC-MS analysis results were partial. Nevertheless, we were at least able to closely identify HPLC fraction 3 main peaks as strongly related to the benzoquinones class. Terfez could, therefore, contain a multitude of antimicrobial compounds present in the gleba and/or in the mycelium and vice versa or are only synthesized under certain ecological conditions [59].
CONCLUSION
The spore germination study shows that the ascospores of T. claveryi have an exogenous dormancy, likely to be lifted by the presence of chemical factors in the culture medium and an endogenous dormancy, which would be linked to the impermeability of the envelope’s spores. The mycelium resulting from ascospores germination grows slowly on liquid or solid media, which constitutes a major obstacle for the cultivation development attempts of this mushroom. The massive production of mycelium in Malt extract liquid medium allows the extraction of antimicrobial substances and the carry out of antibiotic tests. The results show that the extracts of mycelium and culture broth had a broad spectrum of action against bacteria, fungi, and yeasts. Terfezia claveryi active principles concentration is higher in the mycelium than in the culture broth or gleba. Gleba extracts were only antibacterial. Antagonism tests show that T. claveryi could act as a good antagonist against specific fungal species, particularly Fusarium genera. Furthermore, we developed a fractionation protocol for the active compounds synthesized by T. claveryi mycelium. The outcomes of the various chromatographic techniques and antimicrobial activity tests of the fractions of each technique enabled us to select the LH-20 method, which gave better product separation. HPLC analysis of the active fractions from the LH-20 column reveals the presence of at least two active products probably belonging to Quinones. Ample active fraction amounts are needed to isolate pure products to elucidate their complete chemical structure and to evaluate their pharmacological properties.
ACKNOWLEDGMENTS
The authors are sincerely grateful to Professor Jean-Francois Biard, formerly Lecturer at the Faculty of Pharmacy of the University of Nantes (France) and specialist in Pharmacognosy, for his precious scientific support throughout the realization of this Research and for sharing his insights and expertise in shaping the direction of this Project.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Morte A, Arenas F, Marqués-Gálvez JE, Andrino A, Guarnizo ÁL, Gutiérrez A, et al. Desert Truffles (Terfezia spp.) breeding. In: Al-Khayri JM, Jain SM, Johnson DV, editors. Advances in plant breeding strategies: vegetable crops: volume 10: leaves, flowerheads, green pods, mushrooms and truffles [Internet]. Cham: Springer International Publishing; 2021 [cited 2023 Aug 23]. pp. 479–504. Available from: https://doi.org/10.1007/978-3-030-66969-0_13
2. Zitouni-Haouar FEH, Fortas Z, Chevalier G. Morphological characterization of mycorrhizae formed between three Terfezia species (desert truffles) and several Cistaceae and Aleppo pine. Mycorrhiza. 2014;24:397–403. Available from: https://doi.org/10.11646/phytotaxa.334.2.7
