INTRODUCTION
Atherosclerosis is a severe public health issue responsible for a relatively high mortality rate, particularly in the coronary arteries. Atherosclerosis is expected to be a significant health burden in the future [1]. Atherosclerosis is mainly caused by various causes, one of which is dyslipidemia. Dyslipidemia, caused mainly by an increase in low-density lipoprotein (LDL) cholesterol, may lead to endothelial dysfunction [2] and is susceptible to oxidative stress and the formation of oxidized LDL, which promotes atherosclerosis [3].
The levels of high-density lipoprotein (HDL), apoprotein A-I (ApoA-I), and apoprotein E (ApoE) levels have previously been utilized as indicators of inflammation caused by dyslipidemia [4]. Netrin-1, a novel protein utilized as a marker of atherosclerosis, has been discovered [5]. Netrin-1 relates to the laminin protein family, which was shown to be an essential protein in neuronal proliferation during embryonic development [6]. Following the recent identification of the Netrin-1 receptor in cells other than the central nervous systems Netrin-1 was shown to be implicated in the pathological process that controls multiple degenerative illnesses, such as atherosclerosis [7]. Netrin-1 prevents cardiovascular disease by inhibiting the inflammation process in patients with atherosclerosis. This inflammation inhibition occurs due to the release of macrophages from the endothelium lesion and the entrance of the monocyte into the lesion [8].
Previous research has shown that Netrin-1 decreased inflammation in various oxidative stress approaches. The low levels of Netrin-1, which is frequently observed in the blood sample of type 2 diabetes patients, impacted inflammation [9]. The low level of plasma Netrin-1 in individuals with atherosclerosis and blood vessel wall inflammation was effectively restored by utilizing the recombinant Netrin-1 [10]. The role of Netrin-1 in blood vessels is to maintain the optimum endothelial function as an anti-inflammatory by preventing the adhesion and migration of monocyte [11].
ApoE is a required encoded protein for lipid metabolism [12]. ApoE acts as a mediator for particular cell surface receptors, including those of the LDL family and heparin sulfate proteoglycans which control lipoprotein clearance from plasma [13]. According to a recent study, ApoE in very-low-density lipoprotein can influence lipoprotein lipase activity and has a role in the etiology of metabolic diseases, such as type II diabetes, metabolic syndrome, and cardiovascular disease [14]. According to gene function studies, the mutation of the ApoE gene causes lipid metabolic problems in the rat’s liver [15].
Based on the statement above, it is essential to look for natural remedies that can increase blood levels of Netrin-1 and ApoE while lowering total cholesterol. Purple sweet potato is an economic crop with important value in many countries, including Indonesia [16]. Interestingly, apart from being used as a staple food. Purple sweet potato can also act as a source of bioactive compounds that have attracted much attention for development [17]. Recent reports revealed that more than 135 bioactive compounds have been isolated from this plant and those compounds are able to provide good pharmacological activity. Polysaccharides and flavonoids have been developed as bioactive sources for drug-delivery systems [18]. Based on in vitro study, purple sweet potato extract (PSPE) has been widely used as an antitumor in HepG2, LOVO, and MCF-7 cell models [17]. Polyphenols isolated from PSPE could promote intestinal epithelial differentiation by upregulating PGC-1α and increasing the mitochondrial biogenesis of Caco-2 cells [19]. Likewise, in vivo studies showed that PAPER has an antihyperuricemia effect by inhibiting xanthine oxidase [20]. Anthocyanin-rich of PSPE can also promote liver function against damage caused by exposure to CCl4 [21]. In our previous study conducted on laboratory animals, PSPE at a dose of 200 mg/day/rat has been shown to reduce oxidative stress and inflammation and was not significantly different compared to the simvastatin group (standard control) [22,23].
However, the revealed effect of purple sweet potato status on atherosclerotic rats fed a high-cholesterol diet was limited. A previous study showed that PSPE could decrease the significantly increased oxidative stress caused by a high-cholesterol diet [23]. This study aims to investigate how PSPE influenced lipid profile protein levels of Netrin-1 and ApoE in rats administered with a high-cholesterol diet by decreasing oxidative causes of atherosclerosis.
MATERIALS AND METHODS
Ethical clearance
The Research Ethics Committee of the Faculty of Medicine, Udayana University, had permitted this research ethical clearance statement with Protocol No. 2021.03.1.1078.
Research design
This research was included in an experimental laboratory study with a randomized pre-and post-test design. A total of 32 male Wistar rats were adapted for 2 weeks based on inclusion criteria (aged 4 months, weighing 200–300 g, and in healthy condition). Additionally, for 3 months, 32 rats were separated into two groups (16 rats each), the control group (high cholesterol diet) and the PSPE group (high cholesterol diet + PSPE 200 mg/day/rats).
Preparation of PSPE
PSPE was produced by macerating 1 kg of purple sweet potato flour in 3 l of 70% ethanol and acidifying this with 3% citric acid for 24 hours. The maceration performance was obtained and concentrated using a rotating vacuum evaporator at a temperature of 40°C and a pressure of 70–80 mbar to produce a concentrated extract. The concentration of the extract was determined to be 200 mg/ml [22,24].