3. Chatin A. La truffe. Paris, France: Lib. J.B. Baillère et Fils; 1892.
4. Alsheikh AM, Trappe JM. Desert truffles: the genus Tirmania. Trans Br Mycol Soc [Internet]. 1983 Aug [cited 2023 Aug 24];81(1):83–90. Available from: https://www.sciencedirect.com/science/article/pii/S0007153683802071
5. Fortas Z, Chevalier G. Effet des conditions de culture sur la mycorhization de l’Helianthemum guttatum par trois espèces des genres Terfezia et Tirmania d’Algérie. Canad J Bot. 1992;70:2453–60. Available from: https://doi.org/10.1139/b92-303
6. Morte MA, Cano A, Honrubia M, Torres P. In vitro mycorrhization of micropropagated Helianthemum almeriense plantlets with Terfezia claveryi (desert truffle). Agric Food Sci [Internet]. 1994 May [cited 2023 Aug 31];3(3):309–14. Available from: https://journal.fi/afs/article/view/72700
7. Khanaqa A. Truffle production in the Kingdom of Saudi Arabia – potential and limitation. J Appl Bot Food Qual [Internet]. 2006 [cited 2023 Aug 24];80(1):14–8. Available from: https://ojs.openagrar.de/index.php/JABFQ/article/view/2184
8. Bradai L, Bissati S, Chenchouni H. Desert truffles of the north algerian sahara: diversity and bioecology. Emir J Food Agric [Internet]. 2014 [cited 2023 Aug 24];26:425–35. Available from: https://www.ejfa.me/index.php/journal/article/view/430
9. Khabar L. Mediterranean Basin: North Africa. In: Kagan-Zur V, Roth-Bejerano N, Sitrit Y, Morte A, editors. Desert truffles: phylogeny, physiology, distribution and domestication (Soil Biology). [Internet]. Berlin, Heidelberg: Springer; 2014 [cited 2023 Aug 29]. pp. 143–58. Available from: https://doi.org/10.1007/978-3-642-40096-4_10
10. Bouzadi M, Grebenc T, Turunen O, Kraigher H, Taib H, Alafai A, et al. Characterization of natural habitats and diversity of Libyan desert truffles. 3 Biotech [Internet]. 2017 Oct [cited 2023 Aug 29];7(5):328. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5602791/
11. Fortas Z, Dib-Bellahouel S, Aibeche C. Characterization of a rare habitat of Terfezia boudieri Chatin in the coastal dunes of northwestern Algeria. South Asian J Exp Biol [Internet]. 2022 Sep [cited 2023 Aug 24];12(5):651–60. Available from: https://sajeb.org/index.php/sajeb/article/view/698
12. Bradai L, Neffar S, Amrani K, Bissati S, Chenchouni H. Ethnomycological survey of traditional usage and indigenous knowledge on desert truffles among the native Sahara Desert people of Algeria. J Ethnopharmacol [Internet]. 2015 [cited 2023 Aug 24];162:31. Available from: https://doi.org/10.1016/j.jep.2014.12.031
13. Zitouni-Haouar F, Bidartondo M, Moreno G, Carlavilla J, Manjón J, Neggaz S, et al. Bioclimatic origin shapes phylogenetic structure of Tirmania (Pezizaceae): new species and new record from North Africa. J Fungi. 2023;9:532. Available from: https://doi.org/10.3390/jof9050532
14. Haloubi A, Denizot M. Les plantes des terrains salés et désertiques, vues par les anciens Arabes: confrontation des données historiques avec la classification des végétaux, leur état et leur répartition actuels en Proche-Orient. Montpellier, France: Atelier duplication U.S.T.L; 1988.
15. Lee H, Nam K, Zahra Z, Farooqi MQU. Potentials of truffles in nutritional and medicinal applications: a review. Fungal Biol Biotechnol [Internet]. 2020 Jun [cited 2023 Aug 31];7(1):9. Available from: https://doi.org/10.1186/s40694-020-00097-x
16. Al-Shabibi MMA, Toma SJ, Haddad BA. Studies on Iraqi Truffles. I. Proximate analysis and characterization of lipids. Can Inst Food Sci Technol J [Internet]. 1982 Jan [cited 2023 Aug 26];15(3):200–2. Available from: https://www.sciencedirect.com/science/article/pii/S0315546382725386
17. Bokhary HA, Suleiman AAA, Basalah MO, Parvez S. Chemical composition of desert truffles from Saudi Arabia. Can Inst Food Sci Technol J [Internet]. 1987 Dec [cited 2023 Aug 26];20(5):336–41. Available from: https://www.sciencedirect.com/science/article/pii/S0315546387713285
18. Bokhary HA, Parvez S. Chemical composition of desert truffles Terfezia claveryi. J Food Compos Anal. 1993;6(3):285–93. Available from: https://doi.org/10.1006/jfca.1993.1031
19. Kivrak S, Kivrak I. Investigation of chemical composition and nutritional value of truffle mushroom (Tuber nitidum Vittad.). Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi. 2018;22:10. Available from: https://dergipark.org.tr/en/download/article-file/552979
20. Wang S, Marcone MF. The biochemistry and biological properties of the world’s most expensive underground edible mushroom: truffles. Food Res Int [Internet]. 2011 Nov [cited 2023 Aug 31];44(9):2567–81. Available from: https://www.sciencedirect.com/science/article/pii/S0963996911003711
21. Hamurcu M, Özcan MM, Dursun N, Fahad AJ. Mineral contents of truffles (Terfezia boudieri chatin and Terfezia claveryi chatin). Asian J Chem. 2014;26:6693–4. Available from: https://doi.org/10.14233/ajchem.2014.16985
22. Rougieux R. Antibiotic and stimulating actions of the desert truffle (Terfezia boudieri chatin). Ann Inst Pasteur (Paris). 1963;105:315–8.
23. Neggaz S, Fortas Z. Tests of antibiotic properties of Algerian Desert Truffles against bacteria and fungi. T-I-T J Life Sci. 2013;7(3):259–66. Available from: https://www.researchgate.net/publication/292146222_Tests_of_antibiotic_properties_of_Algerian_Desert_Truffles_against_bacteria_and_fungi
24. Chellal A. Terfezia boudieri et Tirmania pinoyi: composition chimique et effet des traitements thermiques, pouvoir antibiotique [Thèse de Magister]. [Sétif]: Ferhat Abbas; 1995.
25. Dennouni N. Mise en évidence des activités antibactériennes et antifongiques chez deux espèces de terfez d’Algérie (Tirmania nivea et T.pinoyi) [Internet] [Thèse de Magister]. [Tlemcen Algeria]: Abou Bakr Belkaid; 2014 [cited 2023 Aug 26]. Available from: http://dspace.univ-tlemcen.dz//handle/112/6737
26. Neggaz S, Fortas Z, Chenni M, Abed DE, Ramli B, Kambouche N. In vitro evaluation of antioxidant, antibacterial and antifungal activities of Terfezia claveryi Chatin. Phytothérapie [Internet]. 2018 Feb [cited 2023 Aug 29];16(1):20–6. Available from: https://phyto.revuesonline.com/articles/lvphyto/abs/2018/01/lvphyto2018161p20/lvphyto2018161p20.html
27. Chevalier G. Obtention de cultures de mycélium de truffe à partir des carpophores et des mycorhizes. Comptes rendus des séances de l’Académie d’agriculture de France. 1972;12:981–9.
28. De Araújo MF, Júnior FEAC, Alvarez MG, Durantini EN, Carvalho MGD. Antifungal Activity of extracts from two Ouratea species on Candida albicans. J App Pharm Sci [Internet]. 2013 Jun 27 [cited 2023 Oct 12]. Available from: http://japsonline.com/abstract.php?article_id=940
29. Ines Ben Chobba. In vitro evaluation of antimicrobial and cytotoxic activities of Rosmarinus officinalis L. (Lamiaceae) essential oil cultivated from South-West Tunisia. J App Pharm Sci [Internet]. 2012 Nov [cited 2023 Oct 12];2:34–9. Available from: http://japsonline.com/counter.php?aid=692
30. Dennis C, Webster J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans Br Mycol Soc [Internet]. 1971 Jan [cited 2023 Aug 25];57(1):25–39. Available from: https://www.sciencedirect.com/science/article/pii/S0007153671800773
31. Kope HH, Fortin JA. Inhibition of phytopathogenic fungi in vitro by cell free culture media of ectomycorrhizal fungi. New Phytologist. 1989;113(1):57–63. Available from: https://doi-org.sndl1.arn.dz/10.1111/j.1469-8137.1989.tb02395.x
32. Nisa TU, Wani AH, Bhat MY, Pala SA, Mir RA. In vitro inhibitory effect of fungicides and botanicals on mycelia growth and spore germination of Fusarium oxysporum. J Biopes. 2011;4:53–6. Available from: http://www.jbiopest.com/users/LW8/efiles/Vol_4_1_238.pdf
33. Janex-Favre MC, Janex-Favre MC, Parguey-Leduc A. Les asques et les ascospores du Terfezia claveryi Ch. (Tubérales). Cryptogamie Mycologie [Internet]. 1985;6(2):87–99. Available from: https://www.biodiversitylibrary.org/part/354200
34. Malençon G. Champignons hypogés du nord de l’Afrique—I. Ascomycètes. Persoonia [Internet]. 1973 [cited 2023 Aug 27];7:261–79. Available from: https://www.semanticscholar.org/paper/Champignons-hypog%C3%A9s-du-nord-de-l%E2%80%99Afrique%E2%80%94I.-Malen%C3%A7on/1adc6900b88b3746fd433e3d07f7de42d56f8938