Rats models and treatment
During the acclimation phase, rats were fed a regular diet that consisted of protein (20%–25%), fat (5%), carbohydrates (45%–50%), crude fiber (5%), ash (4%), and vitamins and minerals. As pretest data, total cholesterol, lipid profile, Netrin-1, Apo-E, malondialdehyde (MDA), and blood superoxide dismutase (SOD) were measured on day 15. It was then followed by random allocation, in which 32 rats were divided into two groups of 16 rats each/groups. The high-cholesterol diet was made using a combination of lard oil (10%), duck egg yolks (5%), and standard feed [25]. The control group was given a high-cholesterol diet. The PSPE group was given a high-cholesterol diet and 200 mg/day/rats of PSPE. For 3 months, a high-cholesterol diet was administered ad libitum orally. Total cholesterol, lipid profile, Netrin-1, Apo-E, MDA, and blood SOD levels were measured again 3 months later as posttest data. Blood samples for testing these parameters were taken through the orbital sinus [25].
Lipid profiles
Determination of total cholesterol levels
Total cholesterol level was determined using the Cholesterol FS Kit (DyaSys, USA) (Cat No. 1 1300 99 10 030). In summary, 10 μl of Wistar rat serum and a standard was prepared. Furthermore, as much as 10 μl of distillate water was prepared blank reagent for control. 1,000 μl of the reagent and standard mixtures were prepared, mixed, and incubated for 10 minutes at 20°C–25°C. The absorbance value of the sample was read by UV-Vis spectrophotometry at a wavelength of 500 nm for 60 minutes and compared with the blank reagent. The calculation of total cholesterol levels using the following equation:
Triglycerides (TG)
Determination of serum TG levels was performed by using TG FS (DyaSys, USA) (Cat. No. 1-5700-99-10-030). In summary, 10 μl of Wistar rat serum and 1 μl of distillate water were prepared. Similarly, blank and standard reagents were prepared, 1,000 μl of each. The mixture of distillate water and reagent was used as a blank. Each blank and sample was mixed and incubated for 10 minutes at 20°C–25°C. The absorbance value of the sample was read by UV-Vis spectrophotometry at a wavelength of 500 nm for 60 minutes and compared with the blank reagent. The calculation of total cholesterol levels using the following equation:
High-density lipoprotein
Determination of HDL levels was performed by using HDL Precipitant (DiaSys, USA) (Cat No. 1: 3540 99 90 885). The first test procedure was precipitation: namely, as much as 200 μl of sample or standard and 500 μl of precipitation reagent were mixed and incubated for 15 minutes at room temperature, then centrifuged for 20 minutes at 2,500 g. Within 2 hours after centrifugation, 0.1 ml of the clear supernatant was transferred to the reaction solution to determine HDL cholesterol levels. The determination of HDL cholesterol was carried out by preparing 100 μl of sample supernatant and 100 μl of standard, respectively. A total of 1,000 μl of cholesterol reagent was added to the standard and sample, mixed, and incubated for 10 minutes at room temperature. Then the absorbance value of the sample or standard was measured using a UV-Vis spectrophotometer at a wavelength of 500 nm. The results were then compared with the reagent blank value within 45 minutes. The calculations are carried out using the following equation:
The standard concentration is the total cholesterol concentration in a standard cholesterol solution.
Low-density lipoprotein
Determination of LDL levels was performed by using the LDL Precipitant Kit (DyaSys, USA) (Cat No. 1: 4330 99 90 885). The first test procedure was through precipitation; namely, a 100 μl sample and 1,000 μl of precipitation reagent were prepared, mixed, and incubated for 15 minutes at room temperature, then centrifuged for 20 minutes at 2,500 g. Within 1 hour after centrifugation, 100 μl of the clear supernatant was transferred to the reaction solution to determine LDL cholesterol. The determination of LDL cholesterol was carried out similarly to HDL cholesterol. The results were compared with the reagent blank value within 45 minutes. The calculations are carried out using the following equation:
Oxidative stress assay
Malondialdehide
Rat Malondialchechyche ELISA Kit (Bioassay Tech Laboratory, China) MDA levels (Cat. No. E0156Ra) was used to perform the MDA assay. All of the reagents, samples, and standards were prepared, and then the samples and ELISA reagents were added to each well and incubated at 37°C for 1 hour. The ELISA plate was cleaned five times before adding substrate solutions A and B and incubating for 10 minutes at 37°C. When a stop solution was introduced, the color changed from blue to yellow. The optical density (OD value) of each well was assessed using a microplate reader set to 450 nm for 10 minutes.
Superoxide dismutase
SOD levels were determined using the Rat Super Oxidase Dismutase ELISA Kit (Bioassay Tech Laboratory, China) (Cat. No. E0168Ra) was used to perform the SOD assay. All the reagents, samples, and standards were prepared following the manufacturer’s procedure. Samples and ELISA reagent were added to each well and incubated for 1 hour at 37°C. The ELISA plate was washed five times, and substrate solutions A and B were added and incubated for 10 minutes at 37°C. A stop solution was added, and the color change was observed. The OD values for each well were read using a microplate reader set up at 450 nm for 10 minutes.
Netrin-1
The Rat Netrin-1 ELISA Kit (MyBioSource, USA) (Cat No. MBS163009) was used in this study. Each well was filled with samples and ELISA reagent and incubated for 1 hour at 37°C. The ELISA plate was then rinsed five times before substrate solutions A and B were added. After 10 minutes at 37°C, the stop solution was added, and the color changed from blue to yellow. Each well OD value was determined using a microplate reader set to 450 nm for 10 minutes.