35. Fortas Z, Chevalier G. Effet des conditions de culture sur la mycorhization de l’Helianthemum guttatum par trois espèces de terfez des genres Terfezia et Tirmania d’Algérie. Can J Bot [Internet]. 1992 Dec [cited 2023 Aug 24];70(12):2453–60. Available from: https://cdnsciencepub.com/doi/10.1139/b92-303
36. Splivallo R, Ottonello S, Mello A, Karlovsky P. Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol [Internet]. 2011 [cited 2023 Aug 31];189(3):688–99. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1469-8137.2010.03523.x
37. Slama A, Neffati M, Boudabous A. Biochemical composition of desert truffle Terfezia boudieri chatin. Acta Hortic [Internet]. 2010 Feb [cited 2023 Aug 24];(853):285–90. Available from: https://www.actahort.org/books/853/853_33.htm
38. Awameh MS, Alsheikh A. Features and analysis of spore germination in the brown Kamé Terfezia Claveryi. Mycologia [Internet]. 1980 May [cited 2023 Aug 24];72(3):494–9. Available from: https://doi.org/10.1080/00275514.1980.12021210
39. Graziosi S, Hall IR, Zambonelli A. The mysteries of the white truffle: its biology, ecology and cultivation. Encyclopedia [Internet]. 2022 Dec [cited 2023 Aug 26];2(4):1959–71. Available from: https://www.mdpi.com/2673-8392/2/4/135
40. Rouquerol T, Payre H. Obeservations sur le comportement de Tuber melanosporum dans un site naturel. Rev Mycol. 1975;39(2):107–17.
41. Parguey-Leduc A, Janex-Favre MC, Montant C. Formation et évolution des ascospores de Tuber melanosporum (truffe noire du Périgord, Discomycètes). Can J Bot [Internet]. 1987 [cited 2023 Aug 31];65(7):1491–503. Available from: https://www.lissa.fr/rep/articles/PF_7645347
42. Mischiati P, Fontana A. In vitro culture of Tuber magnatum mycelium isolated from mycorrhizas. Mycol Res. 1993;97(1):40–4. Available from: https://doi.org/10.1016/S0953-7562(09)81110-6
43. Paolocci F, Rubini A, Riccioni C, Arcioni S. Reevaluation of the life cycle of Tuber magnatum. Appl Environ Microbiol [Internet]. 2006 Apr [cited 2023 Aug 26];72(4):2390–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1449033/
44. Arenas F, Navarro-Ródenas A, Chávez D, Gutiérrez A, Pérez-Gilabert M, Morte A. Mycelium of Terfezia claveryi as inoculum source to produce desert truffle mycorrhizal plants. Mycorrhiza [Internet]. 2018 Oct [cited 2023 Aug 31];28(7):691–701. Available from: https://doi.org/10.1007/s00572-018-0867-3
45. López-Nicolás JM, Pérez-Gilabert M, García-Carmona F, Lozano-Carrillo MC, Morte A. Mycelium growth stimulation of the desert truffle Terfezia claveryi chatin by β-cyclodextrin. Biotechnol Prog. 2013;29(6):1558–64. Available from: https://doi.org/10.1002/btpr.1791
46. Navarro-Ródenas A, Lozano-Carrillo MC, Pérez-Gilabert M, Morte A. Effect of water stress on in vitro mycelium cultures of two mycorrhizal desert truffles. Mycorrhiza. 2011;21(4):247–53. Available from: https://doi.org/10.1007/s00572-010-0329-z
47. Chellal A, Lukasova E. Evidence for antibiotics in the two Algerien truffles Terfezia and Tirmania. Pharmazie. 1995;50(3):228–9.