Apolipoprotein-E
The ApoE levels were measured using the ApoE ELISA Kit (MyBioSource, USA) (Cat No. MBS263133) was used to perform the ApoE levels. The standards, samples, and reagents were well prepared. The well was incubated at 37°C for 90 minutes. The biotinylated antibody solution was prepared and incubated for 30 minutes. The ELISA plate was cleaned twice. Then the biotinylated antibodies were applied to each well. The ELISA plate was cleaned three times more, and the enzyme conjugate was applied to each empty well for 30 minutes at 37°C. The ELISA plate was washed more than five times. The color reagent was added to each empty well and then incubated at 37°C in the dark. The standard turned dark and a color gradient developed. The color reagent C was well mixed into each well and read at 450 nm OD for 10 minutes.
Data analysis
The provided data was analyzed using the SPSS 23.0 software program (IBM Corporation, Armonk, NY). The Kolmogorov–Smirnov and Levene tests were used to determine the normality of the data. The data were examined using a paired t-test (pre-and posttest) and an independent test between groups with a confidence interval of p < 0.05 to assess the significant difference between treatments. Figures and tables were used to describe the results of the observations. GraphPad Prism 8.0 was used to create the graphical display (GraphPad Software, Inc., San Diego, CA).
RESULTS
Lipid profile
Total cholesterol level
The total cholesterol level of control rats was higher in the pretest than that in the post-test. However, there was no significant difference (p > 0.05) between pre- and posttest PSPE rats (Table 1). This suggests that PSPE can maintain total cholesterol levels even when impacted by a high-cholesterol diet (Figure 1).
TG, HDL and LDL levels
The mean TG, HDL, and LDL levels of Wistar rats did not differ significantly (p > 0.05) between the groups in the pretest circumstances (Table 2). However, TG, HDL, and LDL levels differed significantly (p > 0.05) between the control and PSPE groups (Table 2). Due to high-cholesterol diets, PSPE intervention lowered TG levels, maintained HDL levels, and reduced LDL levels, which eventually resulted in total cholesterol levels that were comparable (Figure 2).
Oxidative stress
MDA and SOD levels
In the pretest conditions, the mean levels of MDA and SOD in rats fed a high-cholesterol diet did not show significant differences (p > 0.05). (Figure 3). However, as compared to the control group, there was a significant decrease (p < 0.05) in the posttest condition of the treatment group, which was given a combination of high cholesterol and PSPE diet for 3 months (Table 3).
Netrin-1 levels
The investigation found that rats fed a high-cholesterol diet for 3 months had decreased Netrin-1 protein levels. Netrin-1 protein concentration was 111 ng/l in the pretest condition and decreased to 97 ng/l after 3 months on a high-cholesterol diet. The PSPE rats group substantially increased Netrin-1 protein levels (p < 0.05). The pretest Netrin-1 protein level in the PSPE group was 82.44 ng/l and increased to 115 ng/l after 3 months of treatment (Table 4). Being compared to the posttest Netrin-1 protein levels in the control group, these findings demonstrated more significantly increased Netrin-1 protein levels in the PSPE group (Figure 4).
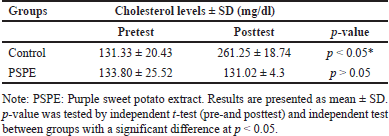 | Table 1. Total blood cholesterol levels before (pretest) and after (posttest) treatment. [Click here to view] |
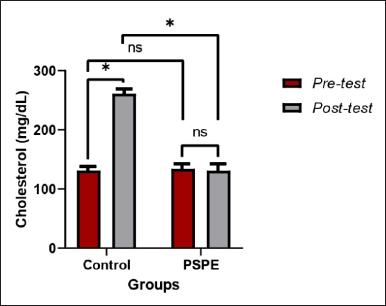 | Figure 1. Total cholesterol levels of hypercholesterolemic Wistar rats. Based on the paired t-test (pre-and posttest) and an independent test between groups, the * sign indicates a significant difference (p < 0.05), whereas NS indicates no significant difference (p > 0.05). [Click here to view] |
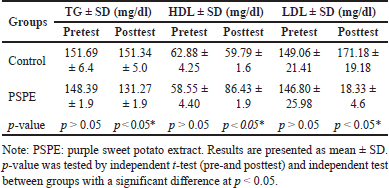 | Table 2. TG, HDL, and LDL levels before (pretest) and after (posttest) treatment. [Click here to view] |
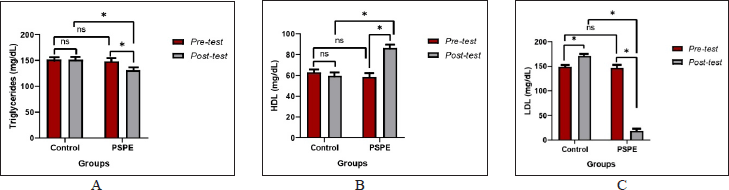 | Figure 2. (A) TG, (B) HDL, and (C) LDL levels of hypercholesterolemic Wistar rats. Based on the paired t-test (pre-and posttest) and an independent test between groups, the * sign indicates a significant difference (p < 0.05), whereas NS indicates no significant difference (p > 0.05). [Click here to view] |
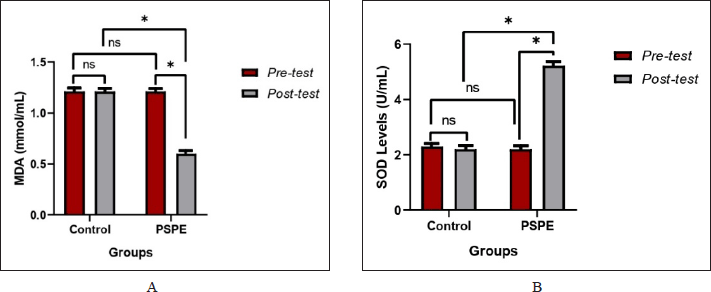 | Figure 3. (A) MDA and (B) SOD levels of hypercholesterolemic Wistar rats. Based on the paired t-test (pre-and posttest) and an independent test between groups, the * sign indicates a significant difference (p < 0.05), whereas NS indicates no significant difference (p > 0.05). [Click here to view] |
Apo-E levels
In the pretest and posttest conditions, Apo-E levels in the control group showed no significant decrease (p > 0.05) even after 3 months of a high-cholesterol diet (Figure 5). Similar to the pretest control conditions, the group of PSPE rats showed a significant increase in Apo-E levels (p < 0.05) after 3 months of PSPE treatment (Table 5).
DISCUSSION
The research aimed to examine the antiatherogenic impact of a high-cholesterol diet by consuming PSPE for 3 weeks and investigate the mechanism of reducing lipid profile, Netrin-1 level, and Apo-E level by decreasing oxidative stress biomarkers. The positive impact of PSPE in hypercholesterolemic subjects has not been explored, particularly as indicated by Netrin-1 and Apo-E protein levels. Several sweet potato cultivars, including purple sweet potato, have previously been studied for their chemical and bioactive content, and this raw material has the potential to be developed as a therapy in both the pharmaceutical and biomedical industries [26].
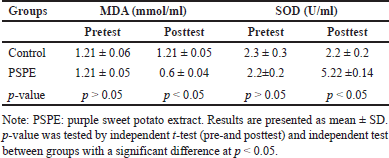 | Table 3. Comparison of MDA and SOD levels in this study group. [Click here to view] |
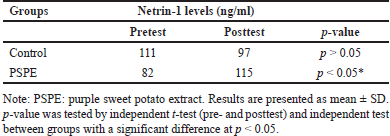 | Table 4. Netrin-1 levels in the serum in this study. [Click here to view] |
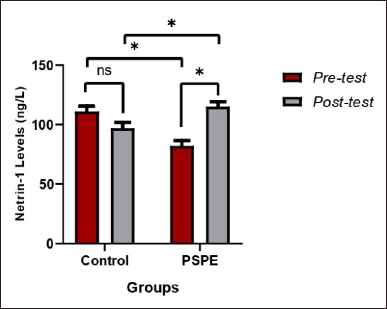 | Figure 4. Netrin-1 protein levels in hypercholesterolemic Wistar rats. Based on the paired t-test (pre-and posttest) and an independent test between groups, the * sign indicates a significant difference (p < 0.05), whereas NS indicates no significant difference (p > 0.05). [Click here to view] |
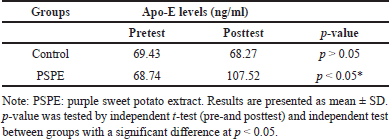 | Table 5. Apo-E levels in this study group. [Click here to view] |
The findings in this study indicated that PSPE treatment significantly improved the lipid profile and demonstrated effectiveness in the posttest. Rats on a high-cholesterol diet may accumulate body fat by enhancing their lipid profiles and promoting an increase in adipocyte size and quantity [27,28]. Because LDL accounts for 60% of total cholesterol, it is also known as “bad cholesterol” and is a major risk factor for atherosclerosis [29]. More cholesterol will be transported from the liver to peripheral tissues when LDL cholesterol levels increase. As a result of cholesterol accumulating in the blood vessels, atherosclerosis develops [30]. Previous research revealed that PSPE supplementation at a dose of 30% was able to reduce body weight and fat accumulation, improve lipid profiles, and modulate energy expenditure, as well as being able to maintain liver and kidney function in obese rats given a high-fat diet [31]. Related research also revealed that the performance function of PSPE, which was able to induce the expression of AMP-activated protein kinase in the liver, increased type 2 glucose transporter, glucokinase protein levels, and insulin-α receptors significantly in hyperglycemic rats [32].
Previous studies have shown the efficacy of garlic and ginger in reducing dyslipidemia by improving fat metabolism [33,34]. The extracted material stimulates bile acid production, decreasing cholesterol [35]. Our data here show that the decrease in cholesterol was followed by an increase in HDL in the PSPE groups, a substance known to participate in the reverse cholesterol transport (RCT) process [36]. Interestingly, a previous study had shown that lowering LDL cholesterol by 2 mg/dl could reduce the risk of cardiovascular disease and atherosclerosis by 1% compared to a high-cholesterol diet [37,38]. In contrast to LDL, HDL is referred to as “good cholesterol” and is required for the RCT mechanism to function correctly [39–41]. Consequently, elevated LDL levels can allocate excessive cholesterol from peripheral tissues to the liver for processing [42].