48. Casarica A, Moscovici M, Daas M, Nicu I, Panteli M, Rasit I. A purified extract from brown truffles of the species Terfezia claveryi chatin and its antimicrobial activity. Farmacia (Bucure?ti) [Internet]. 2016 [cited 2023 Aug 31];64(2):298–301. Available from: https://farmaciajournal.com/wp-content/uploads/2016-02-art-24-Moscovici_Misu_Casarica_298-301.pdf
49. Owaid MN, Muslim RF, Hamad HA. Mycosynthesis of silver nanoparticles using Terminia sp. Desert truffle, Pezizaceae, and their antibacterial activity. Jordan J Biol Sci [Internet]. 2018 [cited 2023 Aug 31];11(4):401–5. Available from: https://jjbs.hu.edu.jo/files/v11n4/Paper%20Number%209.pdf
50. Shaw TM, Dighton J, Sanders FE. Interactions between ectomycorrhizal and saprotrophic fungi on agar and in association with seedlings of lodgepole pine (Pinus contorta). Mycol Res [Internet]. 1995 Feb [cited 2023 Aug 27];99(2):159–65. Available from: https://www.sciencedirect.com/science/article/pii/S0953756209808800
51. Chakravarty C, Peterson RL, Ellis BE. Interaction between the ectomycorrhizal fungus Paxillus involutus, damping-off fungi and Pinus resinosa seedlings. J Phytopathol [Internet]. 1991 [cited 2023 Aug 26];132(3):207–18. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1439-0434.1991.tb00113.x
52. Silambarasan S, Murugan T, Saravanan D, Balagurunathan R. Antibacterial and antifungal activities of actinobacteria isolated from Rathnagiri hills. J App Pharm Sci [Internet]. 2012 Oct [cited 2023 Oct 12];2:99–103. Available from: http://japsonline.com/abstract.php?article_id=675
53. Sabine RD. Antibiotiques et antibiogrammes/Sabine Robert-Dernuet. Montréal, Canada; Paris, France: Décarie Vigot; 1995.
54. Baute MA, Deffrieux G, Vercautern J, Baute R, Badoc A. Bioconvertions fongiques produisant des composés pyroniques inhabituels à partir de glucides, XVII.- Production d’ascopyrones P et T par des Ascomycètes appartenant aux Pézizales et Tubérales. Bordeaux: Bull Soc Pharm. 1993;138:29–39.
55. Devi SS, Mehendale HM. Quinone. In: Wexler P, editor. Encyclopedia of toxicology. 3rd ed. [Internet]. Oxford: Academic Press; 2014 [cited 2023 Aug 28]. pp. 26–8. Available from: https://www.sciencedirect.com/science/article/pii/B978012386454300350X
56. Christiansen JV, Isbrandt T, Petersen C, Sondergaard TE, Nielsen MR, Pedersen TB, et al. Fungal quinones: diversity, producers, and applications of quinones from Aspergillus, Penicillium, Talaromyces, Fusarium, and Arthrinium. Appl Microbiol Biotechnol. 2021 Nov;105(21–22):8157–93. Available from: https://doi.org/10.1007/s00253-021-11597-0
57. Paris R, Moyse H. Précis de matière médicale. Paris, France; 1976.
58. Ahmed AA, Mohamed MA, Hami MA. Libyan truffles “Terfezia boudieri Chatin:” chemical composition and toxicity. J Food Sci [Internet]. 1981 [cited 2023 Aug 26];46(3):927–9. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2621.1981.tb15383.x
59. G. Gomathy. Phytochemical screening and GC-MS analysis of Mukia maderaspatana (L.) leaves. J App Pharm Sci [Internet]. 2012 Dec [cited 2023 Oct 12];2:104–6. Available from: http://japsonline.com/counter.php?aid=734