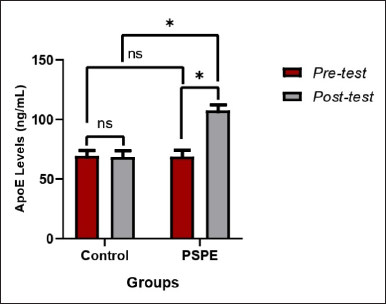 | Figure 5. Apo-E levels of hypercholesterolemic Wistar rats based on the paired t-test (pre-and posttest) and an independent test between groups, the * sign indicates a significant difference (p < 0.05), whereas NS indicates no significant difference (p > 0.05). [Click here to view] |
Antihypercholesteromic activity from PSPE is linked to its flavonoid concentration, which is thought to influence the lipid profile by interacting with several enzymes involved in lipid metabolism in the liver [28,38]. Preclinical studies using cell culture and animal models established the role of flavonoids (anthocyanins, anthocyanidins, flavonols, and flavones) in influencing RCT and HDL performance in forms other than essentially regulating HDL cholesterol concentrations by regulating macrophage-derived cholesterol release and hepatic paraoxonase one expression [43]. Furthermore, the consumption of anthocyanin-rich foods was related to significant changes in blood biomarkers associated with HDL function in various human populations (hyperlipidemia, hypertension, and diabetes) in clinical investigations [44,45]. Because of a chemical structure known as delpenidine, anthocyanins improved lipid profiles. The chemical structure of anthocyanin affected TG, LDL, and HDL [46].
Oxidative stress caused by high cholesterol diet also seems to be reduced by PSPE treatment. We hypothesized that PSPE flavonoids protected against oxidative stress, as demonstrated by decreased MDA and increased SOD levels in the PSPE group. Flavonoids can also prevent thrombus formation [47], enhance endothelial function [48], modify lipid levels [49], and regulate glucose metabolism [50]. Consequently, dietary content plays a role in regulating oxidative stress caused by the consumption of oxidants and antioxidants [51]. Thus, atherosclerosis can be reduced by improving endothelial function by administering cholesterol-lowering drugs or antioxidant treatment. Interestingly, our previous study showed a linear relationship between the dose of flavonoid produced from PSPE and its antihyperglycemic effect in streptozotocin-induced rats [52].
The distribution of the protein Netrin-1 is essential to determine its effect on atherosclerosis development [53,54]. Our finding of an increase in the Netrin-1 protein level after PSPE administration supports the notion that it is required for optimal endothelial function due to the increased serum Netrin-1 levels. In contrast, under oxidative stress situations, particularly, those associated with elevated LDL levels due to high-cholesterol diets may result in reduced endothelial function and lower Netrin-1 levels [10]. According to its function, the protein Netrin-1 inhibits the inflammatory process by preventing monocytes from reaching the endothelium. Thus, decreasing Netrin-1 levels could increase the inflammation caused by monocyte entrance into blood vessels [55]. Thus, Netrin-1 in the artery lumen is atheroprotective, whereas Netrin-1 generated by macrophages on plaque locations causes macrophage retention and smooth muscle cell migration within plaques [1].
Apo-E functions as a ligand for LDL receptors in liver cells, boosting or optimizing blood LDL absorption by liver cells for metabolism [56]. Interestingly, Apo-E inhibits atherosclerosis in hyperlipidemic circumstances by increasing plasma ApoA1-HDL, which is proven to reduce intracellular lipid accumulation, circulating leukocyte activation, and endothelial activation [57]. An increase in Apo-E levels in this study could be associated with a decrease in oxidative stress enzymes such as MDA [58]. These results are supported by previous studies utilizing aged garlic extract, which is capable of slowing down atherosclerotic processes involving inflammatory processes by decreasing serum CRP, XB2, TNF-α, IRAK4 protein levels, and AMP-activated protein kinase activity in the liver in Apo-E-knockout mice [59].
In summary, the findings of this study demonstrate the importance of PSPE with high anthocyanin content in maintaining a healthy lipid profile under normal conditions, consistent with previous research. Purification of bioactive compounds and other components is necessary to ascertain whether bioactive substances function in atherosclerosis. The reduced oxidative stress improves endothelial function and higher Netrin-1 synthesis by the endothelium. These findings suggest that PSPE supports the protein Netrin-1 in circulation as a protective endothelium, particularly in reducing inflammation and avoiding atherosclerosis.
CONCLUSIONS AND FURTHER RESEARCH
This research demonstrates that PSPE has antiatherogenic properties. It has been shown to regulate the lipid profile, decrease oxidative stress by lowering MDA levels and boosting SOD levels, and increase Netrin-1 and Apo-E protein levels in rats given a high-cholesterol diet for 3 months. We suggest that supplementation with 200 mg/ml PSPE with a healthy diet may help to control atherogenesis and might be developed as a dietary supplement.
ACKNOWLEDGMENT
The authors like to express their gratitude to the Study and Community Service Institute (LPPM) at Udayana University (UNUD) for supporting and funding this research under the DIPA PNBP Udayana University with Contract No. B/96-73/UN14.4.A/PT.01.05/2021.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Research Ethics Committee of the Faculty of Medicine, Udayana University, had approved this research protocol with approval No. 2021.03.1.1078, dated 07/12/2021.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bruikman CS, van Gils JM. Netrin-1 in coronary artery disease (CAD): mechanism of action and potential as a therapeutic target. Expert Opin Ther Targets [Internet]. 2019 Sep 2;23(9):729–31. Available from: https://www.tandfonline.com/doi/full/10.1080/14728222.2019.1653280
2. Wengrofsky P, Lee J, Makaryus AN. Dyslipidemia and its role in the pathogenesis of atherosclerotic cardiovascular disease: implications for evaluation and targets for treatment of dyslipidemia based on recent guidelines. In: McFarlane SI, editor. Dyslipidemia [Internet]. London, UK: IntechOpen; 2019. Available from: https://www.intechopen.com/books/dyslipidemia/dyslipidemia-and-its-role-in-the-pathogenesis-of-atherosclerotic-cardiovascular-disease-implications
3. Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal [Internet]. 2010 Jul;13(1):39–75. Available from: http://www.liebertpub.com/doi/10.1089/ars.2009.2733
4. Ito F, Sono Y, Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants [Internet]. 2019 Mar 25;8(3):72. Available from: https://www.mdpi.com/2076-3921/8/3/72
5. Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat Rev Immunol [Internet]. 2021 Aug 13;22(4):251–65. Available from: https://www.nature.com/articles/s41577-021-00584-1
6. Rajasekharan S, Kennedy TE. The netrin protein family. Genome Biol [Internet]. 2009;10(9):239. Available from: http://genomebiology.biomedcentral.com/articles/10.1186/gb-2009-10-9-239
7. Layne K, Ferro A, Passacquale G. Netrin-1 as a novel therapeutic target in cardiovascular disease: to activate or inhibit? Cardiovasc Res [Internet]. 2015 Sep 1;107(4):410–9. Available from: https://academic.oup.com/cardiovascres/article-lookup/doi/10.1093/cvr/cvv201
8. Schlegel M, Sharma M, Brown EJ, Newman AAC, Cyr Y, Afonso MS, et al. Silencing myeloid netrin-1 induces inflammation resolution and plaque regression. Circ Res [Internet]. 2021 Aug 20;129(5):530–46. Available from: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.121.319313
9. Liu C, Ke X, Wang Y, Feng X, Li Q, Zhang Y, et al. The level of netrin-1 is decreased in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord [Internet]. 2016 Dec 2;16(1):33. Available from: http://bmcendocrdisord.biomedcentral.com/articles/10.1186/s12902-016-0112-z
10. Bruikman CS, Vreeken D, Hoogeveen RM, Bom MJ, Danad I, Pinto-Sietsma SJ, et al. Netrin-1 and the grade of atherosclerosis are inversely correlated in humans. Arterioscler Thromb Vasc Biol [Internet]. 2020 Feb;40(2):462–72. Available from: https://www.ahajournals.org/doi/10.1161/ATVBAHA.119.313624
11. Lin Z, Jin J, Bai W, Li J, Shan X. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol Immunol [Internet]. 2018 Nov;103:166–72. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0161589018306904
12. Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis [Internet]. 2014 Dec;72:3–12. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0969996114002563
13. Vella F. The metabolic and molecular bases of inherited disease seventh edition. Biochem Educ [Internet]. 1996 Jan;24(1):65. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0307441296800197
14. Whitacre BE, Howles P, Street S, Morris J, Swertfeger D, Davidson WS. Apolipoprotein E content of very low-density lipoprotein limits lipoprotein lipase-mediated triglyceride hydrolysis. J Lipid Res [Internet]. 2021 Dec;12:100157. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022227521001401
15. Xu J, Guo Y, Huang X, Ma X, Li P, Wang Y, et al. Effects of DHA dietary intervention on hepatic lipid metabolism in apolipoprotein E-deficient and C57BL/6J wild-type mice. Biomed Pharmacother [Internet]. 2021 Dec;144:112329. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332221011136
16. Silvana Arianti Y, Wahyu Harinta Y. Sweet potatoes: development and potential as alternative food ingredients in Karanganyar Regency, Indonesia. E3S Web Conf [Internet]. 2021 Jan 5;226:00050. Available from: https://www.e3s-conferences.org/10.1051/e3sconf/202122600050
17. Amagloh FC, Yada B, Tumuhimbise GA, Amagloh FK, Kaaya AN. The potential of sweetpotato as a functional food in Sub-Saharan Africa and its implications for health: a review. Molecules [Internet]. 2021 May 17;26(10):2971. Available from: https://www.mdpi.com/1420-3049/26/10/2971
18. Jiang T, Ye S, Liao W, Wu M, He J, Mateus N, et al. The botanical profile, phytochemistry, biological activities and protected-delivery systems for purple sweet potato (Ipomoea batatas (L.) Lam.): an up-to-date review. Food Res Int [Internet]. 2022 Nov;161:111811. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0963996922008699
19. Sun Q, Bravo Iniguez A, Tian Q, Du M, Zhu MJ. PGC-1α in mediating mitochondrial biogenesis and intestinal epithelial differentiation promoted by purple potato extract. J Funct Foods [Internet]. 2022 Nov;98:105291. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464622003619
20. Yang Y, Zhang Z, Zhou Q, Yan J, Zhang J, Su G. Hypouricemic effect in hyperuricemic mice and xanthine oxidase inhibitory mechanism of dietary anthocyanins from purple sweet potato (Ipomoea batatas L.). J Funct Foods [Internet]. 2020 Oct;73:104151. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1756464620303753
21. Wang L, Zhao Y, Zhou Q, Luo CL, Deng AP, Zhang ZC, et al. Characterization and hepatoprotective activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8). J Food Drug Anal [Internet]. 2017 Jul;25(3):607–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1021949816301582
22. Jawi IM, Indrayani W, Arijana IGK, Subawa AAN, Suprapta DN. Aqueous extract of purple sweet potato increased SOD-2 and SOD-3 expression on human umbilical vein endhothelial cells in vitro. J Biol Agric Healthc. 2016;6(2):103–10.
23. Jawi IM, Putu Sutirta Yasa IW, Mahendra AN, Sumardika IW. Effective dose and safety profile of purple sweet potato tablet preparation in rats with high cholesterol diet. Biomed Pharmacol J [Internet]. 2020 Jun 30;13(02):665–71. Available from: https://biomedpharmajournal.org/vol13no2/effective-dose-and-safety-profile-of-purple-sweet-potato-tablet-preparation-in-rats-with-high-cholesterol-diet/
24. Subawa AAN, Yasa IWPS, Jawi IM, Mahendra AN. Antioxidant and hypolipidemic effects of Ipomoea batatas L and Pandanus conoideus Lam combination on rats fed with high cholesterol diet. Open Access Maced J Med Sci [Internet]. 2021 Jul 10;9(A):473–6. Available from: https://oamjms.eu/index.php/mjms/article/view/6390
25. Widhiantara IG, Permatasari AAAP, Rosiana IW, Wiradana PA, Widiastini LP, Jawi IM. Antihypercholesterolemic and antioxidant effects of Blumea balsamifera L. leaf extracts to maintain luteinizing hormone secretion in rats induced by high-cholesterol diets. Indones Biomed J [Internet]. 2021 Dec 31;13(4):396–402. Available from: https://inabj.org/index.php/ibj/article/view/1694
26. Krochmal-Marczak B, Zagórska-Dziok M, Michalak M, Kie?tyka-Dadasiewicz A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J King Saud Univ Sci [Internet]. 2021 Sep;33(6):101532. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1018364721001932
27. Widhiantara IG, Putri Permatasari AAA, Rosiana IW, Sari NKY, Sudyadnyana IMGS, Wiradana PA, et al. The role of biopolymers as candidates for promoting health agents: a review. J Appl Pharm Sci [Internet]. 2022;13(1):001–14. Available from: https://japsonline.com/abstract.php?article_id=3819&sts=2
28. Widhiantara IG, Putri Permatasari AAA, Sutirta Yasa IWP. Spermatogenic and leydig cells induced hyperlipidemia: a review. Res J Pharm Technol [Internet]. 2021 Oct 31;14(10):5573–8. Available from: https://rjptonline.org/AbstractView.aspx?PID=2021-14-10-89
29. Oksal E, Pangestika I, Muhammad TST, Mohamad H, Amir H, Kassim MNI, et al. In vitro and in vivo studies of nanoparticles of chitosan-Pandanus tectorius fruit extract as new alternative treatment for hypercholesterolemia via scavenger receptor class B type 1 pathway. Saudi Pharm J [Internet]. 2020 Oct;28(10):1263–75. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1319016420301973
30. Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Circ Physiol [Internet]. 2020 Sep 1;319(3):H613–31. Available from: https://journals.physiology.org/doi/10.1152/ajpheart.00220.2020
31. Ju R, Zheng S, Luo H, Wang C, Duan L, Sheng Y, et al. Purple sweet potato attenuate weight gain in high fat diet induced obese mice. J Food Sci [Internet]. 2017 Mar;82(3):787–93. Available from: https://onlinelibrary.wiley.com/doi/10.1111/1750-3841.13617
32. Jiang T, Shuai X, Li J, Yang N, Deng L, Li S, et al. Protein-bound anthocyanin compounds of purple sweet potato ameliorate hyperglycemia by regulating hepatic glucose metabolism in high-fat diet/streptozotocin-induced diabetic mice. J Agric Food Chem [Internet]. 2020 Feb 12;68(6):1596–608. Available from: https://pubs.acs.org/doi/10.1021/acs.jafc.9b06916
33. Osaretin AE, Bukola CA. Orlistat and garlic curtailed antioxidant enzymes’ activities and ameliorated high fat diet induced obese Sprague-Dawley rats. Int J Biol Med Res. 2016;7(3):5635–9.
34. Nazish I, Ansari SH, Arora P, Ahmad A. Antiobesity activity of Zingiber officinale. Pharmacogn J [Internet]. 2016 Jul 1;8(5):440–6. Available from: http://phcogj.com/article/188
35. Adegbola PI, Fadahunsi OS, Ajilore BS, Akintola AO, Olorunnisola OS. Combined ginger and garlic extract improves serum lipid profile, oxidative stress markers and reduced IL-6 in diet induced obese rats. Obes Med [Internet]. 2021 May;23:100336. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2451847621000191
36. Gu X, Zhu YZ. Therapeutic applications of organosulfur compounds as novel hydrogen sulfide donors and/or mediators. Expert Rev Clin Pharmacol [Internet]. 2011 Jan 10;4(1):123–33. Available from: http://www.tandfonline.com/doi/full/10.1586/ecp.10.129
37. Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev [Internet]. 2019 Jul 1;2019:1–32. Available from: https://www.hindawi.com/journals/omcl/2019/8563845/
38. Sun J, Wang Z, Chen L, Sun G. Hypolipidemic effects and preliminary mechanism of Chrysanthemum flavonoids, its main components luteolin and luteoloside in hyperlipidemia rats. Antioxidants [Internet]. 2021 Aug 20;10(8):1309. Available from: https://www.mdpi.com/2076-3921/10/8/1309
39. März W, Kleber ME, Scharnagl H, Speer T, Zewinger S, Ritsch A, et al. HDL cholesterol: reappraisal of its clinical relevance. Clin Res Cardiol [Internet]. 2017 Sep 24;106(9):663–75. Available from: http://link.springer.com/10.1007/s00392-017-1106-1
40. von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J [Internet]. 2022 Nov 7;44(16):1394–407. Available from: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehac605/6807326
41. Jomard A, Osto E. High density lipoproteins: metabolism, function, and therapeutic potential. Front Cardiovasc Med [Internet]. 2020 Mar 31;7:39. Available from: https://www.frontiersin.org/article/10.3389/fcvm.2020.00039/full
42. Takahashi Y, Konishi T, Yamaki K. Tofu and fish oil independently modulate serum lipid profiles in rats: analyses of 10 class lipoprotein profiles and the global hepatic transcriptome. PLoS One [Internet]. 2019 Jan 17;14(1):e0210950. Available from: https://dx.plos.org/10.1371/journal.pone.0210950
43. Boesch-Saadatmandi C, Niering J, Minihane AM, Wiswedel I, Gardeman A, Wolffram S, et al. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis [Internet]. 2010 Jul;211(1):110–3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0021915010001681
44. Ockermann P, Headley L, Lizio R, Hansmann J. A review of the properties of anthocyanins and their influence on factors affecting cardiometabolic and cognitive health. Nutrients [Internet]. 2021 Aug 18;13(8):2831. Available from: https://www.mdpi.com/2072-6643/13/8/2831
45. Sapian S, Taib IS, Katas H, Latip J, Zainalabidin S, Hamid ZA, et al. The role of anthocyanin in modulating diabetic cardiovascular disease and its potential to be developed as a nutraceutical. Pharmaceuticals [Internet]. 2022 Oct 30;15(11):1344. Available from: https://www.mdpi.com/1424-8247/15/11/1344
46. Araki R, Yada A, Ueda H, Tominaga K, Isoda H. Differences in the effects of anthocyanin supplementation on glucose and lipid metabolism according to the structure of the main anthocyanin: a meta-analysis of randomized controlled trials. Nutrients [Internet]. 2021 Jun 10;13(6):2003. Available from: https://www.mdpi.com/2072-6643/13/6/2003
47. Vallance TM, Ravishankar D, Albadawi DAI, Osborn HMI, Vaiyapuri S. Synthetic flavonoids as novel modulators of platelet function and thrombosis. Int J Mol Sci [Internet]. 2019 Jun 25;20(12):3106. Available from: https://www.mdpi.com/1422-0067/20/12/3106
48. Fu Y, Jia Y, Sun Y, Liu X, Yi J, Cai S. Dietary flavonoids alleviate inflammation and vascular endothelial barrier dysfunction induced by advanced glycation end products in vitro. Nutrients [Internet]. 2022 Feb 28;14(5):1026. Available from: https://www.mdpi.com/2072-6643/14/5/1026
49. Sandoval V, Sanz-Lamora H, Arias G, Marrero PF, Haro D, Relat J. Metabolic impact of flavonoids consumption in obesity: from central to peripheral. Nutrients [Internet]. 2020 Aug 10;12(8):2393. Available from: https://www.mdpi.com/2072-6643/12/8/2393
50. Neri-Numa IA, Cazarin CBB, Ruiz ALTG, Paulino BN, Molina G, Pastore GM. Targeting flavonoids on modulation of metabolic syndrome. J Funct Foods [Internet]. 2020 Oct;73:104132. Available from: https://linkinghub.elsevier.com/retrieve/pii/S175646462030356X
51. Watiniasih NL, Budiarsa IN, Antara ING, Wiradana PA. Propolis extract as a green bacterial corrosion inhibitor on three types of metals. Biodiversitas J Biol Divers [Internet]. 2022 Sep 23;23(9):4852–60. Available from: https://smujo.id/biodiv/article/view/11763
52. Yustiantara PS, Warditiani NK, Sari PMNA, Dewi NLKAA, Ramona Y, Jawi IM, et al. Determination of TLC fingerprint biomarker of Ipomoea batatas (L.) Lam leaves extracted with ethanol and its potential as antihyperglycemic agent. Pharmacia [Internet]. 2021 Dec 10;68(4):907–17. Available from: https://pharmacia.pensoft.net/article/71334/
53. Fiorelli S, Cosentino N, Porro B, Fabbiocchi F, Niccoli G, Fracassi F, et al. Netrin-1 in atherosclerosis: relationship between human macrophage intracellular levels and in vivo plaque morphology. Biomedicines [Internet]. 2021 Feb 8;9(2):168. Available from: https://www.mdpi.com/2227-9059/9/2/168
54. Claro V, Ferro A. Netrin-1: focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc Dis [Internet]. 2020 Jan 25;9:204800402095957. Available from: http://journals.sagepub.com/doi/10.1177/2048004020959574
55. Kang H, Li X, Xiong K, Song Z, Tian J, Wen Y, et al. The entry and egress of monocytes in atherosclerosis: a biochemical and biomechanical driven process. Cardiovasc Ther [Internet]. 2021 Jul 8;2021:1–17. Available from: https://www.hindawi.com/journals/cdtp/2021/6642927/
56. Husain MA, Laurent B, Plourde M. APOE and Alzheimer’s disease: from lipid transport to physiopathology and therapeutics. Front Neurosci [Internet]. 2021 Feb 17;15:630502. Available from: https://www.frontiersin.org/articles/10.3389/fnins.2021.630502/full
57. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J [Internet]. 2020 Jun 21;41(24):2313–30. Available from: https://academic.oup.com/eurheartj/article/41/24/2313/5735221
58. Sharma C, Kim SR. Linking oxidative stress and proteinopathy in Alzheimer’s disease. Antioxidants [Internet]. 2021 Jul 30;10(8):1231. Available from: https://www.mdpi.com/2076-3921/10/8/1231
59. Morihara N, Hino A, Miki S, Takashima M, Suzuki J. Aged garlic extract suppresses inflammation in apolipoprotein E-knockout mice. Mol Nutr Food Res [Internet]. 2017 Oct;61(10):1700308. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.201700308